Abstract
Esophageal adenocarcinoma is increasing in the US and Western countries and frequent gastresophageal reflux or gastresophageal reflux disease carrying gastric acid and bile acid could contribute to esophageal adenocarcinogenesis. This study was designed to detect the expression of gastric acid-inducing gene Na + /H + exchanger-1 (NHE-1) ex vivo and then to explore targeting of NHE-1 expression or activity to control esophageal cancer cell viability in vitro and in nude mouse xenografts. The data showed that NHE-1 was highly expressed in esophageal adenocarcinoma tissues (66 of 101 cases [65.3%], but not in normal esophageal squamous cell epithelium (1 of 26 cases [3.8%]). Knockdown of NHE-1 expression using NHE-1 shRNA or inhibition of NHE-1 activity using the NHE-1 inhibitor amiloride suppressed viability and induced apoptosis in esophageal cancer cells. Molecularly, amiloride inhibited expression of cyclooxygenase-2 and matrix metallopeptidase-9 but not NHE-1 mRNA in esophageal cancer cells. A combination of amiloride and guggulsterone (a natural bile acid receptor inhibitor) showed more than additive effects in suppressing esophageal cancer cell growth in vitro and in nude mouse xenografts. This study suggests that inhibition of NHE-1 expression or activity or combination of amiloride and guggulsterone could be useful in control of esophageal adenocarcinoma.
Keywords: esophageal cancer, NHE-1, amiloride, guggulsterone, cell viability
Introduction
Esophageal adenocarcinoma accounts for more than two-thirds of esophageal cancer cases in the United States, and its incidence is continuously increasing although esophageal squamous cell carcinoma accounts for 95% of esophageal cancer cases worldwide (Blot, 1994; Chen and Yang, 2001; Spechler, 2005). Frequent gastresophageal reflux or gastresophageal reflux disease (GERD) carrying gastric acid, bile acid, and protease could be responsible for esophageal adenocarcinoma development because this condition usually results in Barrett esophagus (a premalignant lesion) and/or esophageal adenocarcinoma (Blot, 1994; Chen and Yang, 2001; Menges et al., 2001; Spechler, 2005; Xu, 2009; Falk, 2009; Guan et al., 2013). Barrett esophagus is characterized by replacement of the squamous cell epithelium with columnar epithelium (incomplete intestinal metaplasia with or without dysplastic lesions) in the lower to middle esophagus (Fitzgerald et al., 1997, 1998; Yamada et al., 1999; Xu, 2009). Thus, studying the role of gastric acid, bile acid, and protease in the development of esophageal adenocarcinoma and targeting these risk factors could help us to prevent or delay esophageal adenocarcinogenesis.
An acidic cell environment could induce expression of sodium-hydrogen exchanger 1 (NHE-1) (Slepkov and Fliegel, 2002; Putney et al., 2002; Masereel et al., 2003), a ubiquitous and integral cell membrane protein that regulates cellular pH level. NHE-1 removes intracellular acid, exchanging a proton for an extracellular sodium ion. NHE-1 also plays a role in cell proliferation, inhibition of apoptosis, cell migration, and promotion of carcinogenesis. Expression and activity of NHE1 are mediated through response to acid, growth factors, hormones, and osmotic stress. Activation of receptor tyrosine kinases increases NHE1 activity through the Ras-mediated ERK cascade (Slepkov and Fliegel, 2002; Putney et al., 2002; Masereel et al., 2003). In esophageal cancer, expression of NHE-1 mRNA was shown to be increased from normal to Barrett esophageal and cancerous tissues (Our search of Oncomine database).
Two major classes of pharmacological agents are currently used to inhibit NHE1 activity: (1) amiloride and its 5′ alkyl-substituted derivatives and (2) the benzoylguanidine derivatives. Amiloride was reported to suppress cancer development and metastasis in the lungs, stomach, colon, and mammary glands (Sparks et al., 1983; Newell et al., 1992; Tatsuta et al., 1993, 1997; Evans et al., 1998; Evans and Sloan Stakleff 2004; Li et al., 2009). Amiloride was first approved for use in 1967 for the management of hypertension and congestive heart failure (Slepkov and Fliegel, 2002; Putney et al., 2002; Masereel et al., 2003). An effective inhibitor of NHE-1, amiloride has been used to inhibit cell growth in various tumor cell lines (Slepkov and Fliegel, 2002; Putney et al., 2002) and is usually well tolerated in the clinic (Official FDA information: Amiloride, side effects and usage. http://www.drugs.com/pro/amiloride.html).
