Abstract
BACKGROUND
Reduction in pulmonary exacerbations is an important efficacy endpoint for CF clinical studies. Powering exacerbation endpoints requires estimation of the future exacerbation incidence in CF study populations, but rates differ across the population.
METHODS
We have estimated exacerbation rates for Epidemiologic Study of CF subpopulations stratified by age, FEV1 % predicted, sex, weight-for-age percentile, respiratory signs and symptoms, and history of exacerbation and bacterial culture. Sample sizes required to attain 80% power to detect exacerbation reductions of 20% to 80% in 1:1 randomized studies of 3 to 12 month duration were determined. Exacerbation treatments with “any” antibiotic (new oral quinolone, new inhaled antibiotic, or intravenous (IV) antibiotic) and with IV antibiotics were studied.
RESULTS
At all ages, decreased FEV1, female sex, exacerbation history, and Pseudomonas aeruginosa culture history were associated with increased treatment for exacerbation.
CONCLUSIONS
These data should assist investigators in the design of future CF exacerbation studies.
Keywords: study design, pulmonary exacerbation, sample size
INTRODUCTION
Cystic fibrosis (CF) is a life-shortening genetic disease characterized by chronic pulmonary bacterial infections, local inflammation, and progressive loss of pulmonary function [1]. As their lung disease progresses, persons with CF experience more frequent episodic increases in respiratory signs and symptoms that require aggressive intervention, commonly termed pulmonary exacerbations [2-6]. Pulmonary exacerbations adversely impact health-related quality of life [7-9] and survival [10-13] and lead to significant resource utilization [14, 15]. Despite the lack of a consensus definition of pulmonary exacerbation, they are clinically meaningful [2, 5] and reduction in exacerbations is an important efficacy endpoint for clinical studies of chronic CF therapies [16-21].
In order to adequately power CF clinical trials employing exacerbation as an efficacy endpoint, investigators must be able to predict the relative risk of exacerbation or median time to exacerbation for untreated subjects in their study population. This task is complicated because the risk that an individual will develop signs and symptoms resulting in exacerbation diagnosis and treatment is not uniform across the CF population. For example, diagnoses of pulmonary exacerbation increase with decreasing pulmonary function [2]. We used data from the Epidemiologic Study of CF [22] to model relative risk of exacerbation and median time to exacerbation for different CF subpopulations. We then estimated the effects of duration of measure and magnitude of proposed treatment effects on sample size requirements for randomized clinical trials using relative risk of pulmonary exacerbation as an efficacy endpoint.
METHODS
Data were obtained from ESCF, a prospective, encounter-based, multicenter, observational study designed to evaluate the natural history of CF patients in North America from 1994 to 2005 [22]. Informed consent was obtained based on decisions by local human subjects review boards. Pulmonary function test results were reported as measured values and converted to percent predicted using reference equations from Wang et al. [23] for females through age 15 and males through age 17, and Hankinson et al. [24] at older ages.
To be included in this analysis, patients had to have had a routine (i.e., stable) clinic encounter (index visit) at least twelve months after enrollment in ESCF during which pulmonary function testing was performed within ± 7 days of the visit and no treatment for exacerbation had been administered within ± 14 days. In addition, the patient had to have had at least one routine clinic encounter within the calendar year prior to the index visit (the baseline period) and to have had at least 4 encounters spaced roughly quarterly (± 45 days) within a 13.5 month follow up period after the index visit. Patients could be included more than once in the analysis provided that their subsequent index visits did not occur during the follow up period from previous analyses. Patients missing their date of ESCF enrollment, date of birth, or sex were excluded from the analysis.
Two definitions of treatment associated with pulmonary exacerbations were employed in analyses: intravenous (IV) treatment (treatment with any IV antibiotic) and “any” treatment (defined as treatment with any IV antibiotic, any new inhaled antibiotic, or any new oral fluoroquinolone) within a −7 to +28 day period around an encounter. The first treatment for pulmonary exacerbation after the index visit during the follow up period was characterized for patient subgroups stratified at their index visit by age in years (< 6, 6-12, 13-17, 18-24, or ≥ 25), FEV1 % predicted (≥ 100, 70 - < 100, 40- < 70, < 40), sex, weight-for-age percentile (WFA) (< 25th, ≥ 25th), presence of signs and symptoms (daily cough, daily sputum production, clubbing, crackles, or wheeze), number of IV treatments for exacerbation during the baseline period (0, 1, 2, ≥ 3), and presence of Pseudomonas aeruginosa, Staphylococcus aureus, or Haemophilus influenzae on respiratory tract culture during the baseline period (any positive, no positive). P. aeruginosa culture history during the baseline year was further stratified by modified Leeds criteria [25] as either “chronic” infection (2 or more cultures and > 50% of cultures positive for P. aeruginosa), “intermittent” infection (2 or more cultures and ≤ 50% positive), or “indeterminate” infection (one positive culture). Patients with no record of IV antibiotic treatment for an exacerbation during the baseline period were considered to have had zero exacerbations, and bacterial culture results obtained at the index visit were used for patients lacking culture results during the baseline period.
Analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC). Median times to treatment for exacerbation during the follow up period were determined for all patient subgroups using Kaplan-Meier estimates generated from the SAS LIFETEST procedure. We also determined proportions of patients treated at least once for exacerbations at 3, 6, 9, and 12 months after the index visit. We used this information to calculate the number of subjects per arm required in 1:1 randomized controlled studies to attain 80% power for a variety of treatment effect sizes, study durations, and patient subgroups based on a log-rank test using the SAS POWER procedure assuming a two-sided alpha of 0.05. Reductions of 20%, 30%, 40%, 50%, 60%, 70% and 80% in the proportion of subjects treated for pulmonary exacerbation were evaluated for studies of 3, 6, 9, and 12 month durations using both exacerbation treatment definitions. Sample sizes were calculated for patient subgroups by age, FEV1, sex, WFA, signs and symptoms, previous exacerbation history, and bacterial culture status.
RESULTS
A total of 39,326 unique analysis periods were captured among 16,082 eligible ESCF patients, with 10,018 patients contributing at least twice to analyses. Distributions of patients by age, pulmonary function, and other variables are provided in Table 1. Due to inclusion criteria, none of the 9,653 patients under 6 years of age were less than 1 year of age at their index visit; the group had a median age of 3.69 years.
Table 1.
Numbers of Patients Contributing to Subgroup Analyses
| FEV1 Range (% predicted) | ||||||
|---|---|---|---|---|---|---|
| All | Age < 6 yrs | ≥ 100 | 70 to <100 | 40 to <70 | <40 | |
| All patients | 39,326 | 9,653 | 5,573 | 12,752 | 7,991 | 3,357 |
|
| ||||||
| Age Group | ||||||
| 6-12 yrs | 13,398 | - | 4,038 | 6,926 | 2,117 | 317 |
| 13-17 yrs | 7,698 | - | 1,293 | 3,686 | 2,171 | 548 |
| 18-24 yrs | 4,280 | - | 180 | 1,364 | 1,823 | 913 |
| ≥ 25 yrs | 4,297 | - | 62 | 776 | 1,880 | 1,579 |
|
| ||||||
| Sex, Male | 19,930 | 4,827 | 2,998 | 6,497 | 3,761 | 1,847 |
|
| ||||||
| WFA Percentilea | ||||||
| <25th | 18,228 | 3,911 | 1,791 | 5,469 | 4,643 | 2,414 |
| ≥25th | 20,988 | 5,711 | 3,772 | 7,255 | 3,320 | 930 |
|
| ||||||
| Signs and Symptomsa | ||||||
| Daily Cough | 18,016 | 2,460 | 1,683 | 5,605 | 5,457 | 2,811 |
| Daily Sputum | 11,087 | 640 | 678 | 3,094 | 4,156 | 2,519 |
| Clubbing | 20,190 | 2,139 | 2,356 | 6,935 | 5,880 | 2,880 |
| Crackles | 6,739 | 392 | 221 | 1,338 | 2,619 | 2,169 |
| Wheeze | 1,778 | 288 | 148 | 483 | 500 | 359 |
|
| ||||||
| Pulmonary Exacerbation Historyb | ||||||
| None | 24,777 | 7,149 | 4,566 | 8,793 | 3,395 | 874 |
| One | 8,438 | 1,700 | 757 | 2,703 | 2,335 | 943 |
| Two | 3,384 | 504 | 167 | 816 | 1,163 | 734 |
| Three or more | 2,727 | 300 | 83 | 440 | 1,098 | 806 |
|
| ||||||
| P. aeruginosa Culture Historyc | ||||||
| All Negative | 15,572 | 5,761 | 2,846 | 4,885 | 1,647 | 433 |
| Any Positive | 20,269 | 2,732 | 2,295 | 6,749 | 5,765 | 2,728 |
| Indeterminated | 4,818 | 685 | 607 | 1,797 | 1,216 | 513 |
| Intermittente | 4,155 | 1311 | 626 | 1,303 | 684 | 231 |
| Chronicf | 11,296 | 736 | 1,062 | 3,649 | 3,865 | 1,984 |
As recorded at the index visit
Number of pulmonary exacerbations treated with IV antibiotics in the 12 months prior to the index visit
Recorded in the 12 months prior to the index visit
Only one culture result reported
≤50% of cultures positive
>50% of cultures positive
During the follow up period, patients were much more likely to be treated with “any” antibiotic (including oral fluoroquinolones and inhaled antibiotics) than to be treated with IV antibiotics for exacerbation. The median time to receive “any” treatment for all patients was 294 days, with 55.0% having been treated at least once during the follow up period. In contrast, only 34.1% of patients had been treated at least once with IV antibiotics during the same period.
