Abstract
Reactive gliosis, also known as glial scar formation, is an inflammatory response characterized by the proliferation of microglia and astrocytes as well as astrocytic hypertrophy following the injury in the central nervous system (CNS). The glial scar forms a physical and molecular barrier to isolate the injured area from adjacent normal nervous tissue for re-establishing the integrity of the CNS. It prevents the further spread of cellular damage but represents an obstacle to regrowing axons. In this review, we integrated the current findings to elucidate the tightly reciprocal modulation between activated microglia and astrocytes in reactive gliosis, and propose that modification of cellular response to the injury or cellular reprogramming in the glial scar could lead advances in axon regeneration and functional recovery after the CNS injury.
Keywords: reactive gliosis, glial scar, microglia, astrocyte, central nervous system
1. Introduction
Reactive gliosis, also known as glial scar (GS) formation, is a reactive cellular process that occurs after injury in the central nervous system (CNS), involving reactive astrocytes, activated microglia, fibroblast, endothelial cells, infiltrating immune cells, and extracellular matrix surrounding the damaged region. The inflammation seems to be a critical step in secondary degeneration after the CNS injury and causal to the GS formation. Growing evidence suggest that cytokines released from microglia, macrophages, and infiltrating immune cells during the acute phase of CNS damage may function as either initial molecular inducers (e.g. IL-6, TNF-α, IFN-γ) or repressors (e.g. IL-10) of astrocyte proliferation and GS formation [1–4]. On the other hand, molecules released from reactive astrocytes in turn maintain a persistent inflammatory response and modulate the microglial activation during the chronic phase of the CNS injury. For the regenerative studies of spinal cord injury (SCI), reactive astrogliosis has become an important therapeutic target for axonal regrowth and functional recovery [6–7]. In the primary lesion stage of SCI, astrocytes first provide support to the injured area, maintain blood-cord barrier, secrete cytokines and prevent excitotoxicity. In the secondary lesion stage, astrocytes enter the hypertrophic state (reactive astrocytes) with increased synthesis of intermediate filaments such as GFAP and vimentin, which form a physical wall and produce inhibitory proteoglycans (e.g. CSPGs and KSPGs) to drive back axonal regeneration [6]. Although the GS represents a physical and molecular barrier to axonal regrowth, it also isolates the injury site from healthy tissue, which prevents further damage due to uncontrolled expansion of inflammation [8, 9]. However, the reciprocal impact of microglia and astrocytes as well as how it determines the progression of CNS injury are still poorly understood. In this review, we will attempt to address this complex issue by integrating current findings in microglial and astrocytic activation after the CNS injury, which may aid in understanding the fine balance between inflammation and the GS formation.
2. Origin, development and physiological functions of microglia and astrocyte in the CNS
2.1. Microglia
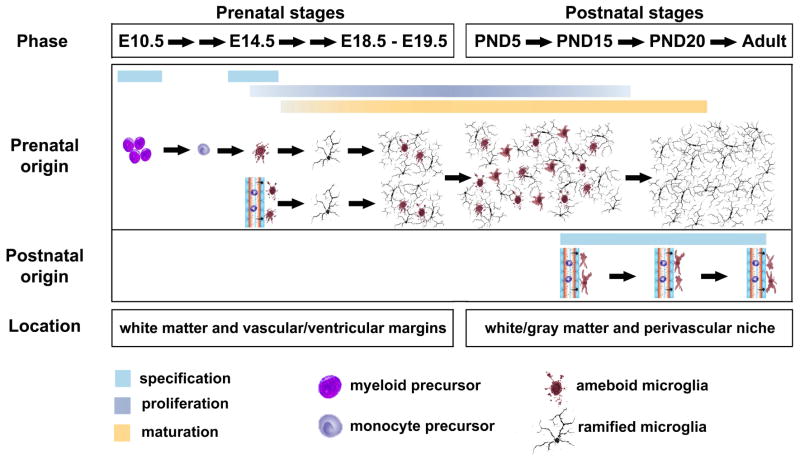
Microglia are widely regarded as the resident mononuclear phagocytes distributed ubiquitously throughout the nervous system, which are typically characterized by ramified morphology in a ‘resting’ state and express certain cell surface antigens, such as CD11b/c, CD14, major histocompatibility complex molecules, chemokine receptors, and several other markers [10]. In mice, microglial progenitors with amoeboid/phagocytic morphology start to colonize in neural tube around E10.5 (i.e. embryonic day 10.5) [11]. Three days later, they are significantly detected within superficial mantle layer of spinal cord as well as subventricular zone in brain [12]. The precise origin and cell lineage of microglia remain debated. At least two separate ‘populations’ of microglial progenitors exist during the prenatal CNS development. One mainly comes from extravascular progenitors that are of myeloid/mesenchymal progressively developing until the adulthood. The other derives from circulating progenitors – monocytes and/or fetal macrophages that are seeded within the CNS after the fetal circulation has been established at E14. They may also be derived from neuroectoderm similar to oligodendrocytes and astrocytes. However, in the early postnatal and adult CNS, blood-borne precursors only give rise to a small number of perivascular ameboid-like macrophages/microglia, not most of ramified microglia that are widely and stably distributed in the CNS [12, 13]. Microglial progenitors are differentiated and localized along vascular/ventricular margins and white matter during prenatal stages. Around five days after birth (~PND5), these microglia are observed in both white matter and gray matter regions, which are dramatically proliferate between PND5 and PND15. By PND20, the adult microglia are well matured and distributed throughout the CNS (Fig. 1). Traditionally, microglia are thought to be in a ‘resting’ state to maintain homoeostatic activity in the normal CNS. Recently, accumulating evidence revealed that microglia are highly dynamic to communicate with neurons, astrocytes, oligodendrocytes, and immune cells, which are proposed to be renamed as ‘surveying’ microglia [13, 14].
Figure 1.
Origin and development of microglia in the rodent CNS. The myeloid/mesenchymal-derived microglial progenitors start to colonize in neural tube around E10.5. Four days later, the second population of microglial progenitors originates from the circulating blood monocytes and/or fetal macrophages. The proliferating progenitors are differentiated and localized along vascular/ventricular margins and white matter during prenatal stages. Around PND5, these microglia are observed in both white matter and gray matter region, which are dramatically proliferate between PND5 and PND15. By PND20, the microglia are well matured with ramified morphology and stably distributed throughout the CNS. In the early postnatal and adult CNS, blood-borne precursors also generate a small number of perivascular ameboid-like macrophages/microglia.
