Abstract
Spinocerebellar ataxia type 14 (SCA14) is an autosomal, dominant neurodegenerative disorder caused by mutations in PKCγ. The objective of this study was to determine effects of PKCγ H101Y SCA14 mutation on Purkinje cells in the transgenic mouse. Results demonstrated that wild type PKCγ-like Purkinje cell localization of HA-tagged PKCγ H101Y mutant proteins, altered morphology and loss of Purkinje cells were observed in the PKCγ H101Y SCA14 transgenic mouse at four weeks of age. Failure of stereotypical clasping responses in the hind limbs of transgenic mice was also observed. Further, PKCγ H101Y SCA14 mutation caused lack of total cellular PKCγ enzyme activity, loss of connexin 57 phosphorylation on serines, and activation of caspase-12 in the PKCγ H101Y SCA14 transgenic mouse. Results clearly demonstrate a need for PKCγ control of gap junctions for maintenance of Purkinje cells. This is the first transgenic mouse to our knowledge which models a human SCA14 mutation.
Introduction
Spinocereballar ataxias (SCAs) are autosomal, heterogeneous, dominant neurodegenerative disorders. SCAs are classified at least into 27 types. Fourteen out of 27 SCAs are linked to particular gene mutations [1]. Spinocerebellar ataxia type 14 (SCA14) is caused by mutations in the PKCγ gene with onset age as early as three years [2–27]. SCA14 is a newly identified neurodegenerative disorder, the mechanistic aspects of this disease remain unknown.
PKC is a family of phospholipid-dependent serine/threonine kinases that participates in many cellular functions. PKCγ is a classical PKC, primarily found in the central and peripheral nervous systems. It is particularly abundant in cerebellar Purkinje cells and in hippocampal pyramidal cells [28]. SCA14 mutations occur throughout the PKCγ gene from the regulatory to catalytic domains and also include a six-pair in-frame deletion and a splice site mutation in the C1B region, where most mutations occur [2–27]. Verbeek et al. [10] reported that PKCγ SCA14 mutations have increased kinase activity and altered membrane targeting in kidney COS-7 cells with overexpression of SCA14 mutants. No significant mutant protein aggregation is observed. However, Seki et al. reported that SCA14 mutants are susceptible to aggregation in the Chinese hamster ovary CHO-K1 [7]. These contradictory conclusions, by two separate groups, indicates that irrelevant cell lines, such as kidney COS-7 and Chinese hamster ovary CHO-K1, may not be appropriate in vitro cell systems to study the molecular mechanism of a neurodegenerative disorder, such as SCA14.
Since SCA14 is rare and not fatal in most cases, it is difficult to investigate the pathogenesis due to lacking of human brain tissue [2]. Thus, it is critical to generate an animal model with SCA14 phenotype. In our lab, we have previously used well-characterized, hippocampal neuronal HT22 cells [6,29]. We have demonstrated that these mutant PKCγ’s cause a caspase-3 linked apoptosis in culture and PKCγ SCA14 mutants are not activated by an oxidative signal such as H2O2 when expressed in neuronal HT22 cells in culture [6]. Endogenous wild type PKCγ is negatively affected by the presence of the mutations (ie., a dominant effect). Here we reported the generation of the PKCγ H101Y SCA14 transgenic mouse which expresses loss of Purkinje cells and neurological phenotype.
Materials and Methods
Generation of the transgenic mouse
The HA-tagged PKCγ H101Y mice were generated in a C57BL/6J background by the standard transgenic strategy. The experiments were done at the University of Missouri-Columbia Transgenic Animal Facility using the pronuclear injection method. Injected DNA fragments in a length of 3.14 kb consisted of the CMV promoter, PKCγ H101Y SCA14 mutant with N-terminal HA tags, and a SV40 poly A terminus. Positive pups were confirmed by PCR using tail genomic DNA. Primers used for PCR screen were:
Primer 1. HAFOR, Tm= 68 C, 35 mers:
5′ CC ATG TAC CCA TAC GAT GTT CCA GAT TAC GCT CTT 3′
Primer 2. SCAREV, Tm=70 C, 28 mers:
5′ GGT CGC AGA AGG TGG GAC TGC TGT AGC T 3′
Predicted PCR products were 403 bp in length containing the HA tag and a partial N-terminal portion of PKCγ H101Y SCA14 mutants.