Patient stratification identified subgroups at greater risk for exacerbation treatment during the follow up period. Table 2 shows median times to “any” antibiotic treatment for different subgroups (data not shown for IV treatment), and Table 3 shows the percentages of patients treated with “any” and with IV antibiotics during the follow up period. Likelihood of treatment was strongly influenced by FEV1 as measured at the index visit. Patients < 6 years of age and patients 6-12 and 13-17 years of age with FEV1 ≥ 100% predicted had estimated median times to “any” treatment > 365 days: only 35.3%, 36.5%, 46.1%, respectively, were treated at least once during the one-year follow up period (Tables 2 and 3). Median time to IV antibiotic treatment was less than 6 months for patients with FEV1 < 40% predicted and about 9 months for patients with FEV1 between 40 and < 70% predicted; other patients with higher FEV1 % predicted had median times to IV treatment of more than 12 months. Older patients tended to have lower median times to treatment with “any” antibiotics than younger patients (Table 2), with the exception that patients ≥ 25 years old at their index visit had a slightly longer median time to treatment with any antibiotic (155 days) than patients 18 to 24 years old (143 days).
Table 2.
Median Time (Days) to Treatment during the Follow Up Period with “Any” Antibiotics for a Pulmonary Exacerbation by Patient Subgroup.
| FEV1 Range (% predicted) | ||||||
|---|---|---|---|---|---|---|
| All | |Age <6 yrs| | ≥ 100 | 70 to <100 | 40 to <70 | <40 | |
| All patients | 294 | (>365)a | (>365) | 299 | 147 | 112 |
|
| ||||||
| Age Group | ||||||
| 6-12 yrs | 364 | - | (>365) | 365 | 179 | 98 |
| 13-17 yrs | 202 | - | (>365) | 258 | 133 | 96 |
| 18-24 yrs | 143 | - | 312 | 203 | 127 | 98 |
| ≥ 25 yrs | 155 | - | 302 | 252 | 161 | 125 |
|
| ||||||
| Sex | ||||||
| Male | 329 | (>365) | (>365) | 343 | 168 | 120 |
| Female | 266 | (>365) | (>365) | 268 | 133 | 98 |
|
| ||||||
| WFA Percentileb | ||||||
| <25th | 238 | (>365) | (>365) | 299 | 133 | 106 |
| ≥25th | 357 | (>365) | (>365) | 299 | 168 | 121 |
|
| ||||||
| Signs and Symptomsb | ||||||
| Daily Cough | 189 | (>365) | 364 | 224 | 133 | 107 |
| Daily Sputum | 154 | 317 | 280 | 210 | 128 | 108 |
| Clubbing | 203 | (>365) | (>365) | 266 | 135 | 110 |
| Crackles | 133 | 273 | (>365) | 203 | 121 | 103 |
| Wheeze | 218 | (>365) | (>365) | 308 | 136 | 126 |
|
| ||||||
| Pulmonary Exacerbation Historyc | ||||||
| None | 398 | (>365) | (>365) | (>365) | 231 | 189 |
| One | 193 | (>365) | 280 | 193 | 147 | 124 |
| Two | 119 | 259 | 168 | 121 | 98 | 102 |
| Three or more | 77 | 168 | 134 | 84 | 69 | 65 |
|
| ||||||
| P. aeruginosa Culture Historyd | ||||||
| All Negative | (>365) | (>365) | (>365) | (>365) | 197 | 139 |
| Any Positive | 187 | (>365) | 316 | 216 | 131 | 105 |
| Indeterminatee | 222 | (>365) | 308 | 252 | 175 | 133 |
| Intermittentf | 282 | (>365) | (>365) | 271 | 159 | 91 |
| Chronicg | 147 | 268 | 276 | 189 | 119 | 98 |
When time >365 days, less than half of subjects were treated with “any” antibiotics during the follow up period
As recorded at the index visit
Number of pulmonary exacerbations treated with IV antibiotics in the 12 months prior to the index visit
Recorded in the 12 months prior to the index visit
Only one culture result reported
≤50% of cultures positive
>50% of cultures positive
Table 3.
