Abstract
In pathological scenarios, such as tumor growth and diabetic retinopathy, blocking angiogenesis would be beneficial. In others, such as myocardial infarction and hypertension, promoting angiogenesis might be desirable. Due to their putative influence on endothelial cells, vascular pericytes have become a topic of growing interest and are increasingly being evaluated as a potential target for angioregulatory therapies. For example, the strategy of manipulating pericyte recruitment to capillaries could result in anti- or pro-angiogenic effects. However, our current understanding of pericytes is limited by knowledge gaps regarding pericyte identity and lineage. To use a music analogy, this review is a “mash-up” that attempts to integrate what we know about pericyte functionality and expression with what is beginning to be elucidated regarding their regenerative potential. We explore the lingering questions regarding pericyte phenotypic identity and lineage. The expression of different pericyte markers (e.g., SMA, Desmin, NG2 and PDGFR-β) varies for different subpopulations and tissues. Previous use of these markers to identify pericytes has suggested potential phenotypic overlaps and plasticity toward other cell phenotypes. Our review chronicles the state of the literature, identifies critical unanswered questions, and motivates future research aimed at understanding this intriguing cell type and harnessing its therapeutic potential.
Keywords: pericytes, stem cell, angiogenesis, therapy
INTRODUCTION
Pericytes are elongated mural support cells that extend along endothelial cells (41, 46). First identified by French physiologist and anatomist Charles–Marie–Benjamin Rouget in 1873 and termed “Rouget cells,” pericytes are present in every vascularized tissue in the body (32). Although it has been suggested that pericytes may reside in large vessels (5), pericytes are most commonly associated with the microvasculature. At the capillary level, pericytes regulate vessel permeability, vessel diameter, and endothelial cell proliferation through both paracrine signaling (43, 47, 64) and direct contact with endothelial cells (41), (101). Through cell-matrix and cell-cell interactions, pericytes play an essential role in contractile force transmission (59, 60, 63), vessel diameter regulation (81), vessel stabilization (41), and endothelial cell survival (38). Pericytes can even directly control capillary diameter, as their presence has been shown to be necessary for constriction (81). The functional effects of pericyte-endothelial cell signaling have previously been highlighted by comprehensive reviews (30, 112). Pericytes are considered “angioregulators” in that they can both stabilize and promote angiogenesis. Their importance in angiogenesis is evident when considering the cases of diabetic retinopathy and tumor growth, in which pericyte loosening from the endothelium is associated with uncontrollable angiogenesis (31, 117). But importantly, their function can be dependent on the type of stimulus. For example, angiogenesis in skeletal muscle has been correlated with both the withdrawal of pericytes and an increase in pericyte number (32, 33). In recent years, new and intriguing roles have emerged for the pericyte. These include mediating leukocyte trafficking (10, 100), contributing to fibrosis (87), and functioning as a tissue-resident stem or progenitor cell (107, 108). The multi-faceted role for pericytes emphasizes the need to both carefully identify their structural alterations during different pathological scenarios and better understand how their specific phenotypes relate to their functions. Meanwhile, pericytes have been convincingly established as critical players in angiogenesis and as potential therapeutic targets in inflammation and tissue regeneration. In addition to serving as putative drug targets, pericytes have been implicated as tools for cell-based therapies due to their angioregulatory capabilities.
As pericytes receive increasing attention from a wider array of research domains, it has become even more apparent that advancing our knowledge about pericytes and our ability to therapeutically manipulate their function will require answering fundamental questions about pericyte ancestry, progeny, and phenotypic differentiation capacity that have confounded studies since Rouget’s time. Even within the field of pericyte biology, varying terminology has created confusion (7). The perplexity of pericyte identity can be attributed to pericyte phenotypes being cell and tissue specific and is best exemplified by considering Ito cells in the liver. Ito cells, also known as hepatic stellate cells, are thought to be mesenchymally derived and display smooth muscle-like, pericyte-like characteristics (42). Ito cells, like pericytes, can exist in a quiescent or activated state depending on the local environment (42, 93). Ito cells interact with the sinusoid endothelium and control blood flow. They also can express multiple pericyte markers and participate in fibrogenesis. Based on the similarities to pericytes, Ito cells are, unsurprisingly, classified as the pericytes of the liver (83). In this review we further identify sources of potential confusion regarding pericyte phenotypes based on their overlaps with other cell types. Then, we focus on the plastic relationships between pericytes and mesenchymal stem cells and the potential for therapeutically using stem cells to play the roles of pericytes. Pericytes represent a scientifically intriguing and therapeutically exciting cell type, but there are far more questions than answers. We approach some of these questions by convolving some of the basic science understanding about pericyte form and function with emerging evidence that suggest multipotency and points to their application as a cell-based therapy.
PERICYTE DYNAMICS
Much of what constitutes our understanding about a cell population, and more importantly, the “identity” of a given cell population, are the behaviors and functionality that cells and their environment impart on one another. This is particularly true for pericytes, for which no cell type-specific expression markers exist. Even more informative than their behaviors during homeostasis is their behavior during dynamic processes. In the case of pericytes, the dynamic process of reference is angiogenesis, defined as the sprouting of new capillaries off of existing vessels. The role of pericytes in regulating angiogenesis has been reviewed extensively (12, 59), so we briefly summarize some of the landmark findings that have shaped the field’s understanding about pericyte function during this dynamic process.
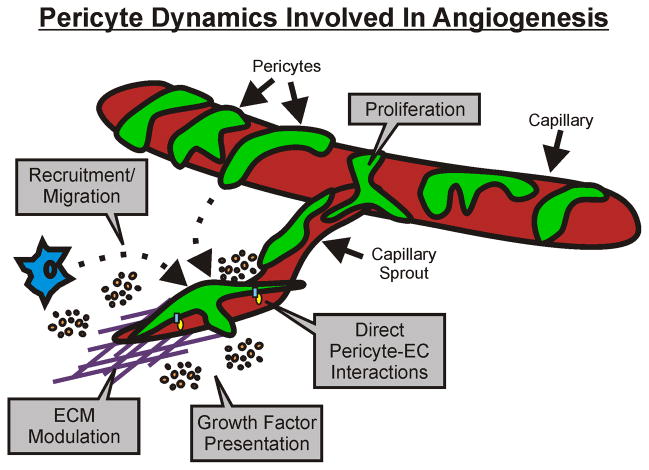
Pericyte dynamics during angiogenesis have been shown to include alterations in cell-to-cell contacts with endothelial cells, migration, growth factor presentation, proliferation, and extracellular matrix modulation (Figure 1) (6, 41). Pericytes are present along capillary sprouts and have been shown to both lag and lead endothelial cells at the sprout tip (11, 84). In some cases, pericytes can even bridge the gaps between two sprouting endothelial cell segments (75). Subsequently, the integration of pericyte dynamics during capillary sprouting logically can be linked to endothelial cell guidance. While the full scope of communication between pericytes and endothelial cells remains to be elucidated, work in this area has established that the pericyte-endothelial cell interactions during angiogenesis are regulated in part by Ang-1/Tie2, TGF-β and PDGFB/PDGFR-β signaling (36).
Figure 1.
Pericyte dynamics involved in angiogenesis. The main dynamics include cell-to-cell contacts with endothelial cells, migration, growth factor presentation, and extracellular matrix modulation. In some cases, pericyte proliferation has also been implicated. Each cellular interaction represents a target to manipulate capillary sprouting.
Perhaps the most convincing evidence for a requirement of endothelial cell-pericyte dynamic interaction during angiogenesis has been shown through work with PDGFB and PDGFR-β (43, 44, 47, 64). Manipulating proper pericyte investment by altering recruitment to the endothelium through PDGFB-PDGFR-β signaling can cause lethal microvascular dysfunction, affect vascular patterning during development, and affect the formation of new vessels during physiological and pathological angiogenesis (41). Indeed, tremendous research efforts using transgenic mice and co-culture in vitro assays have generated a molecularly detailed picture of the proteins that regulate pericyte dynamics during angiogenesis (60).
