Summary
The protein nucleobindin 2 (NUCB2) or NEFA (DNA binding/EF-hand/acidic amino acid rich region) was identified over a decade ago and implicated in intracellular processes. New developments came with the report that post-translational processing of hypothalamic NUCB2 may result in nesfatin-1, nesfatin-2 and nesfatin-3 and convergent studies showing that nesfatin-1 and full length NUCB2 injected in the brain potently inhibit the dark phase food intake in rodents including leptin receptor deficient Zucker rats. Nesfatin-1 also reduces body weight gain, suggesting a role as a new anorexigenic factor and modulator of energy balance. In light of the obesity epidemic and its associated diseases, underlying new mechanisms regulating food intake may be promising targets in the drug treatment of obese patients particularly as the vast majority of them display reduced leptin sensitivity or leptin resistance while nesfatin-1’s mechanism of action is leptin independent. Although much progress on the localization of NUCB2/nesfatin-1 in the brain and periphery as well as on the understanding of nesfatin-1’s anorexic effect have been achieved during the past three years, several important mechanisms have yet to be unraveled such as the identification of the nesfatin-1 receptor and the regulation of NUCB2 processing and nesfatin-1 release.
Keywords: Food intake, hypothalamus, nesfatin-1/NUCB2, X/A-like cells
Introduction and historical note on nucleobindin 2
In the early 1990s, a protein was identified in mouse (1) and human cell lines (2) and termed nucleobindin or NEFA (DNA binding/EF-hand/acidic amino acid rich region). This protein contains multiple functional domains including a signal peptide on the N-terminal side, a leucine/isoleucine rich region, a DNA binding domain and a putative nuclear targeting signal, while the second half contains two Ca2+-EF-hand motifs and a leucine zipper motif in the C-terminal region (2). Until now, two nucleobindins have been identified, namely nucleobindin 1 (NUCB1 or CALNUC, rat: NM 053463.1) (3) and nucleobindin 2 (NUCB2 or NEFA, rat: NM 021663.2) (4). Both are part of a homologous gene family and likely derived from one four-domain EF-hand ancestor (5). NUCB2 contains a 24-amino acid (aa) N-terminal signal peptide and a 396 aa sequence that is highly conserved in rodents and humans (4) pointing towards its physiological relevance.
Localization of nucleobindin 2 and functions as intracellular signal
Nucleobindin 2 was localized on the plasma membrane and in the cytoplasm (2). Based on NUCB2 mutants, it has been established that the N-terminal leucine/isoleucine rich region is essential for NUCB2 to be retained in the Golgi complex although its action within the Golgi is still not well understood (2,6). Early on, it has been suggested that NUCB2 may be involved in autoimmunity as indicated by the increased production of anti-DNA antibodies following treatment of B-cells from lupus-prone MRL/1 mice with NUCB2 in vitro, whereas no effect was detected when B-cells from BALB/c mice were used (1). In line with these findings, NUCB2 and nucleosomal DNA were detected in vitro in the supernatant of KML1-7 cells obtained from lupus-prone MRL/1 mice (1) suggesting the release of nucleosomal DNA and binding to NUCB2 resulting in the formation of immunogenic DNA in vivo. These DNA-NUCB2 complexes may trigger the increased production of anti-DNA antibodies by activated B-cells (4) and therefore point towards a pathophysiological role of NUCB2 in the maintenance of autoimmune diseases such as lupus erythematosus under already primed conditions. Studies using mutation or over-expression of NUCB2 will help to clarify this role.
Processing of nucleobindin 2 into nesfatin-1, nesfatin-2 and nesfatin-3
In 2006, Oh-I and colleagues were the first to describe that putative post-translational processing of NUCB2 by the enzyme pro-hormone convertase (PC)-1/3 results in nesfatin-1 (aa 1–82), nesfatin-2 (aa 85–163) and nesfatin-3 (aa 166–396) (7). So far, a biological activity has only been demonstrated for nesfatin-1 and the fragment, nesfatin-124– 53. The initial study was the only one to report mature nesfatin-1 (aa 1–82) in the cerebrospinal fluid of rats (7), whereas the 82 aa polypeptide was not detectable in hypothalamic protein extracts in the initial report and confirmed by an independent group (7,8). Likewise, two other studies were unable to detect endogenous mature nesfatin-1 (9.7 kDa) in the stomach, pancreas or plasma, whereas full length NUCB2 was present (9,10). These negative data do not seem to be due to the lack of sensitivity in the detection method as the synthetic nesfatin-1 peptide (50 ng) was detectable on the Western Blot (9). Whether rapid peptide ex vivo degradation contributes to the lack of detectability of nesfatin-1 will have to be investigated. However, the absence of circulating mature nesfatin-1 in the blood may also point towards an autocrine or paracrine mode of action. In addition, the vast number of reports on the expression of nesfatin-1 immunoreactivity in brain or other tissues used an antibody raised against full length nesfatin-1 that also recognizes NUCB2 and thus does not distinguish between nesfatin-1 and NUCB2 (9–11). Therefore, so far it is not clear whether nesfatin-1 or full length NUCB2 is the biologically active molecule because NUCB2 also displays biological activity upon third ventricular injection in rats (7).
Central nervous system distribution of nesfatin-1/nucleobindin 2 and co-localization with other transmitters
The initial report described the expression of NUCB2 mRNA substantiated by nesfatin-1 immunohistochemistry in rat hypothalamic and brainstem nuclei involved in the regulation of ingestive behaviour, such as the paraventricular nucleus of the hypothalamus (PVN), supraoptic nucleus, arcuate nucleus, lateral hypothalamic area, zona incerta and the nucleus of the solitary tract (7). These findings were confirmed and extended in a number of studies reporting nesfatin-1 immunopositive cells in additional hypothalamic, midbrain and hindbrain nuclei, namely the dorsomedial hypothalamic nucleus, tuberal hypothalamic area, periventricular nucleus, Edinger-Westphal nucleus, locus coeruleus, medullary raphe nuclei and the dorsal motor nucleus of the vagus nerve (8,12–14). This brain mapping was recently completed by our group with the detection of nesfatin-1 immunoreactivity in the rat insular cortex, central amygdaloid nucleus, ventrolateral medulla, cerebellum and pre-ganglionic sympathetic as well as parasympathetic neurons of the thoracic, lumbar and sacral spinal cord (11).
Nesfatin-1 immunopositive neurons co-localize with a number of brain transmitters (8,12–17). A major proportion of hypothalamic nesfatin-1 immunoreactive neurons co-express melanin-concentrating hormone (MCH), cocaine-and amphetamine-regulated transcript (CART)/pro-opiomelanocortin (POMC), α-melanocyte-stimulating hormone (α-MSH), mammalian target of rapamycin (m-TOR), vasopressin, oxytocin, neuropeptide Y (NPY), somatostatin, growth hormone-releasing hormone (GHRH), thyrotropin-releasing hormone (TRH), corticotropin-releasing factor (CRF) and neurotensin (8,12–17) (Table 1). In midbrain nuclei, nesfatin-1 immunoreactivity was detected in cholinergic and urocortin-1 containing neurons of the Edinger-Westphal nucleus (12,17) (Table 1) and in the brainstem, in serotonin (5-HT)-positive neurons of the medullary raphe (12). Collectively, nesfatin-1 immunoreactivity is localized in forebrain and hindbrain nuclei that are also immunoreactive for several peptidergic transmitters regulating food intake (POMC/CART, α-MSH, MCH, oxytocin, NPY, CRF), pituitary hormone regulation (TRH, GHRH, CRF, somatostatin) and stress (CRF) giving rise to potentially expanded biological actions of nesfatin-1 including neuroendocrine regulation, autonomic control of viscera, pain and stress.
