Abstract
Head and neck squamous cell carcinoma (HNSCC) patients with human papillomavirus (HPV) infection have better prognosis than those without HPV infection. Although p16INK4a expression is used as a surrogate marker for HPV infection, there is controversy as to whether p16INK4a reliably indicates HPV infection. Here, to evaluate the accuracy of p16INK4a expression for determining HPV infection and the prognostic value of HPV infection and p16INK4a expression for HNSCC survival, especially oropharyngeal squamous cell carcinoma (OPSCC) survival, 150 fresh-frozen HNSCC samples were analyzed for HPV DNA, E6/E7 mRNA and p16INK4a expression by polymerase chain reaction and immunohistochemistry. p16INK4a expression was scored from 0 to 4 according to the percentage of p16INK4a-positive cells, with overexpression defined as >40% positive cells. Of the 150 tumor samples tested, 10 tumors were nasopharyngeal, 53 oropharyngeal, 39 hypopharyngeal, 24 laryngeal and 24 were located in the oral cavity. HPV DNA was detected in 47 (31.3%) samples, but only 21 also exhibited HPV mRNA expression. Inter-rater agreement was low between p16INK4a expression and HPV DNA presence and between p16INK4a expression and HPV mRNA expression, but was good between the combination of HPV DNA status and p16INK4a overexpression and HPV mRNA expression. Three-year recurrence-free survival was significantly higher for OPSCC patients who were HPV DNA-positive than for OPSCC patients who were HPV DNA-negative (P=0.008) and for OPSCC patients over-expressing p16INK4a than for without overexpressing p16INK4a (P=0.034). Multivariate analysis revealed that T1-3 stage and the combination of HPV DNA positivity and p16INK4a overexpression predicted significantly better recurrence-free survival. This combination is a more accurate marker for active HPV infection in HNSCC than HPV DNA status or general p16INK4a-positive status alone and offers a useful and reliable method for detecting and determining the prognosis of HPV-related HNSCC.
Keywords: head and neck cancer, human papillomavirus, HPV E6, E7 mRNA, p16INK4a, prognosis
Introduction
Each year, 600,000 new cases of head and neck squamous cell carcinoma (HNSCC) are diagnosed worldwide (1). Common risk factors for most forms of HNSCC include heavy consumption of tobacco and/or alcohol (2), although the oropharyngeal squamous cell carcinoma (OPSCC) is less likely to be associated with tobacco and alcohol exposure and more often correlated with human papillomavirus (HPV) infection (3). The incidence of OPSCC associated with HPV infection is increasing; for example, among cases of tonsillar cancer in Stockholm, HPV-positive cases rose from 23% in the 1970s to 57% in the 1990s and 79% from 2000 to 2007 (4). Moreover, alongside tobacco and alcohol, high-risk HPV variants (HR-HPVs) have emerged as risk factor for HNSCC, including OPSCC (5).
HNSCC patients who are HPV positive have substantially better prognosis than those who are HPV negative (6–10). Although the detection of E6/E7 mRNA transcripts is regarded as the gold standard for the presence of clinically relevant (active) HPV (11), the requirement of unfixed (fresh frozen) tissue and the cost of polymerase chain reaction (PCR) make direct detection of E6/E7 impractical for cancer diagnostics at present. Accordingly, many studies have attempted to identify an easily measured surrogate maker for the diagnosis of HPV-associated HNSCC.
Expression of the tumor suppressor p16INK4a has been proposed as a surrogate marker for HPV infection: its over-expression is thought to reflect the presence of biologically active HPV infection given that functional inactivation of pRb by viral E7 induces p16INK4a upregulation. Detection of p16INK4a expression can also be performed using formalin-fixed, paraffin-embedded (FFPE) samples (11–13). However, there is controversy as to whether p16INK4a expression reliably indicates HPV infection (11,12).
Klaes et al classified p16INK4a staining as negative (<1% of cells positive), sporadic (<5% of cells positive), focal (<25% of cells positive) or diffuse (>25% of cells positive) (14). Other studies have defined p16INK4a expression in tumors as strong and diffuse when ≥70% of cells (cytoplasm and nuclei) are stained (15–17), while Fischer et al assessed tumors as p16INK4a positive when ≥5% of cells were immunopositive (18). These diverse scoring systems may lead to significant discrepancies across studies in the relationship between HPV infection and p16INK4a expression. Furthermore, p16INK4a expression has been observed in tumor-free tonsillar tissue without HPV infection, implicating other mechanisms in p16INK4a upregulation (19). Bussu et al concluded that it is unnecessary to measure a surrogate, such as p16INK4a expression, for objective, reliable, and direct diagnosis because HPV nucleic acids can be detected by PCR without requiring subjective assessments by histopathologists (17).
