Abstract
Objective:
In a previous, double-blind, placebo-controlled study, patients with posttraumatic stress disorder (PTSD) showed lower physiological response during script-driven traumatic imagery 1 week after receiving a single dose of propranolol given after the retrieval of a traumatic memory. We hypothesized that this effect would extend beyond 1 week using a modified treatment approach.
Method:
Twenty-eight participants with PTSD read an account of their traumatic event once weekly for 6 consecutive weeks under the influence of open-label propranolol. One week and 4-months later, skin conductance, heart rate, and left corrugator electromyogram responses were measured while participants engaged in script-driven mental imagery of their traumatic event. Results from the 22 study participants were compared with results from treated and untreated participants in a previously published trial.
Results:
Most participants in our study were classified as non-PTSD cases at posttreatment and follow-up according to a psychophysiological discriminant function analysis. Posttreatment skin conductance and heart rate responses of the current (propranolol-treated) participants were lower than those of placebo participants from the previous study. No difference was observed between physiological responding measured posttreatment and at follow-up.
Conclusions:
Low physiological responding during script-driven traumatic imagery after treatment extends up to 4 months, demonstrating the durability of the treatment effect’s. Limitations include the absence of a placebo-controlled group and lack of physiological baseline measures. Despite these limitations, results point to the need for future trials examining the clinical efficacy of trauma reactivation plus propranolol, as it has the potential to become a novel, cost- and time-effective treatment for PTSD.
Keywords: posttraumatic stress disorder, treatment, reconsolidation, memory, propranolol, traumatic stress
Abstract
Objectif :
Dans une étude précédente à double insu contrôlée par placebo, les patients souffrant du trouble de stress post-traumatique (TSPT) ont montré une réponse physiologique plus faible durant l’imagerie traumatique par scénario, une semaine après avoir reçu une seule dose de propranolol, administrée après la récupération d’un souvenir traumatique. Nous avons émis l’hypothèse que cet effet durerait plus d’une semaine à l’aide d’une approche de traitement modifiée.
Méthode :
Vingt-huit participants souffrant du TSPT ont lu un récit de leur événement traumatique une fois par semaine pendant 6 semaines consécutives sous l’effet du propranolol en étiquetage ouvert (open label). Une semaine et 4 mois plus tard, les réponses de la conduction cutanée, des battements de cœur, et de l’électromyogramme du muscle sourcilier gauche ont été mesurées pendant que les participants assistaient à une imagerie mentale par scénario de leur événement traumatique. Les résultats des 22 participants à l’étude ont été comparés avec les résultats de participants traités et non traités à une étude publiée précédemment.
Résultats :
La plupart des participants à notre étude ont été classés comme étant des cas de non-TSPT post-traitement et au suivi, d’après une analyse de fonction discriminante psychophysiologique. Les réponses post-traitement de la conduction cutanée et des battements de cœur des participants actuels (traités par propranolol) étaient plus faibles que celles des participants au placebo de l’étude précédente. Aucune différence n’a été observée entre les réponses physiologiques mesurées post-traitement et au suivi.
Conclusions :
Les réponses physiologiques faibles durant l’imagerie traumatique par scénario après le traitement se prolongent jusqu’à 4 mois, démontrant la durabilité de l’effet du traitement. Les limitations incluent l’absence d’un groupe contrôle par placebo et le manque de mesures physiologiques de départ. Malgré ces limitations, les résultats révèlent le besoin de futures études qui examineraient l’efficacité clinique de la réactivation du traumatisme avec propranolol, car elle a le potentiel de devenir un traitement du TSPT nouveau et rentable tant sur le plan des coûts que du temps.
In a double-blind, placebo-controlled study,1 a 1-day dose of propranolol following retrieval of a traumatic memory produced lower physiological responding 1 week later during script-driven traumatic imagery in participants with PTSD. One explanation for this finding is that propranolol blocked the reconsolidation of the reactivated memory,2–6 thereby decreasing its strength as indexed by lower physiological responding during recall. Our study had a small sample size and there was no follow-up, leaving open the possibilities that the observed effect is not replicable or durable. We addressed these possibilities by analyzing physiological data obtained in a previously described sample of 28 participants7 who had undergone 6 weekly traumatic memory retrieval plus open-label propranolol treatment sessions for PTSD.