Table 1.
Co-localization of nesfatin-1 immunoreactivity with other brain transmitters in forebrain and hindbrain nuclei
| Nucleus | % of neurons positive for other marker co-labelling with nesfatin-1 immunoreactivity |
Reference |
|---|---|---|
| Hypothalamus Supraoptic nucleus |
59% with arginine vasopressin 49% with oxytocin |
(14) |
| Paraventricular nucleus | 47–57% with arginine vasopressin 40–45% with oxytocin 18–27% with thyrotropin-releasing hormone 13–24% with corticotropin-releasing factor |
(8,14) |
| Periventricular nucleus | 38% with somatostatin | (8) |
| Lateral hypothalamic area | 82–84% with melanin-concentrating hormone 70% with cocaine-and amphetamine-regulated transcript |
(8,13) |
| Arcuate nucleus | 60–65% with cocaine-and amphetamine-regulated transcript 64% with α-melanocyte-stimulating hormone 59% with mammalian target of rapamycin 46% with neuropeptide Y 29% with growth hormone-releasing hormone 14% with neurotensin |
(8,16) |
| Midbrain Edinger-Westphal nucleus |
90% with cocaine-and amphetamine-regulated transcript 90% with urocortin-1 |
(17) |
Secretion of nesfatin-1/nucleobindin 2
Based on the structure of NUCB2, it was suspected that the protein acts as an intracellular signal. However, early reports indicated protein secretion either in vitro by detection in the culture medium (2,18) or in vivo in the serum of lupus prone MRL lpr−1 mice (19). Likewise, nesfatin-1 was suggested to be secreted based on the initial Western blot detection of a 9.7 kDa band corresponding to nesfatin-1 in the cerebrospinal fluid of rats (7). However, in the brain, nesfatin-1 immunoreactivity has only been localized in the cytoplasm of the neuronal soma including membrane bound Golgi structures and proximal processes but not varicosities and axon terminals (8,11,12,15) pointing towards an action of nesfatin-1/NUCB2 as an intracellular transmitter. In light of the finding that cell bodies can also release cellular contents (20,21), nesfatin-1/NUCB2 may as well have extracellular regulatory properties. This assumption is also supported by a recent electron microscopy study showing nesfatin-1 immunoreactivity in secretory granules in neurons of the rat hypothalamic PVN (15).
Central action of nesfatin-1 to reduce food intake and body weight
Nesfatin-1 reduces dark phase food intake
The initial report showed that nesfatin-1, but not nesfatin-2 or nesfatin-3, reduced dark phase food intake from 1–6 h post third ventricular (3v) injection at low doses (5 and 25 pmol = 0.05 and 0.25 µg rat−1, respectively) in freely fed rats (7). This effect was not restricted to one species as it could be reproduced in chronically 3v cannulated mice following injection of nesfatin-1 (1 µg mouse−1) (15). Similarly, injection of nesfatin-1 into the lateral brain ventricle (intracerebroventricular, icv) of ad libitum fed rats reduced the dark phase food intake with a delayed onset and a long-lasting action (22,23). Likewise, microinjection of nesfatin-1 directly into the PVN suppresses the dark phase food intake in male Wistar rats during the first 3 h post injection (15). When administered at the level of the hindbrain either into the fourth ventricle (4v) or the cisterna magna (ic), nesfatin-1 induces a rapid in onset and sustained reduction of dark phase food intake (22). This differential onset of food intake reduction upon icv vs. 3v/4v/cisterna magna injection points towards distinct forebrain and hindbrain sites of action for nesfatin-1. This is also supported by immunohistochemical studies indicating that nesfatin-1 injected into the 3v increases the number of Fos-positive neurons in the PVN in the forebrain and the nucleus of the solitary tract in the hindbrain (15). However, additional microinjection studies are required to localize, in addition to the PVN, other specific forebrain and hindbrain nuclei responsive to nesfatin-1 and, more importantly, the identification of the cognate receptor will help to identify the sites of nesfatin-1’s action.
Divergent results have been reported when nesfatin-1 was investigated during the light phase. There was no reduction of food consumption during the light phase in overnight fasted rats when injected icv at a similar dose reducing the dark phase food intake in freely fed rats (22). However, when a higher dose was injected icv (1.8 µg rat−1) nesfatin-1 reduced food ingestion during the light phase re-feeding period after an overnight fast between 3 h and 5 h post injection (23). Taken together, these data suggest a lower efficacy of nesfatin-1 to reduce food intake under conditions outside of the physiological feeding period in rats. This could indicate an interaction between nesfatin-1 and clock genes or other transmitters specifically activated during the dark phase and/or reflect differential intensity of drive to eat in response to fasting compared with nocturnal physiological feeding which warrant further investigations. Collectively, independent reports indicate that nesfatin-1 injected into the cerebrospinal fluid at low picomolar doses either at the level of lateral, 3rd or 4th ventricle, cisterna magna or into the brain parenchyma at the level of the PVN reproducibly induces a sustained reduction of nocturnal feeding in freely fed rats and less potently reduces the hyperphagia to a fast in rodents.
In addition to the effects on food ingestion, continuous central infusion of nesfatin-1 into the 3v reduced body weight gain in rats (7). Conversely, 3v administration of a NUCB2 antisense oligonucleotide increased body weight gain in rats suggesting a physiological role for brain nesfatin-1/NUCB2 as inhibitory regulator of body weight (7). In line with these findings, we reported that an acute injection of nesfatin-1 icv (5 pmol rat−1) reduced body weight by 1.8 ± 0.5% at 24 h post injection in the absence of alterations in the 24-h cumulative food intake (22). These data point towards a possible stimulatory effect of nesfatin-1 on energy expenditure which needs to be characterized.
Implication of leptin independent hypothalamic signalling systems in the anorexigenic action of central nesfatin-1
Peptides regulating food intake often act in concert or series with other neurotransmitters to exert their actions (24) and NUCB2/nesfatin-1 is co-localized with a number of hypothalamic peptides regulating food intake (8,12–17). Consistent with these premises, several interactions have been described to underlie the central anorexic effect of nesfatin-1. In vitro, electrophysiologic experiments demonstrated that nesfatin-1 directly inhibits arcuate hypothalamic neurons containing the orexigen NPY as shown by the hyperpolarization of NPY neurons in the arcuate nucleus (Fig. 1) (25). This effect could involve an autocrine or intracellular mode of action as nesfatin-1 immunoreactivity has been described in a portion of NPY-positive neurons in the arcuate nucleus (Table 1) (16). Recently, we demonstrated that 59% of the pm-TOR-positive neurons in the arcuate nucleus of the hypothalamus were also immunoreactive for nesfatin-1 (16). As m-TOR signalling has been established to exert an inhibitory tone on NPY mRNA expression (26), nesfatin-1 may also influence this circuitry in an autocrine or intracellular fashion. In contrast, nesfatin-1’s action is independent of central leptin signalling as suggested by the finding that nesfatin-1’s central food intake reducing effect is retained in leptin-receptor deficient Zucker rats (7). Conversely, leptin injected into the 3v does not modulate 3v nesfatin-1’s inhibitory effect on dark phase food intake (7).
