Abstract
For more than a decade, the high threshold dual process (HTDP) model has served as a guide for studying the functional neuroanatomy of recognition memory. The HTDP model's utility has been that it provides quantitative estimates of recollection and familiarity, two processes thought to support recognition ability. Important support for the model has been the observation that it fits experimental data well. The continuous dual process (CDP) model also fits experimental data well. However, this model does not provide quantitative estimates of recollection and familiarity, making it less immediately useful for illuminating the functional neuroanatomy of recognition memory. These two models are incompatible and cannot both be correct, and an alternative method of model comparison is needed. We tested for systematic errors in each model's ability to fit recognition memory data from four independent data sets from three different laboratories. Across participants and across data sets, the HTDP model (but not the CDP model) exhibited systematic error. In addition, the pattern of errors exhibited by the HTDP model was predicted by the CDP model. The findings were the same at both the group and individual levels of analysis. We conclude that the CDP model provides a better account of recognition memory than the HTDP model.
1. Introduction
Dual-process theorists hold that recognition memory depends on two components: familiarity and recollection. Familiarity involves knowing only that an item is old or new, and recollection involves accessing specific details about the episode in which the item was encountered. The relative contribution of these two processes to individual recognition decisions is debated. On one hand, the recognition decision for a particular item may be based on one process or the other, varying from one decision to the next. On the other, the recognition decision for a particular item may be based on both familiarity and recollection. These possibilities are formalized in two models that have been used to characterize recognition memory function, the high-threshold dual-process model (HTDP; Yonelinas 1994; Yonelinas, 1999) and the continuous dual-process model (CDP; Wixted & Mickes, 2010). In many cases, the CDP model is mathematically equivalent to the single process unequal variance signal detection (UVSD) model (Wixted & Mickes, 2010). However, because of the large body of evidence indicating the existence of separate processes in recognition memory (Diana, Reder, & Arndt, 2006), we focus on the dual process interpretation of the UVSD model (namely, the CDP model).
The HTDP model provides quantitative estimates of familiarity and recollection from confidence ratings made on a standard old/new recognition task, but the CDP model holds that recollection and familiarity cannot be disentangled on the basis of old/new recognition decisions alone. The HTDP model's ability to quantify recollection and familiarity may explain the notable role it has played in guiding investigations of the neural basis of recognition memory. However, it is important to consider that the HTDP model's ability to make these estimates and the CDP model's corresponding inability are derived from the assumptions made by the two models about recognition. If the assumptions that a model makes about recognition memory are accurate, then, when it is fit to recognition data, the only source of error in the fit should be randomly distributed noise. However, if the assumptions that a model makes about recognition memory are inaccurate, then errors in the model's ability to fit data are likely to be systematic (even if the model provides a good fit to the data). Here, we investigate whether the HTDP model or the CDP model produces systematic errors, that is, deviations from what is observed in recognition memory data.
The assumptions of the HTDP model differ from the CDP model in two important respects. First, the HTDP model assumes that recollection is a high-threshold process (Yonelinas, 1994; Yonelinas, 1999; Macmillan & Creelman, 2005), such that recollection is either successful (yielding recognition decisions made with high confidence and high accuracy) or unsuccessful. The CDP model (Wixted & Stretch, 2004; Wixted, 2007; Wixted & Mickes, 2010), by contrast, assumes that recollection can vary continuously (yielding recognition decisions made with a wide range of confidence and accuracy).
A second difference between the two models follows from the HTDP model's assumption that recollection is a high-threshold process. The HTDP model predicts that if recollection is successful, then familiarity does not contribute to the recognition decision because recollection provides unambiguous evidence of a previous encounter. If recollection is unsuccessful, then the recognition decision is based wholly on the strength of the familiarity signal. By contrast, the CDP model posits that familiarity and recollection are combined during recognition memory decision-making. This feature of the model arises from the proposition that both recollection and familiarity are assumed to be imperfect continuous processes, and combining them can yield a more diagnostic memory signal than relying on either one alone.
A number of studies have compared the CDP model to the HTDP model using receiver operating characteristic (ROC) analysis, a technique based on confidence ratings that allows model-based inferences about the nature of the underlying memory-strength distributions across items (Macmillan & Creelman, 2005). The validity of model-based inferences is typically assessed in ROC analysis by comparing a model's fit to the observed data in order to calculate a goodness-of-fit statistic. Although there have been many studies (e.g. Yonelinas, 1994; Glanzer, Hilford, Kim, & Adams, 1999; Healy, Light, & Chung, 2005; Heathcote, 2003; Glanzer, Hilford, & Kim, 2004; Kelley & Wixted, 2001; Slotnick & Dodson, 2005), the evidence based on goodness-of-fit statistics alone has been mixed, with some studies favoring the HTDP model and some favoring the CDP model. The CDP model often provides the better fit to typical recognition memory data (e.g. Slotnick & Dodson, 2005; for review see Wixted, 2007), but some results are better accounted for by the HTDP model (e.g. Yonelinas, 1999; Howard, Bessette-Symons, Zhang, & Hoyer, 2006).