Internal control primer pairs (ZP3-1 and ZP3-2) were used as a DNA quality control to amplify genomic DNA:
ZP3-1 CAG CTC TAC ATC ACC TGC CA
Zp3-2 CAC TGG GAA GAG ACA CTC AG
The predicted products were 500 bp.
Western blot was performed to further confirm the positive generations using anti-HA antibody. Founders were mated with wild type C57BL/6J mice to create the next generation. After the 6th generation, mice from same littermates were used for studies.
Mouse cerebellar slice culture
Cerebellar slice culture was modified from the previous reports [28, 30]. Briefly, after euthanization, sagittal cerebellar slices were obtained immediately from the wild type and/or PKCγ H101Y transgenic mice at age of 4 weeks. The cerebellar slices were sectioned at 250 μm and cultured in serum-free BME medium supplemented with 2.5 mM glutamine, 5 mM glucose and 5 ng/mL nerve growth factor at 5 % CO2 in an incubator at 37 °C for up to 1 hour. H2O2/oxidative stress treatments were conducted in the slice culture system. After that, slices were homogenized for further analyses.
Cell cultures
HT22 cells were cultured in DMEM (high glucose, 4.5 g/L) (Invitrogen, CA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, GA) and 50 μg/ml gentamicin, 0.05 U/ml penicillin, 50 μg/ml streptomycin, pH 7.4 at 37 °C in an atmosphere of 95% air and 5% CO2.
Western blot and immunoprecipitation
Western blotting and immunoprecipitation were performed as described previously [4]. For immunoprecipitation assay, cells or tissue homogenates were lysed. After 8,000 x g centrifugation for 15 min the supernatants are used for immunoprecipitation. Anti-HA antibody was purchased from Covance (Berkeley, CA), anti-PKCγ was from BD Biosciences (San Jose, CA), anti-Cx57 was purchased from Diatheva (Italy), and antibodies against caspase-3, caspase-12, and α-tubulin were purchased from Cell Signaling (Danvers, MD).
PKCγ enzyme activity assays
PKCγ enzyme activity was measured as previously described [4]. Briefly, equal protein amounts of cerebellar slice extracts from the wild type or PKC γ H101Y transgenic mice were incubated with PKC γ antisera at 4 °C for 4 h to immunoprecipitate endogenous wild type PKCγ and/or H101Y mutants. Immunoprecipitated PKCγ and/or PKCγ H101Y-agarose bead complexes were recovered and incubated with PKC reaction mixture according to the manufacturer’s instructions. The fluorescent phospho-PepTag peptides (phosphorylated by PKCγ and/or PKCγH101Y) were resolved by 0.8% agarose gel electrophoresis and visualized under UV light. The phosphorylated peptide bands were excised, and their fluorescence intensities were quantified by spectrophotometry at 570 nm.
Immunohistochemistry and confocal microscopy
To determine the Purkinje cell localization of PKCγ, PKC γ H101Y, and Cx57 in the wild type and transgenic mice, cerebellar tissues were fixed in 2 % paraformaldehyde and sagittally sectioned at 20 μm in thickness using a cryostat. The cerebellar sections were labeled with primary antisera including anti-Cx57, anti-HA, and/or anti-PKCγ for overnight at 4 °C. After washing in PBS, the slice sections were further incubated with secondary antisera with Alexa Fluor 568 or 488 conjugation for 2 hours at room temperature. Then Purkinje cell localization of desired proteins was determined using a Nikon C1 confocal microscope.
Light microscopy
For the Purkinje cell pathogenesis study, whole cerebellum was fixed in 2% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M cacodylate and then post–fixed in osmium tetroxide. Dehydration was in 70 % ethanol for 12 h, overnight with 100 % ethanol, and 12 h with 100 % acetone and finally embedding in epon resin at room temperature. Sagittal cerebellar sections were made with an ultramicrotome and thick sections (1 μm) were cut with a diamond knife and stained with 1% toluidine blue. Purkinje cell layers including soma and dentrites were viewed and images were captured by a Nikon light microscope.