2.2. Astrocyte
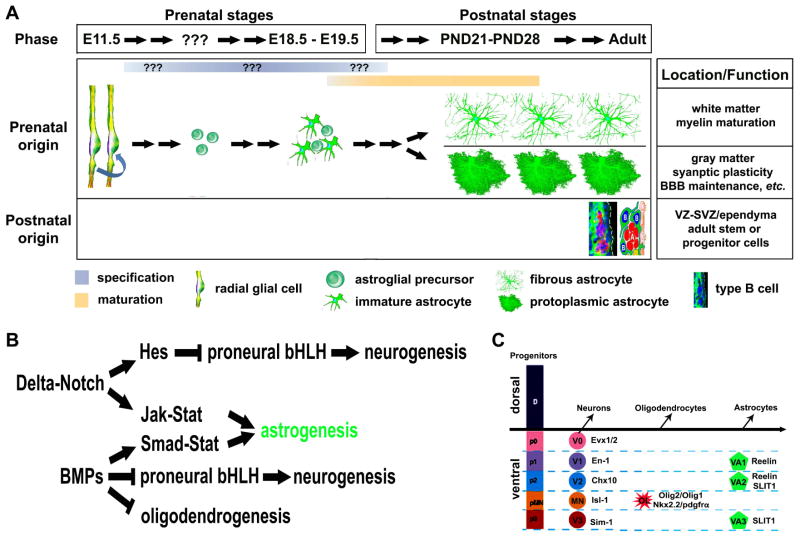
Astrocytes, known as astroglia, are the most abundant cells in the CNS. Astrocytes are classically identified as cells expressing the intermediate filament GFAP, a marker of terminally differentiated astrocytes. Although originally defined as gap fillers for the neuronal network, astrocytes have strategic locations, being in closely contact with CNS-resident cells (neurons, microglia, oligodendrocytes and other astrocytes) and with blood vessels. The initiation of glial specification occurs after the neurogenesis at E11.5 in the rodent CNS. Radial glial cells derived from the neuroepithelium are the primary precursor cells at embryonic stages to generate neurons first, followed by glia. The timing of this neuron-glia switch is temporally-spatially controlled by extrinsic and intrinsic factors [15, 16]. The bone morphogenetic proteins (BMPs), Delta-Notch and Jak-Stat pathways are well-known signaling to activate a set of transcription factors that determine the cell fate of astrocytes in the populations of the ventricular zone (VZ). However, the precise timing of astroglial specification remains unclear. Patterning domains in the ventral spinal cord that generate astrocyte have been established in the p1, p2, and p3 domains at the VZ along the dorsal-ventral axis which specify three subtypes of ventral white matter astrocytes - VA1, VA2, and VA3, respectively [16, 17]. All newly born astrocytes could be essentially identical, but differentiate into different ‘shape’ due to their final residental area. The morphology of astrocyte appears to be mature by the third to fourth postnatal week. Two types of astrocytes are identified based on location in the white versus gray matter [18–21]. Fibrous astrocytes typically showing more classic ‘star-like’ processes with dense GFAP staining populate the white matter. Protoplasmic astrocytes having more thinner and spongiform processes reside in gray matter (Figure 2). The lack of reliable markers is a major limitation for astrocyte study. GFAP, as a terminally-differentiated astrocyte marker, is mainly expressed in the late development of fibrous astrocytes and activated astrocytes under pathological condition. It is also synthesized in type B multipotent cells at the subventricular zone (SVZ) in the adult rodents. Since neural cells are generated from the neuroepithelium, astrocytes share some markers the same as either neurons (e.g. NFI A/B, FABP7/BLBP, FGFR3, and Sox9) or oligodendrocytes (e.g. Glast, NFI A/B, FGFR3, Sox9, Id3, and S-100β) at specific embryonic stages [22]. Astrocytes now have been found not only to participate in neurotransmitter regulation, ion homeostasis, blood–brain barrier maintenance, immune responses modulation, and the production of extracellular matrix (ECM) molecules [23, 24], but also to play a number of active roles in cell migration, differentiation, and maturation in the developing CNS, not just as a supportative cell [25]. More recent studies further showed that astrocytes were involved in regulating synaptic plasticity [26], and myelin maturation [14]. In the adult rodent brain, GFAP-positive astrocyte-like cells at the VZ-SVZ (type B cells) serve as stem/progenitor cells that give rise to adult-born neurons in the olfactory bulb [27].
Figure 2.
Origin and development of astrocyte in the rodent CNS. (A) Generation, morphological changes, and physiological functions of astrocyte across developmental time. The initiation of glial specification occurs after the neurogenesis at E11.5 in the rodent CNS. Radial glial cell-derived astroglial progenitors are generated after the neuron-glia switch at E11.5. The precise timing of astroglial specification remains unclear. All newly born astrocytes could be essentially identical, but differentiate into fibrous or protoplasmic astrocyte with respective functions due to their final location in the white matter or gray matter. (B) Signaling pathways that determine the astroglial specification. (C) A schematic illustration of neuronal, oligodendroglial, and astroglial domains in the ventral spinal cord development. The homeodomain code controls the generation of neuron, oligodendrocyte and white matter astrocyte.
3. Reactive gliosis following the CNS injury – from inflammation to glial scar
3.1. Microglia activation and inflammatory response
Microglia are considered ‘the tissue macrophages’ in the nervous system, owing to their phenotype and reactivity following any disturbance or loss of homeostasis that indicates real or potential danger to the nervous system. It has been reported that a subpopulation of monocytes enters the neural tissue and transforms into microglia after blood-brain barrier damage [28]. Under the pathological conditions of the CNS injury such as infection, ischemia, neurodegenerative disease, and trauma etc., microglia are readily activated and undergo a dramatic transformation from their ‘surveying’ ramified state into an amoeboid morphology [29]. The ‘surveying’ microglia are able to extend or retract cytoplasmic processes within seconds or minutes and reorient their processes within a few minutes. The transformed microglia (activated state) migrate towards the site of lesion in the CNS and form a dense border that seems to seal the lesion and block the spread of damage [30]. In their activated state, they can up-regulate or express de novo distinct profiles of cell surface ‘phenotypic’ markers, which are also found on other mononuclear phagocytes such as macrophages. They serve diverse beneficial functions essential to neuron survival, which include cellular maintenance and innate immunity [31]. Meanwhile, activated microglia are also involved in regulating the CNS development and neurogenesis through the release of trophic and anti-inflammatory factors [13]. However, under the over-activated state, microglia induce detrimental neurotoxic effects by releasing a diverse set of cytotoxic substances, including pro-inflammatory factors such as TNF-α, PGE2, and INF-γ and oxidative stress factors which are toxic to neurons [32–34]. Some experiments have shown that two kinds of functional subsets of monocyte-derived macrophages exist in peripheral blood and may contribute to distinct biological performance in inflammatory diseases [35]. Similarly, different stimulus to microglia may lead to diverse phenotype, referred to as microglial polarization, which results in cells with either pro- or anti-inflammatory properties [36]. In the SCI, the classically-activated M1 macrophages/microglia activated by Lipopolysaccharide (LPS) and pro-inflammatory cytokine IFN-γ produce high levels of oxidative metabolites (e.g., nitric oxide, superoxide) and pro-inflammatory cytokines (IL-12, IL-23, IL-1β and TNF-α), and increase their phagocytic and antigen-presenting capacity [37]. M1 macrophages/microglia not only play essential roles in host defense but also cause the damage to peripheral healthy cells and tissue [38]. Conversely, alternative M2 macrophages/microglia activated by cytokines interleukin-4 (IL-4) or IL-13 promote angiogenesis, matrix remodeling, and expression of MHCII molecules. They also suppress destructive immunity, nitric oxide (NO) and pro-inflammation cytokines (TNF-α, IL-1β, IL-2, IL-8, IL-12 and CXCL10) release [39–41]. M1 macrophages/microglia express specific antigens such as CD86, CD32, inducible NO synthase while M2 can be identified by arginase 1, mannose receptor, CD206 [38, 39].
Generally speaking, short-term microglial activation is not considered to be detrimental, even plays beneficial effects in CNS injury or diseases. Microglia produce a number of neuroprotective substances in response to injury, including anti-inflammatory cytokines and neurotrophic factors. TGF-β and IL-10 down-regulate the expression of molecules associated with antigen presentation and decrease the production of pro-inflammatory cytokines, chemokines, and nitric and oxygen free radicals [42, 43]. Microglia can also release brain-derived growth factor (BDNF), insulin-like growth factor1 (IGF1), which led to improved neuronal cell viability [44]. Recent evidence indicates that different cytokines released from activated microglia can stimulate T immune cells to acquire diverse phenotypes with detrimental [45] or beneficial [36, 46] effects in the CNS. In the acute stage of SCI, macrophages/microglia are activated (Fig. 3A) and become the primary source of the pro-inflammatory cytokines IL-1, IL-6, and TNF-α [47]. Most macrophages/microglia are M1 cells, with only a transient and small number showing M2 polarization. cDNA microarray and quantitative real-time PCR analyses showed that M1 and M2 markers were rapidly unregulated after spinal cord injury. The M2 marker - arginase 1 had only transient increase and returned to normal levels by 7 days post injury. In contrast, M1 markers CD16/32, CD86 expression was maintained for up to one month post-injury [38]. The in vivo and in vitro studies indicate that M1 macrophages can directly induce neuronal death and correlate with tissue damage in spinal cord injury as those anti-inflammatory M2 macrophages/microglia probably contributes to the prolonged pro-inflammatory response that has detrimental effects on tissue preservation and cell viability. Furthermore, M1 macrophages may have a negative impact on axon regeneration possibly due to 17-fold higher expression of CSPGs in M1 than that in M2 cells [48]. In culture, M1-conditioned medium induces stunted, short neurites with multiple branches, whereas M2-conditioned medium promotes extensive, long neurites from dorsal root ganglion cells [38], suggesting M2 macrophages/microglia may provide a more permissive axon regeneration microenvirment than M1 macrophages in spinal cord injury. Thus, it is very important to understand that the diverse phenotype acquired and their regulatory signals of microglial cells responding to the diverse stimulations. It may provide a new therapeutical strategy for treatment of the CNS injury via adjusting the shift of microglial subtypes.