These and other landmark studies are foundational to the field’s current understanding of what pericytes are and the how they function during angiogenesis. Advancing our knowledge requires identifying how and when pericyte behaviors change during the transition from quiescent to angiogenic states. This entails not only a description of their spatial and temporal dynamics, but also a prospective method for identifying pericytes and potential specific subpopulation cell types. As we explore in the next section, this has proven to be a challenge that has created insights, as well as some degree of confusion, about pericyte lineage and plasticity. Indeed, the scope of pericyte dynamics broadens upon considering the potential of transdifferentiation of pericytes into or from other cell types (73, 74, 88). The putative links between interstitial cell populations (including macrophages, fibroblasts, and progenitor cells), pericytes, and SMCs necessitates discussion of how cell phenotypes, rather than cell nomenclature per se, relate to specific functions.
A CASE OF MISTAKEN IDENTITY?
As researchers recognize the importance and therapeutic potential for vascular pericytes, more questions than answers have been generated. Reflection on the literature reveals some historical confusion about pericytes – confusion propagated by a lack of cell type-specific markers, potential for transdifferentiation, and visualization in histological cross-sectional views that may obscure their true identity.
Pericyte Morphology
Cell types by are typically identified by their morphology and expression of certain genes and/or proteins. A quintessential hallmark of pericyte identity has long been the characteristic wrapping of cell processes, or filopodia, around capillary endothelium. Another morphological trait is the sharing of a common basal lamina with the endothelium (61, 95). Pericytes are most convincingly identified by their morphology when using electron microscopy of sectioned tissues (50) or confocal microscopy of whole-mounted tissues (70, 105). However, in most cases pericytes are identified using lower resolution techniques, which limits the classification to their general localization next to an endothelial cell. At low magnification (e.g., 20x or lower), it is impossible to determine where the ablumenal membrane of the endothelium ends and where the pericyte membrane starts, creating the possibility for one to mistake an extravasating monocyte, a proliferating endothelial cell, or any number of other cell types in the niche for a pericyte. Moreover, other cell types that can occupy space in the perivascular niche (e.g. macrophages, fibroblasts) may also express the same cell surface marker(s) being used to denote a pericyte phenotype. While morphology alone may be insufficient for ascribing a pericyte identity to an observed cell, marker expression is similarly inadequate for distinguishing pericytes from other cell types.
Pericyte Phenotypic Markers
Unlike some cell types, such as the endothelial cell, that are classically and specifically identified by their expression of cell type-specific genes or proteins (e.g. VE-cadherin and PECAM-1), a cell-type specific marker for pericytes has yet to be identified. Pericytes are known to express a battery of different proteins that can be visualized using off-the-shelf antibodies, but expression is shared by other cells, many of which occupy similar locations in tissues, further exacerbating the challenge of distinguishing pericytes from non-pericytes. Although this has the potential to create uncertainty, this challenge is an opportunity to gain new insights into pericyte lineage and identity.
Commonly used pericyte markers include SMA, Desmin, NG2 and PDGFR-β (41); however, their expression patterns are not pericyte-specific and differ depending on species, tissue, and even developmental stage (6, 41). The question remains: How do we unequivocally identify a pericyte? The use of the above mentioned markers and morphological characteristics provide a partial solution, but as Table 1 highlights, the “marker equals phenotype” approach can create confusion. Table 1, which lists the various pericyte markers that have been used by investigators interested in identifying pericytes, also raises the question whether the combination of different pericyte markers can be used to identify different pericyte subpopulations. As seen in Figure 2, the expression of known markers (NG2, Desmin, SMA) can identify two neighboring cells. Expression differences highlight what we still do not know regarding how local cues might regulate pericyte phenotypes. We postulate that observations like these implicate a possibility of pericyte phenotype specialization and motivate a new area of research that might be analogous to tip-cell versus stalk-cell sub-classifications for endothelial cells along a capillary sprout.
Table 1.
Expression Markers Used for Pericyte Identification
| Cell Surface Markers | Physiological Location | Detection Method | Citation |
|---|---|---|---|
| Aminopeptidace N, Aminopeptidase A, Nestin | Adult Mouse Brain | IHC | Alliot, et al. J. Neurosci Res. 1999. (1) |
| Endosialin, Mayer’s Hemalaun | Human Primary Pericytes | IHC, RT-PCR, Western Blot | Christian, et al. Am J Pathol. 2008. (21) |
| NG2 | Rat Mesentery | IHC | Murfee, et al. Microcirculation. 2006. (69) |
| NG2 | Mouse Retina | IHC | Taylor, et al. Microvasc Res. 2010. (106) |
| NG2, Alkaline Phosphatase | Human Skeletal Muscle | IHC, RT-PCR | Dellavalle, et al. Nat Cell Bio. 2007. (27) |
| NG2, Class III β-Tubulin | Rat Mesentery | IHC | Stapor, Murfee. Microvasc Res. 2012. (99) |
| NG2, PDGFRβ, CD29, CD90, CD146 | Mouse Femoral Artery | IHC | Tigges, Stallcup. J Vasc Res. 2012. (107) |
| NG2, PDGFRβ, RGS5 | Mouse Brain, Lung, Gut, Kidney, Artery, Vein | IHC, RNA Microarray, in situ Hybridization | Bondjers, et al. Am J Pathol. 2003. (15) |
| NG2, SMA | Rat Mesentery, Spinotrapezius, Dorsal Subcutaneous Tissue | IHC | Taylor, et al. Microciruation. 2007. (105) |
| NG2, SMA 3G5 | Bovine Retina | IHC | Kutcher, et al. Am J Pathol. 2007. (60) |
| NG2, SMA, Desmin | Mouse Choroid | IHC | Condren, et al. PLOS ONE. 2013. (23) |
| NG2, SMA, PDGFRβ | Mouse Kidney Interstitium | IHC | Schrimpf, Duffield. Curr Opin Nephrol Hypertens. 2011. (94) |
| NG2, SMA, PDGFRβ, Calponin I, smMHC | Murine Infantile Hemangioma | IHC, qRT-PCR, Western Blot | Boscolo, et al. Arterioscler Thromb Vasc Biol. 2011.(16) |
| NG2, SMA, PDGFRβ, Nestin | Rat Aorta | IHC, RT-PCR | Howson, el al. Am J Physiol Cell Physiol. 2005. (48) |
| NG2, SMA, TLR2, TLR4, FPR2, TNFR1, NLRP3, ICAM-1 | Human Placental Pericytes | IHC, RT-PCR | Stark, et al. Nat Immunol. 2012. (100) |
| SMA, 3G5 | Human Neonatal Foreskin | IHC, Phase-Contrast Microscopy, Magnetic Bead Isolation, FACS | Helmbold, et al. Microvasc Res. 2001.(45) |
Figure 2.
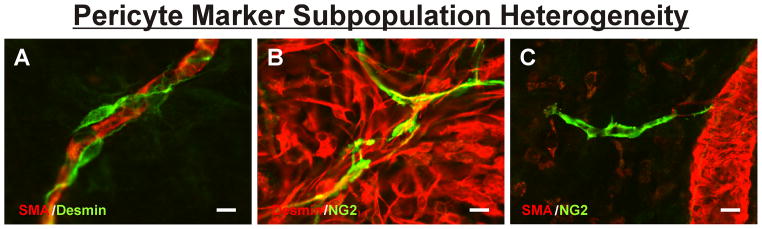
Pericyte marker subpopulation heterogeneity between neighboring cells in adult rat mesenteric microvascular networks. (A) Example of Desmin-positive cells wrapping around a SMA covered capillary in a quiescent scenario. (B) Example of NG2-positive pericytes along capillaries that co-localize to a sub-population of Desmin-positive cells during a wound healing response. (C) Example of an NG2-positive pericyte apparently along a capillary sprout off a SMA covered venule. Scale bars = 10 μm.