One reason for these inconclusive results may be that both models are able to fit recognition memory data quite well. Indeed, an earlier analysis of a typical data set found that the HTDP model accounted for 99.91% of the variance, and the CDP model accounted for 99.97% of the variance (Glanzer et al., 1999; Yonelinas, 1999b). Similarly, across four data sets analyzed below, which involved 65 participants, the average percent of variance accounted for was above 90% for both models (HTDP = 91%, CDP = 96%). The fact that both models fit the data well may explain why both are given credence despite their fundamental differences.
The assumption is often made that models that fit data well are good models. However, this assumption is not necessarily valid (Roberts & Pashler, 2000). Accordingly, model comparisons based on goodness-of-fit may have difficulty deciding which model is best. An alternative, more promising, way to distinguish between the merits of the two models is to first ask whether the models generate systematic errors in their ability to account for recognition memory data. Second, if a model generates systematic errors, then one can ask whether the other model, in fact, predicts these errors.
Figure 1 illustrates the essential differences between the two models. The models make the same assumptions about the distractor distribution (i.e., the distribution of memory strength signals generated by the foils), but they differ in their assumptions about the target distribution (i.e., the distribution of memory strength signals generated by the targets). The HTDP model has two target distributions, a high-threshold distribution for items that are recollected (these targets have essentially infinite memory strength) and a separate continuous distribution for items judged on the basis of familiarity. The familiarity and distractor distributions are assumed to have equal variance. In contrast, the CDP model has a single target distribution, and that distribution has greater variance than the distractor distribution.
Figure 1.
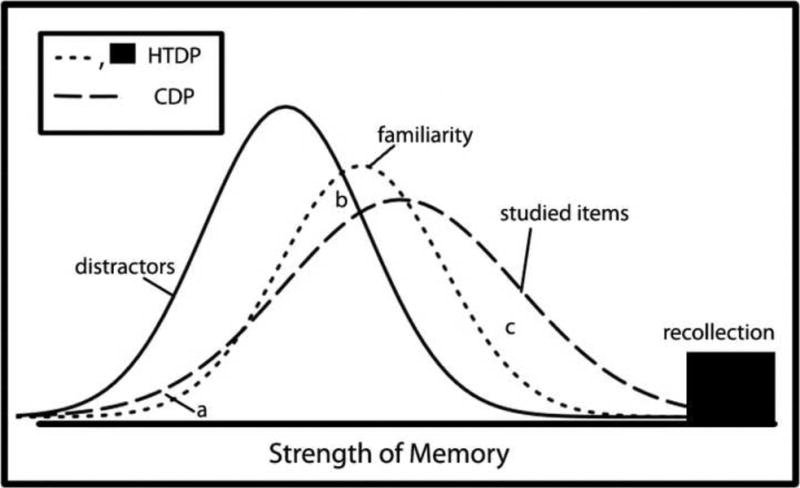
Schematic representation of the theoretical distributions of items in memory according to both the high threshold dual process model (HTDP; dotted lines) and the continuous dual process model (CDP; dashed lines). The two models share a distribution for new items (distractors; solid line). The HTDP model has separate distributions for study items supported by recollection and familiarity. The CDP model has a single distribution for study items. The X-axis represents the strength of memory, proceeding from low memory strength at the left to high memory strength at the right. Areas a, b, and c show areas of non-overlap between the two models where the predicted data differ systematically.
Moving from low memory strength (Figure 1; left) to high memory strength (Figure 1; right), visual inspection of the models' target distributions reveals areas where the models do not overlap and where the predicted data differ systematically. At low levels of memory strength (area a), the HTDP model predicts a lower frequency of target items than does the CDP model. At medium levels of memory strength (b), the HTDP model predicts a higher frequency of target items than does the CDP model. At moderately high levels of memory strength (c), the HTDP model predicts a lower frequency of target items than does the CDP model. Lastly, at the highest levels of memory strength (represented in the HTDP model by the distribution of recollection responses and in the CDP model by the rightmost tail of the target distribution), the HTDP model predicts a higher frequency of target items than does the CDP model. Thus, if the assumptions of the HTDP model are correct, then one might expect to find that the best-fitting CDP model predicts too many low-confidence responses to targets, too few medium confidence responses to targets, and too many moderately high confidence ratings to targets. If, instead, the assumptions of the CDP model are correct, then the best-fitting HTDP model should exhibit the opposite pattern of systematic error.
Note that Figure 1 is simply an example illustrating systematic errors that might be observed for a particular set of model parameter values. We chose these parameter values because they correspond to values typically observed in recognition memory experiments. Still, the actual systematic errors could differ across individuals depending on the model parameters that characterize the performance of each individual.