Tail suspension photography
Neurological dysfunction of transgenic mice was exhibited as hindlimb and/or forelimb clasping determined by tail suspension test [31, 32]. A tape was applied to the surface of the tail to set a metal clip which is anchored to the fixed lever. Mice first struggled to escape but sooner or later attained a posture of immobility. The response of each mouse to 1 min of vertical suspension from the tail was tested and photographed. Total 12 transgenic and 12 wild type mice at 12 months of age were tested. Of this, over 90 % of the transgenic mice exhibited a clasping response as illustrated in Figure 1D.
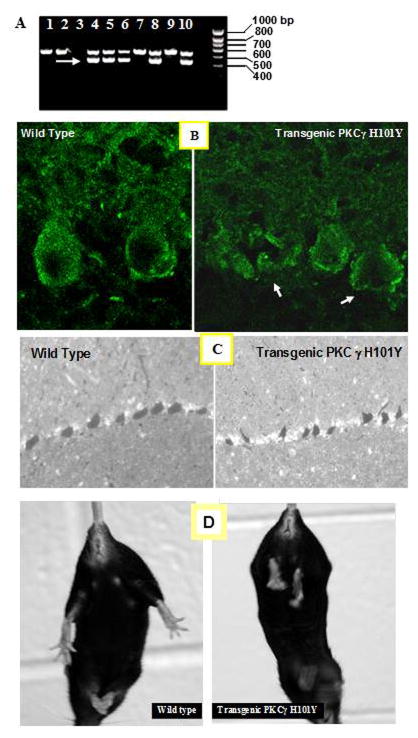
Fig. 1. Characterization of PKCγ H101Y SCA14 transgenic mice.
A. Five founders were identified by PCR using PKCγ H101Y specific primers. Transgenic PCR products are arrowed. B. Endogenous PKCγ is mainly localized in Purkinje cells of the wild type mouse (left panel), and a similar Purkinje cell localization of HA-tagged PKCγ H101Y proteins in the PKCγ H101Y transgenic mouse at the age of 4 weeks (right panel). Altered Purkinje cell body is observed in transgenic mice (as arrowed). C. Light microscopy of Purkinje cell layers in the wild type (left panel) and PKCγ H101Y transgenic mice (right panel) at four weeks of age, showing an altered morphology of Purkinje cell layer in the transgenic mice. D. Tail suspension photography. Almost all 12-month old nontransgenic mice demonstrated splaying out of hind limbs (Left panel). However, PKCγ H101Y transgenic mice manifested stereotypical clasping responses (Right panel)
Measurement of cell surface Cx57 gap junction plaques
HT22 cells were treated with 200 nM 12-O-tetradecanoylphorbol-13-acetate (TPA), 100 μM H2O2, or phosphate-buffered saline (PBS) for 30 min. Endogenous Cx57 gap junctions was determined as described previously [4]. Briefly, the cells were fixed with 2.5 % paraformaldehyde for 5 min and labeled with anti-Cx57 overnight at 4 °C. After washing, the fixed cells were incubated with the secondary antibody with Alexa Fluor 568 conjugation. The cells were then examined using a Nikon confocal microscope. We photographed ten points per slide, three slides for each treatment. For quantitation, the cell surface Cx57 gap junctions from single cells in single sections in each image were counted. The number of gap junction plaques was expressed as mean ± SEM.
Statistical analysis
The statistical analysis employed in this paper is the unpaired Student’s t-Test. All analyses represent at least triplicate experiments. The level of significance (*) was considered at p ≤ 0.05. All data are mean ± S.E.M.
Results and Discussion
Neurological phenotype of the PKCγ H101Y transgenic mouse
According to our in vitro cell culture study, PKCγ H101Y mutant cells have milder cell apoptosis phenotype while S119P and G128D mutants are more severe [4, 6]. Therefore, we expected that PKCγ H101Y mutation would not be lethal to the transgenic mutant mouse. In the current study, we have successfully created five PKCγ H101Y transgenic founders as determined by PCR (Fig. 1A, arrowed). We also determined the expression of PKCγ H101Y mutant proteins in the transgenic cerebellum by immunohistology/confocal microscopy (Fig. 1B). Confocal microscopy results demonstrated that endogenous PKCγ is mainly localized in Purkinje cells of the wild type C57BL/6J at 4 weeks of age (Fig. 1B, left panel). Compared to expression pattern of the wild type of PKCγ, a similar Purkinje cell localization of HA-tagged PKCγ H101Y was observed in transgenic mice (Fig. 1B, right panel). Of interest, altered Purkinje cell soma were found in the transgenic mice at 4 weeks of age (Fig. 1B, arrowed).