Percentage of Patients Treated with Either “Any” Antibiotics or IV Antibiotics during the Follow Up Period by Patient Subgroup
| FEV1 Range (% predicted) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients | Age < 6 yrs | ≥ 100 | 70 to <100 | 40 to <70 | <40 | |||||||
| “Any” | IV | “Any” | IV | “Any” | IV | “Any” | IV | “Any” | IV | “Any” | IV | |
| All Patients | 55.0 | 34.1 | 35.3 | 18.8 | 39.5 | 16.0 | 55.0 | 29.2 | 76.8 | 57.0 | 85.7 | 72.7 |
|
| ||||||||||||
| Age Group | ||||||||||||
| 6-12 yrs | 49.8 | 27.6 | - | - | 36.5 | 14.5 | 49.2 | 25.2 | 71.8 | 54.1 | 84.5 | 72.5 |
| 13-17 yrs | 65.5 | 41.0 | - | - | 46.1 | 19.9 | 60.7 | 32.0 | 79.4 | 59.0 | 89.0 | 79.8 |
| 18-24 yrs | 76.0 | 56.1 | - | - | 54.7 | 21.9 | 65.6 | 39.5 | 80.6 | 61.7 | 86.4 | 76.5 |
| ≥ 25 yrs | 75.8 | 54.6 | - | - | 51.6 | 12.9 | 60.5 | 33.6 | 75.7 | 53.1 | 84.3 | 68.2 |
|
| ||||||||||||
| Sex | ||||||||||||
| Male | 52.3 | 31.2 | 33.3 | 17.9 | 37.9 | 14.4 | 51.7 | 25.2 | 73.6 | 53.2 | 84.1 | 69.2 |
| Female | 57.8 | 37.1 | 37.3 | 19.8 | 41.4 | 17.7 | 58.4 | 33.3 | 79.6 | 60.3 | 87.6 | 77.0 |
|
| ||||||||||||
| WFA Percentilea | ||||||||||||
| <25th | 60.5 | 40.9 | 39.0 | 23.3 | 40.6 | 17.1 | 55.2 | 30.1 | 78.9 | 59.9 | 86.8 | 74.9 |
| ≥25th | 50.2 | 28.2 | 32.7 | 15.8 | 39.0 | 15.4 | 54.7 | 28.4 | 74.0 | 53.1 | 82.6 | 67.1 |
|
| ||||||||||||
| Signs and Symptomsa | ||||||||||||
| Daily Cough | 67.9 | 46.7 | 44.8 | 26.2 | 49.5 | 21.5 | 62.6 | 36.3 | 79.8 | 60.4 | 86.8 | 74.2 |
| Daily Sputum | 74.8 | 54.8 | 52.9 | 33.3 | 57.5 | 25.7 | 65.9 | 40.0 | 80.3 | 61.9 | 87.0 | 74.6 |
| Clubbing | 65.5 | 43.5 | 43.8 | 24.0 | 42.7 | 16.7 | 59.5 | 32.2 | 79.3 | 59.7 | 86.4 | 74.0 |
| Crackles | 78.1 | 60.9 | 58.0 | 41.6 | 46.0 | 25.6 | 67.0 | 42.7 | 82.3 | 64.8 | 86.7 | 74.5 |
| Wheeze | 61.5 | 43.2 | 40.0 | 27.3 | 40.2 | 18.5 | 53.6 | 31.7 | 75.2 | 54.3 | 79.3 | 66.3 |
|
| ||||||||||||
| Pulmonary Exacerbation Historyb | ||||||||||||
| None | 42.4 | 19.4 | 28.9 | 12.7 | 34.4 | 11.1 | 46.2 | 19.3 | 64.0 | 37.8 | 72.1 | 49.3 |
| One | 68.9 | 47.4 | 48.6 | 30.3 | 58.0 | 32.6 | 69.3 | 43.1 | 80.2 | 60.1 | 85.5 | 71.0 |
| Two | 82.8 | 67.9 | 61.1 | 45.3 | 73.2 | 48.7 | 82.1 | 61.3 | 89.4 | 76.4 | 90.2 | 81.9 |
| Three or more | 92.0 | 84.4 | 68.9 | 55.5 | 81.4 | 69.4 | 93.0 | 81.9 | 95.6 | 89.0 | 96.2 | 91.8 |
|
| ||||||||||||
| P. aeruginosa Culture Historyc | ||||||||||||
| All Negative | 38.4 | 22.5 | 29.5 | 16.1 | 29.2 | 12.8 | 41.4 | 22.3 | 66.4 | 50.2 | 77.9 | 66.0 |
| Any Positive | 69.1 | 44.6 | 48.4 | 25.1 | 53.0 | 20.2 | 65.3 | 35.0 | 80.8 | 60.5 | 87.8 | 75.1 |
| Indeterminated | 63.9 | 36.3 | 45.2 | 22.9 | 53.5 | 20.2 | 61.7 | 31.3 | 74.8 | 50.0 | 82.8 | 64 |
| Intermittente | 56.5 | 32.8 | 44.6 | 21.7 | 44.7 | 17.9 | 58.2 | 38.9 | 76.3 | 55.7 | 86.9 | 77.3 |
| Chronicf | 75.9 | 52.5 | 58.3 | 33.3 | 57.6 | 21.6 | 69.6 | 50.0 | 83.5 | 64.6 | 89.1 | 77.7 |
As recorded at the index visit
Number of pulmonary exacerbations treated with IV antibiotics in the 12 months prior to the index visit
Recorded in the 12 months prior to the index visit
Only one culture result reported
≤50% of cultures positive
>50% of cultures positive
Females were consistently more likely to receive antibiotic treatment than males irrespective of treatment criteria or subgroup studied (Tables 2 and 3). Overall, patients with lower WFA at their index visit were at a modestly higher risk for treatment, with the greatest differences observed among patients with FEV1 < 70% predicted and those < 6 years old. Patients with signs and symptoms of daily cough, daily sputum production, clubbing, crackles, or wheeze at their index visits generally were more often treated with antibiotics than the entire population (“all patients” in Table 3), an exception being patients with wheeze and lower FEV1.