To differentiate between the HTDP and CDP models, we first examined the ability of each model to fit recognition memory data in four data sets from three different laboratories and then investigated whether any systematic errors were evident in their fits to target items. Lure items were also examined but yielded no systematic errors for either model. We then tested whether the systematic errors generated by one model (if any) could be predicted by the other model. We performed this analysis at the individual level (i.e., fitting the two models to each individual's data separately, and generating predictions of systematic error based on each participant's performance individually). We found that only the HTDP model generated systematic errors in its fit to target items. Moreover these errors were predicted by the CDP model. By contrast, the CDP model did not yield systematic errors, and the HTDP model predicted errors for the CDP model that were not observed. In other words, the predictions of the CDP model were confirmed (validating its assumptions about recognition memory), whereas the predictions of the HTDP model were disconfirmed (invalidating its assumptions about recognition memory). This pattern was observed even though both models fit the data well (as is usually true), underscoring the fact that a good fit does not necessarily imply a valid model (Roberts & Pashler, 2000).
2. Methods
2.1 Data
Four data sets were used involving 65 participants, 32 of whom were tested under two different conditions. All data sets were collected using similar word recognition memory tests. Participants were asked in each case to rate their confidence that a word had been previously presented from 1 (sure new) to 6 (sure old). These data sets were selected because they are based on sufficient data to allow for individual model fitting and because the methods were comparable. The data represent results from a laboratory that has generally supported the CDP model (Dede, Wixted, Hopkins & Squire, 2013), a laboratory that has generally supported the HTDP model (Koen & Yonelinas, 2010), and a neutral laboratory (Van Zandt, 2000).
Dede, Wixted, Hopkins and Squire (2013)
Participants were five memory-impaired patients with bilateral lesions limited to the hippocampus. Eleven age and education-matched controls were also tested. Participants were given three tests of recognition memory. In each test, participants were presented with 50 study words and asked to make pleasantness ratings. After a 3-5 minute delay, participants were presented with 100 test words (50 new words and 50 old words). An additional group of seven age and education-matched controls were tested using an identical procedure but with the delay interval between study and test extended to one week.
Koen and Yonelinas(2010)
Thirty-two undergraduate participants were presented with a mixed list of 80 words presented for four seconds and 80 words presented for one second. Immediately afterwards, participants were presented with 320 test words (160 old words and 160 new words). The data were analyzed as two separate sets, one based on the 80 study words presented for four seconds (plus 160 new words), and the other based on the 80 study words presented for one second (plus 160 new words).
Van Zandt(2000)
Ten undergraduate participants were presented with 32 study words. Immediately afterwards, participants were presented with 20 of the study words and 20 new words. This procedure was repeated a total of 20 times, using different lists.
2.2 Analysis of Systematic Error
First, we fit the HTDP and CDP models to the data sets from the three laboratories to determine whether either model yielded a pattern of systematic error. All data were fit with both the HTDP and CDP models at the individual subject level using maximum likelihood estimation. These fits yielded predictions of the frequency with which a participant used each confidence-level response (1-6) for the study items. The observed frequencies of different confidence ratings to target items were then subtracted from the corresponding predicted frequencies derived from the model fits to calculate errors in each model's predictions. If errors for a particular confidence rating are random and non-systematic, then they should have a mean of zero across participants. If errors are systematic, then they should deviate systematically from zero. To test for such systematic error, a series of one-sample t-tests determined whether there was significant non-zero error at each confidence level within each of the four data sets. Errors were deemed systematic if they were identified as significant in all four data sets (Dede et al., 2013; Koen & Yonelinas, 2010, 4-sec condition; Koen & Yonelinas, 2010, 1-sec condition; Van Zandt, 2000).
2.3 Individual Prediction Analysis
In this analysis, we created predictions of model error that were based on each participant's performance. This analysis was computationally similar to the parametric bootstrap analysis used by Wagenmakers et al. (2004) to assess model mimicry. To understand this analysis conceptually, consider the systematic errors that are produced when the HTDP model is fit to real data. If the same systematic errors are generated when the HTDP model is fit to data generated by the CDP model in simulation, then the inference can be made that the CDP model, having accurately predicted the HTDP model's error, is likely to accurately reflect the phenomenon that produced the real data. Six steps were applied to each participant individually. Step 1. 500 non-parametric bootstrap samples were taken. Step 2. These samples were fit with both the HTDP and CDP models using maximum likelihood estimation. The average error generated in these fits across the 500 bootstrap samples was used to measure systematic error. Step 3. Using the parameters obtained in Step 2, simulated data were created by both the HTDP and CDP model simulators described in Section A.1. This step yielded 500 simulated data sets for each model. Step 4. The HTDP model was fit to the CDP model simulation data, and the CDP model was fit to the HTDP model simulation data. Step 5. The fits from Step 4 were used to derive an error prediction at each confidence level for each model. The error prediction was the mean error value for each confidence rating, as predicted by each model individually across the 500 simulated data sets. Step 6. The predicted error values for each model's fit were correlated with the observed error values in each individual's data. This was done in two ways. The predicted error values were correlated with the observed error values found when each model was directly fit to the original data and with the mean error values found when each model was fit to the non-parametric bootstrapped data (Step 2). The bootstrapping procedure was used to obtain a pattern of observed systematic error that was more robust to noise. The histograms of the correlation values across participants were plotted for visual inspection, and the correlation distribution produced by each model was compared to 0 using one-sample t-tests (see Section A.2 for a detailed example based on an individual participant and Section A.3 for further analyses of model flexibility).