Light microscopy of sections from saggital cerebella of the control and PKCγ H101Y transgenic mice is shown in Fig. 1C. As predicted from the cell culture studies, transgenic mice overexpressing the PKCγ H101Y SCA14 mutants were observed with altered morphology of and loss of Purkinje cells at four weeks of age (Fig. 1C, right panel). Further, we determined motor function using tail suspension photography. In current experiments 11 out of 12, 12-month old wild type C57BL/6J mice showed splaying out of hind limbs (Fig. 1D, left panel). However, over 90 % of the PKCγ H101Y transgenic mice (11 out of 12 mice) manifested the stereotypical clasping response (Fig. 1D, right panel) in the hind limbs, indicating that the presence of a H101Y SCA14 mutant may confer a neurological phenotype which is more severe than a knockout for PKCγ which has a normal Purkinje cell layer (data not shown).
Although the PKCγ H101Y SCA14 transgenic mouse expressed a HA-tagged SCA14 mutant PKCγ H101Y under CMV promoter control, a universal promoter, PKCγ H101Y was expressed specifically in the Purkinje cell layers particularly in the soma and dentrites. We chose this promoter after discussion with others who had failed to find an effect using a Purkinje cell promoter. We have the only colony showing Purkinje neuron degeneration and others are not currently commercially available.
Dominant negative effect of PKCγ H101Y mutation on wild type endogenous PKCγ and Cx57 phosphorylation in the transgenic mice
We have previously demonstrated that PKCγ H101Y mutation causes dominant negative effects on endogenous wild type PKCγ enzyme activity which leads to uncontrolled, open gap junctions in the HT22 cells overexpressing PKCγ SCA14 mutants [4, 6]. In this study we used HT22 cells and cerebellar slice culture in vitro to determine control of Cx57 gap junctions by PKCγ. Almost 90 % confluence of HT22 cells were treated with 200 nM TPA or 100 μM H2O2 for 30 min, cell surface Cx57 gap junction plaques were visualized by immunocytochemistry/confocal microscopy as demonstrated previously [4]. Plaque number was counted and graphed (Fig. 2A). Results indicated that application of PKCγ activators, such as TPA and H2O2, caused decreases in the Cx57 gap junction plaques (Fig. 2A, graph). TPA or H2O2-activation of PKCγ caused Cx57 phosphorylation on serines in HT22 cells as determined by immunoprecipation/Western blotting. Inhibition of Cx57 gap junction plaques and phosphorylation of Cx57 on serines results in inhibition of gap junction activity.
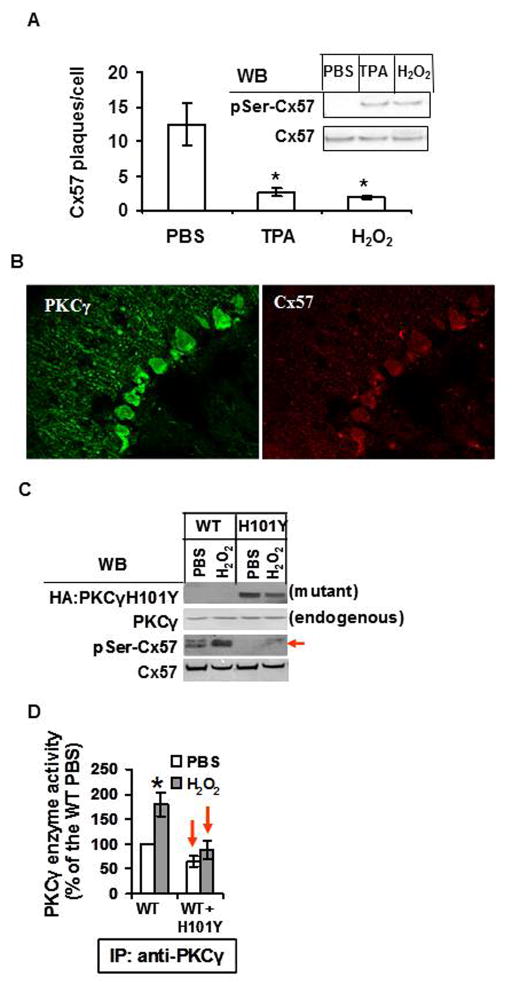
Fig. 2. The dominant negative effect of PKCγ H101Y on Cx57 phosphorylation and endogenous PKCγ activation in PKCγ H101Y SCA14 transgenic mice.