Pericyte Marker Overlap with Other Cell Types
As mentioned above, a pericyte-specific marker has not been identified by current research. Consequently, we must resort to identifying pericytes using a combination of markers, each of which is expressed by a multitude of other cell types that can occupy the perivascular niche (Figure 3). Recognition of labeling overlaps offers a potential opportunity to gain fundamental insight into both the lineage and plasticity of pericytes. Although the shared expression of one or more markers does not confirm that two different cells share the same lineage, it may suggest the possibility of a common lineage and should not be ruled out without further investigation. The idea that pericytes and other cell types resident in the perivascular-niche share a common lineage, which is evidenced by overlapping marker expression, is provocative.
Figure 3.
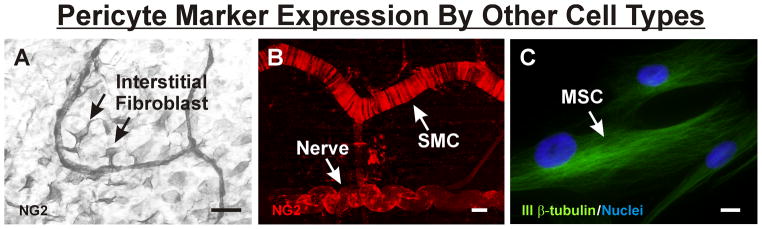
Pericyte marker expression by other cell types. (A) Example of NG2 identification of interstitial fibroblasts in remodeling adult rat mesenteric networks. The fibroblasts apparently interact with NG2-positive pericytes along capillaries. (B) Example of NG2 identification of tightly wrapped SMCs along an arteriole and a nerve in unstimulated mouse spinotrapezius muscle. (C) Example of class III β-tubulin identification of MSCs in vitro. Scale bars = 50 μm (A), 20 μm (B), and 10 μm (C).
For example, SMA is expressed by pericytes, immature SMCs, which express SMA but not smMHC, and mature SMCs, which express both contractile proteins (73, 85, 110, 115). Myofibroblasts, which can also reside in the perivascular niche, also can express SMA. For example, SMA expression identifies myofibroblasts in scenarios of wound healing and fibrosis (89). Recent studies have further suggested that SMA-positive myofibroblasts in the kidney originate from pericytes (49), lending support to the idea that coincident expression of the same marker in pericytes and other cell types may, in fact, signify a common lineage. Desmin and PDGFR-β are also expressed by mature SMCs and interstitial fibroblasts (72).
Perhaps the most common marker used to identify pericytes in recent years has been neuron-glia antigen 2, or, more commonly, NG2 (75, 76). We have demonstrated that NG2 is dramatically upregulated along venules during capillary sprouting, implicating its involvement and potential as an angiogenic specific marker (69). But like other pericyte markers, NG2 is not pericyte specific. During development, NG2 is expressed by oligodendrocyte progenitor cells, immature chondroblasts, skeletal myoblasts, and cardiomyocytes (97). NG2-positive cells in the CNS have been referred to as O2A cells because they can be differentiated into either oligodendrocytes or another type of glial cell in vitro. In the adult, NG2-positive cell populations increase following CNS injury, in part due to upregulation of NG2 by glial cells and macrophages (97). NG2 expressing cells also include Schwann cells, at least in mouse, and can be found near the nodes of Ranvier in the peripheral nervous system. This suggests a role for NG2-positive cells in regulation of myelination (97). NG2 was also found to be the same as HMP, previously associated with tumor cells, further confirming the concept that NG2 is expressed by highly active cell types (18). As mentioned above, NG2 is expressed by pericytes throughout the microvasculature in most, if not all, quiescent tissues. This above evidence suggests that pericyte NG2 expression pattern in the microvasculature mimics that in the nervous system, wherein NG2 expression contributes to both homeostasis and regeneration. Indeed, NG2-positive pericytes acquired from the central nervous system and exposed to bFGF in culture can acquire phenotypes that overlap with glial cell lineages (29). The common use of NG2 as a pericyte marker and its analogous expression pattern – and possibly function -- in neural and vascular support cells raises the question of whether other neural phenotypic markers also identify pericytes during angiogenesis.
Class III β-tubulin, which like NG2 has been identified as a marker of neural progenitor cells in the CNS and peripheral nerves in the adult, is another candidate pericyte marker. In contrast to other markers, class III β-tubulin might offer a temporal and spatial marker of angiogenic pericytes in vivo (99). In unstimulated adult rat mesenteric networks, class III β-tubulin is nerve specific and absent along arterioles, venules, and capillaries. After the networks are stimulated to undergo angiogenesis, class III β-tubulin is upregulated by pericytes along these vessel types and is subsequently down regulated to unstimulated levels after capillary sprouting (99). Class III β-tubulin is one of seven β-tubulin isotypes that forms α/β-tubulin heterodimers with six α-tubulin isotypes during microtubule assembly and is most commonly used as a marker of neural phenotypes (54). Much like NG2, during development in the central nervous system class III β-tubulin is transiently expressed by glial precursor cells; in the adult it is expressed by peripheral nerves (54). Outside the nervous system, class III β-tubulin expression by tumor cells correlates with increased metastasis and resistance to tubulin binding agents (40, 55). The positive expression of class III β-tubulin by tumor cells and human pericytes in vitro (unpublished data) highlights a potential issue with using class III β-tubulin expression to indicate a neural phenotype. For example, stem cell differentiation into nerves has been confirmed, in part, based on class III β-tubulin expression (13, 37). However, data from our laboratories, suggests that class III β-tubulin can be expressed by pericytes and is not nerve specific. We have also confirmed that human placenta-derived pericytes, human mesenchymal stem cells (Figure 3), and mouse embryonic stem cells also express class III β-tubulin in vitro (data not shown). Since mesenchymal stem cells might be a source of vascular pericytes in adult tissues (9) and, vice versa, vascular pericytes can be induced to exhibit multipotent stem cell activity (29), we speculate that the transient class III β-tubulin in vivo identifies a precursor cell population.
Both NG2 and class III β-tubulin expression by pericytes and neural cells highlight an emerging area of microvascular research focused on the link between neural and vascular patterning (34, 35, 113). This link is supported at the molecular level when considering growth inhibitors in the CNS such as ephrins, semaphorins, NG2, and Nogo (91), and is commonly presented in the context of either endothelial cell tip cells or arterial/venous identity. The overlap between neural and vascular patterning offers an exciting new perspective on the study of adult microvascular remodeling. The shared expression of NG2 and class III β-tubulin by pericytes and neural cells motivates the need to understand if and how perineural cells and pericytes are related.
Indeed, these findings motivate an even broader question: are pericytes able to differentiate into other cell types? Emerging evidence suggests the answer is “yes,” and a putative mechanism is via a bridging cell type that has well known multipotent differentiation capabilities: the MSC. For example, G. Paul et al. (80), recently isolated, purified and characterized a progenitor cell population from the ventricular wall and the neocortex in the adult human brain. They confirmed that these cells co-express markers for MSCs and pericytes in vivo and in vitro and have multilineage potential towards both mesodermal and neuroectodermal phenotypes. As discussed above, it is intriguing that the brain harbors a cell population with the ability to both modulate angiogenesis as pericytes and regenerate neural tissues as neural progenitor cells. Evidence from other tissues corroborates this interesting finding in the brain, and in the following section we use recent reports to conceptualize the putative linkage between pericytes and MSCs (Figure 4).
Figure 4.