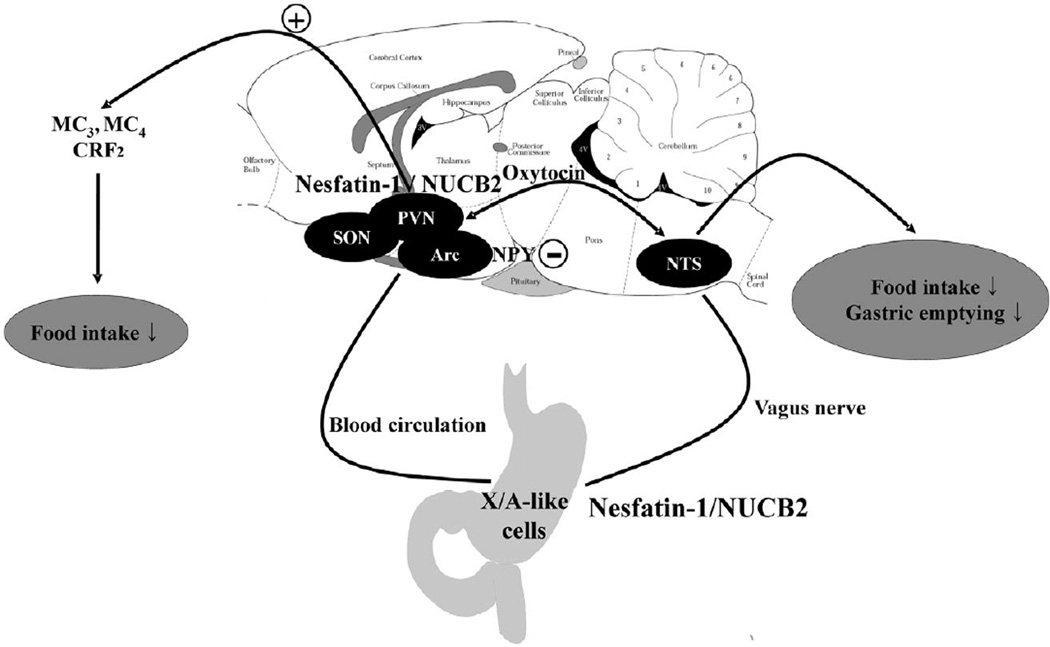
Figure 1.
Potential pathways by which nesfatin-1 induces a sustained and prolonged reduction of dark phase food intake and delays gastric emptying in rats as suggested by pharmacological interventions and neuroanatomical approaches. Central nesfatin-1/NUCB2 mediates its anorexigenic effect via activation of melanocortin3/4 and CRF2 signalling and also by hyperpolarizing neurons containing the orexigenic peptide, neuropeptide Y. Nesfatin-1 also activates the hypothalamic magnocellular oxytocinergic system which could reduce food intake and delay gastric emptying. Peripheral nesfatin-1 can reach the brain via the circulation and crossing the blood-brain barrier and/or direct action on circumventricular organs as well as modulation of vagal afferent activity. +, stimulation; −, inhibition; ↓, reduction; Arc, arcuate nucleus; NPY, neuropeptide Y; NTS, nucleus of the solitary tract; PVN, paraventricular nucleus of the hypothalamus; SON, supraoptic nucleus (7,15,22,23,25).
A recent study provided compelling evidence for the involvement of an oxytocin pathway in nesfatin-1’s inhibitory effect on food intake (15). First, oxytocin injected into the 3v reduces food intake via a leptin-independent mechanism (15). Second, nesfatin-1 injected into the 3v activates oxytocin-positive neurons in the magnocellular part of the PVN as assessed by double labelling for Fos/oxytocin immunoreactivity and in vitro it stimulates the release of oxytocin from PVN neurons (15). Third, there is pharmacologic and anatomical support for oxytocinergic projections from the PVN to the nucleus of the solitary tract to be involved in the anorexigenic signalling of nesfatin-1. An oxytocin receptor antagonist injected into the hindbrain at the level of the 4v blocked the food intake reducing effect of nesfatin-1 injected into the PVN and tracing studies showed synaptic contacts between oxytocinergic nerve terminals and POMC neurons in the nucleus of the solitary tract that can be activated by oxytocin (15) (Fig. 1). Likewise, an independent group also showed that an oxytocin antagonist injected icv blocks the food intake suppressing effects of icv nesfatin-1 and α-MSH (27). Whether the hypothalamic/nesfatin-1-oxytocin-brainstem/POMC signalling is the predominant pathway or an intrahypothalamic nesfatin-1-POMC/oxytocin network exists as well, warrants further investigation.