3. Results
The first objective was to identify systematic errors in the fits of each model to recognition memory data. Accordingly, we fit both models to four sets of data from three studies of recognition memory (Dede et al., 2013; Koen & Yonelinas, 2010; Van Zandt, 2000). Fits were performed using maximum likelihood estimation on an individual participant basis (see Methods). For the data from Dede et al. (2013), there were no significant differences in the error patterns across groups, so data from the different groups were combined (patients, controls tested with no delay, controls tested with a one-week delay).
3.1 The HTDP model but not the CDP model generated systematic error
Figure 2A shows the pattern of error when the recognition memory data were fit with the HTDP model. Figure 2B shows the pattern of error when the same data were fit with the CDP model. The four sets of data indicate that the HTDP model consistently underestimated the frequency of low memory strength responses to target items (i.e., confidence ratings of 1 on the 6-point scale), consistently overestimated the frequency of medium-strength responses to target items (confidence ratings of 3), and consistently underestimated the frequency of high-strength responses to target items (confidence ratings of 5)(Figure 2A). There was no trend towards systematic error in the fit of the CDP model, and no instance where all four data sets identified a significant error (Figure 2B).
Figure 2.
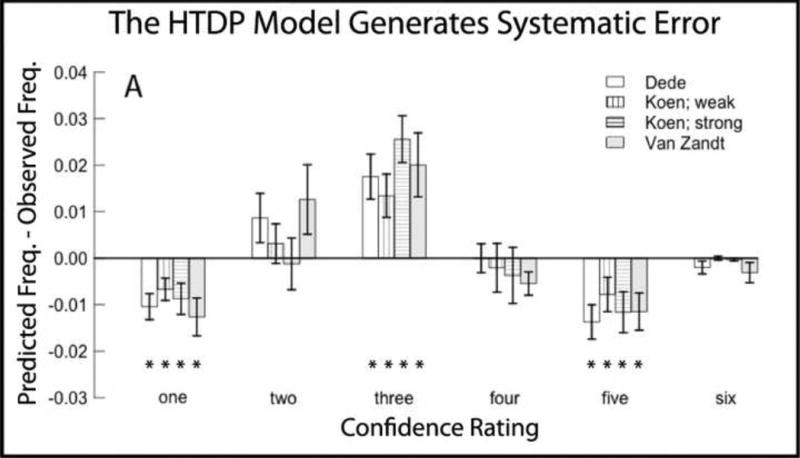
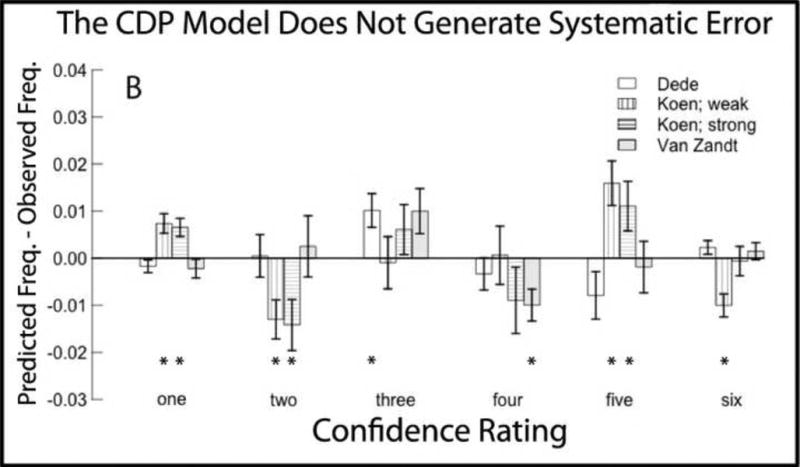
Errors in the fits of the high threshold dual process model (HTDP) and the continuous dual process (CDP) to study items from four sets of data. A. The HTDP model consistently underestimated the frequency of 1 and 5 responses and consistently overestimated the frequency of 3 responses. B. For the CDP model there was no instance where all four data sets identified a systematic error. Error bars indicate SEM. * denotes p<.05 in single sample t-tests compared to zero.