Strong positive relationships were observed across all lung disease stages between history of exacerbation in the baseline year and antibiotic treatment during the follow up period (Table 3). History of any respiratory culture positive for P. aeruginosa in the baseline period increased the probability of treatment for exacerbation during the follow up period among all subgroups. The median time to treatment with “any” antibiotic for patients with a positive P. aeruginosa culture was about 6 months, and over two-thirds were treated during the follow up period, compared with less than 40% of patients with no positive P. aeruginosa cultures in the prior year (Tables 2 and 3). Similarly, 44.6% of patients with a positive P. aeruginosa culture in the prior year were treated with IV antibiotics in the follow up period, compared to 22.5% of patients with no positive cultures during the period. Within the P. aeruginosa culture-positive subpopulation, patients identified as having chronic infection in their baseline year generally had shorter median times to treatment with “any” antibiotics than other patients (Table 2), and were uniformly more likely to be treated with IV or “any” antibiotics than were intermittently infected patients (Table 3). Patients with history of S. aureus culture in the baseline period had only modestly lower probability of treatment in the follow up period relative to the entire population (less than a 2% difference). History of H. influenzae culture in the baseline period had a more pronounced negative influence, with “any” antibiotic treatment lower than “all patients” by about 10 percentage points and IV antibiotic treatment lower by about 7 percentage points. This effect was most pronounced in children less than 6 years old and older patients with less advanced lung disease (data not shown).
Sample sizes required to attain 80% power to detect a given reduction in treatment for exacerbation in a 1:1 randomized study of all patients varied as a function of antibiotic treatment definition (“any” antibiotics or IV antibiotics) and duration of observation (Figure 1). Because fewer patients had been treated with IV antibiotics than “any” antibiotics at any given time during the follow up, correspondingly more subjects are required in order to adequately power randomized studies using IV treatment than studies using “any” antibiotic treatment as endpoints (Figure 1). Similarly, the increase in the proportion of patients treated for exacerbation as a function of time elapsed from their index visit results in fewer subjects per arm being required to detect a given treatment effect as study observation periods increase from 3 months to 1 year (Figures 1, 2). Studies limited to patient subgroups at greater risk for exacerbation (e.g., with lower FEV1 % predicted at their index visit, prior history of exacerbation or positive P. aeruginosa culture history) require fewer subjects per arm to detect a given treatment effect than studies in patients without these risk factors (Table 4 and Figure 2). Numbers of subjects stratified by subgroups required per study arm to attain 80% power to detect a 40% reduction in the risk of treatment (i.e., hazard ratio = 0.6) with “any” antibiotics and IV antibiotics for exacerbation in a 1:1 randomized 6 month study are provided in Table 4.
Figure 1. Effects of Treatment Definition and Duration of Observation on Sample Sizes Required to Detect a Given Treatment Effect in a Randomized Controlled Study.
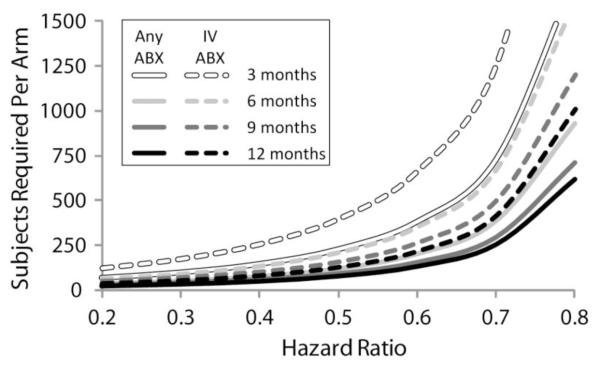
Sample sizes per arm required to assure 80% power to detect reductions in exacerbation treatment (as hazard ratios) in 1:1 randomized studies of 3 month (clear lines), 6 month (light gray lines), 9 month (dark gray lines), or 12 month (black lines) durations. Results for exacerbation treatment defined as administration of “any” antibiotics are shown as solid lines and those for treatment defined as administration of IV antibiotics are shown as dashed lines.
Figure 2. Sample Sizes Required per Study Arm to Retain 80% Power to Detect a 40% Reduction in Risk of Treatment for Exacerbation as a Function of Lung Disease Stage and Study Duration.
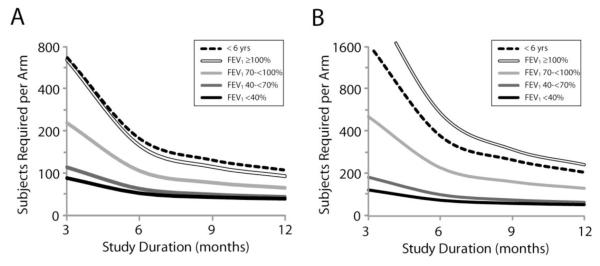
Panel A: Sample sizes required to detect a reduction in treatment with “any” antibiotics. Panel B: Sample sizes required to detect a reduction in treatment with IV antibiotics. Note that the scale of the vertical axis of panel B is twice the magnitude of that of panel A.
Table 4.