In this study, we evaluated the relationship between HPV infection and p16INK4a expression and the value of both HPV-positive status and p16INK4a expression levels for HNSCC prognosis using tissue samples from a well-characterized cohort of Japanese patients with HNSCC receiving curative treatment. We measured the presence of HPV DNA, HPV E6/E7 mRNA expression using fresh-frozen samples and measured p16INK4a expression using FFPE samples.
Materials and methods
Subjects and study design
The eligibility criteria for this study were as follows: the presence of previously untreated, pathologically confirmed primary HNSCC without distant metastasis (M0); receiving curative treatment of surgery alone, surgery combined with radiation therapy (RT) or chemoradiotherapy (CRT), concurrent chemoradiotherapy (CCRT) or RT alone with >66 Gy of total dosage; and complete remission after primary treatment. The treatment modalities were determined according to tumor location, tumor stage, response to induction chemotherapy, and general physical condition, not by the results of HPV status and p16INK4a in tumor tissue. Based on these criteria, 150 patients treated by the Department of Otorhinolaryngology, Head and Neck Surgery, University of the Ryukyus, Japan were recruited between October 2006 and June 2013. In contrast to our previous study on HPV status and squamous cell carcinoma antigen (SCCA), this study involved a greater total number of cases who met the inclusion criteria and we also updated the prognostic information of some of the patients reported previously (8). Each patient gave written informed consent before enrolment. The research protocol was approved by the Ethics Committee of the University of the Ryukyus. Tissue samples were snap-frozen in liquid nitrogen during biopsy or surgical excision and stored in liquid nitrogen until further analysis. Demographic and clinicopathologic parameters for each patient were collected at scheduled intervals during the follow-up period.
Cell lines and culture
The cervical cancer cell lines CaSki (harboring ∼600 copies of integrated HPV-16 DNA/genome) and SiHa (1–2 copies of integrated HPV-16 DNA/genome) were purchased from the European Collection of Animal Cell Cultures (Salisbury, UK) and the American Type Culture Collection (Tokyo, Japan), respectively, and cultured according to the suppliers’ instructions.
DNA and RNA extraction
Genomic DNA and total RNA were extracted from frozen tumor samples, SiHa cells and CaSki cells using the Gentra Purification Tissue kit (Qiagen, Germantown, MD) and the ToTALLY RNA™ kit (Ambion, Austin, TX), respectively, according to the manufacturers’ protocols. The extracted RNA was suspended in 50 μl ultra-high quality diethyl pyrocarbonate-treated water.
PCR for detection of HPV DNA
The presence and integrity of the DNA in all samples was verified by PCR β-globin gene amplification using the primers PC04 and GH20 (20). Water (negative control) and DNA from HPV-16-positive CaSki cells (positive control) were included in each amplification series. The presence of HPV DNA was analyzed by PCR using the general consensus primer sets GP5+/GP6+ and MY09/11 (21,22). DNA samples that were negative for HPV using GP5+/GP6+ or MY09/11 PCR were re-amplified by (auto-) nested PCR using the GP5+/GP6+ primer pair as previously described (23). PCR products were purified and directly sequenced with an ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA). Obtained sequences were aligned and compared with those of known HPV types in the GenBank database using the BLAST program.
Detection of HPV E6/E7 mRNA by reverse transcription PCR
Before cDNA synthesis, any residual DNA was removed by incubation with 1 U DNase I (Ambion) at room temperature for 25 min. cDNA was then synthesized from DNA-free total RNA using the RETROscript® kit (Ambion) according to the manufacturer’s instructions. To examine the presence of contaminating DNA in RNA samples, all the assays were performed both with and without reverse transcriptase.
To detect high-risk E6/E7 mRNA transcripts, PCR was performed with the cDNA from HPV DNA-positive samples using the Takara PCR Human Papillomavirus Typing Set (Takara, Bio Inc., Otsu, Shiga, Japan), which can identify high-risk HPV types 16, 18, 31, 33, 35, 52 and 58. To verify the HPV-16 E6/E7 mRNA transcripts, the HPV-16 DNA-positive samples were also examined using a half-nested PCR approach with cDNA as previously described by Wiest et al (24). Positive PCR products were purified and directly sequenced using an ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems).