The systematic errors generated by the HTDP model suggest that the HTDP model did not accurately describe how responses of different memory strength would be distributed in tests of recognition memory. Note that the errors generated by the HTDP model were the same errors predicted from Figure 1, as outlined in Section 1. That is, the HTDP model generated the errors indicated by areas a, b, and c in Figure 1.
3.2 The CDP model predicted the errors that were generated by the HTDP model at an individual level
Before presenting the results of this analysis, it is useful to explain the logic of our technique. Consider models A and B. When model A generates simulated data and model B is fit to that simulated data, there will be a certain pattern of systematic errors in the fit of model B. This pattern will reflect the differences between models A and B and can be thought of as the pattern of predicted errors in the fit of model B. Most importantly, the predicted error pattern in the fit of model B is conditional on model A producing the data. Turning to the fitting of real data, it is unknown which model best approximates the phenomenon under study, but if the predicted pattern of error in the fit of model B is similar for real data and for data simulated by model A then model A likely reflects the phenomenon that produced the real data. This entire process and logic can be reversed to provide predictions of the errors in the fit of model A when model B produces the data.
For each participant, we correlated the pattern of error that was generated when each model was fit to the data with the pattern of error that was generated when each model was fit to data simulated by the other model. We also correlated the average pattern of error that was generated when each model was fit to a set of 500 non-parametric bootstrapped samples with the pattern of error that was generated when each model was fit to data simulated by the other model. This analysis resulted in two sets (one based on fits to raw data and one based on fits to bootstrapped data) of 97 correlations for each model (65 participants, 32 of whom were tested in two different conditions), based on the frequency of ratings at each confidence level (1-6). Figure 3A shows the distribution of correlations (one correlation for each participant) between the errors generated by fitting the HTDP model to the data and the CDP model's prediction of errors. The average correlation was .44, a value greater than zero (t(96) = 9.5, p<.001). When this analysis was based on bootstrapped error patterns, which should be less susceptible to noise, the average correlation value increased to .54 (t(96) = 12.9, p<.001). Thus, the CDP model predicted the (systematic) errors made by the HTDP model when the HTDP model was fit to individual data. Figure 3B shows the corresponding distribution of correlations between the errors generated by fitting the CDP model to the data and the HTDP model's prediction of errors. The average correlation was .02, which was not different from zero (t(96)=.3, p=.75). When this analysis was based on bootstrapped error patterns, the average correlation increased to .08 (t(96)=1.2, p=.22), a smaller increase than was seen for the CDP model. Thus, the HTDP model did not predict the (nonsystematic) errors made by the CDP model when the CDP model was fit to individual data.
Figure 3.
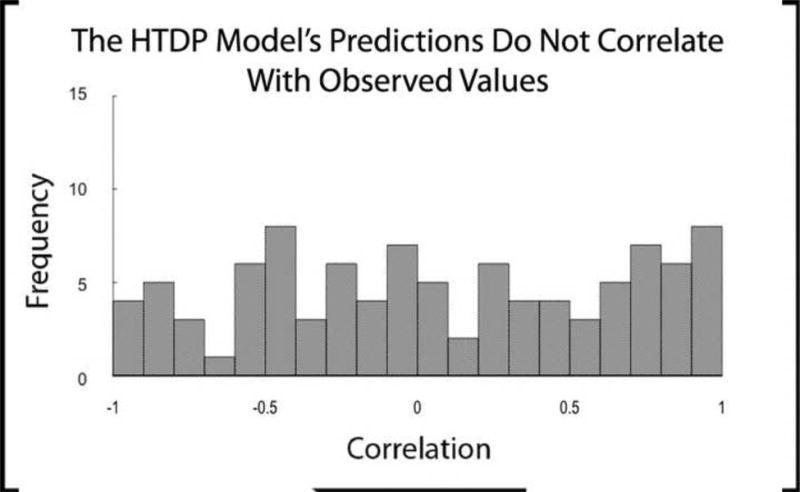
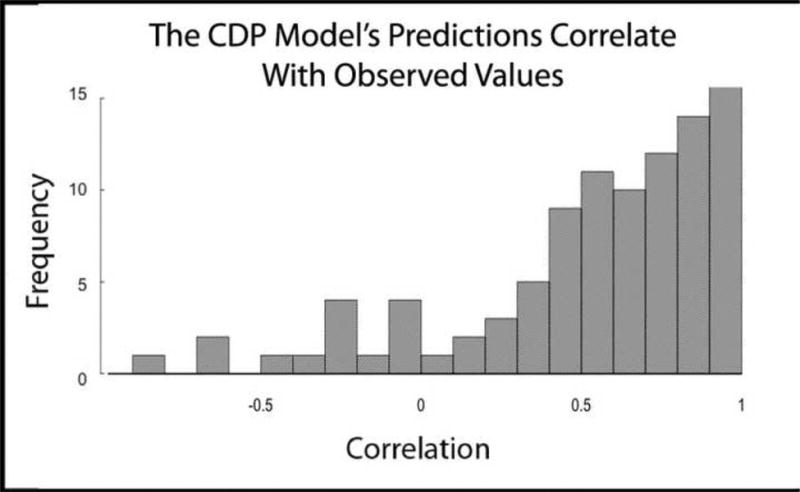
Distribution of correlation values (one value for each of 97 participants) between the errors generated by fitting one model to the data and the errors predicted by the other model. A. The CDP model's predictions of error are well correlated with the errors generated by the HTDP model. B. The HTDP model's predictions of error are not well correlated with the errors generated by the CDP model.