A. HT22 cells were treated with 200 nM TPA, 100 μM H2O2, or PBS for 30 min, cell surface Cx57 gap junction plaques were measured by immunocytochemistry/confocal microscopy. Gap junction plaques per cell were graphed. Phosphorylation of Cx57 on serines was determined by immunoprecipitation/Western blotting (see insert at right). B. Saggittal sections of C57BL/6J mouse cerebella at age of 4 weeks were made and endogenous PKCγ and Cx57 were labeled and visualized by immunohistology/confocal microscopy. C. Four-week old cerebellar slices in culture from the wild type and transgenic mice were treated with 250 μM H2O2 for 30 min. After treatments, whole tissue extracts were used to determine phosphorylation of Cx57 by Western blotting. PKCγ H101Y SCA14 mutant proteins are revealed by anti-HA antisera. Total cellular PKCγ is revealed by anti-PKCγ antisera. α-tubulin is used as a loading control. D. The whole tissue extracts from C were used to immunoprecipitate both endogenous PKCγ and HA-tagged PKCγ H101Y by anti-PKCγ antibodies. The precipitates (wild type and mutant H101Y PKCγ together, see C) were used for the enzyme sources to determine total cellular PKCγ enzyme activity. WT= control mouse cerebellar slice cultures; H101Y= PKCγ H101Y transgenic mouse slices in culture; WT+H101Y= transgenic mouse cerebellar slice cultures which have both endogenous PKCγ and the H101Y mutated PKCγ. Note: We can not make a quantitative estimate of mutant PKCγ expression vs endogenous wild type PKCγ since PKCγ and HA antisera can not be compared to each other. WB= Western blot
Cx57 was newly characterized in the Purkinje cell layers of wild type C57BL/6B mouse (Fig. 2B). To determine whether control of Cx57 gap junctions was altered in the cerebella of PKCγ transgenic mice, we treated four-week old mouse cerebellar slices in culture with 250 μM H2O2 for 30 min and then determined Cx57 phosphorylation. As shown in Fig. 2C, PKCγH101Y was only expressed in the transgenic mice though endogenous PKCγ were present in both types of animals. Lack of Cx57 phosphorylation on serines was observed in the transgenic mice. Enzyme activity assay further confirmed that overexpression of PKCγ H101Y mutant in vivo caused lack of PKCγ enzyme activity in the transgenic cerebella (Fig. 2D). Taken together, overexpression of PKCγ H101Y mutants caused malfunction of endogenous wild type PKCγ which may further lead to loss of control of gap junctions such as Cx57 in vivo, with or without stress stimuli.
Activation of caspase-12 in the transgenic mouse
Based on the observation from cell cultures [6, 7, 18, 33], we next determined endoplasmic reticulum (ER) stress by activation of caspase-12 in the PKCγ H101Y transgenic mouse. Results from Fig. 3 showed that caspase-12 was greatly activated in the PKCγ H101Y mutant cerebellar tissues. In vitro application of 250 μM H2O2 for 30 min significantly enhanced activation of caspase-12, suggesting that ER stress may be a key event for phathogenesis of SCA14.
Fig. 3. Activation of caspase-12 in the transgenic mouse.

Four-week old mouse cerebellar slices in culture were treated with 250 μM H2O2 for 30 min. The samples were collected after treatments. The whole tissue extracts were used to determine active caspase-3 and caspase-12 by Western blotting. WT= control mouse cerebellar slice cultures; H101Y= PKCγ H101Y transgenic mouse slices in culture.
These data suggest that overexpression of PKCγ SCA14 mutants, e.g., H101Y, triggered cellular ER stress and Purkinje cell apoptosis in vivo. Loss of control of gap junctions may further propagate ER stress-linked cell death signals to adjacent Purkinje cells. This is a novel transgenic mouse model which provides an important model system to study ER stress-initiated, dysfunction of gap junctions-mediated neurodegenerative disorders. This may be a suitable model for developing potential treatments that will prevent or delay neurodegeneration through restoration of control of gap junction and/or elimination of ER stress in the brain.