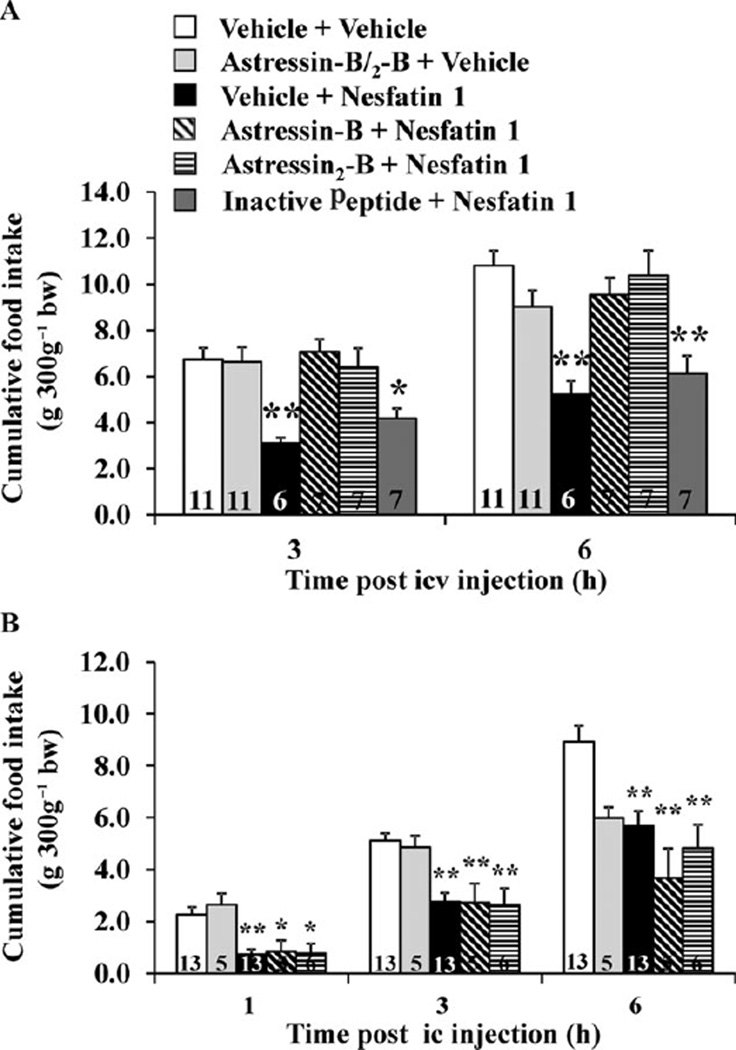
Based on the observation of a delayed and long lasting anorexigenic effect following icv injection of nesfatin-1 which mimics the characteristics of the food intake reducing effect of CRF2 receptor agonists, urocortins (28,29), we investigated the possible involvement of the CRF2 receptors in the mediation of nesfatin-1’s effect. The CRF2 antagonist, astressin2-B (30) injected icv completely abolished the dark phase food intake reduction induced by icv nesfatin-1 (22). By contrast, a control peptide of similar structure as astressin2-B but without affinity to the CRF2 receptor did not influence icv nesfatin-1’s action (22). These data indicate a specific blockade on the CRF2 receptor rather than a direct peptide-peptide interaction and therefore a role of CRF2 signalling pathways in nesfatin-1’s action (Figs 1 and 2). However, astressin2-B injected ic did not modulate the rapid onset reduction of food intake observed after ic injection of nesfatin-1 (Fig. 2) (22) supporting differential forebrain and hindbrain sites and mechanisms of nesfatin-1’s food intake inhibitory action. In contrast to the effect on food intake, the CRF2 antagonist, astressin2-B injected icv did not alter the icv nesfatin-1 induced delayed gastric emptying (22) giving rise to different downstream signalling pathways mediating icv nesfatin-1’s inhibitory effects on food intake and gastric transit. Lastly, the melanocortin 3/4 receptor antagonist, SHU9119 injected icv diminished (23), and into the 3v abolished (7), the anorexigenic effect of nesfatin-1. As hypothalamic CRF is a well-established downstream effector of α-MSH- or melanocortin4 receptor agonist-induced suppression of the dark phase feeding (31,32) with CRF being responsible for the acute reduction and urocortins mediating the delayed suppression of feeding (32), nesfatin-1 is likely to act in series through the recruitment of the central melanocortin and CRF2 signalling systems to reduce food intake (Fig. 1).
Figure 2.
Corticotropin-releasing factor receptor antagonists injected intracerebroventricularly (icv) prevent the icv nesfatin-1 induced reduction of dark phase food intake in freely fed rats (A). Chronically icv cannulated rats were injected with 30 µg rat−1 of astressin-B astressin2-B or astressin2-B analog devoid of CRF2 binding affinity (inactive peptide) or vehicle before icv nesfatin-1 (0.05 µg rat−1) or vehicle; cumulative food intake was monitored for 6h. *P<0.02 and **P< 0.001 vs. vehicle/vehicle. CRF receptor antagonists injected intracisternally (ic) do not prevent the ic nesfatin-1 induced reduction of dark phase food intake (B). Rats under brief anesthesia were injected ic with 30 µg rat−1 of astressin-B, astressin2-B or vehicle before ic injection of nesfatin-1 (0.5 µg rat−1) or vehicle and cumulative food intake monitored for 6h. *P<0.05 and **P < 0.005 vs. vehicle/vehicle. Bars represent the mean ± SEM of number of rats indicated at the bottom. Reproduced with permission from reference (22); Copyright 2009, The Endocrine Society.
Regulation of nesfatin-1/nucleobindin 2 pathways under different metabolic conditions
Besides the potent inhibitory effect observed after exogenous brain administration of nesfatin-1, 3v injection of a NUCB2 antisense oligonucleotide increases food intake in rats (7). Moreover, hypothalamic NUCB2/nesfatin-1 expression varies under different metabolic conditions, which also supports a physiological role as regulator of food intake. NUCB2 mRNA expression in the PVN decreased after a 24-h fast resulting in reduced NUCB2 protein expression (Table 2) (7). In addition, NUCB2 mRNA expression in the supraoptic nucleus was downregulated after 24 h fasting and restored after re-feeding (Table 2) (14). Lastly, re-feeding after a 24-h fasting period activated nesfatin-1 immunopositive neurons in the supraoptic nucleus as assessed by double staining for Fos and nesfatin-1 immunoreactivity (Table 2) (22) suggesting the activation of those neurons may be involved in satiety signalling. The regulation of NUCB2/nesfatin-1 expression by changes in metabolic status seems to be specific for the hypothalamus as no changes have been observed in the Edinger-Westphal nucleus after two days of fasting in rats (33). There is also evidence that NUCB2 mRNA expression in the hypothalamus is up-regulated by anorexigenic compounds such as the serotonin 5-HT1B/2C receptor agonist, m-chlorophenylpiperazine (34) or α-MSH (7) (Table 2). In addition, the anorexigenic gut-brain hormone, cholecystokinin injected intraperitoneally activated nesfatin-1 immunopositive cells in forebrain (PVN) and hindbrain nuclei (nucleus of the solitary tract) of rats (Table 2) (22,35) pointing towards a role for central nesfatin-1 in the mediation of gut peptides’ satiety signalling.
Table 2.
Regulation of central nesfatin-1/NUCB2 under different metabolic conditions and signals associated with alterations of food intake behaviour
| Condition | Species | Effect | Reference |
|---|---|---|---|
| 24 h fasting | Rat | ↓ NUCB2 mRNA and protein in PVN | (7) |
| 48 h fasting | Rat | ↓ NUCB2 mRNA in SON | (14) |
| 48 h fasting + 2 h re-feeding | Rat | ↑ NUCB2 mRNA in SON | (14) |
| 24 h fasting + 2 h re-feeding | Rat | Activation of nesfatin-1 ir SON neurons assessed by Fos | (22) |
| m-chlorophenylpiperazine (5–10 mg kg−1, ip) | Mouse | ↑ NUCB2 mRNA in the hypothalamus | (34) |
| α-melanocyte-stimulating hormone (1.5 µg rat−1, 3v) | Rat | ↑ NUCB2 mRNA in the hypothalamus | (7) |
| Cholecystokinin-8S (3–10 µg kg−1, ip) | Rat | Activation of nesfatin-1 ir PVN and NTS neurons assessed by Fos | (22,35) |
↑ increase; ↓, decrease; 3v, third brain ventricle; ip, intraperitoneal; ir, immunoreactive; NTS, nucleus of the solitary tract; NUCB2, nucleobindin 2; PVN, paraventricular nucleus of the hypothalamus; SON, supraoptic nucleus.