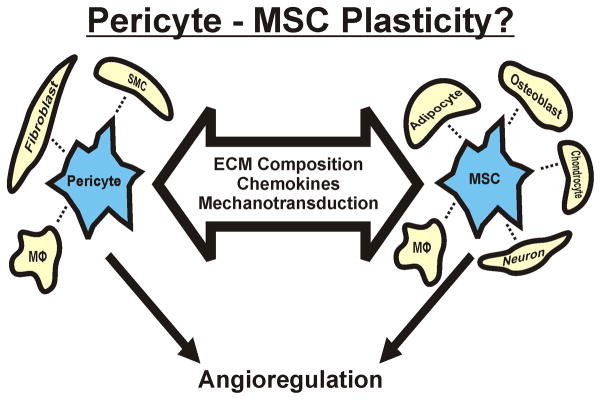
Pericyte and MSC phenotypic plasticity. Recent studies have suggested that pericytes (or a subset therein) and MSCs share a common lineage and possess phenotypic plasticity that can be modulated by different environmental factors (large arrow). A functional intersection between the different phenotypes is their potential to participate in angioregulation by either stimulating angiogenesis or stabilizing new vessel formation.
PERICYTES OR MESECHYMAL STEM CELLS (OR BOTH)?
Within the past ten years, a number of studies have demonstrated that MSCs and pericytes co-localize within the perivascular niche and express many of the same cell surface markers. Implying that the perivascular niche may serve as a systemic reservoir of tissue-resident stem cells (20, 52), these findings have generated a number of provocative questions around the topic of MSC and pericyte identity and lineage, and their possible overlap (Figure 4). In this section we will briefly review the literature in this area with respect to two fundamental questions that have yet to be completely answered: 1) are pericytes MSCs? and 2) do MSCs give rise to pericytes?
Are pericytes MSCs?
Initially isolated from the bone marrow, MSCs have been defined by the International Society for Cellular Therapy as a population of cells that, upon removal from tissue depots, is adherent to plastic, expresses a panel of defined surface markers (CD73, CD90, and CD105, and lack of CD11b or CD14, CD19 or CD79, CD45, and HLA-DR), and have the ability to differentiate into adipocytes, chondrocytes, and osteoblasts (28). Emerging evidence confirms the presence of MSCs within the perivascular niche in a wide array of tissues throughout the body, including fat, muscle, bone, and brain (65, 90, 96, 102, 103). Indeed, several studies have suggested that blood vessels throughout the circulation contain multi-lineage precursors and contribute to tissue repair/regeneration (25, 39). Dr. Bruno Péault and colleagues (26) were the first to identify, purify, and characterize distinct populations of MSCs from the vasculature of multiple human organs (19, 104). MSCs in the perivascular niche have been classified into subsets that include pericytes (26), adventitial cells (24), and myogenic endothelial cells (119). Each subset is able undergo adipogenesis, osteogenesis, and chondrogenesis in culture, and can elicit substantial regenerative capabilities when injected back in vivo. The regenerative capacity of multipotent pericytes, isolated by flow cytometry selection of CD34–/CD146+/CD45–/CD56– cells from adipose and other tissues, has been evaluated in skeletal muscle (79), lung (68), dermal (118), and nervous tissues (17). The lack of CD34 expression (CD34–) is thought to be a hallmark of multipotent pericytes in most tissues (26), but a rare CD34+/CD146+/CD45–/CD31– population of adipose-derived pericytes has also been identified in adipose tissue (109, 114, 120–123) using stringent rare-event strategies for its detection and isolation by flow cytometry (116, 121). So in many ways, detection of a multipotent subpopulation of pericytes, as is the case for pericyte identification in general, is still invariably elusive using the currently available techniques, such as flow cytometry and immunohistochemistry (65).
The extent to which pericytes share a common identity or lineage with MSCs may differ from tissue to tissue and depend on how quiescent or stimulated the tissue may be (67). There are likely a number of different mechanisms that control the endogenous phenotype of a pericyte at any given point in time (Figure 4). An alteration in chemical cues—either diffusible or matrix bound, is likely a key contributor to pericyte-MSC differentiation in situ. The source of these chemical cues is likely variable but attributable to cells in and around the perivascular niche, like endothelial cells, fibroblasts, and macrophages. Lee et al. (62) found that IL-1β –activated macrophages promote differentiation of perivascular-resident MSCs in adipose tissue to vascular SMCs through a prostaglandin F2α –mediated paracrine mechanism. It is also likely that changes in the extracellular matrix composition itself are candidates for regulators of MSC and pericyte differentiation (71). Mechanical stiffness of the underlying substrate has also been shown to influence de-activation of stem cells and myofibroblasts (57), so there are likely mechanical cues, in addition to chemical cues, that guide pericyte differentiation in situ. A disturbance in the endothelial-pericyte interaction has also been proposed as a driver for osteogenic and adipogenic differentiation of pericytes. In settings where endothelial-pericyte interactions are disrupted, like heterotopic ossification and atherosclerosis, pathological bone mineralization and adipogenesis has been observed, suggesting that disruption of endothelial-pericyte interactions may be another key driver of pericyte/MSC differentiation (75, 78).
Do MSCs differentiate into pericytes?
The relevance of this perivascular stem cell niche to tissue homeostasis and regeneration is only beginning to be explored, but one of its more obvious implications is as a sustainable source for new pericytes in vivo. As an example, MSCs derived from excised bone marrow and co-cultured with endothelial cells have been shown to differentiate into pericytes that can support engineered vessels in three-dimensional collagen gels implanted in vivo for more than 130 days (58). In another study where MSCs derived from the bone marrow of GFP transgenic mice were intravenously injected into mice that had received cutaneous wounds, GFP-positive bone marrow cells in the wound expressed pericyte markers (92).
Support for MSC differentiation into vascular pericytes is also provided by bone marrow lineage studies. Ozerdem et al. showed that NG2-expressing pericytes were recruited from the bone marrow when the mouse cornea was treated with bFGF to induce angiogenesis (77). Over ninety percent of these pericytes expressed CD45 or CD11b, indicating their hematopoietic origin and mesenchymal lineage overlap. Similar findings were presented by Rajnate et al. during tumor and VEGF induced angiogenesis. Song et al., also demonstrated that PDGFR-β expressing pericyte progenitors from the bone marrow reside in the tumor interstitium and are capable of differentiating into NG2, Desmin, and SMA-positive pericytes. Jung et al. identified what they termed “multipotent pericyte-like cells” (51) in the circulating blood based on fluorescence or magnetic activated cell sorting with anti-PDGFR-β antibody.
While compelling evidence seems to support that pericytes can be derived from MSCs and contribute to vascular growth and remodeling, the dynamics of this process remain an open avenue for discovery. Whether or not MSCs are derived from the bone marrow and circulate systemically to tissues throughout the body, or if they self-renew within tissue-resident perivascular niches, or both, have yet to be determined and will likely require some of the tools presented in the final section of this review.
STEM CELL APPLICATIONS FOR PERICYTE TARGETED ANGIOGENIC THERAPIES
Clearly, confusion remains: do pericytes and MSCs belong in the same pool, or are they distinct populations that share no such common lineage? Regardless of the answer, the fields of tissue engineering and regenerative medicine have continued to move forward, focusing on isolating and injecting these cells, despite their uncertain lineage, for therapeutic gain. While the mechanisms influencing their recruitment out of the circulation, differentiation, and dominant function are undefined, their application and our developing ability to manipulate their multifaceted role during angiogenesis offers promising potential for future therapies. This section will overview just a small sampling of the many studies (pre-clinical and clinical) that have explored cell-based angioregulation therapies where a pericyte-like role has either been explicitly mentioned or indirectly implicated (Figure 5; Table 2).