Acknowledgments
The authors would like to thank Dr. Denis Medeiros for critical reading of the manuscript. The transgenic production was done at the University of Missouri-Columbia Transgenic Animal Facility. The work was supported by NIH R01 EY013421 to D.J.T., NIH COBRE Grant P20-RR-017686, NIH K-INBRE Major Starter grant P20-RR-016475, and the National Organization for Rare Disorders grant to D.L. The publication contribution number from K-State Research and Extension is.
References
- 1.Duenas AM, Goold R, Giunti P. Molecular pathogenesis of spinocerebellar ataxias. Brain. 2008;129:1357–1370. doi: 10.1093/brain/awl081. [DOI] [PubMed] [Google Scholar]
- 2.Chen DH, Brkanac Z, Verlinde CL, Tan XJ, Bylenok L, Nochlin D, Matsushita M, Lipe H, Wolff J, Fernandez M, Cimino PJ, Bird TD, Raskind WH. Missense mutations in the regulatory domain of PKC gamma: a new mechanism for dominant nonepisodic cerebellar ataxia. Am J Hum Genet. 2003;72:839–849. doi: 10.1086/373883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vlak MH, Sinke RJ, Rabelink GM, Kremer BP, van de Warrenburg BP. Novel PRKCG/SCA14 mutation in a Dutch spinocerebellar ataxia family: Expanding the phenotype. Mov Disord. 2006;21:1025–1028. doi: 10.1002/mds.20851. [DOI] [PubMed] [Google Scholar]
- 4.Lin D, Takemoto DJ. Oxidative activation of protein kinase Cgamma through the C1 domain: Effects on gap junctions. J Biol Chem. 2005;280:13682–13693. doi: 10.1074/jbc.M407762200. [DOI] [PubMed] [Google Scholar]
- 5.Lin D, Takemoto DJ. Loss of control of gap junctions by protein kinase C γ spinocerebellar ataxia type 14 mutations leads to neural cell apoptosis. Annals Neurol. 2006;60 (S10):S33. [Google Scholar]
- 6.Lin D, Takemoto DJ. Protection from ataxia-linked apoptosis by gap junction inhibitors. Biochem Biophy Res Comm. 2007;362:982–987. doi: 10.1016/j.bbrc.2007.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seki T, Adachi N, Ono Y, Mochizuki H, Hiramoto K, Amano T, Matsubayashi H, Matsumoto M, Kawakami H, Saito N, Sakai N. Mutant protein kinase Cgamma found in spinocerebellar ataxia type 14 is susceptible to aggregation and causes cell death. J Biol Chem. 2005;280:29096–29106. doi: 10.1074/jbc.M501716200. [DOI] [PubMed] [Google Scholar]
- 8.Stevanin G, Hahn V, Lohmann E, Bouslam N, Gouttard M, Soumphonphakdy C, Welter ML, Ollagnon-Roman E, Lemainque A, Ruberg M, Brice A, Durr A. Mutation in the catalytic domain of protein kinase C gamma and extension of the phenotype associated with spinocerebellar ataxia type 14. Arch Neurol. 2004;61:1242–1248. doi: 10.1001/archneur.61.8.1242. [DOI] [PubMed] [Google Scholar]
- 9.van de Warrenburg BP, Verbeek DS, Piersma SJ, Hennekam FA, Pearson PL, Knoers NV, Kremer HP, Sinke RJ. Identification of a novel SCA14 mutation in a Dutch autosomal dominant cerebellar ataxia family. Neurol. 2003;61:1760–1765. doi: 10.1212/01.wnl.0000098883.79421.73. [DOI] [PubMed] [Google Scholar]
- 10.Verbeek DS, Knight MA, Harmison GG, Fischbeck KH, Howell BW. Protein kinase C gamma mutations in spinocerebellar ataxia 14 increase kinase activity and alter membrane targeting. Brain. 2005;128:436–442. doi: 10.1093/brain/awh378. [DOI] [PubMed] [Google Scholar]