Immunohistochemistry for p16INK4a and scoring of results
Serial 4-μm-thick sections from FFPE tumor samples were deparaffinized in a graded alcohol series. Epitope retrieval was performed by heating at 95–99° C for 10 min in Tris/EDTA buffer (pH 9.0). Endogenous peroxidase activity was quenched by incubating the sections in 3% hydrogen peroxide plus 15 mM sodium azide for 5 min. The sections were subsequently incubated overnight at 4°C with primary monoclonal mouse anti-p16INK4a antibody (MTM Laboratories AG, Heidelberg, Germany). After extensive washing in phosphate-buffered saline, the slides were incubated for 30 min at room temperature with a horseradish peroxidase-conjugated goat anti-mouse secondary antibody (MTM Laboratories). Immunolabeling was visualized by incubation in 3-3′-diaminobenzidine for 10 min. Stained slides were counterstained with hematoxylin.
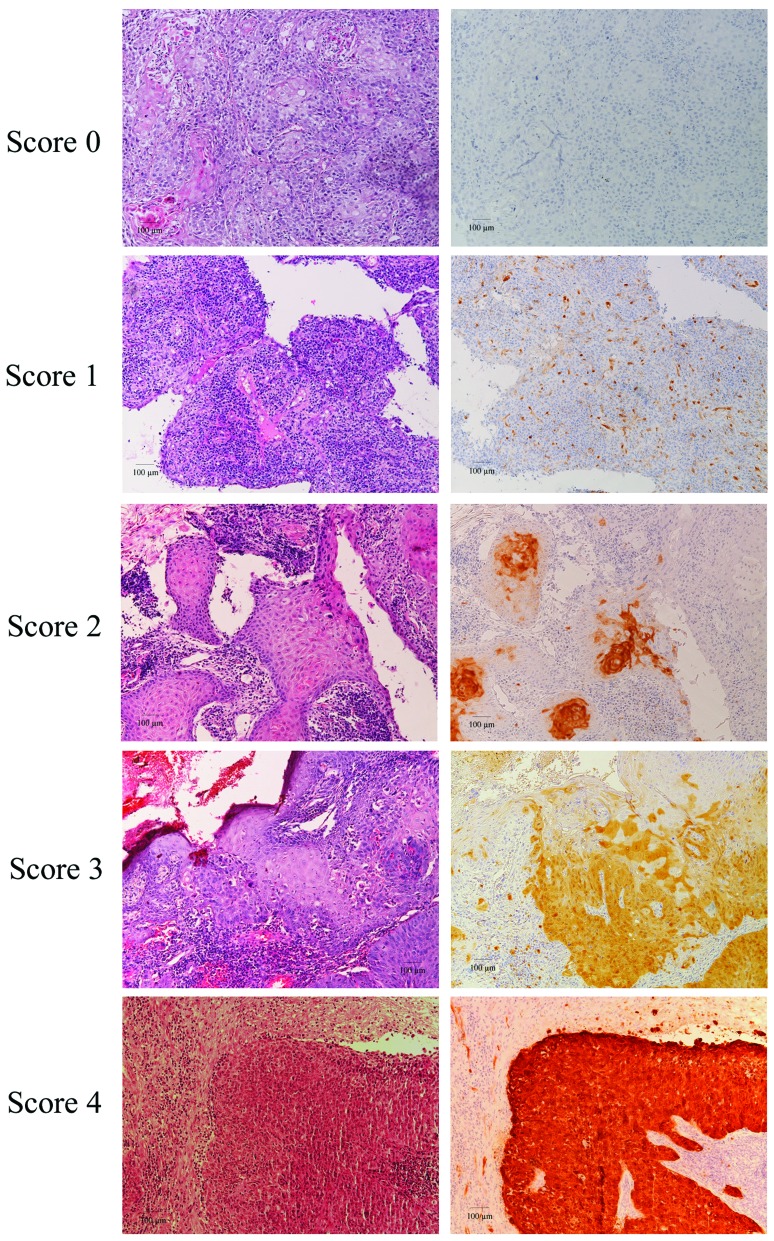
Cases were considered p16INK4a-positive when intense nuclear and/or cytoplasmic reactivity was present. The scoring criteria for p16INK4a immunoreactivity (p16INK4a expression) were defined for this study based on previous scoring methods (14,15): 0 (no staining), 1 (1–10% of tumor cells positive), 2 (11–40% positive), 3 (40–70% positive) and 4 (>70% positive). The term ‘p16INK4a Overexpression’ is defined as a score of 3 or 4.
Survival analysis
Descriptive statistics were used to characterize patient baseline characteristics. The Mann-Whitney U-test or Kruskal-Wallis test was used for continuous variables, and Pearson’s χ2 test or Fisher’s exact test was used for categorical variables. The inter-rater agreements between HPV-DNA presence and p16INK4a expression and between HPV mRNA expression and p16INK4a expression were measured by calculating Cohen’s κ coefficient. A κ-value <0.20 was considered slight agreement, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as good and 0.81–0.99 as excellent agreement (17,25–27).