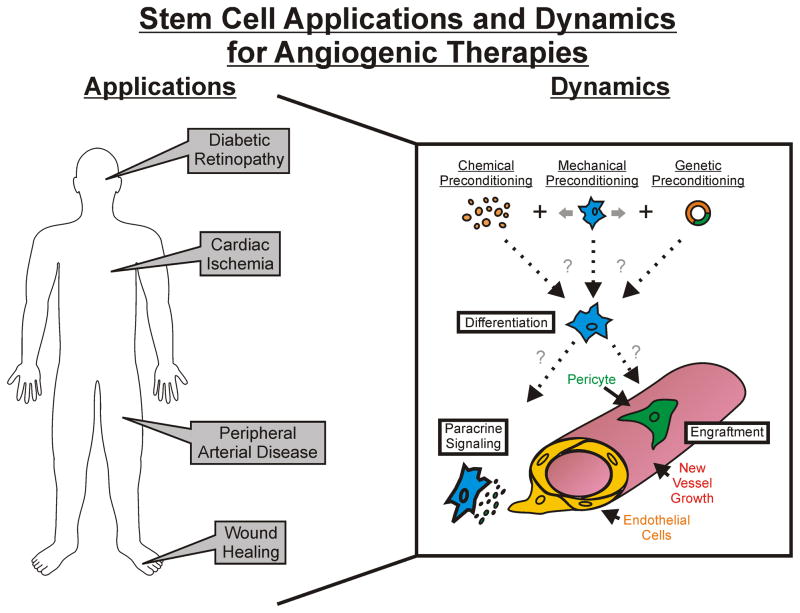
Figure 5.
The application of MSCs in cell based therapies to manipulate angiogenesis. The integrated schematic highlights the wide range of disease scenarios that can be targeted and the approaches to influence MSC fate and function for capillary sprouting. We hypothesize that optimal therapies will result from a combination of chemical, mechanical, and genetic preconditioning modalities. The embedded Table 2 details the pathology and delivery method for recent examples of stem cell therapeutic use.
Table 2.
MSC/Pericyte Therapeutic Uses
| Pathology | Delivery Method | Fate/Function | Citation |
|---|---|---|---|
| Infantile Hemangioma | Subcutaneous Matrigel | Pervascular Niche/Endothelial Cell Contact Mediated Vessel Formation | Boscolo, et al. Arterioscler Thromb Vasc Biol. 2011.(16) |
| Diabetic Retinopathy | Intravitreal Injection | Perivascular Niche/Vessel Stabilization | Mendel, et al. PLOS ONE. 2013. (66) |
| Myocardial Infarction | Intracardiac Injection | Perivascular Niche/Paracrine Signaling | Katare, et al. Circ Res. 2011. (53) |
| Bone Defects | Ectopic Intramusclar Implant | Osteoblast/Vascularized Bone Regeneration | Askarinam, et al. Tissue Eng Part A. 2013. (8) |
| Multiple Sclerosis | Intravenous Injection | Undetermined/CNS Inflammation Reduction | Cohen. Neurology. 2012. (22) |
| Malignant Gliomas | Intravenous Injection | Perivascular Niche/Tumor-Directed Migration | Bexell. Mol Ther. 2009. (14) |
| Bronchopulmonary Dysplasia | Intratracheal Injection | Perivascular Niche/Paracrine Signaling | Pierro, et al. Thorax. 2013. (82) |
As mentioned above, it has been suggested that MSCs can be derived from multiple tissue sources, including circulating blood. Our group was among the first to attribute a pericyte-like role to MSCs harvested from adipose tissue (Figure 6). Passaged MSCs from adipose tissue (also known as hASCs) were injected I.P. into Nude mice that had been stimulated by Compound 48/80 to invoke angiogenesis in the mesenteric tissues (2). Ten days later, the injected cells exhibited pericyte-like morphologies, expressed NG2 and SMA, and significantly increased vascular density. These effects persisted out to sixty days, suggesting that the injected MSCs may have differentiated into vessel-stabilizing pericytes. In the setting of diabetic retinopathy, Mendel el al. (66) also very recently demonstrated that MSCs obtained from human and mouse adipose tissue, operating in a pericyte-like capacity, stabilized the compromised vasculature in different murine models of retinal vasculopathies, including oxygen-induced retinopathy and Akimba diabetic mice. The authors showed that injected MSCs integrated alongside host endothelial cells in a vasculoprotective mechanism that was strengthened with TGF-β1 treatment, across all three murine models of disease.
Figure 6.
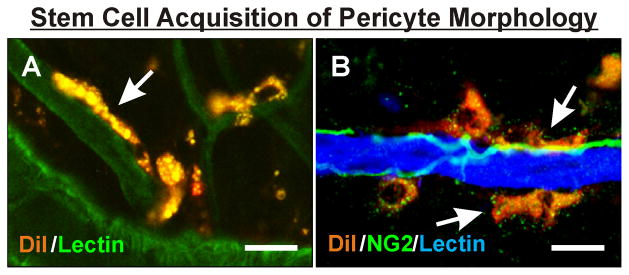
MSCs from adipose tissue (hASCs) adopt a putative pericyte fate in vivo following therapeutic delivery. DiI-labeled MSCs from human adipose tissue were injected intraperitoneally and identified in the inflamed Nude rat mesentery (A; (4)) or injected intravenously and identified in the ischemic spinotrapezius muscle of NOD-SCID mice (B). In both tissues, injected MSCs (arrows) were observed aligning along BSI-Lectin-positive capillaries in a manner similar to native pericytes and exhibited morphologies similar to that of native pericytes. NG2 labeling in (B) also identifies nerves. Scale bar = 10 μm.
Cardiac and peripheral ischemic diseases also represent potential uses for MSC-pericytes. Katare et al. (53) evaluated the effects of BMSC pericyte progenitor cells in an infarcted heart model and concluded that the delivered cells worked through a paracrine mechanism to reduce myocardial scaring, apoptosis, and fibrosis, while increasing vascular stability and attenuating permeability. In the setting of peripheral ischemic disease, Rehman et al. (86) reported that MSCs from adipose tissue increased endothelial cell growth and reduced endothelial apoptosis in a pericyte-like manner. In a model of hindlimb ischemia, perfusion recovery was accelerated when MSCs from adipose tissue were injected.
Another promising application of MSCs is in cutaneous wound healing. Kim et al. (56) demonstrated that topical transplantation of allogeneic MSCs in canine cutaneous wounds increased the rate of wound closure and degree of collagen production, cell proliferation, and angiogenesis primarily through paracrine effects on the local cell population. Using a model of delayed diabetic wound healing, Amos et al. (3) demonstrated that topically applied MSCs from adipose tissue also increased rate of wound closure through the production of extracellular matrix proteins and soluble factors. Similar results were obtained in a clinical trial by Vojtassak et al. (111) using autologous MSCs derived from bone marrow. In this study, a chronic non-healing diabetic ulcer was treated with BMSCs and autologous skin fibroblasts delivered in a collagen membrane. The ulcer experienced increased vascularity and dermis thickness, as well as significant wound closure over the 29 days of treatment. While pericytes were not explicitly monitored in any of these wound healing studies, each reported increases in vascularity, which is consistent with pericyte contributions.
Future studies like the examples detailed above will serve to transcend our understanding of MSC and pericyte functionality, especially in a therapeutic context. Whether or not MSCs and pericytes share a common lineage, the ease of culture expansion and transplantation make MSCs an attractive cell source for therapeutic angioregulation in different disease settings.
FUTURE PERSPECTIVE
Pericytes have recently attracted a lot of attention from the research community at large. Moving forward, new model systems and imaging approaches will greatly advance our understanding of their dynamics, functionality, and phenotypic flexibility. The ability to monitor and track endogenously-labeled pericytes in vivo using confocal imaging and other high-resolution intravital imaging approaches enables us to observe the dynamic behaviors of cells in tissues, such as the ear (100). Ex vivo explant systems where pericytes can be labeled and dynamically visualized will also help us learn how they interact with the microvasculature and other cell types throughout the tissue space and over time (98). Using these and other new tools will help us learn more about pericyte identity and lineage, which in turn will help us leverage our understanding of this unique cell population for therapeutic means. Meanwhile, fundamental questions remain to be answered regarding where pericytes come from, where they go, what they do, and what they can become.
Acknowledgments
Funding: NIH EY022063-01 to S.M.P; NIH 5-P20GM103629-02 to W.L.M.