Activation of nesfatin-1/nucleobindin 2 pathways by various stressors
The expression of nesfatin-1 immunoreactivity in CRF containing neurons (8) and the mediation of its anorexigenic effects via a CRF2 dependent pathway (22) led to the assumption that central nesfatin-1 may play a role in the response to stress. Abdominal surgery is well established to induce postoperative gastric ileus in rats and reduces food intake (36). As we observed that icv nesfatin-1 induced a dose-dependent inhibition of gastric emptying (22), we applied abdominal surgery to investigate whether this visceral stressor would activate nesfatin-1 immunoreactive neurons assessed by Fos immunohistochemistry as marker for neuronal activation. Abdominal surgery that encompassed laparotomy and cecal palpation for 1 min performed in rats under short anesthesia activated nesfatin-1 immunopositive neurons in the magnocellular neuroendocrine system, anterior parvicellular part of the PVN, Edinger-Westphal nucleus and nuclei of the catecholaminergic and serotonergic system (Table 3) (37). Likewise, intraperitoneal injection of lipopolysaccharide originating from gram-negative bacterial cell walls activated nesfatin-1 immunoreactive neurons in the hypothalamic supraoptic, PVN and arcuate nuclei as well as in the nucleus of the solitary tract in the hindbrain (38) giving rise to a role of nesfatin-1 in the reduction of food intake observed after infections or surgery. In contrast to physical/immunological stressors, emotional stressors require processing and integration by the brain, particularly in cortical limbic and pontine structures and also hypothalamic nuclei to result in a stress response (39). Restraint, a model of emotional stress (40) also activates nesfatin-1 immunopositive neurons in various hypothalamic and hindbrain nuclei (Table 3) (17,41). In addition, when injected icv at higher doses compared with those effective to reduce dark phase food intake, nesfatin-1 induces anxiogenic and fear-related behaviours. This is supported by the reduction of time spent in the open arms in the elevated plus maze test and the startle response and freezing behaviour in an emotional response test without overall changes in locomotor activity (42), effects similar to those exerted by central injection of CRF (43,44) and urocortins 1 and 2 (45,46). As the anorexigenic effect of icv nesfatin-1 is dependent on downstream activation of CRF2 receptors, the central CRF signalling system may be involved in the anxiogenic and fear-related behaviours induced by nesfatin-1 as well. As nesfatin-1 immunoreactivity was also described in the central amygdaloid nucleus (11), a brain area primarily implicated in the mediation of fear and anxiety (47), central nesfatin-1 may also directly recruit this circuitry. Based on the consistent activation of nesfatin-1 neurons in key brain areas involved in processing the efferent limb of the stress response following exposure to visceral and psychological stressors (Table 3), nesfatin-1 may have an implication in the pathophysiology of stress including related eating disorders.
Table 3.
Activation of brain nesfatin-1 immunopositive neurons assessed by double immunohistochemistry for Fos/nesfatin-1 induced by visceral and psychological stressors in rats
| Brain nucleus | Abdominal surgery |
Wrap restraint |
||||||
|---|---|---|---|---|---|---|---|---|
| Fos |
Fos + nesfatin-1 |
Fos |
Fos + nesfatin-1 |
|||||
| Sham | Surgery | Sham | Surgery | Control | Restraint | Control | Restraint | |
| Supraoptic nucleus | + | ++++*** | + | ++++*** | + | +++* | + | +++* |
| Anterior parvicellular PVN | + | +++*** | + | +++*** | + | +++*** | − | ++*** |
| Lateral magnocellular PVN | ++ | ++++*** | + | +++*** | − | ++++* | − | ++* |
| Medial magnocellular PVN | ++ | ++++*** | + | ++++*** | − | ++++** | − | ++** |
| Medial parvicellular PVN | +++ | ++++*** | + | ++*** | + | ++++*** | − | ++** |
| Locus coeruleus | + | +++* | + | +++* | − | +++*** | − | ++*** |
| Rostral raphe pallidus | + | ++* | + | ++* | + | +++*** | − | ++*** |
| Edinger-Westphal nucleus | +++ | ++++** | ++ | +++** | ND | ND | ND | ND |
| Ventrolateral medulla | ++ | +++*** | + | +++** | − | ++*** | 1 | ++*** |
| Nucleus of the solitary tract† | +++ | ++++** | + | ++** | + | +++*** | − | ++*** |
Data are numbers of Fos-positive or double-labelled nesfatin-1/Fos immunoreactive neurons expressed as − 0 positive cells, + 1–4 positive cells, ++ 5–10 positive cells, +++ 11–20 positive cells and ++++ >20 positive cells.
P < 0.05;
P < 0.01;
P < 0.001 vs. sham/control.
ND, not detected; NUCB2, nucleobindin 2; PVN, paraventricular nucleus of the hypothalamus.
Distribution and effects of peripheral nesfatin-1
Distribution of nesfatin-1/nucleobindin 2 in peripheral organs
Recently, two independent groups reported that nesfatin-1 can cross the blood-brain barrier bidirectionally from the peripheral circulation to the brain and brain to the peripheral circulation in a non-saturable manner (48,49). As several peptides expressed in the gut are also produced in the brain (50), we hypothesized that nesfatin-1 is also produced in the periphery. We found that NUCB2 mRNA expression in the rat gastric oxyntic mucosa was 20-fold higher compared with the brain and 12-fold compared with other viscera such as the heart as assessed by microarray analysis and substantiated by RT-qPCR (9). Immunostaining in peripheral tissues confirmed the expression of nesfatin-1/NUCB2 protein in the rat stomach and additionally in pancreatic endocrine islets of Langerhans, in testis and the pituitary gland (9). Nesfatin-1 immunoreactivity was mainly co-expressed with ghrelin in X/A-like cells of the gastric oxyntic mucosa although in different populations of vesicles (9). Similarly, nesfatin-1 immunopositive cells of the endocrine pancreas exclusively co-localize with insulin in β-cells in mouse, rat and human (10,51). These findings suggest a differential release of nesfatin-1 and ghrelin from the stomach and nesfatin-1 and insulin from the pancreas which warrants further investigation. The prominent and exclusive endocrine distribution of nesfatin-1/NUCB2 in cells of the stomach and pancreas support that nesfatin-1 may act as a gut-brain peptide to influence food intake and glucose homeostasis.