- 11.Chen DH, Cimino PJ, Ranum LP, Zoghbi HY, Yabe I, Schut L, Margolis RL, Lipe HP, Feleke A, Matsushita M, Wolff J, Morgan C, Lau D, Fernandez M, Sasaki H, Raskind WH, Bird TD. The clinical and genetic spectrum of spinocerebellar ataxia 14. Neurol. 2005;64:1258–1260. doi: 10.1212/01.WNL.0000156801.64549.6B. [DOI] [PubMed] [Google Scholar]
- 12.Yabe I, Sasaki H, Chen DH, Raskind WH, Bird TD, Yamashita I, Tsuji S, Kikuchi S, Tashiro K. Spinocerebellar ataxia type 14 caused by a mutation in protein kinase C gamma. Arch Neurol. 2003;60:1749–1751. doi: 10.1001/archneur.60.12.1749. [DOI] [PubMed] [Google Scholar]
- 13.Alonso I, Costa C, Gomes A, Ferro A, Seixas AI, Silva S, Cruz VT, Coutinho P, Sequeiros J, Silveira I. A novel H101Q mutation causes PKCgamma loss in spinocerebellar ataxia type 14. J Human Genet. 2005;50:523–529. doi: 10.1007/s10038-005-0287-z. [DOI] [PubMed] [Google Scholar]
- 14.Klebe S, Durr A, Rentschler A, Hahn-Barma V, Abele M, Bouslam N, Schols L, Jedynak P, Forlani S, Denis E, Dussert C, Agid Y, Bauer P, Globas C, Wullner U, Brice A, Riess O, Stevanin G. New mutations in protein kinase Cgamma associated with spinocerebellar ataxia type 14. Ann Neurol. 2005;58:720–729. doi: 10.1002/ana.20628. [DOI] [PubMed] [Google Scholar]
- 15.Fahey MC, Knight MA, Shaw JH, Gardner RJ, du Sart D, Lockhart PJ, Delatycki MB, Gates PC, Storey E. Spinocerebellar ataxia type 14: study of a family with an exon 5 mutation in the PRKCG gene. J Neurol Neurosurg Psychiatry. 2005;76:1720–1722. doi: 10.1136/jnnp.2004.044115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adachi N, Kobayashi T, Takahashi H, Kawasaki T, Shirai Y, Ueyama T, Matsuda T, Seki T, Sakai N, Saito N. Enzymological analysis of mutant protein kinase Cgamma causing spinocerebellar ataxia type 14 and dysfunction in Ca2+ homeostasis. J Biol Chem. 2008;283:19854–19863. doi: 10.1074/jbc.M801492200. [DOI] [PubMed] [Google Scholar]
- 17.Doran G, Davies KE, Talbot K. Activation of mutant protein kinase Cgamma leads to aberrant sequestration and impairment of its cellular function. Biochem Biophys Res Commun. 2008;372:447–453. doi: 10.1016/j.bbrc.2008.05.072. [DOI] [PubMed] [Google Scholar]
- 18.Seki T, Takahashi H, Adachi N, Abe N, Shimahara T, Saito N, Sakai N. Aggregate formation of mutant protein kinase C gamma found in spinocerebellar ataxia type 14 impairs ubiquitin-proteasome system and induces endoplasmic reticulum stress. Eur J Neurosci. 2007;26:3126–3140. doi: 10.1111/j.1460-9568.2007.05933.x. [DOI] [PubMed] [Google Scholar]
- 19.Miura S, Nakagawara H, Kaida H, Sugita M, Noda K, Motomura K, Ohyagi Y, Ayabe M, Aizawa H, Ishibashi M, Taniwaki T. Expansion of the phenotypic spectrum of SCA14 caused by the Gly128Asp mutation in PRKCG. Clin Neurol Neurosurg. 2008 Nov 3; doi: 10.1016/j.clineuro.2008.09.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Verbeek DS, Goedhart J, Bruinsma L, Sinke RJ, Reits EA. PKC gamma mutations in spinocerebellar ataxia type 14 affect C1 domain accessibility and kinase activity leading to aberrant MAPK signaling. J Cell Sci. 2008;121:2339–2349. doi: 10.1242/jcs.027698. [DOI] [PubMed] [Google Scholar]
- 21.Doran G, Davies KE, Talbot K. Activation of mutant protein kinase Cgamma leads to aberrant sequestration and impairment of its cellular function. Biochem Biophys Res Commun. 2008;372:447–453. doi: 10.1016/j.bbrc.2008.05.072. [DOI] [PubMed] [Google Scholar]