We hypothesized that low physiological responding during script-driven imagery would be observed at 1-week and 4-month posttreatment assessments. First, we applied a discriminant function,8 derived from previously studied people with and without PTSD, to each participant’s physiological responses. We selected this outcome measure because heightened physiological responding to trauma cues is a reliable and frequently replicated biological finding in PTSD,9 and it allowed for replication and extension of our previous finding. Second, we compared the physiological responses of the current participants with those of the propranolol and placebo groups in the previously reported double-blind, placebo-controlled study.1 Because we were initially concerned about the possible habituation of physiological responses, the script-driven imagery procedure was administered only after treatment,9 although recent findings suggest that habituation effects may be negligible.10
Highlights
The low physiological responses in reaction to the script-driven imagery suggest that reconsolidation blockade’s effects are sustained.
However, the study design cannot rule out that the participants were low physiological responders to start with.
Overall, this study suggests that research on reconsolidation blockade as a treatment for PTSD should be pursued vigorously.
Methods
Participants
We recruited, via newspaper advertisements, 9 men and 19 women aged 18 to 65 years (mean 37.9, SD 9.5) with PTSD (mean 17.1 years, SD 14.2) as assessed by a structured clinical interview. Exclusion criteria included the following: systolic blood pressure of less than 100 mmHg; asthma, heart failure, heart block, certain cardiac arrhythmias, or insulin-requiring diabetes; previous adverse reaction to a beta blocker; current use of another beta blocker; use of medication that could adversely interact with propranolol; pregnancy or breast feeding; recovered memory of traumatic events; and a mean score of more than 20 on the Dissociative Experiences Scale.12 Participants included French- or English-speaking Caucasian participants with a mean of 14.9 years (SD 4.2) of education and of which 41% were in a relationship. The traumatic events met criterion A and included the following: motor vehicle accident (n = 3), participation in a peacekeeping mission (n = 3), physical assault (n = 5), assault with a weapon (n = 2), sexual abuse (n = 3), incest (n = 5), physical abuse in childhood (n = 3), or other events (n = 4). Comorbid current Axis I disorders13 included the following: major depressive disorder (n = 8), social phobia (n = 8), obsessive–compulsive disorder (n = 6), generalized anxiety (n = 5), panic disorder with (n = 2) and without (n = 5) agoraphobia, agoraphobia without panic (n = 2), bulimia (n = 3), and anorexia nervosa (n = 1). Our study received ethics approval from the Douglas Mental Health University Institute in Montreal, Quebec. Participants gave written informed consent and were reimbursed up to $250 on a pro-rated basis.
Procedure
While under the influence of 0.67 mg/kg of short-acting plus 1mg/kg of long-acting propranolol hydrochloride (Inderal), participants read an account of their traumatic event for 5 to 10 minutes once during each of 6 weekly visits based on a procedure described in detail elsewhere.7 One week after the treatment, a clinical psychologist conducted a semi-structured diagnostic interview, the results of which have been presented elsewhere.7 Prior to the clinical interview, an independent research assistant conducted a script-driven imagery procedure.14 After a 30-second baseline period, each participant listened to 4 audio-recorded scripts (2 portraying the participant’s traumatic event and 2 portraying neutral events). After listening to each script, participants imagined the event as if it were happening for 30 seconds while SC, HR, and left corrugator EMG were recorded. Change scores were calculated by subtracting the preceding baseline mean value for each physiological measure from the mean value of the imagery period that followed it. Responses to the trauma scripts were averaged and square-root transformed to reduce heteroskedasticity. The 26-week follow-up repeated the posttreatment assessment.
Among the 28 people having completed the treatment, 2 were lost to follow-up and 4 had unusable SC data. These participants were not included in the analyses, resulting in a final sample of 22. For each participant, a composite measure of psychophysiological reactivity during trauma-related imagery was obtained by applying an a priori discriminant function to their HR, SC, and EMG responses, yielding a single score (posterior probability) reflecting a person’s overall psychophysiological reactivity during trauma-related imagery and the likelihood that their score belonged to the calibration sample’s PTSD group.16 The discriminant function was derived from the HR, SC, and EMG responses of previously studied trauma-exposed people with and without PTSD.14,17–20 Participants with a posterior probability greater than 0.5 were deemed to have a greater than 50% likelihood of having a current diagnosis of PTSD and were assigned to the PTSD group. Participants with a posterior probability less than 0.5 were deemed to have a lower likelihood of having a current diagnosis of PTSD and were assigned to the non-PTSD group.
Posttreatment HR, SC, and EMG responses from the 22 present participants were compared with responses from the propranolol- (n = 9) and placebo- (n = 10) treated participants from our previous study1 in a 3-group (current propranolol-treated [propranolol-II], compared with past propranolol-treated [propranolol-I], compared with placebo [placebo]) MANOVA, with SC, HR, and EMG as dependent measures, followed by univariate ANOVAs. These analyses were conducted using the IBM SPSS Statistics, version 21 (IBM SPSS Inc, Armonk, NY).