Subjects Required per Arm to Detect a 0.6 Hazard Ratio in a 6-Month Controlled Study using Either Treatment with “Any” or IV Antibiotics for Exacerbation as an Endpoint
| FEV1 Range (% predicted) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients | Age < 6 yrs | ≥ 100 | 70 to <100 | 40 to <70 | <40 | |||||||
| “Any” | IV | “Any” | IV | “Any” | IV | “Any” | IV | “Any” | IV | “Any” | IV | |
| All Patients | 202 | 355 | 360 | 745 | 332 | 959 | 212 | 450 | 129 | 195 | 105 | 138 |
|
| ||||||||||||
| Age Group | ||||||||||||
| 6-12 yrs | 239 | 473 | - | - | 377 | 1,031 | 248 | 556 | 143 | 213 | 103 | 139 |
| 13-17 yrs | 161 | 288 | - | - | 261 | 851 | 188 | 401 | 121 | 183 | 95 | 121 |
| 18-24 yrs | 127 | 191 | - | - | 214 | 630 | 157 | 288 | 119 | 175 | 102 | 127 |
| ≥ 25 yrs | 134 | 204 | - | - | 197 | 686 | 193 | 389 | 136 | 216 | 112 | 152 |
|
| ||||||||||||
| Sex | ||||||||||||
| Male | 217 | 398 | 374 | 754 | 348 | 1,101 | 234 | 535 | 139 | 217 | 111 | 152 |
| Female | 188 | 320 | 347 | 736 | 315 | 833 | 193 | 386 | 121 | 179 | 99 | 124 |
|
| ||||||||||||
| WFA Percentilea | ||||||||||||
| <25th | 175 | 283 | 315 | 572 | 317 | 868 | 211 | 429 | 123 | 183 | 103 | 131 |
| ≥25th | 231 | 455 | 399 | 939 | 339 | 1010 | 214 | 469 | 138 | 214 | 113 | 159 |
|
| ||||||||||||
| Signs and Symptomsa | ||||||||||||
| Daily Cough | 151 | 241 | 260 | 480 | 242 | 666 | 175 | 336 | 121 | 180 | 103 | 134 |
| Daily Sputum | 132 | 198 | 206 | 352 | 207 | 514 | 164 | 298 | 119 | 174 | 103 | 132 |
| Clubbing | 160 | 263 | 272 | 528 | 305 | 887 | 192 | 392 | 123 | 185 | 103 | 134 |
| Crackles | 123 | 177 | 192 | 279 | 273 | 615 | 163 | 299 | 114 | 168 | 102 | 132 |
| Wheeze | 165 | 264 | 315 | 557 | 324 | 912 | 197 | 376 | 126 | 190 | 116 | 163 |
|
| ||||||||||||
| Pulmonary Exacerbation Historyb | ||||||||||||
| None | 305 | 778 | 471 | 1258 | 406 | 1529 | 282 | 807 | 185 | 370 | 152 | 267 |
| One | 155 | 260 | 251 | 436 | 204 | 439 | 155 | 294 | 126 | 200 | 111 | 154 |
| Two | 109 | 150 | 181 | 268 | 133 | 252 | 113 | 173 | 94 | 124 | 96 | 120 |
| Three or more | 86 | 103 | 135 | 188 | 117 | 168 | 88 | 111 | 81 | 96 | 78 | 87 |
|
| ||||||||||||
| P. aeruginosa Culture Historyc | ||||||||||||
| All negative | 327 | 608 | 458 | 901 | 496 | 1321 | 302 | 620 | 162 | 241 | 127 | 166 |
| Any positive | 150 | 257 | 243 | 535 | 229 | 708 | 170 | 362 | 120 | 179 | 101 | 131 |
| Indeterminated | 176 | 358 | 271 | 679 | 240 | 740 | 190 | 482 | 140 | 244 | 120 | 170 |
| Intermittente | 198 | 374 | 273 | 643 | 290 | 825 | 197 | 418 | 131 | 199 | 99 | 121 |
| Chronicf | 130 | 207 | 187 | 355 | 199 | 639 | 154 | 309 | 112 | 162 | 97 | 125 |
As recorded at the index visit
Number of pulmonary exacerbations treated with IV antibiotics in the 12 months prior to the index visit
Recorded in the 12 months prior to the index visit
Only one culture result reported
≤50% of cultures positive
>50% of cultures positive
DISCUSSION
There is little question that chronic therapies capable of reducing the incidence of CF exacerbations have the potential to reduce health care costs [14, 15], improve patient quality of life [7-9], spare loss of lung function [26], and possibly improve survival [10-13]. However, the use of change in risk of pulmonary exacerbation as an efficacy endpoint for randomized CF trials is challenging. For example, powering of trials employing risk of exacerbation as an endpoint requires knowledge of the underlying risk of exacerbation in the intended study population, but risk of exacerbation is not uniform across the CF population [2].