- 22.Adachi N, Kobayashi T, Takahashi H, Kawasaki T, Shirai Y, Ueyama T, Matsuda T, Seki T, Sakai N, Saito N. Enzymological analysis of mutant protein kinase Cgamma causing spinocerebellar ataxia type 14 and dysfunction in Ca2+ homeostasis. J Biol Chem. 2008;283:19854–19863. doi: 10.1074/jbc.M801492200. [DOI] [PubMed] [Google Scholar]
- 23.Klebe S, Faivre L, Forlani S, Dussert C, Tourbah A, Brice A, Stevanin G, Durr A. Another mutation in cysteine 131 in protein kinase C gamma as a cause of spinocerebellar ataxia type 14. Arch Neurol. 2007;64:913–914. doi: 10.1001/archneur.64.6.913. [DOI] [PubMed] [Google Scholar]
- 24.Lin D, Shanks D, Prakash O, Takemoto DJ. Protein kinase C gamma mutations in the C1B domain cause caspase-3-linked apoptosis in lens epithelial cells through gap junctions. Exp Eye Res. 2007;85:113–122. doi: 10.1016/j.exer.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visser JE, Bloem BR, van de Warrenburg BP. PRKCG mutation (SCA-14) causing a Ramsay Hunt phenotype. Mov Disord. 2007;22:1024–1026. doi: 10.1002/mds.21414. [DOI] [PubMed] [Google Scholar]
- 26.Morita H, Yoshida K, Suzuki K, Ikeda S. A Japanese case of SCA14 with the Gly128Asp mutation. J Hum Genet. 2006;51:1118–1121. doi: 10.1007/s10038-006-0063-8. [DOI] [PubMed] [Google Scholar]
- 27.Hiramoto K, Kawakami H, Inoue K, Seki T, Maruyama H, Morino H, Matsumoto M, Kurisu K, Sakai N. Identification of a new family of spinocerebellar ataxia type 14 in the Japanese spinocerebellar ataxia population by the screening of PRKCG exon 4. Mov Disord. 2006;21:1355–1360. doi: 10.1002/mds.20970. [DOI] [PubMed] [Google Scholar]
- 28.Meller K, Krah K, Theiss C. Dye coupling in Purkinje cells of organotypic slice cultures. Brain Res Dev Brain Res. 2005;160:101–105. doi: 10.1016/j.devbrainres.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Cumming RC, Andon NL, Haynes PA, Park M, Fischer WH, Schubert D. Protein disulfide bond formation in the cytoplasm during oxidative stress. J Biol Chem. 2004;279:21749–21758. doi: 10.1074/jbc.M312267200. [DOI] [PubMed] [Google Scholar]
- 30.Xiang Z, Hrabetova S, Moskowitz HS, Casaccia-Bonnefil P, Young SR, Nimmrich VC, Tiedge H, Einheber S, Karnup S, Bianchi R, Bergold PJ. Long-term maintenance of mature hippocampal slices in vitro. J Neurosci Methods. 2000;98:145–154. doi: 10.1016/s0165-0270(00)00197-7. [DOI] [PubMed] [Google Scholar]
- 31.Ju YI, Sone T, Okamoto T, Fukunaga M. Jump exercise during remobilization restores integrity of the trabecular architecture after tail suspension in young rats. J Appl Physiol. 2008;104:1594–1600. doi: 10.1152/japplphysiol.01004.2007. [DOI] [PubMed] [Google Scholar]
- 32.Jiao Y, Yan J, Zhao Y, Donahue LR, Beamer WG, Li X, Roe BA, Ledoux MS, Gu W. Carbonic anhydrase-related protein VIII deficiency is associated with a distinctive lifelong gait disorder in waddles mice. Genetics. 2005;171:1239–1246. doi: 10.1534/genetics.105.044487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao L, Ackerman Sl. Endoplasmic reticulum stress in health and disease. Current Opinion Cell Biol. 2006;18:444–452. doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]