The authors would like to acknowledge Anthony Bruce for the image in Figure 3B. This work was supported by the Tulane Center for Aging and NIH 5-P20GM103629-02 to W.L.M, as well as NIH EY022063-01 to S.M.P.
ABBREVIATIONS
- Ang1
Angiopoietin 1
- Ang2
Angiopoietin 2
- Tie2
Angiopoietin receptor tyrosine kinase receptor
- TGF-β
Transforming growth factor-β
- PDGFB
Platelet-derived growth factor B
- PDGFR-β
Platelet-derived growth factor receptor-β
- SMA
Smooth muscle alpha-actin
- SMCs
Smooth muscle cells
- NG2
Neuron-glia antigen 2
- HMP
Human melanoma proteoglycan
- PECAM-1
Platelet endothelial cell adhesion molecule-1
- VE-Cadherin
Vascular endothelial cadherin
- VEGF
Vascular endothelial growth factor
- CNS
Central nervous system
- O2A
Oligodendryocyte-type 2 astrocyte
- hASCs
Human adipose-derived stem cells
- bFGF
Basic fibroblast growth factor
- MSC
Mesenchymal stem cell
- GFP
Green fluorescent protein
- IL-1β
Interleukin-1β
- STZ
Streptozotocin
- RT-PCR
Reverse transcription polymerase chain reaction
- BDNF
Brain-derived neurotrophic factor
- CNTF
Ciliary neurotrophic factor
- GDNF
Glial cell line-derived neurotrophic factor
- NGF
Nerve growth factor
- TLR2
Toll-like receptor-2
- TLR4
Toll-like receptor-4
- FPR2
Formyl peptide receptor 2
- TNFR1
Tumor necrosis factor receptor-1
- NLRP3
NOD-like receptor protein-1
- NOD
Nucleotide-binding oligomerization domain-containing protein
- ICAM-1
Intercellular adhesion molecule-1
- smMHC
Smooth muscle Myosin heavy chain
- RGS5
Regulator of G-protein signaling 5
- FACS
Fluorescence-activated cell sorting
- BMSC
Bone marrow-derived stem cell
References
- 1.Alliot F, Rutin J, Leenen PJ, Pessac B. Brain parenchyma vessels and the angiotensin system. Brain Res. 1999;830(1):101–112. doi: 10.1016/s0006-8993(99)01373-6. [DOI] [PubMed] [Google Scholar]
- 2.Amos PJ, Bailey AM, Shang H, Katz AJ, Lawrence MB, Peirce SM. Functional binding of human adipose-derived stromal cells: effects of extraction method and hypoxia pretreatment. Ann Plast Surg. 2008;60(4):437–444. doi: 10.1097/SAP.0b013e318095a771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amos PJ, Kapur SK, Stapor PC, Shang H, Bekiranov S, Khurgel M, Rodeheaver GT, Peirce SM, Katz AJ. Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng Part A. 2010;16(5):1595–1606. doi: 10.1089/ten.tea.2009.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amos PJ, Shang H, Bailey AM, Taylor A, Katz AJ, Peirce SM. IFATS collection: The role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype. Stem Cells. 2008;26(10):2682–2690. doi: 10.1634/stemcells.2008-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreeva ER, Pugach IM, Gordon D, Orekhov AN. Continuous subendothelial network formed by pericyte-like cells in human vascular bed. Tissue Cell. 1998;30(1):127–135. doi: 10.1016/s0040-8166(98)80014-1. [DOI] [PubMed] [Google Scholar]
- 6.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Ashton N, de Oliveira F. Nomenclature of pericytes. Intramural and extramural. Br J Ophthalmol. 1966;50(3):119–123. doi: 10.1136/bjo.50.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Askarinam A, James AW, Zara JN, Goyal R, Corselli M, Pan A, Liang P, Chang L, Rackohn T, Stoker D, Zhang X, Ting K, Peault B, Soo C. Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein. Tissue Eng Part A. 2013;19(11–12):1386–1397. doi: 10.1089/ten.tea.2012.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111(9):4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayres-Sander CE, Lauridsen H, Maier CL, Sava P, Pober JS, Gonzalez AL. Transendothelial migration enables subsequent transmigration of neutrophils through underlying pericytes. PLoS One. 2013;8(3):e60025. doi: 10.1371/journal.pone.0060025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125(9):1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 12.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7(4):452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertani N, Malatesta P, Volpi G, Sonego P, Perris R. Neurogenic potential of human mesenchymal stem cells revisited: analysis by immunostaining, time-lapse video and microarray. J Cell Sci. 2005;118(Pt 17):3925–3936. doi: 10.1242/jcs.02511. [DOI] [PubMed] [Google Scholar]
- 14.Bexell D, Gunnarsson S, Tormin A, Darabi A, Gisselsson D, Roybon L, Scheding S, Bengzon J. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 2009;17(1):183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bondjers C, Kalen M, Hellstrom M, Scheidl SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J, Betsholtz C. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol. 2003;162(3):721–729. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boscolo E, Stewart CL, Greenberger S, Wu JK, Durham JT, Herman IM, Mulliken JB, Kitajewski J, Bischoff J. JAGGED1 signaling regulates hemangioma stem cell-to-pericyte/vascular smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31(10):2181–2192. doi: 10.1161/ATVBAHA.111.232934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho MM, Teixeira FG, Reis RL, Sousa N, Salgado AJ. Mesenchymal stem cells in the umbilical cord: phenotypic characterization, secretome and applications in central nervous system regenerative medicine. Curr Stem Cell Res Ther. 2011;6(3):221–228. doi: 10.2174/157488811796575332. [DOI] [PubMed] [Google Scholar]
- 18.Chekenya M, Immervoll H. NG2/HMP proteoglycan as a cancer therapeutic target. Methods Mol Biol. 2007;361:93–117. doi: 10.1385/1-59745-208-4:93. [DOI] [PubMed] [Google Scholar]
- 19.Chen CW, Corselli M, Peault B, Huard J. Human blood-vessel-derived stem cells for tissue repair and regeneration. J Biomed Biotechnol. 2012;2012:597439. doi: 10.1155/2012/597439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen WC, Park TS, Murray IR, Zimmerlin L, Lazzari L, Huard J, Peault B. Cellular kinetics of perivascular MSC precursors. Stem Cells Int. 2013;2013:983059. doi: 10.1155/2013/983059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christian S, Winkler R, Helfrich I, Boos AM, Besemfelder E, Schadendorf D, Augustin HG. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol. 2008;172(2):486–494. doi: 10.2353/ajpath.2008.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen JA. Mesenchymal stem cell transplantation in multiple sclerosis. J Neurol Sci. 2013 doi: 10.1016/j.jns.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Condren AB, Kumar A, Mettu P, Liang KJ, Zhao L, Tsai JY, Fariss RN, Wong WT. Perivascular mural cells of the mouse choroid demonstrate morphological diversity that is correlated to vasoregulatory function. PLoS One. 2013;8(1):e53386. doi: 10.1371/journal.pone.0053386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21(8):1299–1308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cossu G, Bianco P. Mesoangioblasts--vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13(5):537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 28.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 29.Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26(5):613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- 30.Dulmovits BM, Herman IM. Microvascular remodeling and wound healing: a role for pericytes. Int J Biochem Cell Biol. 2012;44(11):1800–1812. doi: 10.1016/j.biocel.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durham JT, Herman IM. Microvascular modifications in diabetic retinopathy. Curr Diab Rep. 2011;11(4):253–264. doi: 10.1007/s11892-011-0204-0. [DOI] [PubMed] [Google Scholar]
- 32.Edelman DA, Jiang Y, Tyburski J, Wilson RF, Steffes C. Pericytes and their role in microvasculature homeostasis. J Surg Res. 2006;135(2):305–311. doi: 10.1016/j.jss.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Egginton S, Hudlicka O, Brown MD, Graciotti L, Granata AL. In vivo pericyte-endothelial cell interaction during angiogenesis in adult cardiac and skeletal muscle. Microvasc Res. 1996;51(2):213–228. doi: 10.1006/mvre.1996.0022. [DOI] [PubMed] [Google Scholar]
- 34.Eichmann A, Le Noble F, Autiero M, Carmeliet P. Guidance of vascular and neural network formation. Curr Opin Neurobiol. 2005;15(1):108–115. doi: 10.1016/j.conb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005;19(9):1013–1021. doi: 10.1101/gad.1305405. [DOI] [PubMed] [Google Scholar]