Effects of peripheral nesfatin-1 on food intake and glucose control
When administered intraperitoneally, nesfatin-1 (~70 µg mouse−1) also reduces the dark phase food intake in mice during the 3 h post injection (52). Similar to the observations after central injection, peripheral administration of nesfatin-1 reduces food intake under conditions of leptin resistance as assessed in high fat diet-induced obese or leptin receptor deficient db/db mice (52). Structure activity studies established that a fragment of nesfatin-1, consisting of aa 24–53, also reduced the dark phase food intake upon peripheral injection, whereas the N-terminal and C-terminal fragments were inactive giving rise to the active core of nesfatin-1 in this middle fragment (52). Pretreatment with capsaicin to ablate afferent C fibres abolished the ip nesfatin-124–53-induced reduction of food intake (53). These data indicate a potential role of vagal afferents in peripheral nesfatin-1 signalling to the brain. This is further supported by the demonstration that nesfatin-11–82 activates neurons in the nodose ganglion in vitro which contains cell bodies of vagal afferents projecting to the gut and the nucleus of the solitary tract (54) (Fig. 1). In addition, peripherally injected nesfatin-124–53 increases the number of Fos-positive neurons in the nucleus of the solitary tract and up-regulates CART and POMC mRNA expression in this nucleus (52). However, as also pointed out by the authors (52), it is still unknown whether processing of NUCB2/nesfatin-1 leads to this fragment in vivo. Taken together, peripheral nesfatin-1/NUCB2’s anorexic action may be mediated by vagal afferents projecting to the nucleus of the solitary tract in addition to a potential hormonal action through crossing of the blood-brain barrier (Fig. 1). Supportive of this assumption, fasting reduced NUCB2 mRNA expression in an enriched population of gastric endocrine cells as assessed by microarray analysis and substantiated by RT-qPCR (9) resulting in decreased nesfatin-1/NUCB2 plasma levels which were restored after re-feeding (22). One has to keep in mind that peripheral doses required to influence food intake are >1000-fold higher than those effective in the brain suggesting a preferential central mode of action for nesfatin-1. However, as nesfatin-1/NUCB2 is very prominently expressed in the stomach, these levels may be reached in the blood or at local peripheral sites of action. Recent clinical studies showed a reduction in fasting plasma nesfatin-1/NUCB2 in type 2 diabetic patients compared with healthy controls which has been suggested to play a potential role in diabetic hyperphagia (55).
Because of the dense expression of nesfatin-1/NUCB2 in the pancreas and the exclusive co-localization of nesfatin-1 immunoreactivity with insulin in endocrine β-cells (10,51) a potential implication for nesfatin-1 in glucose control has been suspected. In contrast to the acute regulation observed in gastric endocrine cells, overnight fasting did not change the expression of NUCB2 protein in the islets of Langerhans (51). However, Goto-Kakizaki rats, an animal model for type 2 diabetes, had reduced pancreatic islet content of NUCB2 protein compared with non-diabetic Wistar rats (51). An increased expression of NUCB2 mRNA in islets of Langerhans has also been reported in hyperinsulinemic diet induced obese mice as assessed by microarray analysis (56). These data suggest that chronic conditions associated with dys-regulation of glucose homeostasis are associated with alterations of nesfatin-1/NUCB2 expression in the pancreas. As Goto-Kakizaki rats have an impaired β-cell secretory machinery (57) contributing to the compromised release of insulin (58), an impaired release of nesfatin-1/NUCB2 could be suspected as well. However, serum NUCB2 levels did not differ between Wistar and Goto-Kakizaki rats and in vitro the glucose induced NUCB2 release from isolated pancreatic islets of Wistar rats was much less pronounced compared with that of insulin (51). A recent study reported the dose-dependent reduction of blood glucose following intravenous injection of nesfatin-1 under conditions of hyperglycemia as observed in leptin receptor deficient db/db mice, whereas no effects were observed in normoglycemic C57BLKS/J mice (59). Taken together, these data indicate that chronic alterations in glucose concentrations affect pancreatic NUCB2 expression suggesting a regulatory role in glucose homeostasis, especially under conditions of pathologically altered blood glucose levels. Evidence so far points towards an intracellular or auto/paracrine rather than endocrine mode of action of pancreatic nesfatin-1/NUCB2.
Future developments
Nesfatin-1 as a potential target for the drug treatment of obesity
Reduced leptin sensitivity or leptin resistance is a common phenomenon in obese individuals (60). In the light of this and the pre-clinical findings that central and peripheral injection of nesfatin-1 exerts its food reducing effects via a leptin-independent mechanism (7,52), targeting nesfatin-1 may be a promising approach in the drug treatment of obesity and associated diseases. Ongoing pre-clinical data suggest the possible use of subcutaneous and intranasal routes of nesfatin-1 administration (61), which needs to be further explored. Another important aspect to be unraveled is the body weight reduction upon chronic administration of nesfatin-1 and possible related changes in energy expenditure for which reports so far are limited.
Processing and release of nesfatin-1
Although nesfatin-1 and nesfatin-124–53 display biological activity upon peripheral injection, at this point it is not clear whether these polypeptides are expressed endogenously. So far only one study reported the presence of nesfatin-1 in the cerebrospinal fluid of rats (7), whereas nesfatin-124–53 has not been detected yet. As full length NUCB2 also has an anorexigenic effect upon 3v injection (7), this may be the biologically active compound. In addition, in light of the co-localization of nesfatin-1 and ghrelin immunoreactivity in gastric X/A-like cells or nesfatin-1 and insulin signals in pancreatic β-cells, mechanisms involved in their differential regulation and release are largely unknown and need to be investigated.
Identification and characterization of the yet unknown nesfatin-1 receptor
Contrasting with the increasing evidence of nesfatin-1/NUCB2’s biological action to reduce feeding behaviour, the understanding of cellular signalling and receptors involved are unknown. One in vitro study reported that nesfatin-1 increases Ca2+ flux in rat hypothalamic neurons linked with protein kinase A suggesting a mediation by a G-protein coupled receptor (12). The identification of the nesfatin-1 receptor along with its localization in peripheral as well as central tissues will represent an essential leap forward to the understanding of the nesfatin-1 signalling system, site(s) of action and development of pharmacological interventions to further assess nesfatin-1’s physiological role.
Conclusion
Since the seminal report by Oh-I et al. (7), a growing number of consistent reports established that nesfatin-1/NUCB2 injected into the cerebrospinal fluid of brain ventricles (lateral, 3v, 4v) and cisterna magna at low picomolar doses inhibits nocturnal physiological feeding behaviour in rodents. Few reports have explored the impact on body weight gain; however, these studies point towards a body weight loss. Physiological relevance is indicated by the expression of nesfatin-1/NUCB2 in brain areas involved in the regulation of food intake, alterations of expression under different metabolic conditions and inducibility of long-lasting anorexigenic effects following brain injections of low doses of nesfatin-1. However, studies using selective antagonists and/or conditional knockouts will be required to further investigate whether nesfatin-1 is an essential modulator of food intake and to what extent other factors can compensate its function. The additional expression of nesfatin-1/NUCB2 in brain areas outside of feeding regulatory centres, in particular in brain nuclei responsive to various stress paradigms, suggests the involvement in stress-related neuronal circuitries. Consistent with the brain-gut axis, after the initially described central expression of NUCB2, nesfatin-1/NUCB2 was also found to be prominently expressed in peripheral viscera, especially in endocrine cells of stomach and pancreas. The intracellular co-localization of nesfatin-1 immunoreactivity with ghrelin in the stomach and insulin in the endocrine pancreas in different vesicles points towards differential regulation, release and consequently function in the control of food intake and glucose homeostasis. As centrally and peripherally injected nesfatin-1 acts independently of leptin signalling, nesfatin-1 nurtures the hope of representing a new target in the drug treatment of obesity, a condition usually associated with leptin resistance.
Acknowledgement
Supported by: German Research Foundation Grants STE 1765/1-1 (A.S.), GO 1718/1-1 (M.G.), VA Research Career Scientist Award, NIHDK 33061, Center Grant DK-41301 (Animal Core) (Y.T.).
We thank Ms. Eugenia Hu for reviewing the manuscript.