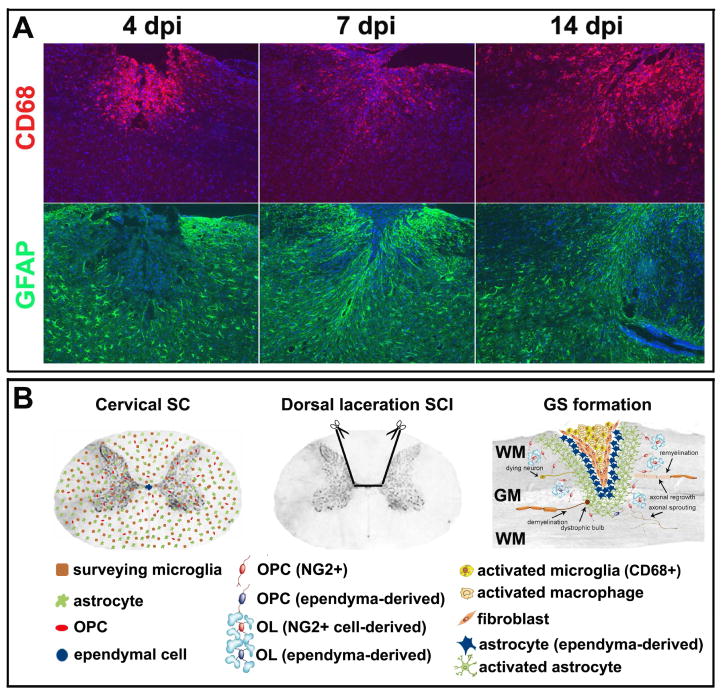
Figure 3.
Reactive gliosis in the mouse spinal cord injury of dorsally-cervical laceration. (A) Activation of microglia (CD68+ cells) and astrocytes (GFAP+ cells) in the acute stage of SCI. dpi: days post injury. (B) Major cellular populations in the adult spinal cord and glial scar formation after the spinal cord injury. In the adult spinal cord, ‘surveying or resting’ microglia (brown) and astrocytes (green) are uniformly distributed as ependymal cells (blue) are confined to the epithelium lining the central canal. More adult oligodendocyte precursor cells (NG2+, red) are located in gray matter than those in white matter. After the injury, the resident microglia, infiltrating macrophages and fibroblasts evenly form the epicenter of the glial scar surrounding by ependymal cell-derived GFAP-negative astrocytes (blue) and activated preexisting GFAP-positive astrocytes (green). This intense inflammatory response leads to a cascade of secondary damage including dystrophic axons and their demyelination. On the other hand, up-regulation of inhibitory extracellular matrix molecules secreted by microglia and astrocytes, such as proteoglycans, are distributed in an increasing concentration gradient from the lesion penumbra to the lesion center. The inhibitory extracellular matrix molecules impede axonal regrowth and remyelination by the surrounding adult oligodendrocytes and precursor cells that are originated mostly from resident adult oligodendrocyte precursor cells (red) and sporadically from ependymal cells (blue). Meanwhile, a few axonal sprouting may appear in the area adjacent to the glial scar. SC: spinal cord; SCI: spinal cord injury; GS: glial scar; WM: white matter; GM: gray matter; OPC: oligodendrocyte precursor cell; OL: mature oligodendrocyte.
3.2. Reactive astrocytes in the GS formation
Reactive astrocytes (also known as astrogliosis or astrocytic scar) are the main cellular component of the GS, which is characterized by the cellular hypertrophy and an abnormal apparent increase in the number of astrocytes. After the injury, astrocytes are likely to react promptly to the damage, which undergo morphological changes, extend their processes, and increase synthesis of intermediate filament proteins. Up-regulation of intermediate filament proteins, in particular GFAP, vimentin and nestin in astrocytes, is regarded as the hallmark of astrogliosis. As a major intermediate filament protein in mature astrocytes, significantly increased expression of GFAP has been found in process of astrogliosis in numerous experimental models (Figure 3A). The levels of vimentin in astrocytes range from very low to intermediate, depending on the subpopulation of astrocytes. It has been suggested that re-expression of vimentin in reactive astrocytes following the injury is indicative of these cells recapitulating developmental migratory processes [49]. Nestin is regarded as a marker of “neural stem/progenitor cells” (NSPCs), which is expressed in both neuronal and glial precursors [50–52]. Nestin-immunopositive cells can be seen in reactive astrocytes in response to the CNS injury [53]. Recent in vivo studies identified two cellular origins of astrocytes in the GS after the SCI, preexisting GFAP-positive astrocytes and ependymal cell-derived GFAP-negative astrocytes [54, 55]. The ependymal cell-derived astrocytes express Sox9 and vimentin but not GFAP. They form the core of the GS surrounding by resident GFAP+ astrocytes activated after SCI (Figure 3B).
Astrocytes perform a serial of protective effects in the CNS injury condition. Activated astrocytes limit the infiltration of peripheral leukocytes/macrophage and activation of local resident microglia by initiating the repair the damaged blood-spinal cord or -brain barrier [56, 57]. They can modulate blood flow by the release of vasoconstrictors [58] and also protect neurons and oligodendrocytes from glutamate excitotoxicity by up-taking excess glutamate in environment [8, 59]. Deactivation of astrocytes via genetic ablation of GFAP resulted in widespread tissue disruption, pronounced cellular degeneration, and severe persisting motor deficits [9]. These findings show that reactive astrocytes provide essential ability that protect tissue loss and preserve function after the CNS injury. On the other hand, reactive astrocytes contribute significantly to the release of the inhibitory ECM components after the CNS injury [60], which form a dense GS around the injured lesion to pose physical and chemical barriers [61, 62]. It suggested that ependymal cell-derived astrocytes do not synthesize those inhibitory ECM components [55]. ECM components such as CSPGs, tenascins, and collagen are dramatically up-regulated in the GS after the CNS injury and inhibit axonal elongation and sprouting [63–65]. It has been found that ChABC, a bacterial enzyme that is able to degrade CSPG gradient, can enhance axonal regeneration through the GS after the SCI [66]. CSPGs also influence the properties of oligodendrocyte precursor cells (OPCs). They inhibit the outgrowth of OPC processes, OPC migration and differentiation [67, 68], which eventually lead to the fail of remyelination for regenerated axons. In addition, astrocytes and matrix components create a scaffold for the vascularization network at the injury site where endothelial cells and fibroblasts are recruited to form new capillaries. Thus, modulation of reactive astrocytes and ECM in the GS may be crucial for axonal regeneration following the CNS injury.
3.3. Reactive gliosis and functional recovery in the CNS injury
The CNS lesion may cause locomotor deficits, sensory impairment, and/or chronic neuropathic pain to the various extents, depending on the location, range, and severity of the injury. Animal studies have shown that anti-inflammatory treatments significantly ameliorated motor and sensory functional recovery [69–73]. Reducing the infiltration of neutrophils, macrophages or T cells with neutralizing antibodies [69, 70], depletion of macrophages [71], or anti-inflammatory cytokine therapy [72] in the acute phase decreased the secondary tissue damage with functional improvement. Repression of microglial/macrophage activation by administration of minocycline or CD25 antibodies during either acute or chronic phases increased the neuroperformance after the traumatic SCI [73–75]. Inhibiting the astroglial activation or remodeling the ECM of astrocytic scar enhanced axonal plasticity and regeneration, and promoted the functional improvement in the rodent SCI models [76–80]. Recently, accumulating evidence suggests that inflammation and reactive gliosis (both microglia and astrocytes) have emerged as key contributors to pathological and chronic neuropathic pain mechanisms in the CNS injury [72, 81–85]. Thus, anti-inflammatory therapy may also relieve the chronic neuropathic pain [86, 87].