4. Discussion
Taking a novel approach to an old problem, we have found support for the CDP model and evidence against the HTDP model. In our first analysis (Figure 2), the HTDP model exhibited systematic errors in its ability to predict the frequency of different confidence responses to target items (despite providing a good fit to the data, which is often taken as evidence of its validity). If the HTDP model accurately accounted for recognition memory, then errors in the predictions made by the best-fitting version of the model for each level of confidence should have been randomly distributed. Instead, the errors were systematic. These systematic errors imply that the HTDP model's assumptions about recognition memory are inaccurate.
By contrast, the CDP model did not exhibit systematic errors. Yet the absence of systematic error alone does not confirm the accuracy of the assumptions about recognition memory that underlie the CDP model. Accordingly, we next asked whether the CDP model could predict the errors generated by the HTDP model. This analysis was performed at the individual level and demonstrated that the CDP model not only fits the data without systematic error but also predicts the systematic errors evident in the fits provided by the HTDP model (Figure 3A). A second analysis (Figure 3B) demonstrated that the HTDP model did not predict the (nonsystematic) errors evident in the fits provided by the CDP model. Taken together, these results suggest that the CDP model accurately accounts for recognition memory decision making and that the HTDP model does not.
There were two potential concerns about our analyses that are worth drawing attention to. First, in order to generate error predictions, the CDP model was always fit to data simulated by the HTDP model, and the HTDP model was always fit to data simulated by the CDP model. Our assumption was that neither model would predict errors in its own fit to the data (because those errors would be random). We tested this assumption by fitting the CDP model to data simulated by the CDP model and by fitting HTDP model to data simulated by the HTDP model. This analysis yielded no systematic errors, confirming our assumption.
Second, the CDP model is known to be slightly more flexible than the HTDP model, and it was unknown what effect this would have on our analyses of systematic error. We addressed this issue in an analysis presented in Section A.3 and found that model flexibility did not play a role in our results.
Within the discipline of cognitive psychology, reservations about the validity of the HTDP model have been expressed by many different researchers (e.g. Slotnick & Dodson, 2005; Qin, Raye, Johnson, & Mitchell, 2001; Glanzer, et al., 1999; Rotello, et al., 2005; Heathcote, 2003; Qin, et al., 2001; Healy, Light, & Chung, 2005; Starns, Rotello, & Ratcliff, 2012; Starns, Ratcliff, & McKoon, 2012). Thus, it is of interest to ask why the HTDP model has nevertheless held favor in guiding research into the neural substrates of recognition memory. An important consideration is that both the HTDP and CDP models virtually always fit experimental data well. Further, investigators often interpret a good fit to imply that a model is valid even though that is not a safe assumption (Roberts & Paschler, 2000). But if one does assume that both models are valid because they fit the data well, there is a choice to be made. On the one hand, the CDP model does not provide a simple way to differentiate between familiarity and recollection on the basis of old/new decisions alone. On the other hand, the HTDP model does. Assuming that a good fit implies a good model, and if the goal is to identify neural substrates of recollection and familiarity, the choice is straightforward: the HTDP model is the one to use.
Yet, considering the fundamentally different ideas about recollection inherent in the HTDP and CDP models, it should be clear that both models cannot be correct. The analyses presented here demonstrate that the CDP model is viable, but that the HTDP model is not (despite the fact that the HTDP model fits the data well). In light of the evidence presented here against the HTDP model, it would make sense to use the CDP model to guide studies of recognition memory (at least when words are used as stimuli, as they often are). It would also make sense to reconsider conclusions about the neuroanatomy of recognition memory that depend on the validity of the HTDP model.
Studies of recognition memory have commonly used fMRI and lesion studies to identify structures important for recollection and familiarity. Many of these studies have relied upon the assumptions of the HTDP model for interpreting the data (e.g. Yonelinas et al., 2002; Aggleton et al., 2005; Ranganath et al., 2004; Yonelinas, et al., 2005; for review see Eichenbaum, Yonelinas, & Ranganath, 2007). These studies have led to the idea that the hippocampus is important for recollection and that the surrounding medial temporal lobe (MTL) cortices are important for familiarity. To reach this conclusion, researchers have had to separate test trials based on recollection from test trials based on familiarity (e.g., in order to compare hippocampal activity for recollection-based vs. familiarity-based decisions), and the assumptions of the HTDP model have been relied upon for this purpose. Studies using the Remember-Know procedure, source memory procedures, and/or confidence rating procedures have all been implicitly or explicitly guided by the HTDP view of recollection. However, if the CDP model is correct (and the HTDP model is incorrect), then all of these studies share a common flaw in that trials assumed to differ only in whether recollection is present or absent also differ in memory strength (strong versus weak; Slotnick & Dodson, 2005; Wixted, 2007; Squire, Clark, & Wixted, 2007).