In practice, many clinical trials have employed physician intervention in their definition of an exacerbation. We have analyzed data collected over 10 years from a large set of CF patients in ESCF to characterize how patient characteristics affect the probability of treatment with antipseudomonal antibiotics for exacerbation and how they affect the corresponding sample size requirements for studies using exacerbation treatment as an efficacy endpoint. We have reported sample sizes required to attain 80% power to detect a 40% reduction in treated exacerbations over a 6 month observation period (Table 4). A treatment benefit of this magnitude (i.e., hazard ratio = 0.6) is consistent with reductions reported in past CF trials of dornase alfa (relative risk = 0.63 for bid use, [16]), inhaled tobramycin (relative risk = 0.64, [17]), chronic azithromycin (relative risk = 0.65, [18]), and inhaled aztreonam (relative risk = 0.55, [21]). The choice of a 6 month observation period was a pragmatic one, in that studies of shorter duration require substantially more subjects to attain adequate statistical power (Figure 1).
Although our data are useful for estimating the likelihood of different subgroups being treated for exacerbation over time, the absolute sample sizes reported in Table 4 are dependent upon several conditions that may not be met in future clinical trials. We have reported results using two indirect measures of pulmonary exacerbation: treatment with “any” antibiotics (defined as new treatment with oral quinolones or inhaled antibiotics or treatment with IV antibiotics) and treatment with IV antibiotics. These measures have been incorporated into exacerbation definitions in the past [16-18, 27], but it should be noted that these treatments tend to be administered in the belief that exacerbation symptoms are caused by P. aeruginosa. In this context, our observation that negative, intermittent, or chronic P. aeruginosa culture histories in the baseline period are associated with different probabilities of treatment with antipseudomonal antibiotics for exacerbation (Table 3) should not be surprising. The extent to which different culture histories actually affect presentation of signs and symptoms of exacerbation or simply increase the probability of being treated with antipseudomonal antibiotics during exacerbation is not clear. Importantly, the proportion of patients with a history of positive P. aeruginosa culture increases with age (Table 1). Clinicians may be less suspicious of P. aeruginosa in younger patients in the absence of definitive culture data, in which case using antipseudomonal antibiotic treatment as a measure would likely underestimate exacerbation rates. Use of an exacerbation definition requiring the presence of a constellation of specific clinical signs and symptoms in addition to specific interventions [16] would presumably reduce the overall event rate, thereby increasing sample size estimates. In contrast, a definition consisting solely of presentation of signs and symptoms and for which intervention is not a requirement [28] would likely increase observed exacerbation rates and correspondingly reduce required sample sizes. The use of patient diaries and patient-reported outcome measures [29, 30] are attractive approaches to avoiding the complication of defining exacerbations based on specific clinical interventions. These approaches are likely to increase event rates and thus reduce required sample sizes for clinical trials.
Observed exacerbation rates may be affected not only by the exacerbation definition, but also by the frequency of encounter. Currently, identification of exacerbation occurs at clinic encounters, so an increased encounter frequency may increase the frequency of exacerbations. To be included in the analysis, patients were required to have at least 4 routine clinic encounters during a year of observation, an encounter frequency consistent with current CF practice guidelines [4]. However, controlled clinical trials are often designed with more frequent (e.g., monthly) study visits, and thus observed rates of exacerbation might be higher and required sample sizes correspondingly lower than our predictions based on quarterly encounter data. For example, 52.0% of placebo subjects were treated with IV antibiotics during the 6 month inhaled tobramycin studies, which included at least 8 clinic encounters after treatment initiation [17]. We analyzed 6,098 ESCF patients employing similar inclusion criteria (≥ 6 years old, FEV1 between 25 and 75% predicted, and chronic P. aeruginosa culture status in the prior year), but our requirement of only 4 quarterly clinic encounters in the follow up period resulted in only 46.1% of these patients treated with IV antibiotics for exacerbation in the 6 months following the index visit. This modest difference in event rates may result in part from differences in encounter frequency.
Even with these caveats, our analyses should be of value to clinical investigators using exacerbation as an efficacy endpoint in CF clinical trials. Despite consistent incremental improvement in the overall health of the CF population [27,31], rates at which patients are treated with antibiotics for pulmonary exacerbations have been remarkably constant over the past two decades [31], suggesting that registry data from the recent past can be useful in predicting future treatment rates. Our data indicate how inclusion/exclusion criteria might be adjusted to select subjects at greater risk for exacerbation (e.g., subjects that are older, have more advanced lung disease, or have a recent history of exacerbation). Conversely, our data suggest that detecting an impact on exacerbation rate in some subpopulations (e.g., subjects with early lung disease) may prove problematic due to sample size requirements.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the participation of the more than 400 site investigators and coordinators in Epidemiologic Study of Cystic Fibrosis (ESCF) in collecting this comprehensive database.