4. Microglial modulation on astrogliosis in the CNS injury
Although astrogliosis is associated with diverse neurological disorders, the cellular and molecular mechanisms leading to astrogliosis are not yet completely understood. As the first line of defense in the CNS, macrophages/microglia respond immediately to the presence of danger signals, react quickly to increase inflammatory signals and destroy the infectious agents before they cause the damage in neural tissue [88]. They can respond within minutes after injury with production of pro-inflammatory cytokines. Growing evidence suggest that activated macrophages/microglia may contribute to the subsequent activation of astrocytes in the CNS injury. A number of cytokines, chemokines, growth factors, and transcription factors have been identified as triggers and modulators for astrogliosis [89], including TNF-α, IL-1β, IL-6, IL-10, TGF-α, TGF-β, CNTF, fibroblast growth factor-2, platelet-derived growth factor, insulin-like growth factor (IGF), leukemia inhibitory factor, monocyte chemoattractant protein-1, endothelin-1, erythropoietin, fibrinogen, matrix metalloproteinase-9, and Sox9. As the most important pro-inflammatory cytokines secreted by macrophages/microglia, IL-1, IL-2, IL-6, TNF-α play important roles as initial triggers to activate the astrocytes via their receptors in the acute phase of CNS injury [90–93]. IL-1, IL-2, IL-6, and TNF-α have been found to increase GFAP immunoreactivity when they were microinjected into brain in the neonatal stab-wound mouse model [94]. IL-1 injected into the cerebral cortex of adult rats not only elicits new blood vessel growth but also stimulates GFAP expression as well as hypertrophy of astrocytes [95], indicating that IL-1-secreting inflammatory cells may mediate astrocyte activation in the CNS injury. IL-6 has been reported to link several neurological disorders such as multiple sclerosis, and Alzheimer’s disease. IL-6 induces the synthesis of neurotrophic factors - nerve growth factors (NGFs) [96] and inhibits the production of the potentially neurotoxic molecule TNF-α [97] in astrocytes. However, excessive expression of IL-6 mice showed marked gliosis and neurological signs even after mild injury of the spinal cord [98]. In IL-6 knockout mice, reactive GFAP-positive stellar astrocytes and gliosis are drastically inhibited [99]. Block the IL-6 signal with IL-6 receptor antibody after the contusive mouse injury model can repress the GS formation at the center of the injured spinal cord by suppressing the transformation of ependymal cells to astrocytes [91]. The in vitro studies showed that TNF-α can promote changes in astrocytes via activation of epidermal growth factor receptor (EGFR) [100] and increase astrocytes proliferation and survival [101]. In transgenic model, overexpression of TNF-α directly enhances the immunoactvity of GFAP and vimentin in hypertrophied astrocytes possessing numerous thick processes via activating the EGFR [102].
5. Effect of reactive astrocytes on microglial activation after the CNS injury
Inflammatory response, mediated largely by macrophages/microglia, has been implicated in several different neurological disorders from acute injuries such as spinal cord injury to chronic neurodegenerative conditions such as AD (Alzheimer’s disease). Compared to the rapid microglial response, the astrocytic response usually occurs as a secondary event. Recent study reported a secondary peak of microglia and macrophage presenting in the injured spinal cord at 60 days, with continued elevation through 180 days after SCI, apart from the primary peak in 3 days to 7 days [103], suggesting that the secondary signals stimulate microglia and eventually cause such long-time chronic inflammation following the SCI. It demonstrated that astrogliosis or GS components may be involved in modulation of inflammation following the SCI. Some studies indicated that disruption of the scar or some of its components reduced numbers of reactive microglia in the lesion area and attenuates monocytic activity [104]. Other studies verified that the glial scar was partially required to maintain inflammatory response under balanced condition. Ablation of active astrocytes inhibits leukocyte infiltration in the spinal lesion area [9, 105]. However, the underlying mechanisms are still ambiguous.
Reactive astrocytes contribute to the release of pro-and anti-inflammatory cytokines such as interleukins (IL-1 and IL-6), TNF-α, TGF-β, and IFN-γ, which may in return activate microglia and cause the secondary injury [106]. The studies in vitro provide certain hints: astrocyte conditioned medium increases ramification of blood monocytes in culture [107], which was prevented by neutralizing antibodies against astrocyte-derived cytokines [108]. Since active microglia also secret the same inflammatory cytokines that exert biochemical effects on themselves by the way of autocrine or paracrine, it is difficult to determine in vivo if the stimulus signals come from reactive astrocytes. Activated astrocytes produce several growth factors and neurotrophic factors, such as IGF, NGF, BDNF, ciliary neurotrophic factor (CNTF) and neurotrophin 3 to support the surrounding cells [109, 110]. They also synthesize and release ATP, glutamate, reactive oxygen species (ROS) and NO, and ECM proteins such as CSPGs [62, 64]. Like microglia, reactive astrocytes can regulate their own activities in an autocrine or paracrine fashion [6, 57]. Meanwhile, they may play important regulatory roles in the activation, survival, and regeneration of adjacent neurons, oligodendrocytes, and microglia by the way of paracrine. ATP, as the second messenger, actually is one of mainly responsible messenger in activation of microglia through purinergic receptors that are expressed prominently on microglia [111, 112]. In response to local brain injury, the ATP released from astrocytes activates morphological changes of local microglia migrating towards the injury site quickly [30, 113]. Another report showed that ATP mediated the calcium signaling between astrocytes and microglia involved in controlling the number and function of microglial cells under pathophysiologic CNS conditions [114]. Ca2+-dependent glutamate released from astrocytes may exacerbate the neuroinflammation in neurodegenerative disorders [115]. Some data implied that glutamate can act on metabotropic glutamate receptor (mGluR) to suppress some facet of the glutamate export mechanism in process of activation of microglia [116]. The effect of glutamate on microglia can be reversed by glutamate receptor antagonists in process of neuroinflammation in some neurodegeneration models [117–119]. All these results indicate that ATP and glutamate released from activated astrocytes directly affect the microglial activity in neuropathlogical condition. In addition, as products of oxidative stress, ROS and NO are other important mediators of inflammatory processes during microglia activation [120]. After the CNS injury, over-reactive astrocytes at the lesion site form the GS and alter the composition of ECM dramatically. ECM components including CSPGs and tenascins are markedly up-regulated in astrocytes [121, 122]. CSPGs were found to adhere to chemoattractive molecules and growth factors which are needed for recruitment and activation of macrophages [123], immune cells [124], and dendritic cells [125]. These findings suggest that CSPGs may capture these factors and increase their focal concentration to attract more microglia migrating towards the lesion area, thereby enlarge immune response to the CNS damage. CD44 functions as a receptor colocalized in astrocytes and microglia. CD44-neutralizing antibodies can suppress CSPG-induced activation of microglia and modulate the release of neurotrophic factors [126, 127]. Furthermore, inhibition of CSPG production leads to a dramatic effect on the spatial organization of the infiltrating macrophages and resident microglia around the lesion site, decrease of IGF-1 expression, and increase of TNF-α level in the acute stage of the SCI, which enhances the motor functional recovery [127]. On the other hand, it has been reported that activated astrocytes can exert inhibitory effects on microglial activation. TGF-β mainly produced by astrocytes [128] can reduce microglial activation by down-regulating the expressions of molecules associated with antigen presentation, pro-inflammatory cytokines, NO and ROS [129]. Additionally, astrocytes can restrain the infiltration of the circulating macrophage and other immune cells by repairing of the damaged blood-brain and -spinal cord barriers [8, 9]. Taken together, accumulative evidence indicate that reactive astrocytes and their products are mostly associated with modulation of inflammatory response by regulating the number, location and activation of infiltrating monocytes-macrophages and resident microglia.