In this study, we first assessed NHE-1 expression in esophageal adenocarcinoma vs. normal tissues and then investigated the effects of amiloride in suppressing esophageal cancer cell growth in vitro and in nude mouse xenografts. We then assessed the combined effects in esophageal cancer cells of amiloride and guggulsterone, a natural inhibitor of the bile acid receptor (farnesoid X receptor [FXR]).
Materials and methods
Immunohistochemical analysis
In this study, we obtained esophageal adenocarcinoma tissue specimens from 101 consecutive patients who had undergone esophagectomy without preoperative chemotherapy or radiotherapy between the years 1986 and 1997 at The University of Texas MD Anderson Cancer Center. The tissue sections from each patient were prepared with use of paraffin blocks and used for immunohistochemical analysis of NHE-1 expression according to our previously described methodology (Guan et al., 2013). The anti-NHE-1 antibody was obtained from Santa Cruz Biotechnologies (Santa Cruz, CA, USA) and used at 1:50 dilution with phosphate-buffered saline (PBS). The sections were then reviewed and categorized under a microscope as positive- or negative-stained (sections with ≥ 10% positively stained tumor cells were considered to be positive-stained). The use of patient samples for this study was approved by our Institutional Review Board.
Cell culture and cell viability MTT assay
Esophageal adenocarcinoma SKGT-4 and SKGT-5 cell lines and esophageal squamous cell cancer TE-3 and TE-12 cell lines were grown in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) with 10% fetal bovine serum at 37°C in a humidified atmosphere of 95% air and 5% CO2. For amiloride or guggulsterone treatment, the cells were plated for 24 h in regular medium and then treated with either control medium (containing the same amount of dimethyl sulfoxide [DMSO]) or medium containing amiloride, guggulsterone, or a combination of both (Sigma Chemicals Co., St. Louis, MO, USA) at various doses or for different periods of time. For the methyl thiazolyl tetrazolium (MTT) assay, 20 μL of MTT (5 mg/mL, Sigma) was added to each well of the 96-well plates and incubated for an additional 2 h, and the growth medium was replaced with 100 μL of DMSO to dissolve MTT in the cells. The optical densities were measured with an automated spectrophotometric plate reader at a single wavelength of 540 nm. The percentage of cell growth was calculated by using the formula: % control = ODt/ODc × 100, where ODt and ODc are the optical densities for treated and control cells, respectively.
NHE-1 shRNA transfection and immunocytochemical staining of Ki67 protein
Esophageal cancer SKGT-4 and SKGT-5 cells were grown in a monolayer overnight and transiently transfected with either pCMS/EGFP (BD Clontech, San Diego, CA, USA) plus negative-control shRNA vector or NHE-1 shRNA (Santa Cruz Biotechnology, Cat# sc-37007 and sc-42650-SH, respectively) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). At 36 h after gene transfection, the cells were treated with 0.25 μg/mL puromycin for an additional 24 h and then fixed with 4% paraformaldehyde for 10 min at room temperature to preserve the green fluorescent protein (GFP) and permeabilized in 0.5% Triton X-100 for 20 min at room temperature. The cells were then immunostained with anti-Ki67, as described previously (Guan et al., 2013). The Ki67 antibody was obtained from Vector Laboratories (Burlingame, CA, USA) and diluted at 1:50 in PBS. The stained cell sections were reviewed with use of fluorescence microscopy, and more than 200 cells in 10 of the 20× objective fields were counted for GFP staining (green) and then for Ki67 staining (red). The percentage of control of cell proliferation was calculated from the equation: % control = NT/NV × 100, where NT and NV are the numbers of Ki67-positive cells in GPF-positive cells of negative control shRNA or NHE-1 shRNA-transfected cells, respectively.
Reverse transcriptase–polymerase chain reaction (RT-PCR) and qRT-PCR
Total cellular RNA was extracted from the cells and subjected to RT-PCR and qRT-PCR analyses of gene expression as described previously (Guan et al., 2013). GAPDH was used as a loading control. The primers for NHE-1 were 5′-CCTTGTTTTTGGGGAGTCCT-3′ and 5′-GGTAAATCGGGAGGTGAAGG-3′ (to generate a 198-bp PCR product); COX-2, 5′-CCTTCTGCCTGACACCTTTC-3′ and 5′-GGTCAATGGAAGCCTGTGAT-3′ (to generate a 194-bp PCR product); MMP-9, 5′-GCACGACGTCTTCCAGTACC-3′ and 5′-GTTTGTATCCGGCAAACTGG-3′ (to generate a 224-bp PCR product); and GAPDH, 5′-CCCTTCATTGACCTCAACTACATGG-3′ and 5′-CATGGTGGTGAAGACGCCAG-3′ (to generate a 192-bp PCR product).