Footnotes
CONFLICT OF INTEREST Donald VanDevanter, Wayne Morgan, and Michael Konstan have received honoraria from Genentech, Inc., for serving as members of the Scientific Advisory Group for the Epidemiologic Study of Cystic Fibrosis (ESCF), and have served as consultants to Genentech. No compensation was provided to these authors in exchange for production of this manuscript. Stefanie Millar and David Pasta are employees of ICON Clinical Research. ICON Clinical Research was paid by Genentech for providing biostatistical services for this study. Ashley Yegin is currently an employee of Genentech. This study is sponsored by Genentech, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ratjen F, Döring G. Cystic fibrosis. Lancet. 2003;361:681–9. doi: 10.1016/S0140-6736(03)12567-6. REVIEW. [DOI] [PubMed] [Google Scholar]
- 2.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62:360–7. doi: 10.1136/thx.2006.060889. REVIEW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey BW. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med. 1996;335:179–88. doi: 10.1056/NEJM199607183350307. Review. [DOI] [PubMed] [Google Scholar]
- 4.Clinical Practice Guidelines for Cystic Fibrosis. Cystic Fibrosis Foundation; Bethesda, MD: 1997. [Google Scholar]
- 5.Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006;148:259–64. doi: 10.1016/j.jpeds.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Flume PA, Mogayzel PJ, Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, Marshall BC, Clinical Practice Guidelines for Pulmonary Therapies Committee Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802–8. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 7.Orenstein DM, Pattishall EN, Nixon PA, Ross EA, Kaplan RM. Quality of well-being before and after antibiotic treatment of pulmonary exacerbation in patients with cystic fibrosis. Chest. 1990;98:1081–4. doi: 10.1378/chest.98.5.1081. [DOI] [PubMed] [Google Scholar]
- 8.Bradley J, McAlister O, Elborn S. Pulmonary function, inflammation, exercise capacity and quality of life in cystic fibrosis. Eur Respir J. 2001;17:712–5. doi: 10.1183/09031936.01.17407120. [DOI] [PubMed] [Google Scholar]
- 9.Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121:64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 10.Liou TG, Adler FR, FitzSimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–52. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166:1550–5. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 12.Emerson J, Rosenfeld M, McNamara S, Ramsey BW, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 13.Ellaffi M, Vinsonneau C, Coste J, Hubert D, Burgel PR, Dhainaut JF, Dusser D. One-year outcome after severe pulmonary exacerbation in adults with cystic fibrosis. Am J Respir Crit Care Med. 2005;171:158–64. doi: 10.1164/rccm.200405-667OC. [DOI] [PubMed] [Google Scholar]
- 14.Lieu TA, Ray GT, Farmer G, Shay GF. The cost of medical care for patients with cystic fibrosis in a health maintenance organization. Pediatrics. 1999;103:e72. doi: 10.1542/peds.103.6.e72. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang L, Grosse SD, Amendah DD, Schechter MS. Healthcare expenditures for privately insured people with cystic fibrosis. Pediatr Pulmonol. 2009;44:989–96. doi: 10.1002/ppul.21090. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 17.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev-K M, Borowitz D, Bowman CM, Marshall BC, Marshall S, Smith AL. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 18.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW, 3rd, Macrolide Study Group Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 19.Murphy TD, Anbar RD, Lester LA, Nasr SZ, Nickerson B, VanDevanter DR, Colin AA. Treatment with tobramycin solution for inhalation reduces hospitalizations in young CF subjects with mild lung disease. Pediatr Pulmonol. 2004;38:314–320. doi: 10.1002/ppul.20097. [DOI] [PubMed] [Google Scholar]
- 20.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT, National Hypertonic Saline in Cystic Fibrosis (NHSCF) Study Group A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 21.McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:921–8. doi: 10.1164/rccm.200712-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan WJ, Butler SM, Johnson CA, Colin AA, FitzSimmons SC, Geller DE, Konstan MW, Light MJ, Rabin HR, Regelmann WE, Schidlow DV, Stokes DC, Wohl ME, Kaplowitz H, Wyatt MM, Stryker S. Epidemiologic study of cystic fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S. and Canada. Pediatr Pulmonol. 1999;28:231–241. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 24.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 25.Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 26.Sanders DB, Hoffman LR, Emerson J, Gibson RL, Rosenfeld M, Redding GJ, Goss CH. Return of FEV(1) after pulmonary exacerbation in children with cystic fibrosis. Pediatr Pulmonol. 2010;45:127–34. doi: 10.1002/ppul.21117. [DOI] [PubMed] [Google Scholar]
- 27.2009 Annual Data Report. Cystic Fibrosis Foundation; Bethesda, MD: 2010. Cystic Fibrosis Foundation Patient Registry. [Google Scholar]
- 28.Rosenfeld M, Emerson J, Williams-Warren J, Pepe M, Smith A, Montgomery AB, Ramsey B. Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr. 2001;139:359–65. doi: 10.1067/mpd.2001.117288. [DOI] [PubMed] [Google Scholar]
- 29.Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128:2347–54. doi: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- 30.Bennett AV, Patrick DL, Lymp JF, Edwards TC, Goss CH. Comparison of 7-day and repeated 24-hour recall of symptoms of cystic fibrosis. J Cyst Fibros. 2010;9:419–24. doi: 10.1016/j.jcf.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanDevanter DR, Rasouliyan LH, Murphy TM, Morgan WJ, Ren CL, Konstan MW, Wagener JS, for the Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis Trends in the clinical characteristics of the U.S. cystic fibrosis patient population from 1995 to 2005. Pediatr Pulmonol. 2008;43:739–44. doi: 10.1002/ppul.20830. [DOI] [PubMed] [Google Scholar]