Unlike the HTDP model, the CDP model does not guide inquiry into the neural basis of recognition memory by providing quantitative estimates of recollection and familiarity. Instead, it suggests novel experimental designs that can be used to test whether (for example) the hippocampus plays a role in recollection and familiarity. A key idea suggested by this model is that it is important to control for memory strength because decisions thought to be based on recollection (e.g., Remember judgments) are typically made with higher confidence and higher accuracy than decisions thought to be based on familiarity (e.g., Know judgments). A difference in memory is not the essence of the theoretical difference between recollection and familiarity. Indeed, recollection can be weak and familiarity can be strong (Ingram, Mickes, & Wixted, 2012). Thus, memory strength is an experimental confound that should be controlled when comparing the two processes. For example, in one study that controlled for memory strength, the hippocampus was active when responses were based on recollection as well as when responses were based on familiarity (Smith et al., 2011). This result does not mean that the hippocampus and surrounding MTL structures provide only an undifferentiated signal of strength. Despite not being informed by the distinction between recollection and familiarity, the different structures of the MTL likely play different roles (Wixted & Squire, 2011). Indeed, a recent study used state-trace analysis, combined with intracranial depth electrode recording, to demonstrate that the hippocampus and perirhinal cortex perform fundamentally different computations (Staresina et al., 2013). For further discussion concerning this issue, see Wixted & Squire, 2011a; Diana & Ranganath, 2011; Montaldi & Mayes, 2011; Wixted & Squire, 2011b; Wixted & Squire, 2011c.
In summary, we have found that the HTDP model does not accurately characterize recognition memory. Although the HTDP model can fit recognition memory data reasonably well, the relatively small errors it makes are systematic in nature. By contrast, the CDP model did not make systematic errors and also accurately predicted the systematic errors generated by the HTDP model. These findings suggest that the assumptions about recollection and familiarity that are underlie the CDP model (e.g., the assumption that recollection is a continuous process) are more accurate than the assumptions that underlie the HTDP model (i.e., e.g., the assumption that recollection is a threshold process). The key implication of these results is that the search for the neuroanatomical basis of recollection and familiarity should not be wedded to theoretical assumptions that are inconsistent with the empirical evidence.
Supplementary Material
Highlights.
Fundamentally different models cannot always be distinguished by their goodness of fit
The high threshold (HTDP) model of recognition memory generates systematic error
The continuous dual process (CDP) model does not generate systematic error
The CDP model predicts the errors generated by the HTDP model
Studies of recognition memory would be better guided by the CDP model
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adam J.O. Dede, Department of Psychology, University of California, San Diego, CA 92093; Veterans Affairs San Diego Healthcare system, San Diego, CA 92161
Larry R. Squire, Veterans Affairs San Diego Healthcare System, San Diego, CA 92161; Departments of Psychiatry, Neuroscience, and Psychology, University of California, San Diego, 92093
John T. Wixted, Department of Psychology, University of California, San Diego, CA 92093
References
- Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, Yonelinas AP. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43:1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Dede AJO, Wixted JT, Hopkins RO, Squire LR. Hippocampal damage impairs recognition memory broadly, affecting both parameters in two prominent models of memory. Proc Natl Acad Sci U S A. 2013;110:6577–6582. doi: 10.1073/pnas.1304739110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Reder LM, Arndt J, Park H. Models of recognition: A review of arguments in favor of a dual-process account. Psychonomic Bulletin & Review. 2006;13:1–21. doi: 10.3758/bf03193807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Ranganath C. Recollection, familiarity and memory strength: confusion about confounds. Trends in Cognitive Science. 2011;15:337–338. doi: 10.1016/j.tics.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzer M, Hilford A, Kim K. Six regularities of source recognition. J Exp Psych: L, M, and C. 2004;30:1176–1195. doi: 10.1037/0278-7393.30.6.1176. [DOI] [PubMed] [Google Scholar]
- Glanzer M, Kim K, Hilford A, Adams JK. Slope of the receiver-operating characteristic in recognition memory. J Exp Psych: L, M, and C. 1999;25:500–513. [Google Scholar]
- Heathcote A. Item recognition memory and the receiver operating characteristic. J Exp Psych: L, M, and C. 2003;29:1210–1230. doi: 10.1037/0278-7393.29.6.1210. [DOI] [PubMed] [Google Scholar]