DNA fragmentation assay
The cells were grown and treated with or without 200 μM amiloride, 25 μM guggulsterone, or a combination of both (using a half of the individual dose) for 3 days; soluble DNA was extracted from both floating and attached cells and then pelleted by centrifugation and resuspended in Tris-EDTA buffer (pH 8.0). The cell pellets were lysed on ice with 400 μl mixture of 10 mM Tris-HCl (pH 8.0), 10 mM EDTA, and 0.5% Triton X-100 for 30 min and then centrifuged at 12000 × g for 15 min to separate soluble (fragmented) from pellet (intact genomic) DNA. Next, soluble DNA was treated with RNase A (50 μg/mL) at 37°C for 1 h and then with proteinase K (100 μg/mL) in 0.5% sodium dodecyl sulfate (SDS) at 50°C for 2 h. The residual material was extracted with phenol/chloroform, precipitated in ethanol, electrophoresed on a 1.8% agarose gel, and stained with ethidium bromide. The gels were then photographed with use of ultraviolet illumination.
Nude mouse experiments
This study was approved by our Institutional Animal Care and Usage protocol. In brief, nu/nu nude mice (6 weeks old) were subcutaneously injected in the right flank through a 22-gauge needle with 3 × 106 SKGT-4 cells in a total volume of 200 μL per mouse. Two days before tumor injection, the mice were given an oral dose of 5 mg/kg amiloride, 50 mg/kg guggulsterone, or a combination of both (at the same individual doses) for a total of 22 days. The mice were monitored daily for tumor formation and growth. At the end of the experiments, the mice were euthanized and tumor xenografts were removed, fixed in 4% paraformaldehyde, weighted, and photographed.
Statistical analysis
The data were summarized from three independent experiments of the triplicate as mean ± SD. Statistical analysis was performed by using the Student t-test with SPSS 11.5 software (Chicago, IL, USA). A probability (p) value of less than 0.05 was considered statistically significant.
Results
Differential expression of NHE-1 protein in esophageal cancer tissues
In this study, we first detected NHE-1 expression in esophageal cancer tissue specimens using immunohistochemistry. We found that NHE-1 was highly expressed in the cytoplasm of esophageal adenocarcinoma tissues in 66 of 101 cases (65.3%) but not in distant normal esophageal squamous cell epithelium (1 of 26 cases [3.8%]) (Fig. 1).
Figure 1.
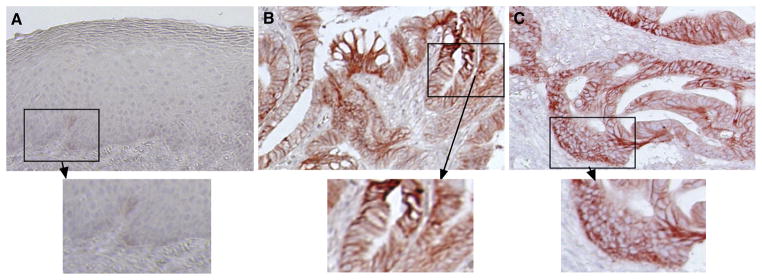
Differential expression of NHE-1 expression in normal and esophageal cancer tissue specimens, which were immunostained with anti-NHE-1 antibody and then viewed and categorized as positive- or negative-stained in each case. A, Normal esophageal squamous cell epithelium; B and C, Esophageal adenocarcinoma. Magnification ×200 (the insert, ×400).