6. Conclusions and Prospects
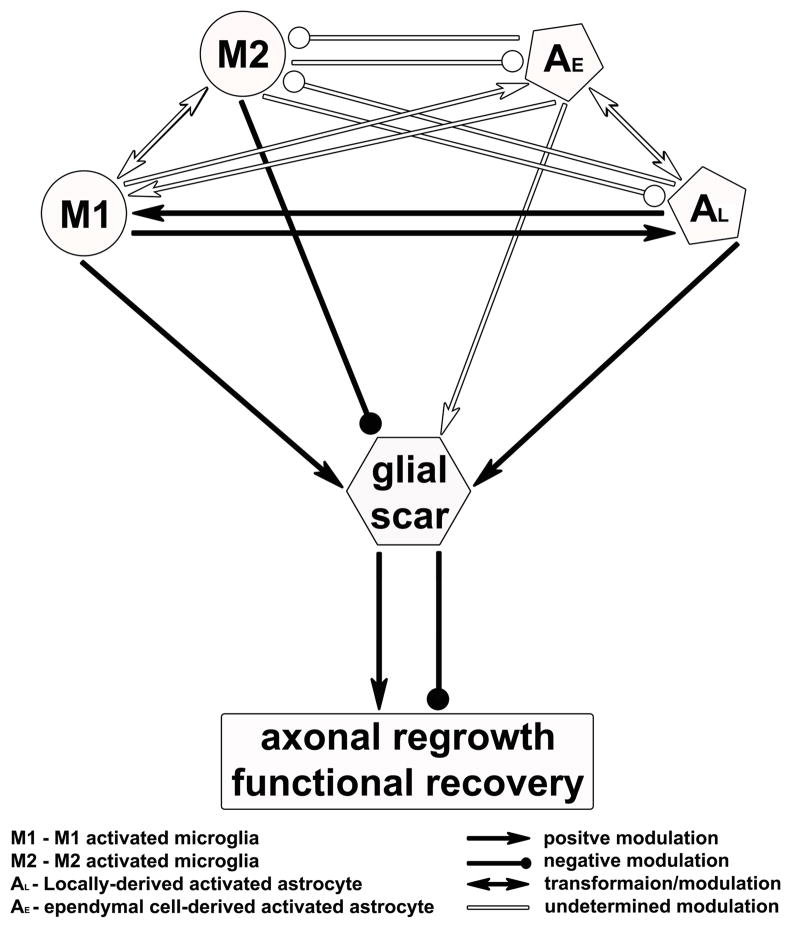
In the CNS, reactive gliosis is a complicated process which functions as both beneficial and detrimental effects on injury recovery. As two major cellular populations of reactive gliosis, microglia and astrocytes can activate each other and have a tightly reciprocal modulation during the GS formation. Either microglia or astrocytes can release a battery of signal molecules to feedback themselves or serve a cross-talk with adjacent brain cells, i.e., neurons, oligodendrocytes, astrocytes, microglia, and infiltrating immune cells. Under the pathological conditions in the CNS, microglia are activated earlier than astrocytes. In acute phase, most subpopulation of macrophages/microglia is pro-inflammatory M1 cells while only a transient and small number are anti-inflammatory M2 cells. The inflammatory molecules produced by activated M1 microglia activate both preexisting GFAP-positive astrocytes and GFAP-negative astrocytes derived from ependymal cells, which form the GS confining the lesion area in the CNS. The products released from reactive astrocytes may contribute to induce a secondary peak of macrophages/microglia presenting in the lesion site and maintain a persistent inflammatory response during the chronic phase of the CNS injury (Figure 4). It remains poorly understood (i) the mechanisms to determine the shift between M1 and M2 microglia; (ii) functional difference in preexisting GFAP-positive astrocytes and ependymal cell-derived GFAP-negative astrocytes, and (iii) how activated microglia and astrocytes synergistically modulate ECM components in the GS formation. A precise understanding of the underlying mechanisms will have significant bearing for potential therapeutic use. Modulation of injury response and cellular function in activated microglia and astrocytes with a new balance of protective and inhibitory effects in the injured CNS will likely become the master key to create a nourishing niche for axonal regeneration. In addition, recent studies in vitro show that lineage-specific transcription factors or microRNA can induce differentiated cells (e.g. fibroblasts and astrocytes) to trans-differentiate into functional neurons without going back to the fully undifferentiated state [130–137], which may alternatively provide an in vivo source of neurons and modify the microenvironment for use in cell-based therapies. Thus, cellular components of the GS, including fibroblasts, astrocytes, and microglia etc., could be reprogramed onsite and driven toward the neuronal lineage for functional repair.
Figure 4.
Hypothetical intermodulation between microglia and astrocytes in reactive gliosis following the CNS injury.
Acknowledgments
This work was supported by an Institutional Development Award (IDeA) from the NIGMS P20GM103453.
Abbreviations
- ATP
adenosine triphosphate
- CD
cluster of differentiation
- ChABC
chondroitinase ABC
- CSPGs
chondroitin sulphate proteoglycans
- CXCL10
C-X-C motif chemokine 10
- FABP7/BLBP
fatty acid binding protein 7, brain (aka, brain lipid binding protein)
- FGFR3
fibroblast growth factor receptor 3
- FNFI A/B
- GFAP
glial fibrillary acidic protein
- Glast
glutamate aspartate transporter
- Id3
inhibitor of DNA-binding/differentiation protein 3
- IFN-γ
interferon gamma
- IL
interleukin
- KSPGs
keratan sulfate proteoglycans
- MHCII
major histocompatibility complex class II
- NFI A/B
nuclear factor I protein A and B
- PGE2
prostaglandin E2
- Sox9
sex determining region Y-box 9
- TGF-α and -β
transforming growth factor alpha and beta
- TNF-α
tumor necrosis factor alpha
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- 1.Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotraum. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- 2.Chi LY, Yu J, Zhu H, Li XG, Zhu SG, Kindy MS. The dual role of tumor necrosis factor-alpha in the pathophysiology of spinal cord injury. Neurosci Lett. 2008;438:174–179. doi: 10.1016/j.neulet.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 3.Yong VW, Moumdjian R, Yong FP, Ruijs TC, Freedman MS, Cashman N, Antel JP. Gamma-interferon promotes proliferation of adult human astrocytes in vitro and reactive gliosis in the adult mouse brain in vivo. P Natl Acad Sci USA. 1991;88:7016–7020. doi: 10.1073/pnas.88.16.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang XF, Huang LD, Yu PP, Hu JG, Yin L, Wang L, Xu XM, Lu PH. Upregulation of type I interleukin-1 receptor after traumatic spinal cord injury in adult rats. Acta Neuropathol. 2006;111:220–228. doi: 10.1007/s00401-005-0016-x. [DOI] [PubMed] [Google Scholar]
- 5.Fehlings MG, Hawryluk GWJ. Editorial. Journal of Neurosurgery: Spine. 2010;13:165–167. doi: 10.3171/2009.11.SPINE09862. [DOI] [PubMed] [Google Scholar]
- 6.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Experimental Neurology. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Zhang W-P, Chen K-D, Qian X-D, Fang S-H, Wei E-Q. Caffeic acid attenuates neuronal damage, astrogliosis and glial scar formation in mouse brain with cryoinjury. Life Sciences. 2007;80:530–537. doi: 10.1016/j.lfs.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 8.Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte Infiltration, Neuronal Degeneration, and Neurite Outgrowth after Ablation of Scar-Forming, Reactive Astrocytes in Adult Transgenic Mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 9.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive Astrocytes Protect Tissue and Preserve Function after Spinal Cord Injury. The Journal of Neuroscience. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rock RB, Gekker G, Hu SX, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942. doi: 10.1128/CMR.17.4.942-964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris L, Graham CF, Gordon S. Macrophages in haemopoietic and other tissues of the developing mouse detected by the monoclonal antibody F4/80. Development. 1991;112:517–526. doi: 10.1242/dev.112.2.517. [DOI] [PubMed] [Google Scholar]
- 12.Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia—New concepts. Brain Research Reviews. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Hanisch U-K, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 14.Nobuta H, Ghiani CA, Paez PM, Spreuer V, Dong HM, Korsak RA, Manukyan A, Li JX, Vinters HV, Huang EJ, Rowitch DH, Sofroniew MV, Campagnoni AT, de Vellis J, Waschek JA. STAT3-Mediated astrogliosis protects myelin development in neonatal brain injury. Ann Neurol. 2012;72:750–765. doi: 10.1002/ana.23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowitch DH. Glial specification in the vertebrate neural tube. Nature reviews Neuroscience. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- 16.Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- 17.Freeman MR. Specification and morphogenesis of astrocytes. Science. 2010;330:774–778. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bignami AEL, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;28:351–354. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- 19.Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. International Journal of Developmental Neuroscience. 2004;22:73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Vaughn JE, Pease A. Electron microscopy of the early postnatal development of fibrous astrocytes. Am J Anat. 1967;121:131–152. doi: 10.1002/aja.1001210109. [DOI] [PubMed] [Google Scholar]