- Healy MR, Light LL, Chung C. Dual-process models of associative recognition in young and older adults: evidence from receiver operating characteristics. J Exp Psych: L, M, and C. 2005;31:768–788. doi: 10.1037/0278-7393.31.4.768. [DOI] [PubMed] [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang YF, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures; Evidence from modeling and receiver operating characteristic curves. Psych and Aging. 2006;21:96–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram KM, Mickes L, Wixted JT. Recollection can be weak and familiarity can be strong. J Exp Psych: L, M, and C. 2012;38:325–339. doi: 10.1037/a0025483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R, Wixted JT. On the nature of associative information in recognition memory. J Exp Psych: L, M, and C. 2001;27:701–722. [PubMed] [Google Scholar]
- Koen JD, Yonelinas AP. Memory variability is due to the contribution of recollection and familiarity, not to encoding variability. J Exp Psych: L, M, and C. 2010;36:1536–1542. doi: 10.1037/a0020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user's guid. Lawrence Erlbaum Associates; Mahwah, New Jersey: 2005. [Google Scholar]
- Montaldi D, Mayes AR. Familiarity, recollection and medial temporal lobe function: an unresolved issue. Trends in Cognitive Science. 2011;15:339–340. doi: 10.1016/j.tics.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Qin JJ, Raye CL, Johson MK, Mitchell KJ. Source ROCs are (typically) curvilinear: comment on Yonelinas (1999) J Exp Psych: L, M, and C. 2001;27:1110–1115. [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Roberts S, Pashler H. How persuasive is a good fit? A comment on theory testing. Psych Rev. 2000;107:358–367. doi: 10.1037/0033-295x.107.2.358. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Macmillan NA, Reeder JA, Wong M. The remember response: subject to bias, graded, and not a process-pure indicator of recollection. Psychonomic Bulletin & Review. 2005;12:865–873. doi: 10.3758/bf03196778. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Dodson CS. Support for a continuous (single-process) model of recognition memory and source memory. Memory & Cognition. 2005;33:151–170. doi: 10.3758/bf03195305. [DOI] [PubMed] [Google Scholar]
- Smith CN, Wixted JT, Squire LR. The hippocampus supports both recollection and familiarity when memories are strong. Journal of Neuroscience. 2011;31:15693–15702. doi: 10.1523/JNEUROSCI.3438-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews Neuroscience. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Fell J, Dunn JC, Axmacher N, Henson RN. Using state-trace analysis to dissociate the functions of the human hippocampus and perirhinal cortex in recognition memory. Proc Natl Acad Sci U S A. 2013;110:3119–3124. doi: 10.1073/pnas.1215710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starns JJ, Ratcliff R, McKoon G. Evaluating the unequal-variance and dual-process explanations of zROC slopes with response time data and the diffusion model. Cognitive Psychology. 2012;64:1–34. doi: 10.1016/j.cogpsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starns JJ, Rotello CM, Ratcliff R. Mixing strong and weak targets provides no evidence against the unequal-variance explanation of zROC slope: a comment on Koen and Yonelinas (2010) Journal of Experimental Psychology: Learning, Memory, and Cognition. 2012;38:793–801. doi: 10.1037/a0027040. [DOI] [PubMed] [Google Scholar]
- Van Zandt T. ROC curves and confidence judgments in recognition memory. J Exp Psych: L, M, and C. 2000;26:582–600. doi: 10.1037//0278-7393.26.3.582. [DOI] [PubMed] [Google Scholar]
- Wagenmakers EJ, Ratcliff R, Gomez P, Iverson GJ. Assessing model mimicry using the parametric bootstrap. J Math Psych. 2004;48:28–50. [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psych Rev. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Mickes L. A continuous dual-process model of remember/know judgments. Psych Rev. 2010;117:1025–1054. doi: 10.1037/a0020874. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends in Cognitive Science. 2011;15:210–217. doi: 10.1016/j.tics.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. Confusion abounds about confounds: response to Diana and Ranganath. Trends in Cognitive Science. 2011b;15:338–339. doi: 10.1016/j.tics.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. The familiarity/recollection distinction does not illuminate medial temporal lobe function: response to Montaldi and Mayes. Trends in Cognitive Science. 2011c;15:340–341. doi: 10.1016/j.tics.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J Exp Psych: L, M, and C. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The contribution of recollection and familiarity to recognition and source-memory judgments: a formal dual-process model and an analysis of receiver operating characteristics. J Exp Psych: L, M, and C. 1999;25:1415–1534. doi: 10.1037//0278-7393.25.6.1415. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Recognition memory ROCs and the dual-process signal-detection model: Comment on Glanzer, Kim, Hilford, and Adams (1999) J Exp Psych: L, M, and C. 1999b;25:514–521. [Google Scholar]
- Yonelinas AP, Kroll NEA, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neuro. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