Inhibition of NHE-1 expression or activity in suppressing esophageal cancer cell viability in vitro
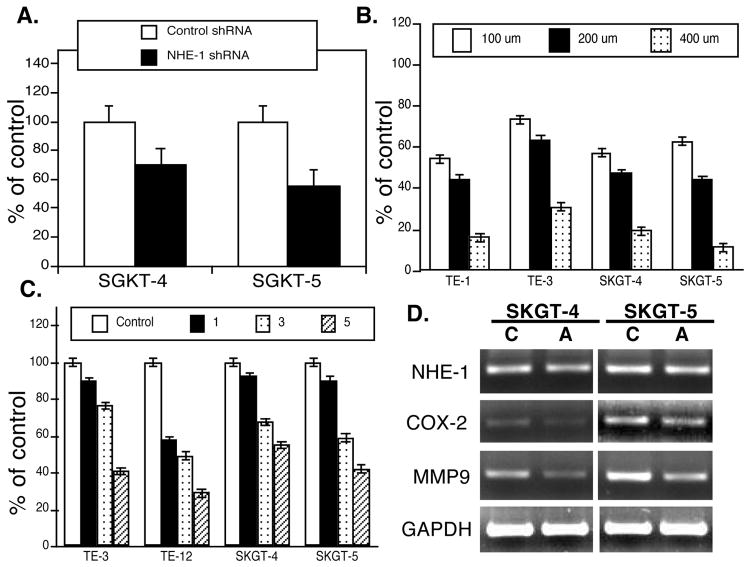
We assessed whether inhibition of NHE-1 expression or activity could control viability and gene expression of esophageal cancer cells by transfecting NHE-1 shRNA into esophageal cancer cells or treating the cells with the NHE-1 inhibitor amiloride for different periods of time and at various concentrations. We found that NHE-1 shRNA transfection reduced tumor cell proliferation (detected by Ki67 immunostaining; Fig. 2A), whereas amiloride treatment reduced tumor cell viability in a dose- and time-dependent manner (Fig. 2B and 2C). At the gene level, amiloride treatment inhibited expression of COX-2 and MMP-9 mRNA (Fig. 2D) but did not affect NHE-1 expression (Fig. 2D). qRT-PCR confirmed these data (data not shown).
Figure 2.
Effect of NHE-1 knockdown or inhibition on regulation of esophageal cancer cell viability and gene expression. A, Immunocytochemical analysis of Ki67 expression. The control shRNA or NHE-1 shRNA plasmid plus pCMS/EGFP was transiently transfected into SKGT-4 and SKGT-5 cells. After Ki67 immunostaining, approximately 200 green fluorescent protein (green)-positive cells from these transfections were counted and then assessed for Ki67 expression (red). The data were summarized as mean ± SD and calculated as % of the control shRNA-transfected cells. *p < 0.05 compared with control cells. B, Cell viability MTT assay. Esophageal cancer cells TE-3, TE-12, SKGT-4, and SKGT-5 were grown and treated with various doses of amiloride for 5 days and then subjected to the MTT assay. *p < 0.05 compared with control cells. C, Cell viability MTT assay. Esophageal cancer cells TE-3, TE-12, SKGT-4, and SKGT-5 were grown and treated with 200 μM amiloride for up to 5 days and then subjected to the MTT assay. *p < 0.05 compared with control cells. D, RT-PCR. SKGT-4 and SKGT-5 cells were grown and treated with 200 μM amiloride for 3 days and then for RNA isolation and RT-PCR analysis of gene expression. We also performed qRT-PCR and the data were similar to RT-PCR data.
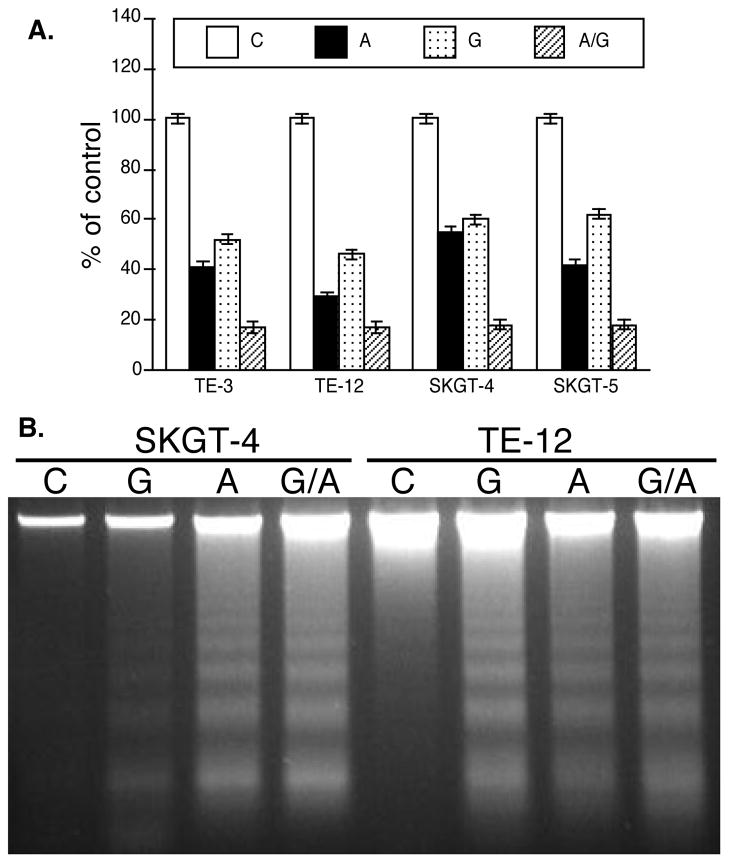
Since gastresophageal reflux carries bile, hydrochloric acid, and proteases that insult esophageal epithelial cells, leading to the development of Barrett esophagus and/or esophageal adenocarcinoma, we combined amiloride with guggulsterone, the natural bile acid receptor FXR inhibitor, to treat esophageal cancer cell lines. The data showed that amiloride and guggulsterone individually or in combination significantly reduced viability of both esophageal adenocarcinoma and squamous cell carcinoma cell lines and induced them to undergo apoptosis (Fig. 3).