- 36.Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87(7):1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 37.Foudah D, Redondo J, Caldara C, Carini F, Tredici G, Miloso M. Human mesenchymal stem cells express neuronal markers after osteogenic and adipogenic differentiation. Cell Mol Biol Lett. 2013;18(2):163–186. doi: 10.2478/s11658-013-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118(10):2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galli D, Innocenzi A, Staszewsky L, Zanetta L, Sampaolesi M, Bai A, Martinoli E, Carlo E, Balconi G, Fiordaliso F, Chimenti S, Cusella G, Dejana E, Cossu G, Latini R. Mesoangioblasts, vessel-associated multipotent stem cells, repair the infarcted heart by multiple cellular mechanisms: a comparison with bone marrow progenitors, fibroblasts, and endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25(4):692–697. doi: 10.1161/01.ATV.0000156402.52029.ce. [DOI] [PubMed] [Google Scholar]
- 40.Gan PP, McCarroll JA, Po’uha ST, Kamath K, Jordan MA, Kavallaris M. Microtubule dynamics, mitotic arrest, and apoptosis: drug-induced differential effects of betaIII-tubulin. Mol Cancer Ther. 2010;9(5):1339–1348. doi: 10.1158/1535-7163.MCT-09-0679. [DOI] [PubMed] [Google Scholar]
- 41.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314(1):15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 42.Hellerbrand C. Hepatic stellate cells--the pericytes in the liver. Pflugers Arch. 2013;465(6):775–778. doi: 10.1007/s00424-012-1209-5. [DOI] [PubMed] [Google Scholar]
- 43.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153(3):543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126(14):3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 45.Helmbold P, Nayak RC, Marsch WC, Herman IM. Isolation and in vitro characterization of human dermal microvascular pericytes. Microvasc Res. 2001;61(2):160–165. doi: 10.1006/mvre.2000.2292. [DOI] [PubMed] [Google Scholar]
- 46.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32(4):687–698. [PubMed] [Google Scholar]
- 47.Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D’Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999;84(3):298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- 48.Howson KM, Aplin AC, Gelati M, Alessandri G, Parati EA, Nicosia RF. The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am J Physiol Cell Physiol. 2005;289(6):C1396–407. doi: 10.1152/ajpcell.00168.2005. [DOI] [PubMed] [Google Scholar]
- 49.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176(1):85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iijima T, Zhang JQ. Three-dimensional wall structure and the innervation of dental pulp blood vessels. Microsc Res Tech. 2002;56(1):32–41. doi: 10.1002/jemt.10007. [DOI] [PubMed] [Google Scholar]
- 51.Jung KH, Chu K, Lee ST, Bahn JJ, Jeon D, Kim JH, Kim S, Won CH, Kim M, Lee SK, Roh JK. Multipotent PDGFRbeta-expressing cells in the circulation of stroke patients. Neurobiol Dis. 2011;41(2):489–497. doi: 10.1016/j.nbd.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 52.Katare R, Oikawa A, Cesselli D, Beltrami AP, Avolio E, Muthukrishnan D, Munasinghe PE, Angelini G, Emanueli C, Madeddu P. Boosting the pentose phosphate pathway restores cardiac progenitor cell availability in diabetes. Cardiovasc Res. 2013;97(1):55–65. doi: 10.1093/cvr/cvs291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami AP, Angelini G, Emanueli C, Madeddu P. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res. 2011;109(8):894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katsetos CD, Draberova E, Smejkalova B, Reddy G, Bertrand L, de Chadarevian JP, Legido A, Nissanov J, Baas PW, Draber P. Class III beta-tubulin and gamma-tubulin are co-expressed and form complexes in human glioblastoma cells. Neurochem Res. 2007;32(8):1387–1398. doi: 10.1007/s11064-007-9321-1. [DOI] [PubMed] [Google Scholar]
- 55.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10(3):194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 56.Kim JW, Lee JH, Lyoo YS, Jung DI, Park HM. The effects of topical mesenchymal stem cell transplantation in canine experimental cutaneous wounds. Vet Dermatol. 2013;24(2):242–e53. doi: 10.1111/vde.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kloxin AM, Benton JA, Anseth KS. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials. 2010;31(1):1–8. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428(6979):138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 59.Kutcher ME, Herman IM. The pericyte: cellular regulator of microvascular blood flow. Microvasc Res. 2009;77(3):235–246. doi: 10.1016/j.mvr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kutcher ME, Kolyada AY, Surks HK, Herman IM. Pericyte Rho GTPase mediates both pericyte contractile phenotype and capillary endothelial growth state. Am J Pathol. 2007;171(2):693–701. doi: 10.2353/ajpath.2007.070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lai CH, Kuo KH. The critical component to establish in vitro BBB model: Pericyte. Brain Res Brain Res Rev. 2005;50(2):258–265. doi: 10.1016/j.brainresrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Lee MJ, Kim MY, Heo SC, Kwon YW, Kim YM, Do EK, Park JH, Lee JS, Han J, Kim JH. Macrophages regulate smooth muscle differentiation of mesenchymal stem cells via a prostaglandin F(2)alpha-mediated paracrine mechanism. Arterioscler Thromb Vasc Biol. 2012;32(11):2733–2740. doi: 10.1161/ATVBAHA.112.300230. [DOI] [PubMed] [Google Scholar]
- 63.Lee S, Zeiger A, Maloney JM, Kotecki M, Van Vliet KJ, Herman IM. Pericyte actomyosin-mediated contraction at the cell-material interface can modulate the microvascular niche. J Phys Condens Matter. 2010;22(19):194115. doi: 10.1088/0953-8984/22/19/194115. [DOI] [PubMed] [Google Scholar]
- 64.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8(16):1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 65.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17(6):1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendel TA, Clabough EB, Kao DS, Demidova-Rice TN, Durham JT, Zotter BC, Seaman SA, Cronk SM, Rakoczy EP, Katz AJ, Herman IM, Peirce SM, Yates PA. Pericytes derived from adipose-derived stem cells protect against retinal vasculopathy. PLoS One. 2013;8(5):e65691. doi: 10.1371/journal.pone.0065691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meury T, Verrier S, Alini M. Human endothelial cells inhibit BMSC differentiation into mature osteoblasts in vitro by interfering with osterix expression. J Cell Biochem. 2006;98(4):992–1006. doi: 10.1002/jcb.20818. [DOI] [PubMed] [Google Scholar]
- 68.Montemurro T, Andriolo G, Montelatici E, Weissmann G, Crisan M, Colnaghi MR, Rebulla P, Mosca F, Peault B, Lazzari L. Differentiation and migration properties of human foetal umbilical cord perivascular cells: potential for lung repair. J Cell Mol Med. 2011;15(4):796–808. doi: 10.1111/j.1582-4934.2010.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murfee WL, Rehorn MR, Peirce SM, Skalak TC. Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation. 2006;13(3):261–273. doi: 10.1080/10739680600559153. [DOI] [PubMed] [Google Scholar]
- 70.Murfee WL, Skalak TC, Peirce SM. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation. 2005;12(2):151–160. doi: 10.1080/10739680590904955. [DOI] [PubMed] [Google Scholar]
- 71.Murphy CM, Matsiko A, Haugh MG, Gleeson JP, O’Brien FJ. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds. J Mech Behav Biomed Mater. 2012;11:53–62. doi: 10.1016/j.jmbbm.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992;270(3):469–474. doi: 10.1007/BF00645048. [DOI] [PubMed] [Google Scholar]
- 73.Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol. 1991;113(1):147–154. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Neill TJ, 4th, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res. 2005;97(10):1027–1035. doi: 10.1161/01.RES.0000189259.69645.25. [DOI] [PubMed] [Google Scholar]