- 21.Vaughn JE, Peters D. Electron microscopy of classically stained astrocytes. J Comp Neurol. 1967;131:143–154. doi: 10.1002/cne.901310206. [DOI] [PubMed] [Google Scholar]
- 22.Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes & development. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walz W. Role of Glial-Cells in the Regulation of the Brain Ion Microenvironment. Progress in Neurobiology. 1989;33:309–333. doi: 10.1016/0301-0082(89)90005-1. [DOI] [PubMed] [Google Scholar]
- 24.Westergaard N, Sonnewald U, Schousboe A. Metabolic Trafficking between Neurons and Astrocytes - the Glutamate Glutamine Cycle Revisited. Dev Neurosci-Basel. 1995;17:203–211. doi: 10.1159/000111288. [DOI] [PubMed] [Google Scholar]
- 25.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navarrete M, Araque A. Basal Synaptic Transmission: Astrocytes Rule! Cell. 2011;146:675–677. doi: 10.1016/j.cell.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Ihrie RA, Alvarez-Buylla A. Lake-Front Property: A Unique Germinal Niche by the Lateral Ventricles of the Adult Brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch U-K, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 29.Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience. 2009;158:1062–1073. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 31.Zhang DHX, Qian L, O’Callaghan JP, Hong J. Astrogliosis in CNS Pathologies: Is There A Role for Microglia? Molecular Neurobiology. 2010;41:232–241. doi: 10.1007/s12035-010-8098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Progress in Neurobiology. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Chatzipanteli K, Garcia R, Marcillo AE, Loor KE, Kraydieh S, Dietrich WD. Temporal and segmental distribution of constitutive and inducible nitric oxide synthases after traumatic spinal cord injury: Effect of aminoguanidine treatment. J Neurotraum. 2002;19:639–651. doi: 10.1089/089771502753754109. [DOI] [PubMed] [Google Scholar]
- 34.Pearse DD, Chatzipanteli K, Marcillo AE, Bunge MB, Dietrich WD. Comparison of iNOS inhibition by antisense and pharmacological inhibitors after spinal cord injury. Journal of neuropathology and experimental neurology. 2003;62:1096–1107. doi: 10.1093/jnen/62.11.1096. [DOI] [PubMed] [Google Scholar]
- 35.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 36.Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147:867–883. doi: 10.1016/j.neuroscience.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 37.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nature reviews Neuroscience. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 38.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of Two Distinct Macrophage Subsets with Divergent Effects Causing either Neurotoxicity or Regeneration in the Injured Mouse Spinal Cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loane DJ, Byrnes KR. Role of Microglia in Neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka T, Huli J, Seder RA, Fazekas De St Groth B, Paul WE. Interleukin-4 Suppresses Interleukin-2 and Interferon-Gamma Production by Naive T-Cells Stimulated by Accessory Cell-Dependent Receptor Engagement. P Natl Acad Sci USA. 1993;90:5914–5918. doi: 10.1073/pnas.90.13.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frei K, Lins H, Schwerdel C, Fontana A. Antigen Presentation in the Central-Nervous-System - the Inhibitory Effect of Il-10 on Mhc Class-Ii Expression and Production of Cytokines Depends on the Inducing Signals and the Type of Cell Analyzed. J Immunol. 1994;152:2720–2728. [PubMed] [Google Scholar]
- 43.O’Keefe GM, Nguyen VT, Benveniste EN. Class II transactivator and class II MHC gene expression in microglia: modulation by the cytokines TGF-beta, IL-4, IL-13 and IL-10. Eur J Immunol. 1999;29:1275–1285. doi: 10.1002/(SICI)1521-4141(199904)29:04<1275::AID-IMMU1275>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 44.Lai AY, Todd KG. Differential regulation of trophic and proinflammatory microglial effectors is dependent on severity of neuronal injury. Glia. 2008;56:259–270. doi: 10.1002/glia.20610. [DOI] [PubMed] [Google Scholar]
- 45.Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: Activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28:9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- 47.Yang LQ, Jones NR, Blumbergs PC, Van Den Heuvel C, Moore EJ, Manavis J, Sarvestani GT, Ghabriel MN. Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J Clin Neurosci. 2005;12:276–284. doi: 10.1016/j.jocn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 49.Wang K, Bekar LK, Furber K, Walz W. Vimentin-expressing proximal reactive astrocytes correlate with migration rather than proliferation following focal brain injury. Brain Res. 2004;1024:193–202. doi: 10.1016/j.brainres.2004.07.086. [DOI] [PubMed] [Google Scholar]
- 50.Andressen C, Stocker E, Klinz FJ, Lenka N, Hescheler J, Fleischmann B, Arnhold S, Addicks K. Nestin-specific green fluorescent protein expression in embryonic stem cell-derived neural precursor cells used for transplantation. Stem Cells. 2001;19:419–424. doi: 10.1634/stemcells.19-5-419. [DOI] [PubMed] [Google Scholar]
- 51.Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003;23:9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura T, Xi GH, Hua Y, Hoff JT, Keep RF. Nestin expression after experimental intracerebral hemorrhage. Brain Res. 2003;981:108–117. doi: 10.1016/s0006-8993(03)02991-3. [DOI] [PubMed] [Google Scholar]
- 53.Frisén JJC, Török C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995;131:453–464. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of New Glial Cells in Intact and Injured Adult Spinal Cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 55.Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J. Spinal cord injury reveals multilineage differentiation of ependymal cells. Plos Biol. 2008;6:1494–1507. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Renault-Mihara F, Katoh H, Ikegami T, Iwanami A, Mukaino M, Yasuda A, Nori S, Mabuchi Y, Tada H, Shibata S, Saito K, Matsushita M, Kaibuchi K, Okada S, Toyama Y, Nakamura M, Okano H. Beneficial compaction of spinal cord lesion by migrating astrocytes through glycogen synthase kinase-3 inhibition. Embo Mol Med. 2011;3:682–696. doi: 10.1002/emmm.201100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 59.Vermeiren C, Najimi M, Vanhoutte N, Tilleux S, de Hemptinne I, Maloteaux JM, Hermans E. Acute up-regulation of glutamate uptake mediated by mGluR5a in reactive astrocytes. J Neurochem. 2005;94:405–416. doi: 10.1111/j.1471-4159.2005.03216.x. [DOI] [PubMed] [Google Scholar]
- 60.Stichel CC, Muller HW. The CNS lesion scar: new vistas on an old regeneration barrier. Cell Tissue Res. 1998;294:1–9. doi: 10.1007/s004410051151. [DOI] [PubMed] [Google Scholar]
- 61.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 63.Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Experimental Neurology. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 64.Mckeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of Neurite Outgrowth in a Model of Glial Scarring Following Cns Injury Is Correlated with the Expression of Inhibitory Molecules on Reactive Astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stichel CC, Hermanns S, Luhmann HJ, Lausberg F, Niermann H, D’Urso D, Servos G, Hartwig HG, Muller HW. Inhibition of collagen IV deposition promotes regeneration of injured CNS axons. Eur J Neurosci. 1999;11:632–646. doi: 10.1046/j.1460-9568.1999.00466.x. [DOI] [PubMed] [Google Scholar]