Figure 3.
Effect of NHE-1 or FXR inhibition on regulation of esophageal cancer cell viability and apoptosis. A, MTT assay. Esophageal cancer cells TE-3, TE-12, SKGT-4, and SKGT-5 were grown and treated with 200 μM amiloride, 25 μM guggulsterone, or a combination of both (a half dose of the individual drug dose) for 5 days and then subjected to the MTT assay. *p < 0.05 compared with control cells. B, DNA fragmentation assay. Esophageal cancer TE-12 and SKGT-4 were grown and treated with 200 μM amiloride, 25 μM guggulsterone, or a combination of both (a half dose of the individual drug dose) for 5 days and then subjected to the DNA fragmentation assay to detect tumor cell apoptosis.
Role of NHE-1 inhibition in suppressing the growth of esophageal cancer cell xenografts
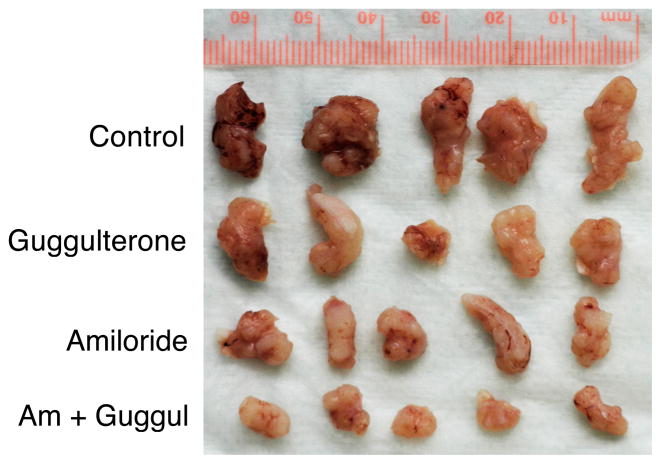
We performed nude mouse xenograft experiments to investigate the effects of NHE-1 inhibition in vivo. We treated the nude mice with daily oral amiloride (5 mg/kg), guggulsterone (50 mg/kg), or a combination of both for 2 days before subcutaneous SKGT-4 cells injection and then continuously treated these mice for an additional 20 days. At the end of the experiments, we resected, weighed, and photographed the xenografts. The data showed that amiloride, guggulsterone, and a combination of both significantly suppressed tumor xenograft formation and growth in nude mice (Table 1 and Fig. 4).
Table 1.
Nude mouse xenograft data
| Mouse bodyweight (g)
|
# of mice
|
Tumor weight (g) | % of control | p value* | |||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | ||||
| Control | 20.4 ± 1.80 | 28.3 ± 1.98 | 5 | 5 | 0.47 ± 0.14 | ||
| Amiloride | 20.2 ± 1.27 | 26.0 ± 2.80 | 5 | 5 | 0.23 ± 0.03 | 48.9 | 0.02 |
| Guggulsterone | 19.1 ± 1.74 | 28.7 ± 1.60 | 5 | 5 | 0.28 ± 0.09 | 59.6 | 0.10 |
| Combination | 18.2 ± 0.85 | 27.3 ± 2.90 | 5 | 5 | 0.12 ± 0.02 | 25.5 | 0.006 |
Student t test compared with control.
Figure 4.
Effect of NHE-1 and FXR inhibition in suppression of growth of nude mouse xenografts. The mice were treated with 5 mg/kg amiloride, 50 mg/kg guggulsterone, or a combination of both (as the same individual dose) for 2 days before they were subcutaneously injected with 3 million SKGT-4 cells. After that, the mice were continuously given oral 5 mg/kg amiloride, 50 mg/kg guggulsterone, or a combination of both (as the same individual dose) daily. Tumor formation and growth were monitored daily. At the end of the experiments, the mice were euthanized and the tumor xenografts were resected, fixed in 4% paraformaldehyde, weighed, and photographed.