Footnotes
Conflict of Interest Statement
A.S., M.G and Y.T. have nothing to disclose.
References
- 1.Kanai Y, Tanuma S. Purification of a novel B cell growth and differentiation factor associated with lupus syndrome. Immunol Lett. 1992;32:43–48. doi: 10.1016/0165-2478(92)90197-v. [DOI] [PubMed] [Google Scholar]
- 2.Barnikol-Watanabe S, Gross NA, Gotz H, Henkel T, Karabinos A, Kratzin H, Barnikol HU, Hilschmann N. Human protein NEFA, a novel DNA binding/EF-hand/leucine zipper protein. Molecular cloning and sequence analysis of the cDNA, isolation and characterization of the protein. Biol Chem Hoppe Seyler. 1994;375:497–512. doi: 10.1515/bchm3.1994.375.8.497. [DOI] [PubMed] [Google Scholar]
- 3.Lin P, Fischer T, Weiss T, Farquhar MG. Calnuc, an EF-hand Ca(2+) binding protein, specifically interacts with the C-terminal alpha5-helix of G(alpha)i3. Proc Natl Acad Sci USA. 2000;97:674–679. doi: 10.1073/pnas.97.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miura K, Titani K, Kurosawa Y, Kanai Y. Molecular cloning of nucleobindin, a novel DNA-binding protein that contains both a signal peptide and a leucine zipper structure. Biochem Biophys Res Commun. 1992;187:375–380. doi: 10.1016/s0006-291x(05)81503-7. [DOI] [PubMed] [Google Scholar]
- 5.Karabinos A, Bhattacharya D, Morys-Wortmann C, Kroll K, Hirschfeld G, Kratzin HD, Barnikol-Watanabe S, Hilschmann N. The divergent domains of the NEFA and nucleobindin proteins are derived from an EF-hand ancestor. Mol Biol Evol. 1996;13:990–998. doi: 10.1093/oxfordjournals.molbev.a025667. [DOI] [PubMed] [Google Scholar]
- 6.Morel-Huaux VM, Pypaert M, Wouters S, Tartakoff AM, Jurgan U, Gevaert K, Courtoy PJ. The calcium-binding protein p54/NEFA is a novel luminal resident of medial Golgi cisternae that traffics independently of mannosidase II. Eur J Cell Biol. 2002;81:87–100. doi: 10.1078/0171-9335-00224. [DOI] [PubMed] [Google Scholar]
- 7.Oh IS, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 8.Foo K, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–579. doi: 10.1016/j.neuroscience.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 9.Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, Taché Y, Sachs G, Lambrecht NW. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150:232–238. doi: 10.1210/en.2008-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez R, Tiwari A, Unniappan S. Pancreatic beta cells colocalize insulin and pronesfatin immunoreactivity in rodents. Biochem Biophys Res Commun. 2009;381:643–648. doi: 10.1016/j.bbrc.2009.02.104. [DOI] [PubMed] [Google Scholar]
- 11.Goebel M, Stengel A, Wang L, Lambrecht NWG, Taché Y. Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic nuclei. Neurosci Lett. 2009;452:241–246. doi: 10.1016/j.neulet.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148:5088–5094. doi: 10.1210/en.2007-0701. [DOI] [PubMed] [Google Scholar]
- 13.Fort P, Salvert D, Hanriot L, Jego S, Shimizu H, Hashimoto K, Mori M, Luppi PH. The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience. 2008;155:174–181. doi: 10.1016/j.neuroscience.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, Dezaki K, Onaka T, Mori M, Yada T. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology. 2008;149:1295–1301. doi: 10.1210/en.2007-1276. [DOI] [PubMed] [Google Scholar]
- 15.Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh IS, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009;10:355–365. doi: 10.1016/j.cmet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Inhoff T, Stengel A, Peter L, Goebel M, Taché Y, Bannert N, Wiedenmann B, Klapp BF, Mönnikes H, Kobelt P. Novel insight in distribution of nesfatin-1 and phospho-mTOR in the arcuate nucleus of the hypothalamus of rats. Peptides. 2010;31:257–262. doi: 10.1016/j.peptides.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okere B, Xu L, Roubos EW, Sonetti D, Kozicz T. Restraint stress alters the secretory activity of neurons co-expressing urocortin-1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 in the mouse Edinger-Westphal nucleus. Brain Res. 2010;1317C:92–99. doi: 10.1016/j.brainres.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi N, Taniura H, Niinobe M, Takayama C, Tominaga-Yoshino K, Ogura A, Yoshikawa K. The postmitotic growth suppressor necdin interacts with a calcium-binding protein (NEFA) in neuronal cytoplasm. J Biol Chem. 2000;275:31674–31681. doi: 10.1074/jbc.M005103200. [DOI] [PubMed] [Google Scholar]
- 19.Kanai Y, Miura K, Uehara T, Amagai M, Takeda O, Tanuma S, Kurosawa Y. Natural occurrence of Nuc in the sera of autoimmune-prone MRL/lpr mice. Biochem Biophys Res Commun. 1993;196:729–736. doi: 10.1006/bbrc.1993.2310. [DOI] [PubMed] [Google Scholar]
- 20.Bustos G, Abarca J, Campusano J, Bustos V, Noriega V, Aliaga E. Functional interactions between somatodendritic dopamine release, glutamate receptors and brain-derived neurotrophic factor expression in mesencephalic structures of the brain. Brain Res Brain Res Rev. 2004;47:126–144. doi: 10.1016/j.brainresrev.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Lambrecht NW, Taché Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009;150:4911–4919. doi: 10.1210/en.2009-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yosten GL, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol. 2009;297:R330–R336. doi: 10.1152/ajpregu.90867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 25.Price CJ, Samson WK, Ferguson AV. Nesfatin-1 inhibits NPY neurons in the arcuate nucleus. Brain Res. 2008;1230:99–106. doi: 10.1016/j.brainres.2008.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu H, Arima H, Ozawa Y, Watanabe M, Banno R, Sugimura Y, Ozaki N, Nagasaki H, Oiso Y. Glucocorticoids increase NPY gene expression in the arcuate nucleus by inhibiting mTOR signaling in rat hypothalamic organotypic cultures. Peptides. 2010;31:145–149. doi: 10.1016/j.peptides.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 27.Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1642–R1647. doi: 10.1152/ajpregu.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zorrilla EP, Taché Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci. 2003;24:421–427. doi: 10.1016/S0165-6147(03)00177-9. [DOI] [PubMed] [Google Scholar]