- 66.Mckeon RJ, Hoke A, Silver J. Injury-Induced Proteoglycans Inhibit the Potential for Laminin-Mediated Axon Growth on Astrocytic Scars. Experimental Neurology. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 67.Siebert JR, Osterhout DJ. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem. 2011;119:176–188. doi: 10.1111/j.1471-4159.2011.07370.x. [DOI] [PubMed] [Google Scholar]
- 68.Siebert JR, Stelzner DJ, Osterhout DJ. Chondroitinase treatment following spinal contusion injury increases migration of oligodendrocyte progenitor cells. Experimental Neurology. 2011;231:19–29. doi: 10.1016/j.expneurol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 69.Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gonzalez R, Hickey MJ, Espinosa JM, Nistor G, Lane TE, Keirstead HS. Therapeutic neutralization of CXCL10 decreases secondary degeneration and functional deficit after spinal cord injury in mice. Regen Med. 2007;2:771–783. doi: 10.2217/17460751.2.5.771. [DOI] [PubMed] [Google Scholar]
- 71.Popovich PG, Guan Z, Wei P, Huitinga I, van RN, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- 72.Sloane E, Ledeboer A, Seibert W, Coats B, van Strien M, Maier SF, Johnson KW, Chavez R, Watkins LR, Leinwand L, Milligan ED, Van Dam AM. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behav Immun. 2009;23:92–100. doi: 10.1016/j.bbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnold SA, Hagg T. Anti-inflammatory treatments during the chronic phase of spinal cord injury improve locomotor function in adult mice. J Neurotrauma. 2011;28:1995–2002. doi: 10.1089/neu.2011.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Festoff BW, Ameenuddin S, Arnold PM, Wong A, Santacruz KS, Citron BA. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. 2006;97:1314–1326. doi: 10.1111/j.1471-4159.2006.03799.x. [DOI] [PubMed] [Google Scholar]
- 75.Lee SM, Yune TY, Kim SJ, Park DW, Lee YK, Kim YC, Oh YJ, Markelonis GJ, Oh TH. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. 2003;20:1017–1027. doi: 10.1089/089771503770195867. [DOI] [PubMed] [Google Scholar]
- 76.Cafferty WB, Yang SH, Duffy PJ, Li S, Strittmatter SM. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27:2176–85. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao Q, Li Y, Shen L, Zhang J, Zheng X, Qu R, Liu Z, Chopp M. Bone marrow stromal cells reduce ischemia-induced astrocytic activation in vitro. Neuroscience. 2008;152:646–55. doi: 10.1016/j.neuroscience.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ito M, Natsume A, Takeuchi H, Shimato S, Ohno M, Wakabayashi T, Yoshida J. Type I interferon inhibits astrocytic gliosis and promotes functional recovery after spinal cord injury by deactivation of the MEK/ERK pathway. J Neurotrauma. 2009;26:41–53. doi: 10.1089/neu.2008.0646. [DOI] [PubMed] [Google Scholar]
- 79.Jeong SR, Kwon MJ, Lee HG, Joe EH, Lee JH, Kim SS, Suh-Kim H, Kim BG. Hepatocyte growth factor reduces astrocytic scar formation and promotes axonal growth beyond glial scars after spinal cord injury. Exp Neurol. 2012;233:312–22. doi: 10.1016/j.expneurol.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 80.Menet V, Prieto M, Privat A, Giménez y Ribotta M. Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci U S A. 2003;100:8999–9004. doi: 10.1073/pnas.1533187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia. 2012;60:1660–70. doi: 10.1002/glia.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gwak YS, Kang J, Unabia GC, Hulsebosch CE. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol. 2012;234:362–72. doi: 10.1016/j.expneurol.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–17. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olechowski CJ, Truong JJ, Kerr BJ. Neuropathic pain behaviours in a chronic-relapsing model of experimental autoimmune encephalomyelitis (EAE) Pain. 2009;141:156–64. doi: 10.1016/j.pain.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Cheng Y, Hitchcock SA. Targeting cannabinoid agonists for inflammatory and neuropathic pain. Expert Opin Investig Drugs. 2007;16:951–65. doi: 10.1517/13543784.16.7.951. [DOI] [PubMed] [Google Scholar]
- 87.Tsuda M, Tozaki-Saitoh H, Inoue K. Purinergic system, microglia and neuropathic pain. Curr Opin Pharmacol. 2012;12:74–9. doi: 10.1016/j.coph.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 88.Nguyen VT, Benveniste EN. Critical role of tumor necrosis factor-alpha and NF-kappa B in interferon-gamma-induced CD40 expression in microglia/macrophages. J Biol Chem. 2002;277:13796–13803. doi: 10.1074/jbc.M111906200. [DOI] [PubMed] [Google Scholar]
- 89.Rohl C, Lucius R, Sievers J. The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res. 2007;1129:43–52. doi: 10.1016/j.brainres.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 90.Lacy M, Jones J, Whittemore SR, Haviland DL, Wetsel RA, Barnum SR. Expression of the Receptors for the C5a Anaphylatoxin, Interleukin-8 and Fmlp by Human Astrocytes and Microglia. J Neuroimmunol. 1995;61:71–78. doi: 10.1016/0165-5728(95)00075-d. [DOI] [PubMed] [Google Scholar]
- 91.Nakamura M, Okada S, Toyama Y, Okano H. Role of IL-6 in spinal cord injury in a mouse model. Clin Rev Allerg Immu. 2005;28:197–203. doi: 10.1385/CRIAI:28:3:197. [DOI] [PubMed] [Google Scholar]
- 92.Norris JG, Tang LP, Sparacio SM, Benveniste EN. Signal-Transduction Pathways Mediating Astrocyte Il-6 Induction by Il-1-Beta and Tumor-Necrosis-Factor-Alpha. J Immunol. 1994;152:841–850. [PubMed] [Google Scholar]
- 93.Sawada M, Itoh Y, Suzumura A, Marunouchi T. Expression of Cytokine Receptors in Cultured Neuronal and Glial-Cells. Neurosci Lett. 1993;160:131–134. doi: 10.1016/0304-3940(93)90396-3. [DOI] [PubMed] [Google Scholar]
- 94.Balasingam V, Tejadaberges T, Wright E, Bouckova R, Yong VW. Reactive Astrogliosis in the Neonatal Mouse-Brain and Its Modulation by Cytokines. J Neurosci. 1994;14:846–856. doi: 10.1523/JNEUROSCI.14-02-00846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giulian D, Young D, Woodward J, Brown D, Lachman L. Interleukin-1 is an astroglial growth factor in the developing brain. The Journal of Neuroscience. 1988;8:709–714. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frei K, Malipiero UV, Leist TP, Zinkernagel RM, Schwab ME, Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989;19:689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- 97.Aderka D, Le JM, Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. The Journal of Immunology. 1989;143:3517–3523. [PubMed] [Google Scholar]
- 98.Brunello AG, Weissenberger J, Kappeler A, Vallan C, Peters M, Rose-John S, Weis J. Astrocytic alterations in interleukin-6/soluble interleukin-6 receptor alpha double-transgenic mice. Am J Pathol. 2000;157:1485–1493. doi: 10.1016/s0002-9440(10)64787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klein MA, Moller JC, Jones LL, Bluethmann H, Kreutzberg GW, Raivich G. Impaired neuroglial activation in interleukin-6 deficient mice. Glia. 1997;19:227–233. doi: 10.1002/(sici)1098-1136(199703)19:3<227::aid-glia5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 100.Junier MP. What role(s) for TGFalpha in the central nervous system? Progress in Neurobiology. 2000;62:443–47. doi: 10.1016/s0301-0082(00)00017-4. [DOI] [PubMed] [Google Scholar]