Third, psychophysiological responses of the present participants [propranolol-II] at follow-up were compared with posttreatment responses using 2-tailed, paired samples Student t tests, separately for each physiological measure (α = 0.05).
Results
According to the discriminant function analysis, 91% of the 22 participants were classified as non-PTSD at posttreatment, and 96% at follow-up, based on their physiological responses during trauma-related imagery.
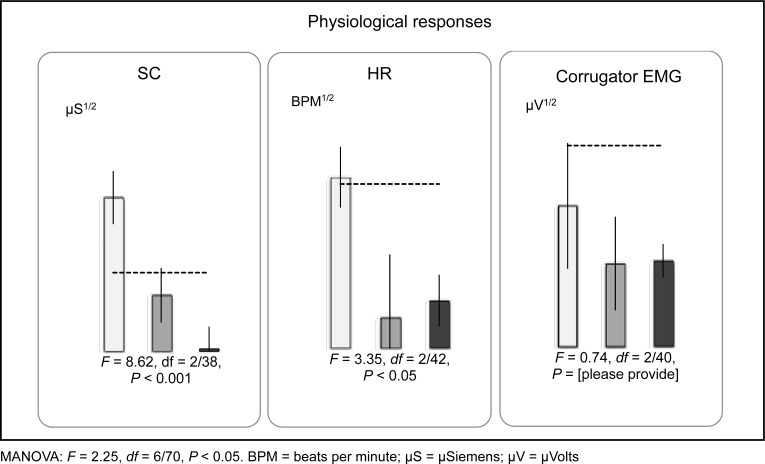
A MANOVA, comparing the 3 groups (current propranolol [propranolol-II], past propranolol [propranolol-I], and placebo [placebo]) revealed a significant main effect for physiological responding, F = 2.3, df = 6/70, P < 0.05 (Wilk’s λ = 0.7). ANOVAs revealed significant group differences for SC (F = 8.6, df = 2/38, P < 0.001) and HR (F = 3.4, df = 2/42, P < 0.05), but not EMG (F < 1.0, df = 2/40, P = 0.48.). Results are presented in Figure 1. Post hoc Tukey honestly significant difference tests revealed that, for SC and HR, the mean score of the present (propranolol-II) group was significantly smaller than that of the previously studied placebo group (for SC, Cohen d = −1.56; for HR, Cohen d = −1.07); these effect sizes were in the predicted direction and considered large.21 In contrast, there were no significant differences between the present (propranolol-II) and previously studied propranolol (propranolol-I) groups: for SC, Cohen d = 0.62; for HR, Cohen d = 0.10. Posttreatment, compared with follow-up, contrasts were not significant for SC (t = 1.16, df = 21, P = 0.26.), HR (t = 0.36, df = 23, P = 0.74.), or EMG (t = 0.44, df = 23, P = 0.67); all Cohen ds were less than 0.33, which is considered small.
Figure 1.
Physiological responses of participants with PTSD during mental imagery of personal traumatic events. Light grey bars (left) placebo (n = 10) and medium grey bars (centre) propranolol-I (n = 9) are from samples from Brunet et al.1 Black bars (right) propranolol-II (n = 22) are from our study. Error bars represent standard error of the mean. Dashed lines represent empirical cut-offs for PTSD based on prior research.
MANOVA: F = 2.25, df = 6/70, P < 0.05. BPM = beats per minute; μS = μSiemens; μV = μVolts
Discussion
After 6 weekly treatments of traumatic memory reactivation combined with open-label propranolol, physiological responding during script-driven imagery at posttreatment and 4-month follow-up was low. According to the discriminant function analysis, this level of responding was characteristic of people without PTSD. These findings replicate and extend, in a larger sample, previous findings from a placebo-controlled, double-blind study by showing that these effects are long-lasting. Physiologically conditioned emotional responses to internal trauma-related cues are a cardinal sign of PTSD22 that is difficult to hide or minimize. Therefore, the reported finding supports a genuine benefit of memory reactivation plus propranolol. Further, these physiological results are consistent with the previously reported clinical data based on a structured clinical interview.7
There are several limitations of our study. First, current participants underwent 6 treatment sessions, whereas the comparison groups underwent a single treatment session. Second, there was no randomization of participants in our study. Third, it is not impossible (although unlikely) that the present participants were weak physiological responders to begin with, and may have shown low physiological responding even if they had not received the treatment. The assessment of physiological responding to trauma reminders prior to treatment is necessary to rule out this possibility. Fourth, although research in animals and humans suggest the contrary, it is possible that the observed effect was unrelated to reconsolidation blockade.5 For example, repetition of the trauma script on 6 occasions might have induced extinction. However, this explanation is unlikely, given the brevity of exposure to trauma cues during treatment. Mechanistic studies and randomized, placebo-controlled trials are needed to disentangle such alternate hypotheses.