- 29.Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szucs A, Koob GF, Zorrilla EP. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 2007;32:1052–1068. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Taché Y, Vale W. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- 31.Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci. 2003;23:7863–7872. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawashima S, Sakihara S, Kageyama K, Nigawara T, Suda T. Corticotropin-releasing factor (CRF) is involved in the acute anorexic effect of a-melanocyte-stimulating hormone: a study using CRF-deficient mice. Peptides. 2008;29:2169–2174. doi: 10.1016/j.peptides.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Xu L, Bloem B, Gaszner B, Roubos EW, Kozicz LT. Sex-specific effects of fasting on Urocortin 1, Cocaine- and Amphetamine-Regulated Transcript peptide and Nesfatin-1 expression in the rat Edinger-Westphal nucleus. Neuroscience. 2009;162:1141–1149. doi: 10.1016/j.neuroscience.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Nonogaki K, Ohba Y, Sumii M, Oka Y. Serotonin systems upregulate the expression of hypothalamic NUCB2 via 5-HT2C receptors and induce anorexia via a leptin-independent pathway in mice. Biochem Biophys Res Commun. 2008;372:186–190. doi: 10.1016/j.bbrc.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Noetzel S, Stengel A, Inhoff T, Goebel M, Wisser AS, Bannert N, Wiedenmann B, Klapp BF, Taché Y, Mönnikes H, Kobelt P. CCK-8S activates c-Fos in a dose-dependent manner in nesfatin-1 immunoreactive neurons in the paraventricular nucleus of the hypothalamus and in the nucleus of the solitary tract of the brainstem. Regul Pept. 2009;157:84–91. doi: 10.1016/j.regpep.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Barquist E, Bonaz B, Martinez V, Rivier J, Zinner MJ, Taché Y. Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. Am J Physiol. 1996;270:R888–R894. doi: 10.1152/ajpregu.1996.270.4.R888. [DOI] [PubMed] [Google Scholar]
- 37.Stengel A, Goebel M, Wang L, Taché Y. Abdominal surgery activates nesfatin-1 immunoreactive brain nuclei in rats. Peptides. 2010;31:263–270. doi: 10.1016/j.peptides.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnet MS, Pecchi E, Trouslard J, Jean A, Dallaporta M, Troadec JD. Central nesfatin-1 expressing neurons are sensitive to peripheral inflammatory stimulus. J Neuroinflammation. 2009;6:27. doi: 10.1186/1742-2094-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 40.Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- 41.Goebel M, Stengel A, Wang L, Taché Y. Restraint stress activates nesfatin-1-immunoreactive brain nuclei in rats. Brain Res. 2009;1300:114–124. doi: 10.1016/j.brainres.2009.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merali Z, Cayer C, Kent P, Anisman H. Nesfatin-1 increases anxiety- and fear-related behaviors in the rat. Psychopharmacology (Berl) 2008;201:115–123. doi: 10.1007/s00213-008-1252-2. [DOI] [PubMed] [Google Scholar]
- 43.Kalin NH, Takahashi LK. Fear-motivated behavior induced by prior shock experience is mediated by corticotropin-releasing hormone systems. Brain Res. 1990;509:80–84. doi: 10.1016/0006-8993(90)90311-x. [DOI] [PubMed] [Google Scholar]
- 44.Heinrichs SC, Menzaghi F, Merlo Pich E, Britton KT, Koob GF. The role of CRF in behavioral aspects of stress. Ann N Y Acad Sci. 1995;771:92–104. doi: 10.1111/j.1749-6632.1995.tb44673.x. [DOI] [PubMed] [Google Scholar]
- 45.Spina MG, Merlo-Pich E, Akwa Y, Balducci C, Basso AM, Zorrilla EP, Britton KT, Rivier J, Vale WW, Koob GF. Time-dependent induction of anxiogenic-like effects after central infusion of urocortin or corticotropin-releasing factor in the rat. Psychopharmacology (Berl) 2002;160:113–121. doi: 10.1007/s00213-001-0940-y. [DOI] [PubMed] [Google Scholar]
- 46.Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides. 2004;25:659–666. doi: 10.1016/j.peptides.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 48.Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides. 2007;28:2223–2228. doi: 10.1016/j.peptides.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Price TO, Samson WK, Niehoff ML, Banks WA. Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptides. 2007;28:2372–2381. doi: 10.1016/j.peptides.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Gardiner JV, Jayasena CN, Bloom SR. Gut hormones: a weight off your mind. J Neuroendocrinol. 2008;20:834–841. doi: 10.1111/j.1365-2826.2008.01729.x. [DOI] [PubMed] [Google Scholar]
- 51.Foo KS, Brauner H, Ostenson CG, Broberger C. Nucleobindin-2/nesfatin in the endocrine pancreas: distribution and relationship to glycaemic state. J Endocrinol. 2010;204:255–263. doi: 10.1677/JOE-09-0254. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu H, Oh IS, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T, Okada S, Yamada M, Yada T, Mori M. Peripheral administration of nesfatin-1 reduces food intake in mice: The leptin-independent mechanism. Endocrinology. 2009;150:662–671. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu H, Ohsaki A, Oh IS, Okada S, Mori M. A new anorexigenic protein, nesfatin-1. Peptides. 2009;30:995–998. doi: 10.1016/j.peptides.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Iwasaki Y, Nakabayashi H, Kakei M, Shimizu H, Mori M, Yada T. Nesfatin-1 evokes Ca2+ signaling in isolated vagal afferent neurons via Ca2+ influx through N-type channels. Biochem Biophys Res Commun. 2009;390:958–962. doi: 10.1016/j.bbrc.2009.10.085. [DOI] [PubMed] [Google Scholar]
- 55.Li QC, Wang HY, Chen X, Guan HZ, Jiang ZY. Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul Pept. 2010;159:72–77. doi: 10.1016/j.regpep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Iguchi H, Ikeda Y, Okamura M, Tanaka T, Urashima Y, Ohguchi H, Takayasu S, Kojima N, Iwasaki S, Ohashi R, Jiang S, Hasegawa G, Ioka RX, Magoori K, Sumi K, Maejima T, Uchida A, Naito M, Osborne TF, Yanagisawa M, Yamamoto TT, Kodama T, Sakai J. SOX6 attenuates glucose-stimulated insulin secretion by repressing PDX1 transcriptional activity and is down-regulated in hyperinsulinemic obese mice. J Biol Chem. 2005;280:37669–37680. doi: 10.1074/jbc.M505392200. [DOI] [PubMed] [Google Scholar]
- 57.Abdel-Halim SM, Guenifi A, Luthman H, Grill V, Efendic S, Ostenson CG. Impact of diabetic inheritance on glucose tolerance and insulin secretion in spontaneously diabetic GK-Wistar rats. Diabetes. 1994;43:281–288. doi: 10.2337/diab.43.2.281. [DOI] [PubMed] [Google Scholar]
- 58.Ostenson CG, Khan A, Abdel-Halim SM, Guenifi A, Suzuki K, Goto Y, Efendic S. Abnormal insulin secretion and glucose metabolism in pancreatic islets from the spontaneously diabetic GK rat. Diabetologia. 1993;36:3–8. doi: 10.1007/BF00399086. [DOI] [PubMed] [Google Scholar]
- 59.Su Y, Zhang J, Tang Y, Bi F, Liu JN. The novel function of nesfatin-1: anti-hyperglycemia. Biochem Biophys Res Commun. 2010;391:1039–1042. doi: 10.1016/j.bbrc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 60.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 61.Shimizu H, Oh IS, Okada S, Mori M. Nesfatin-1: an overview and future clinical application. Endocr J. 2009;56:537–543. doi: 10.1507/endocrj.k09e-117. [DOI] [PubMed] [Google Scholar]