Locoregional control was defined as complete and persistent disappearance of disease at the primary tumor (T site) and regional lymph nodes (N site) after treatment. Recurrence-free survival was defined as the time from the end of treatment to cancer recurrence or last follow-up. Disease-specific survival was defined as the time from the end of treatment to subsidence of disease or last follow-up. Survival curves were evaluated by the Kaplan-Meier method, and survival distributions were compared using the log-rank test. Multivariate Cox proportional hazard analysis was used to identify prognostic parameters and treatments associated with risk of recurrence and disease-specific death. P-values <0.05 were considered significant. All statistical analyses were performed using the SPSS statistical package (SPSS for Windows version 12.0; SPSS, Inc., Chicago, IL).
Results
Characteristics of eligible patients and follow-up
Primary tumor location was the nasopharynx in 10 patients (6.7%), oropharynx in 53 (35.3%), hypopharynx in 39 (26.0%), larynx in 24 (16.0%) and oral cavity in 24 (16.0%). The follow-up period ranged from 6 to 77 months, with a median of 38 months for patients whose data were censored. Sixty-four patients were treated with CCRT, 45 with surgery and postoperative RT or CRT (radiation dosage, 50–54 Gy), 22 with surgery alone and 19 with RT alone. Demographic and clinical characteristics are summarized in Table I.
Table I.
Demographic and clinical characteristics.
| Characteristic | Total no. | HPV+ | HPV− | P-value | p16INK4a overexpression | P-value | |
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| n=47 | n=103 | (+) n=30 | (-) n=120 | ||||
| Gender, n (%)* | |||||||
| Male | 127 | 38 (29.9) | 89 (70.1) | 0.381 | 24 (18.9) | 103 (81.1) | 0.408 |
| Female | 23 | 9 (39.1) | 14 (60.9) | 6 (26.1) | 17 (73.9) | ||
| Age (years) | |||||||
| Mean | 64.1 | 62.6 | 64.8 | 0.286 | 61.8 | 64.7 | 0.216 |
| Range | 28–89 | 39–89 | 28–83 | 39–89 | 28–86 | ||
| ≤50, n (%) | 20 | 9 (45.9) | 11 (55.0) | 0.157 | 7 (35.0) | 13 (65.0) | 0.128 |
| >50, n (%) | 130 | 38 (29.2) | 92 (70.8) | 23 (17.7) | 107 (82.3) | ||
| Smoking, n (%)a | 0.066 | 0.097 | |||||
| Never | 30 | 14 (46.7) | 16 (53.3) | 10 (33.3) | 20 (66.7) | ||
| ≤400 | 21 | 8 (38.1) | 13 (61.9) | 5 (23.8) | 16 (76.2) | ||
| >400 | 99 | 25 (25.3) | 74 (74.7) | 15 (15.2) | 84 (84.8) | ||
| Alcohol use, n (%)b | 0.592 | 0.138 | |||||
| Never | 25 | 10 (40.0) | 15 (60.0) | 6 (24.0) | 19 (76.0) | ||
| ≤50 | 51 | 15 (29.4) | 36 (70.6) | 14 (27.5) | 37 (72.5) | ||
| >50 | 74 | 22 (29.7) | 52 (70.3) | 10 (13.5) | 64 (86.5) | ||
| T classification, n (%) | 0.089 | 0.052 | |||||
| T1 | 18 | 2 (11.1) | 16 (88.9) | 1 (5.6) | 17 (94.4) | ||
| T2 | 58 | 22 (37.9) | 36 (62.1) | 17 (29.3) | 41 (70.7) | ||
| T3 | 41 | 10 (24.4) | 31 (75.6) | 5 (12.2) | 36 (87.8) | ||
| T4 | 33 | 13 (39.4) | 20 (60.6) | 7 (21.2) | 26 (78.8) | ||
| Node status, n (%) | |||||||
| N0 or N1 | 83 | 24 (28.9) | 59 (71.1) | 0.477 | 17 (20.5) | 66 (79.5) | 0.870 |
| N2 or N3 | 67 | 23 (34.3) | 44 (65.7) | 13 (19.4) | 54 (80.5) | ||
| TNM stage, n (%) | |||||||
| Early (I and II) | 42 | 9 (21.4) | 33 (78.6) | 0.103 | 8 (19.0) | 34 (81.0) | 0.856 |
| Advanced (III and IV) | 108 | 38 (35.2) | 70 (64.8) | 22 (20.4) | 86 (79.6) | ||
| Differentiation, n (%) | < 0.001 | 0.030 | |||||
| Well | 68 | 13 (19.1) | 55 (80.9) | 8 (42.1) | 11 (57.9) | ||
| Moderate | 63 | 21 (33.3) | 42 (66.7) | 12 (19.0) | 51 (81.0) | ||
| Poor | 19 | 13 (68.4) | 6 (31.6) | 10 (14.7) | 58 (85.3) | ||
| Tumor location, n (%) | 0.017 | 0.002 | |||||
| Hypopharynx | 39 | 7 17.9) | 32 (82.1) | 3 (7.7) | 36 (92.3) | ||
| Oropharynx | 53 | 25 (47.2) | 28 (52.8) | 20 (37.7) | 33 (62.3) | ||
| Oral cavity | 24 | 8 (33.3) | 16 (66.7) | 2 (8.3) | 22 (91.7) | ||
| Larynx | 24 | 4 (16.7) | 20 (83.3) | 3 (12.5) | 21 (87.5) | ||
| Nasopharynx | 10 | 3 (30.0) | 7 (70.0) | 2 (20.0) | 8 (80.0) | ||
Brinkman index: daily cigarettes x years.