- 101.Sharif A, Prevot V, Renault-Mihara F, Allet C, Studler JM, Canton B, Chneiweiss H, Junier MP. Transforming growth factor alpha acts as a gliatrophin for mouse and human astrocytes. Oncogene. 2006;25:4076–4085. doi: 10.1038/sj.onc.1209443. [DOI] [PubMed] [Google Scholar]
- 102.Rabchevsky AG, Weinitz JM, Coulpier M, Fages C, Tinel M, Junier MP. A role for transforming growth factor alpha as an inducer of astrogliosis. J Neurosci. 1998;18:10541–10552. doi: 10.1523/JNEUROSCI.18-24-10541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rhodes KE, Moon LD, Fawcett JW. Inhibiting cell proliferation during formation of the glial scar: effects on axon regeneration in the CNS. Neuroscience. 2003;120:41–56. doi: 10.1016/s0306-4522(03)00285-9. [DOI] [PubMed] [Google Scholar]
- 105.Faulkner JR, Herrmann JE, Woo MJ, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurotraum. 2003;20:1056–1056. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth F R. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 107.Sievers JPR, Wottge HU. Blood monocytes and spleen macrophages differentiate into microglia-like cells on monolayers of astrocytes: morphology. Glia. 1994;12:245–258. doi: 10.1002/glia.440120402. [DOI] [PubMed] [Google Scholar]
- 108.Schilling TNR, Heinemann U, Haas D, Eder C. Astrocyte-released cytokines induce ramification and outward K channel expression in microglia via distinct signalling pathways. Eur J Neurosci. 2001;14:463–473. doi: 10.1046/j.0953-816x.2001.01661.x. [DOI] [PubMed] [Google Scholar]
- 109.Escartin C, Bonvento G. Targeted Activation of Astrocytes: A Potential Neuroprotective Strategy. Molecular Neurobiology. 2008;38:231–241. doi: 10.1007/s12035-008-8043-y. [DOI] [PubMed] [Google Scholar]
- 110.Wu VW, Nishiyama N, Schwartz JP. A culture model of reactive astrocytes: Increased nerve growth factor synthesis and reexpression of cytokine responsiveness. J Neurochem. 1998;71:749–756. doi: 10.1046/j.1471-4159.1998.71020749.x. [DOI] [PubMed] [Google Scholar]
- 111.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through G(i/o)-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 114.Verderio C, Matteoli M. ATP mediates calcium signaling between astrocytes and microglial cells: Modulation by IFN-gamma. J Immunol. 2001;166:6383–6391. doi: 10.4049/jimmunol.166.10.6383. [DOI] [PubMed] [Google Scholar]
- 115.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 116.McMullan SM, Phanavanh B, Li GG, Barger SW. Metabotropic glutamate receptors inhibit microglial glutamate release. Asn Neuro. 2012;4:323–330. doi: 10.1042/AN20120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Espey MG, Basile AS. Glutamate Augments Retrovirus-Induced Immunodeficiency Through Chronic Stimulation of the Hypothalamic-Pituitary- Adrenal Axis. The Journal of Immunology. 1999;162:4998–5002. [PubMed] [Google Scholar]
- 118.Groom AJ, Smith T, Turski L. Multiple Sclerosis and Glutamate. Annals of the New York Academy of Sciences. 2003;993:229–275. doi: 10.1111/j.1749-6632.2003.tb07533.x. [DOI] [PubMed] [Google Scholar]
- 119.Willard LB, Hauss-Wegrzyniak B, Danysz W, Wenk GL. The cytotoxicity of chronic neuroinflammation upon basal forebrain cholinergic neurons of rats can be attenuated by glutamatergic antagonism or cyclooxygenase-2 inhibition. Exp Brain Res. 2000;134:58–65. doi: 10.1007/s002210000446. [DOI] [PubMed] [Google Scholar]
- 120.Lijia Z, Zhao S, Wang X, Wu C, Yang J. A self-propelling cycle mediated by reactive oxide species and nitric oxide exists in LPS-activated microglia. Neurochemistry international. 2012;61:1220–1230. doi: 10.1016/j.neuint.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 121.Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan Is Upregulated in Injured Brain and in Cytokine-Treated Astrocytes. The Journal of Neuroscience. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gutowski NJ, Newcombe J, Cuzner ML. Tenascin-R and C in multiple sclerosis lesions: relevance to extracellular matrix remodelling. Neuropath Appl Neuro. 1999;25:207–214. doi: 10.1046/j.1365-2990.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- 123.Hayashi K, Kadomatsu K, Muramatsu T. Requirement of chondroitin sulfate/dermatan sulfate recognition in midkine-dependent migration of macrophages. Glycoconjugate journal. 2001;18:401–406. doi: 10.1023/a:1014864131288. [DOI] [PubMed] [Google Scholar]
- 124.Rolls A, Cahalon L, Bakalash S, Avidan H, Lider O, Schwartz M. A sulfated disaccharide derived from chondroitin sulfate proteoglycan protects against inflammation-associated neurodegeneration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:547–549. doi: 10.1096/fj.05-4540fje. [DOI] [PubMed] [Google Scholar]
- 125.Kodaira Y, Nair SK, Wrenshall LE, Gilboa E, Platt JL. Phenotypic and functional maturation of dendritic cells mediated by heparan sulfate. J Immunol. 2000;165:1599–1604. doi: 10.4049/jimmunol.165.3.1599. [DOI] [PubMed] [Google Scholar]
- 126.Bourguignon LY, Gilad E, Rothman K, Peyrollier K. Hyaluronan-CD44 interaction with IQGAP1 promotes Cdc42 and ERK signaling, leading to actin binding, Elk-1/estrogen receptor transcriptional activation, and ovarian cancer progression. J Biol Chem. 2005;280:11961–11972. doi: 10.1074/jbc.M411985200. [DOI] [PubMed] [Google Scholar]
- 127.Rolls A, Shechter R, London A, Segev Y, Jacob-Hirsch J, Amariglio N, Rechavi G, Schwartz M. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS medicine. 2008;5:e171. doi: 10.1371/journal.pmed.0050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ramirez G, Toro R, Dobeli H, von Bernhardi R. Protection of rat primary hippocampal cultures from A beta cytotoxicity by pro-inflammatory molecules is mediated by astrocytes. Neurobiol Dis. 2005;19:243–254. doi: 10.1016/j.nbd.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 129.Herrera-Molina R, von Bernhardi R. Transforming growth factor-beta 1 produced by hippocampal cells modulates microglial reactivity in culture. Neurobiol Dis. 2005;19:229–236. doi: 10.1016/j.nbd.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 130.Addis RC, Hsu FC, Wright RL, Dichter MA, Coulter DA, Gearhart JD. Efficient Conversion of Astrocytes to Functional Midbrain Dopaminergic Neurons Using a Single Polycistronic Vector. Plos One. 2011:6. doi: 10.1371/journal.pone.0028719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 132.Heinrich CGM, Berninger B. Reprogramming of postnatal astroglia of the mouse neocortex into functional, synapse-forming neurons. Methods Mol Biol. 2012;814:485–498. doi: 10.1007/978-1-61779-452-0_32. [DOI] [PubMed] [Google Scholar]
- 133.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, Vanti William B, Moreno H, Abeliovich A. Directed Conversion of Alzheimer’s Disease Patient Skin Fibroblasts into Functional Neurons. Cell. 2011;146:359–371. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xue Y, Ouyang K, Huang J, Zhou Y, Ouyang H, Li H, Wang G, Wu Q, Wei C, Bi Y, Jiang L, Cai Z, Sun H, Zhang K, Zhang Y, Chen J, Fu XD. Direct Conversion of Fibroblasts to Neurons by Reprogramming PTB-Regulated MicroRNA Circuits. Cell. 2013;152:82–96. doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]