Discussion
Clinically, frequent gastresophageal reflux carrying acid and bile acid–containing juice will damage the distal esophagus, causing normal squamous cells around the gastresophageal junction to undergo metaplastic changes with various degrees of incomplete intestinal metaplasia and dysplasia. The metaplastic changes cause cells to become more resistant to acid- and bile-caused injuries; however, Barrett esophagus is thus formed (Chen and Yang, 2001). Acid and bile reflux variably affect Barrett esophagus and may cause dysplasia or adenocarcinoma (Menges et al., 2001; Chen and Yang, 2001). In previous studies, acid and bile acid were found to induce ERK activity, PPAR-γ expression, and cell proliferation in normal esophageal epithelial cells (Jiang et al., 2006). Our previous study demonstrated that bile acid exposure induced FXR and COX-2 expression but suppressed RAR-β2 expression (Guan et al., 2013), whereas other studies have shown that bile acid caused phosphatidyl-inositol-3-kinase–mediated proliferation in Barrett adenocarcinoma cells (Jaiswal et al., 2004) and that deoxycholic acid at neutral pH activated nuclear factor-kappaB (NF-κB) and induced interleukin-8 expression in esophageal cells in vitro (Jenkins et al., 2004).
In our current study, we found that expression of NHE-1 protein was upregulated in esophageal adenocarcinoma tissues compared with that in normal esophageal squamous cell mucosae. This finding suggests that NHE-1 may be responsible for acid-induced altered gene expression in esophageal adenocarcinomas. Our study further demonstrated that targeting of NHE-1 expression or activity can effectively inhibit esophageal cancer cell proliferation and induce tumor cell apoptosis. In addition, we found that a combination of the NHE-1 inhibitor amiloride and the natural FXR inhibitor guggulsterone significantly suppressed esophageal cancer cell growth in vitro and in nude mice. The data from our current study suggest that inhibition of NHE-1 expression or activity and the combination of amiloride with guggulsterone should be further evaluated as a novel strategy in future control of esophageal adenocarcinoma.
Previous studies also showed that acid was able to modulate colon cancer HT29 cell growth and differentiation in an in vitro model for Barrett esophagus (Fitzgerald et al., 1997), whereas altered sodium-hydrogen exchange activity is a mechanism for acid-induced hyperproliferation in Barrett esophagus (Fitzgerald et al., 1998). However, the initial induction of NHE-1 expression is a self-defense mechanism of esophageal epithelium against acid insult; indeed, a previous study showed the role of epidermal growth factor (EGF) and NHE-1 in esophageal epithelial defense against acid-induced injury (Fujiwara et al., 2006). Fujiwara et al. showed that EGF protected esophageal epithelial cells against acid injury in a dose-dependent manner and that the cytoprotective effect of EGF was completely blocked by treatment with NHE-1 inhibitors. Expression of NHE-1 mRNA was increased in esophagitis and upregulated in rats with sialoadenectomy (Fujiwara et al., 2006).
Various growth factors (such as EGF) can activate NHE-1 (Fliegel et al., 1993). NHE-1-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes (Reshkin et al., 2000); other studies showed that overexpression of NHE-1 was directly associated with cellular transformation, invasion, and metastasis (Slepkov and Fliegel, 2002; Putney et al., 2002; Masereel et al., 2003). These observations have heightened the interest in NHE-1 as a promising novel drug target for more effective and selective anticancer therapeutics (Loo et al., 2012). A number of studies showed that NHE-1 inhibitors suppressed development and metastasis of lung, gastric, colon, and breast cancers (Sparks et al., 1983; Newell et al., 1992; Tatsuta et al., 1993, 1997; Evans et al., 1998; Evans and Sloan Stakleff 2004; Li et al., 2009). For example, suppression of NHE-1 expression using NHE-1 antisense cDNA inhibited proliferation and caused induction of apoptosis in drug-resistant human small cell lung cancer cells (Li et al. 2009). Consistent with these previous studies, our current data showed amiloride suppression of esophageal cancer cell growth in vitro and in nude mouse xenografts.