Light drinker ≤50 g alcohol/day; Heavy drinker >50 g alcohol/day. HPV, human papillomavirus.
HPV DNA status and HPV E6/E7 mRNA expression
HPV DNA was detected by PCR in 47 of 150 (31.3%) primary untreated HNSCC specimens, including 30.0% (3/10) of nasopharyngeal, 47.2% (25/53) of oropharyngeal (18/30 of palatine tonsil), 17.9% (7/39) of hypopharyngeal, 16.7% (4/24) of laryngeal and 33.3% (8/24) of oral cavity cases. Among HPV-positive HNSCC samples, 39 (83.0 %) were infected with HPV-16 and the others were infected with other high-risk types (4 with HPV-33, 1 with HPV-35, 2 with HPV-58 and 1 with HPV-56).
As two of these HPV-positive samples were insufficient for RNA assay, E6/E7 mRNA expression by HPV-16, HPV-33, HPV-35, HPV-58 and HPV-56 was examined by reverse transcription PCR in 45 samples. The E6 and E7 mRNA transcripts were detected in 21 of 45 (46.7%) specimens, the majority from OPSCC cases (18/25 HPV-positive cases), as shown in Table II.
Table II.
mRNA expression in HPV-DNA-positive HNSCC samples from various sites.
| HPV | Site
|
Total (%)a | ||||
|---|---|---|---|---|---|---|
| NP (%) | OP (%) | HP (%)a | LC (%)a | OC (%) | ||
| DNA+/mRNA+ (%) | 1 (33.3) | 18 (72.0) | 0 (0) | 1 (33.3) | 1 (12.5) | 21 (46.7) |
| DNA+/mRNA− (%) | 2 (66.7) | 7 (28.0) | 6 (100) | 2 (66.7) | 7 (87.5) | 24 (53.3) |
HPV, human papillomavirus; NP, nasopharynx; OP, oropharynx; HP, hypopharynx; LC, larynx; OC, oral cavity.
Two samples (1 each from HP and LC) were insufficient for RNA assay.
Between the HPV DNA-positive and -negative groups, there were significant differences in the distribution of histological differentiation and tumor location; for example, the HPV DNA-positive group showed poor differentiation in histology and has a higher occurrence of oropharyngeal carcinoma compared with HPV DNA-negative group. The p16INK4a overexpression group showed similar clinical characteristics (Table I).
p16INK4a expression and correlation with HPV status
In this study, the p16INK4a expression scoring system (0–4) was based on the percentage of p16INK4a-positive cells (Fig. 1). Expression of p16INK4a was observed in 40 of 150 (26.7%) HNSCC samples (Table III). The highest frequency of p16INK4a expression was found in OPSCC samples, with 22 of 53 (41.5%) samples demonstrating a p16INK4a staining score ≥1. The sensitivity of p16INK4a staining for detection of HPV DNA in HNSCC was just 61.7%, as only 29 of 47 HPV-positive cases also had detectable p16INK4a staining. The specificity was 89.3% (92/103 HPV-negative samples had a p16INK4a staining score of 0) for all HNSCC cases (Table III). Although the correlation between p16INK4a expression and HPV DNA and the correlation between p16INK4a expression and E6/E7 mRNA expression in all HNSCC cases proved to be significant (both P<0.001), the inter-rater agreements were relatively low (κ=0.53 and 0.54, respectively).
Figure 1.