- 75.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222(2):218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 76.Ozerdem U, Monosov E, Stallcup WB. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res. 2002;63(1):129–134. doi: 10.1006/mvre.2001.2376. [DOI] [PubMed] [Google Scholar]
- 77.Ozerdem U, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004;7(3):269–276. doi: 10.1007/s10456-004-4182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6(3):241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park TS, Gavina M, Chen CW, Sun B, Teng PN, Huard J, Deasy BM, Zimmerlin L, Peault B. Placental perivascular cells for human muscle regeneration. Stem Cells Dev. 2011;20(3):451–463. doi: 10.1089/scd.2010.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paul G, Ozen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E, Jansson K, Dannaeus K, Henriques-Oliveira C, Roybon L, Anisimov SV, Renstrom E, Svensson M, Haegerstrand A, Brundin P. The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS One. 2012;7(4):e35577. doi: 10.1371/journal.pone.0035577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443(7112):700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pierro M, Ionescu L, Montemurro T, Vadivel A, Weissmann G, Oudit G, Emery D, Bodiga S, Eaton F, Peault B, Mosca F, Lazzari L, Thebaud B. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013;68(5):475–484. doi: 10.1136/thoraxjnl-2012-202323. [DOI] [PubMed] [Google Scholar]
- 83.Pinzani M. Hepatic stellate (ITO) cells: expanding roles for a liver-specific pericyte. J Hepatol. 1995;22(6):700–706. doi: 10.1016/0168-8278(95)80227-4. [DOI] [PubMed] [Google Scholar]
- 84.Ponce AM, Price RJ. Angiogenic stimulus determines the positioning of pericytes within capillary sprouts in vivo. Microvasc Res. 2003;65(1):45–48. doi: 10.1016/s0026286202000146. [DOI] [PubMed] [Google Scholar]
- 85.Price RJ, Owens GK, Skalak TC. Immunohistochemical identification of arteriolar development using markers of smooth muscle differentiation. Evidence that capillary arterialization proceeds from terminal arterioles. Circ Res. 1994;75(3):520–527. doi: 10.1161/01.res.75.3.520. [DOI] [PubMed] [Google Scholar]
- 86.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 87.Ren S, Duffield JS. Pericytes in kidney fibrosis. Curr Opin Nephrol Hypertens. 2013;22(4):471–480. doi: 10.1097/MNH.0b013e328362485e. [DOI] [PubMed] [Google Scholar]
- 88.Rhodin JA, Fujita H. Capillary growth in the mesentery of normal young rats. Intravital video and electron microscope analyses. J Submicrosc Cytol Pathol. 1989;21(1):1–34. [PubMed] [Google Scholar]
- 89.Rojas A, Chang FC, Lin SL, Duffield JS. The role played by perivascular cells in kidney interstitial injury. Clin Nephrol. 2012;77(5):400–408. doi: 10.5414/CN107371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 91.Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46(3):225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- 92.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 93.Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28(2):105–112. doi: 10.1247/csf.28.105. [DOI] [PubMed] [Google Scholar]
- 94.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2011;20(3):297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 95.Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7(11):1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 96.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 97.Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2002;31(6–7):423–435. doi: 10.1023/a:1025731428581. [DOI] [PubMed] [Google Scholar]
- 98.Stapor PC, Azimi MS, Ahsan T, Murfee WL. An angiogenesis model for investigating multicellular interactions across intact microvascular networks. Am J Physiol Heart Circ Physiol. 2013;304(2):H235–45. doi: 10.1152/ajpheart.00552.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stapor PC, Murfee WL. Identification of class III beta-tubulin as a marker of angiogenic perivascular cells. Microvasc Res. 2012;83(2):257–262. doi: 10.1016/j.mvr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 100.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14(1):41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 101.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114(24):5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tavian M, Zheng B, Oberlin E, Crisan M, Sun B, Huard J, Peault B. The vascular wall as a source of stem cells. Ann N Y Acad Sci. 2005;1044:41–50. doi: 10.1196/annals.1349.006. [DOI] [PubMed] [Google Scholar]
- 105.Taylor AC, Murfee WL, Peirce SM. EphB4 expression along adult rat microvascular networks: EphB4 is more than a venous specific marker. Microcirculation. 2007;14(3):253–267. doi: 10.1080/10739680601141829. [DOI] [PubMed] [Google Scholar]
- 106.Taylor AC, Seltz LM, Yates PA, Peirce SM. Chronic whole-body hypoxia induces intussusceptive angiogenesis and microvascular remodeling in the mouse retina. Microvasc Res. 2010;79(2):93–101. doi: 10.1016/j.mvr.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tigges U, Komatsu M, Stallcup WB. Adventitial pericyte progenitor/mesenchymal stem cells participate in the restenotic response to arterial injury. J Vasc Res. 2013;50(2):134–144. doi: 10.1159/000345524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Torsney E, Xu Q. Resident vascular progenitor cells. J Mol Cell Cardiol. 2011;50(2):304–311. doi: 10.1016/j.yjmcc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 109.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102(1):77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 110.Van Gieson EJ, Murfee WL, Skalak TC, Price RJ. Enhanced smooth muscle cell coverage of microvessels exposed to increased hemodynamic stresses in vivo. Circ Res. 2003;92(8):929–936. doi: 10.1161/01.RES.0000068377.01063.79. [DOI] [PubMed] [Google Scholar]
- 111.Vojtassak J, Danisovic L, Kubes M, Bakos D, Jarabek L, Ulicna M, Blasko M. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol Lett. 2006;27 (Suppl 2):134–137. [PubMed] [Google Scholar]
- 112.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312(5):623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 113.Weinstein BM. Vessels and nerves: marching to the same tune. Cell. 2005;120(3):299–302. doi: 10.1016/j.cell.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 114.Yamanishi H, Fujiwara S, Soma T. Perivascular localization of dermal stem cells in human scalp. Exp Dermatol. 2012;21(1):78–80. doi: 10.1111/j.1600-0625.2011.01407.x. [DOI] [PubMed] [Google Scholar]
- 115.Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96(3):280–291. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]
- 116.Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208(1):64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 117.You WK, Yotsumoto F, Sakimura K, Adams RH, Stallcup WB. NG2 proteoglycan promotes tumor vascularization via integrin-dependent effects on pericyte function. Angiogenesis. 2013 doi: 10.1007/s10456-013-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zebardast N, Lickorish D, Davies JE. Human umbilical cord perivascular cells (HUCPVC): A mesenchymal cell source for dermal wound healing. Organogenesis. 2010;6(4):197–203. doi: 10.4161/org.6.4.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, Yap S, Pollett JB, Drowley L, Cassino T, Gharaibeh B, Deasy BM, Huard J, Peault B. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25(9):1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 120.Zimmerlin L, Donnenberg VS, Donnenberg AD. Pericytes: a universal adult tissue stem cell? Cytometry A. 2012;81(1):12–14. doi: 10.1002/cyto.a.21168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zimmerlin L, Donnenberg VS, Donnenberg AD. Rare event detection and analysis in flow cytometry: bone marrow mesenchymal stem cells, breast cancer stem/progenitor cells in malignant effusions, and pericytes in disaggregated adipose tissue. Methods Mol Biol. 2011;699:251–273. doi: 10.1007/978-1-61737-950-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77(1):22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zimmerlin L, Donnenberg VS, Rubin JP, Donnenberg AD. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A. 2013;83(1):134–140. doi: 10.1002/cyto.a.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]