Furthermore, since frequent gastresophageal reflux carries both acid and bile acid that damages the distal esophagus, induces formation of Barrett esophagus, and promotes esophageal dysplasia or adenocarcinoma, targeting of these risk factors could more effectively control esophageal cancer cell growth. Thus, we combined amiloride and guggulsterone to treat esophageal cancer cells in vitro and in nude mouse xenografts. We found that their combination had more than additive effects on the suppression of esophageal cancer cell growth in vitro and in nude mouse xenografts. Indeed, previous studies have used combination of amiloride with other agents (such as radiation or morphine) to control glioblastoma or pain (Tang et al., 2013; Ouyang et al., 2012). Thus, future study is needed to further explore the activity of these combined agents in controlling esophageal cancer.
Acknowledgments
We thank the Department of Scientific Publications at MD Anderson Cancer Center for editing the manuscript. This work was supported in part by a grant from National Cancer Institute Grant R01 CA117895 and a grant from the Duncan Family Institute for Cancer Prevention and Risk Assessment, MD Anderson Cancer Center.
Footnotes
Compliance with ethics guidelines
The authors declare no conflict of interest in this work.
References
- Blot WJ. Esophageal cancer trends and risk factors. Semin Oncol. 1994;21(4):403–410. [PubMed] [Google Scholar]
- Chen X, Yang CS. Esophageal adenocarcinoma: a review and perspectives on the mechanism of carcinogenesis and chemoprevention. Carcinogenesis. 2001;22(8):1119–1129. doi: 10.1093/carcin/22.8.1119. [DOI] [PubMed] [Google Scholar]
- Evans DM, Sloan Stakleff KD. Control of pulmonary metastases of rat mammary cancer by inhibition of uPA and COX-2, singly and in combination. Clin Exp Metastasis. 2004;21(4):339–346. doi: 10.1023/B:CLIN.0000046140.19131.19. [DOI] [PubMed] [Google Scholar]
- Evans DM, Sloan-Stakleff KD, Arvan M, Guyton DP. Time and dose dependency of the suppression of pulmonary metastases of rat mammary cancer by amiloride. Clin Exp Metastasis. 1998;16(4):353–357. doi: 10.1023/A:1006517614491. [DOI] [PubMed] [Google Scholar]
- Falk GW. Risk factors for esophageal cancer development. Surg Oncol Clin N Am. 2009;18(3):469–485. doi: 10.1016/j.soc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RC, Bishr Omary M, Triadafilopoulos G. Altered sodium-hydrogen exchange activity is a mechanism for acid-induced hyperproliferation in Barrett’s esophagus. Am J Physiol Gastrointest Liver Physiol. 1998;275:47–55. doi: 10.1152/ajpgi.1998.275.1.G47. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RC, Omary MB, Triadafilopoulos G. Acid modulation of HT29 cell growth and differentiation. An in vitro model for Barrett’s esophagus. J Cell Sci. 1997;110(Pt 5):663–671. doi: 10.1242/jcs.110.5.663. [DOI] [PubMed] [Google Scholar]
- Fliegel L, Dyck JR, Wang H, Fong C, Haworth RS. Cloning and analysis of the human myocardial Na+/H+ exchanger. Mol Cell Biochem. 1993;125(2):137–143. doi: 10.1007/BF00936442. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Higuchi K, Takashima T, Hamaguchi M, Hayakawa T, Tominaga K, Watanabe T, Oshitani N, Shimada Y, Arakawa T. Roles of epidermal growth factor and Na+/H+ exchanger-1 in esophageal epithelial defense against acid-induced injury. Am J Physiol Gastrointest Liver Physiol. 2006;290(4):G665–G673. doi: 10.1152/ajpgi.00238.2005. [DOI] [PubMed] [Google Scholar]
- Guan BX, Li H, Yang ZD, Hoque A, Xu XC. Inhibition of farnesoid X receptor controls esophageal cancer cell growth in vitro and in nude mouse xenografts. Cancer. 2013;119(7):1321–1329. doi: 10.1002/cncr.27910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal K, Tello V, Lopez-Guzman C, Nwariaku F, Anthony T, Sarosi GA., Jr Bile salt exposure causes phosphatidyl-inositol-3-kinase-mediated proliferation in a Barrett’s adenocarcinoma cell line. Surgery. 2004;136(2):160–168. doi: 10.1016/j.surg.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Jenkins GJ, Harries K, Doak SH, Wilmes A, Griffiths AP, Baxter JN, Parry JM. The bile acid deoxycholic acid (DCA) at neutral pH activates NF-kappaB and induces IL-8 expression in oesophageal cells in vitro. Carcinogenesis. 2004;25(3):317–323. doi: 10.1093/carcin/bgh032. [DOI] [PubMed] [Google Scholar]