Immunohistochemical evaluation of p16INK4a expression and scoring in HNSCC. p16INK4a immunoreactivity was scored as 0 (no staining), 1 (1–10% of the tumor cells positive), 2 (11–40% of the tumor cells positive), 3 (40–70% of the tumor cells positive) or 4 (>70 of the tumor cells positive). Each micrograph shows the typical p16INK4a immunoreactivity pattern corresponding to each score (A–F, ×100; bar, 100 μm). Sections were also stained with hematoxylin and eosin (H&E).
Table III.
Scoring of p16INK4a overexpression and its association with HPV DNA in HNSCC and OPSCC.
| Scoring | p16INK4a expression
|
Total | |||||
|---|---|---|---|---|---|---|---|
| p16INK4a no or lower expression (<3)
|
p16INK4a overexpression (≥3)
|
||||||
| 0 | 1 | 2 | 3 | 4 | |||
| HNSCC | HPV DNA+ | 18 | 3 | 1 | 1 | 24 | 47 |
| HPV DNA− | 92 | 3 | 3 | 1 | 4 | 103 | |
| OPSCC | HPV DNA+ | 4 | 1 | 0 | 1 | 19 | 25 |
| HPV DNA− | 27 | 0 | 1 | 0 | 0 | 28 | |
HPV, human papillomavirus; HNSCC, head and neck squamous cell carcinoma; OPSCC, oropharyngeal squamous cell carcinoma.
In contrast to general p16INK4a expression, p16INK4a over-expression demonstrated high specificity for detection of HPV DNA in HNSCC (98/103 HPV-DNA negative cases did not overexpress p16INK4a) and OPSCC (28/28 cases). The sensitivity of p16INK4a overexpression was only 53.2% (25/47 cases) for detection of HPV DNA in HNSCC, but was considerably better for detection of HPV DNA in OPSCC at 80% (20/25 cases) (Table III). However, p16INK4a overexpression indicated the presence of HPV E6/E7 mRNA expression with high sensitivity at 90.5% (19/21) and high specificity at 91.3% (116/127) in HNSCC, and was even more accurate for OPSCC, with sensitivity at 94.4% (17/18) and specificity at 91.4% (32/35) (Table IV). Moreover, the inter-rater agreement of p16INK4a overexpression for HPV E6/E7 mRNA expression status was good for HNSCC (κ=0.69) and excellent for OPSCC (κ=0.84).
Table IV.
Correlation between p16INK4a overexpression and HPV mRNA in HNSCC and OPSCC.
| p16INK4a overexpression | HPV mRNA
|
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| + | - | |||||
| HNSCC | ||||||
| + (≥3) | 19 | 11 | 90.5 | 91.3 | 63.3 | 98.3 |
| − (<3) | 2 | 116 | ||||
| OPSCC | ||||||
| + (≥3) | 17 | 3 | 94.4 | 91.4 | 85.0 | 97.0 |
| − (<3) | 1 | 32 | ||||
HPV, human papillomavirus; HNSCC, head and neck squamous cell carcinoma; OPSCC, oropharyngeal squamous cell carcinoma; PPV, positive predictive value; NPV, negative predictive value.
Based on a combination of HPV DNA status and p16INK4a overexpression, subjects with OPSCC were divided into two groups: a double positive group of patients who were HPV DNA-positive with p16INK4a overexpression and a single positive-negative or double negative group of patients who were either HPV DNA-positive without p16INK4a overexpression, HPV DNA-negative with p16INK4a overexpression, or HPV-DNAnegative without p16INK4a overexpression. The combination of HPV DNA status and p16INK4a overexpression had both high sensitivity (94.4%) and specificity (100%) for detecting HPV E6/E7 mRNA expression in OPSCC (Table V).
Table V.
Relationship between HPV E6/E7 mRNA expression and HPV DNA/p16INK4a expression status in OPSCC.
| HPV mRNA expression
|
P-value | ||
|---|---|---|---|
| HPV DNA/p16INK4a expression status | + | − | |
| HPV DNA+ with | 17 | 0 | <0.001 |
| p16 overexpression | |||
| Others | 1 | 33 | |
| HPV DNA+ without p16 overexpression | 1 | 5 | |
| HPV DNA− with p16 overexpression | 0 | 0 | |
| HPV DNA− without p16 overexpression | 0 | 28 | |
Prognostic value of HPV status and p16INK4a overexpression in OPSCC
Within the observation period, 7 of the 53 patients (13.2%) developed recurrent disease, including 2 of 10 (20.0%) patients with stage I/II OPSCC and 5 of 43 (11.6%) patients with advanced stage OPSCC. However, no patient died with disease during the follow-up period. Since the disease specific rate and overall survival rate in OPSCC were quite fair in the present study, the prognostic analysis of OPSCC focused on recurrence-free survival. Since there were few recurrence-free survival events in OPSCC cases, multivariate analysis of recurrence-free survival was carried out instead for HNSCC cases overall.