Though findings from our study suggest the promise of a new effective treatment for PTSD, it is important to interpret results in light of the limitations described above, particularly concerning the varying treatment dosage of propranolol across groups and the absence of randomization. Therefore, caution should be exercised in the interpretation of results. Nonetheless, results clearly point to the need for further exploration of the clinical utility of traumatic memory reactivation under the influence of propranolol as a treatment for PTSD.
Acknowledgments
Dr Brunet, Dr Ashbaugh, and Ms Thomas received financial support from the Fonds de recherche du Québec while working on this project. Dr Orr received a grant from the United States Army, which supported this study and other projects of which this study was a component.
Abbreviations
- EMG
electromyogram
- HR
heart rate
- PTSD
posttraumatic stress disorder
- SC
skin conductance
References
- 1.Brunet A, Orr SP, Tremblay J, et al. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in posttraumatic stress disorder. J Psychiatr Res. 2008;42(6):503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19(15):6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nature Neurosci. 2009;12(3):256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 4.Besnard A, Caboche J, Laroche S. Reconsolidation of memory: a decade of debate. Prog Neurobiol. 2012;99(1):61–80. doi: 10.1016/j.pneurobio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Schiller D, Phelps EA. Does reconsolidation occur in humans? Front Behav Neurosci. 2011;5:24. doi: 10.3389/fnbeh.2011.00024. doi: 103389/fnbeh.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonergan MH, Olivera-Figueroa LA, Pitman RK, et al. Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in health participants: a meta-analysis. J Psychiatry Neurosci. 2013;38(4):222–231. doi: 10.1503/jpn.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet A, Poundja J, Tremblay J, et al. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J Clin Psychopharmacol. 2011;31(4):547–550. doi: 10.1097/JCP.0b013e318222f360. [DOI] [PubMed] [Google Scholar]
- 8.Orr SP, Lasko NB, Macklin ML, et al. Predicting post-trauma stress symptoms from pre-trauma psychophysiologic reactivity, personality traits and measures of psychopathology. Biol Mood Anxiety Disord. 2012;2(1):8. doi: 10.1186/2045-5380-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orr SP, Roth WT. Psychophysiological assessment: clinical applications for PTSD. J Affect Disord. 2000;61(3):225–240. doi: 10.1016/s0165-0327(00)00340-2. [DOI] [PubMed] [Google Scholar]
- 10.Bauer MR, Ruef AM, Pineles SL, et al. Psychophysiological assessment of PTSD: a potential research domain criteria construct. Psychol Assess. Psychol Assess. 2013;25(3):1037–1043. doi: 10.1037/a0033432. [DOI] [PubMed] [Google Scholar]
- 11.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis. 1986;174(12):727–735. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSMIV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. ; quiz 34–57. [PubMed] [Google Scholar]
- 14.Pitman RK, Orr SP, Forgue DF, et al. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44(11):970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- 15.Orr SP, Metzger LJ, Pitman RK. Psychophysiology of post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25(2):271–293. doi: 10.1016/s0193-953x(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 16.SAS Institute Inc . SAS/STAT 9.3 user’s guide: the DISCRIM procedure, chapter 32 (p 1974–2069) Cary (NC): SAS Institute, Inc; 2011. [Google Scholar]
- 17.Orr SP, Pitman RK, Lasko NB, et al. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. J Abnorm Psychol. 1993;102(1):152–159. doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- 18.Shalev AY, Orr SP, Pitman RK. Psychophysiologic assessment of traumatic imagery in Israeli civilian patients with posttraumatic stress disorder. Am J Psychiatry. 1993;150(4):620–624. doi: 10.1176/ajp.150.4.620. [DOI] [PubMed] [Google Scholar]
- 19.Orr SP, Lasko NB, Metzger LJ, et al. Psychophysiologic assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. J Consult Clin Psychol. 1998;66(6):906–913. doi: 10.1037//0022-006x.66.6.906. [DOI] [PubMed] [Google Scholar]
- 20.Carson MA, Paulus LA, Lansko NB, et al. Psychophysiologic assessment of posttraumatic stress disorder in Vietnam nurse veterans who witnessed injury or death. J Consult Clin Psychol. 2000;68(5):890–897. [PubMed] [Google Scholar]
- 21.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale (NJ): L Erlbaum; 1988. p. p xxi.p. 567. [Google Scholar]
- 22.Harrington JL, Antony MM. Assessment of anxiety disorders. In: Antony MM, Stein MB, editors. Oxford handbook of anxiety and related disorders. New York (NY): Oxford University Press; 2009. pp. 277–291. [Google Scholar]