- Jiang ZR, Gong J, Zhang ZN, Qiao Z. Influence of acid and bile acid on ERK activity, PPARgamma expression and cell proliferation in normal human esophageal epithelial cells. World J Gastroenterol. 2006;12(15):2445–2449. doi: 10.3748/wjg.v12.i15.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bao P, Li Z, Ouyang H, Wu C, Qian G. Inhibition of proliferation and apoptosis induced by a Na+/H+ exchanger-1 (NHE-1) antisense gene on drug-resistant human small cell lung cancer cells. Oncol Rep. 2009;21(5):1243–1249. doi: 10.3892/or_00000347. [DOI] [PubMed] [Google Scholar]
- Loo SY, Chang MK, Chua CS, Kumar AP, Pervaiz S, Clement MV. NHE-1: a promising target for novel anti-cancer therapeutics. Curr Pharm Des. 2012;18(10):1372–1382. doi: 10.2174/138161212799504885. [DOI] [PubMed] [Google Scholar]
- Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na(+)/H(+) exchanger. Eur J Med Chem. 2003;38(6):547–554. doi: 10.1016/S0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- Menges M, Müller M, Zeitz M. Increased acid and bile reflux in Barrett’s esophagus compared to reflux esophagitis, and effect of proton pump inhibitor therapy. Am J Gastroenterol. 2001;96(2):331–337. doi: 10.1111/j.1572-0241.2001.03515.x. [DOI] [PubMed] [Google Scholar]
- Newell K, Wood P, Stratford I, Tannock I. Effects of agents which inhibit the regulation of intracellular pH on murine solid tumours. Br J Cancer. 1992;66(2):311–317. doi: 10.1038/bjc.1992.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang H, Bai X, Huang W, Chen D, Dohi S, Zeng W. The antinociceptive activity of intrathecally administered amiloride and its interactions with morphine and clonidine in rats. J Pain. 2012;13(1):41–48. doi: 10.1016/j.jpain.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Putney LK, Denker SP, Barber DL. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu Rev Pharmacol Toxicol. 2002;42(1):527–552. doi: 10.1146/annurev.pharmtox.42.092001.143801. [DOI] [PubMed] [Google Scholar]
- Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M, Alunni-Fabbroni M, Casavola V, Tommasino M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000;14(14):2185–2197. doi: 10.1096/fj.00-0029com. [DOI] [PubMed] [Google Scholar]
- Slepkov E, Fliegel L. Structure and function of the NHE1 isoform of the Na+/H+ exchanger. Biochem Cell Biol. 2002;80(5):499–508. doi: 10.1139/o02-151. [DOI] [PubMed] [Google Scholar]
- Sparks RL, Pool TB, Smith NKR, Cameron IL. Effects of amiloride on tumor growth and intracellular element content of tumor cells in vivo. Cancer Res. 1983;43(1):73–77. [PubMed] [Google Scholar]
- Spechler SJ. Barrett’s esophagus: a molecular perspective. Curr Gastroenterol Rep. 2005;7(3):177–181. doi: 10.1007/s11894-005-0031-z. [DOI] [PubMed] [Google Scholar]
- Tang JY, Chang HW, Chang JG. Modulating roles of amiloride in irradiation-induced antiproliferative effects in glioblastoma multiforme cells involving Akt phosphorylation and the alternative splicing of apoptotic genes. DNA Cell Biol. 2013;32(9):504–510. doi: 10.1089/dna.2013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta M, Iishi H, Baba M, Uehara H, Nakaizumi A, Taniguchi H. Inhibition by amiloride of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Br J Cancer. 1993;67(5):1011–1014. doi: 10.1038/bjc.1993.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta M, Iishi H, Baba M, Yano H, Sakai N, Uehara H, Nakaizumi A. Chemoprevention by amiloride against experimental hepatocarcinogenesis induced by Wnitrosomorpholine in Sprague-Dawley rats. Cancer Lett. 1997;19(1):109–113. doi: 10.1016/S0304-3835(97)00262-0. [DOI] [PubMed] [Google Scholar]
- Xu XC. Risk factors and altered gene expression in esophageal cancer. In: Verma M, editor. Cancer Epidemiology. New York: Humana press; 2009. pp. 335–360. [DOI] [PubMed] [Google Scholar]
- Yamada T, Alpers DH, Laine L, Owyang C, Powell DW. Textbook of gastroenterology. 3. Lippincott Williams and Wilkins; Philadelphia: 1999. [Google Scholar]