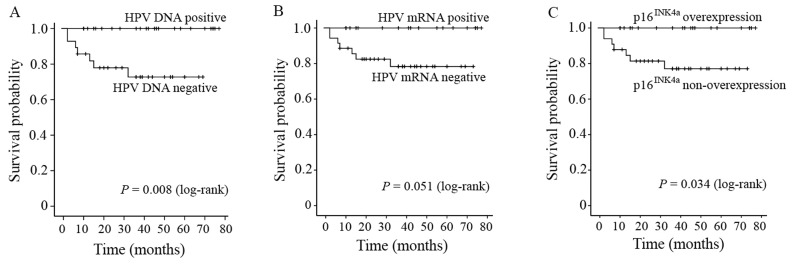
i) Prognostic value of HPV DNA status, E6/E7 mRNA expression and p16INK4a overexpression in OPSCC. HPV DNA-positive patients with OPSCC had better recurrence-free survival than HPV DNA-negative patients with OPSCC (P=0.008) (Fig. 2). The recurrence-free survival after 3 years was 72.7% for patients with HPV DNA-negative OPSCC and 100% for patients with HPV DNA-positive OPSCC. On the contrary, there were no significant differences in recurrence-free survival and disease-specific survival between HPV DNA-positive and -negative patients with non-OPSCC (P=0.139 and 0.144, respectively; Kaplan-Meier curves not shown).
Figure 2.
Kaplan-Meier curves of recurrence-free survival in OPSCC patients according to (A) HPV DNA, (B) HPV mRNA and (C) p16INK4a overexpression. Recurrence-free survival was significantly better in HPV DNA-positive OPSCC patients than HPV DNA-negative OPSCC patients and in OPSCC patients with p16INK4a overexpression than in OPSCC patients without p16INK4a overexpression.
HPV mRNA-positive OPSCC patients exhibited a trend toward improved recurrence-free survival compared with HPV mRNA-negative patients with OPSCC (P=0.051) (Fig. 2). The recurrence-free survival after 3 years was 78.3% for patients with HPV mRNA-negative OPSCC and 100% for patients with HPV mRNA-positive OPSCC.
OPSCC patients with p16INK4a overexpression showed significantly improved recurrence-free survival compared with OPSCC patients without p16INK4a overexpression (P=0.034) (Fig. 2). The recurrence-free survival after 3 years was 100 and 77.1%, respectively.
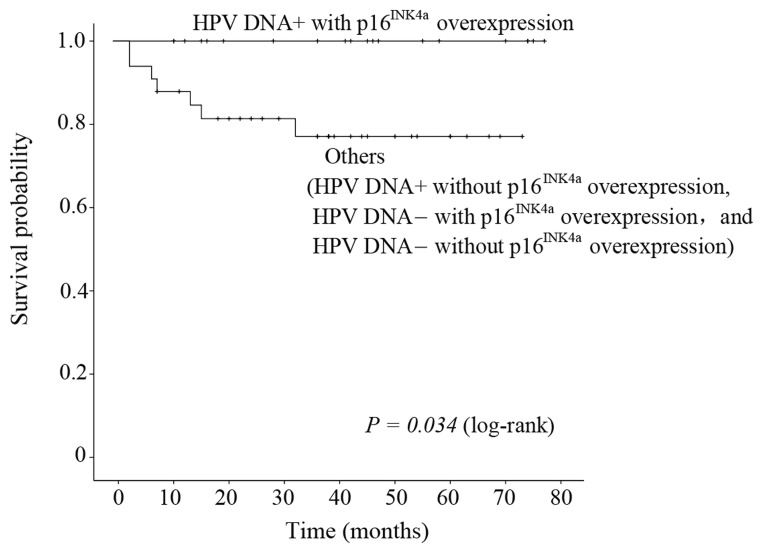
ii) Comprehensive evaluation of survival according to HPV DNA status and p16INK4a overexpression in OPSCC. Patients in the double positive group had better recurrence-free survival than those in a single negative groups or the double negative group (P=0.034) (Fig. 3), with recurrence-free survival after 3 years of 100% and 77.1%, respectively.
Figure 3.
Kaplan-Meier curves of recurrence-free survival in OPSCC patients according to the combination of HPV DNA status and p16INK4a overexpression. OPSCC patients with both HPV DNA-positive status and p16INK4a overexpression showed significantly better recurrence-free survival compared to other patients (HPV DNA-positive without p16INK4a overexpression, HPV DNA-negative with p16INK4a overexpression and HPV DNA-negative without p16INK4a overexpression (P=0.034).
iii) Multivariate analysis using Cox proportional-hazard model in HNSCC. To assess the independent predictive value of all these factors for recurrence-free survival in HNSCC, multivariate analysis using Cox proportional-hazards models was performed. Both HPV DNA presence and p16INK4a over-expression was modeled as one factor. Since no patients with positive HPV E6/E7 mRNA expression had any recurrent lesion, the influence of HPV mRNA expression on recurrence-free survival could not be evaluated.
In univariate analysis, the HNSCC patients with HPV DNA-positive status and p16INK4a overexpression demonstrated significantly higher recurrence-free survival (P=0.015; hazard ratio (HR) = 8.61; 95% confidence interval (CI) = 1.12–66.18) compared with other HNSCC patients (Table VI). The HNSCC patients categorized in T stage 4 and those with oral cavity SCC had significantly lower recurrence-free survival (T4, P=0.003, HR=2.47, 95% CI=1.6–5.76; oral cavity SCC, P=0.031, HR=3.94, 95% CI=1.25–12.41) compared with patients categorized in T stages 1–3 and with OPSCC, respectively.
Table VI.
Univariate and multivariate analysis demonstrating the prognostic impact of HPV DNA and p16INK4a overexpression on recurrence-free survival in HNSCC.
| Variable | P-value | Univariate analysis (n=150) HR (95% CI) |
P-value | Multivariate analysis (n=150) HR (95% CI) |
|---|---|---|---|---|
| HPV DNA/p16INK4a expression (i.e., HPV+ with p16INK4a vs. others) | 0.015 | 8.61 (1.12–66.18) | 0.043 | 7.81 (1.07–57.19) |
| Age (≤50 vs. >50 years) | 0.399 | 0.64 (0.23–1.82) | ||
| Gender (male vs. female) | 0.334 | 0.62 (0.23–1.65) | ||
| T stage (T4 vs. T1–T3) | 0.033 | 2.47 (1.06–5.76) | 0.008 | 2.61 (1.29–5.29) |
| Nodal stage (N2 or N3 vs. N0 or N1) | 0.942 | 0.97 (0.45–2.10) | ||
| Smoking (yes vs. never) | 0.559 | 0.76 (0.30–1.91) | ||
| Alcohol consumption (yes vs. never) | 0.222 | 0.56 (0.22–1.44) | ||
| Differentiation | ||||
| Well | ||||
| Moderate | 0.312 | 0.65 (0.29–1.50) | ||
| Poor | 0.770 | 0.74 (0.22–2.53) | ||
| Tumor location | ||||
| Oropharynx | Reference | Reference | ||
| Nasopharynx | 0.063 | 4.38 (0.98–19.52) | 0.317 | 1.92 (0.54–5.89) |
| Hypopharynx | 0.217 | 1.97 (0.66–5.86) | 0.865 | 1.09 (0.40–3.02) |
| Larynx | 0.500 | 1.73 (0.49–6.13) | 0.870 | 1.10 (0.35–3.50) |
| Oral cavity | 0.031 | 3.94 (1.25–12.41) | 0.107 | 2.27 (0.84–6.17) |
HPV, human papillomavirus; HNSCC, head and neck squamous cell carcinoma; HR, hazard ratio; CI, confidence interval.
The final model of multivariate analysis using a Cox proportional hazards model for identification of fair recurrence-free survival of HNSCC showed that T1–3 stage (P=0.008; adjusted HR=2.61; 95% CI=1.29–5.29) and the combination of HPV DNA-positive status and p16INK4a overexpression (P=0.043; adjusted HR=7.81; 95% CI=1.07–57.19) predicted significantly better recurrence-free survival (Table VI).
Discussion
Over the past two decades, HR-HPV has been firmly established as a common etiologic factor in OPSCC and is now widely used as a prognostic marker for OPSCC. Although there are a number of studies on the epidemiologic role and prognostic value of HPV in OPSCC, some did not distinguish between patients receiving curative treatment from those receiving palliative care (28–31). Moreover, there are few studies on the prognostic value of HPV infection in non-oropharyngeal SCC. In this study, we analyzed the prognostic value of HPV infection in a retrospectively selected cohort of HNSCC patients receiving curative treatment. While the oropharynx was the site of highest prevalence (47.2%), this cohort also included patients with nasopharyngeal, hypopharyngeal, laryngeal and oral cavity tumors. The relatively high prevalence of HPV in cases of nasopharynx and oral cavity SCC suggests that HPV may play an important role in these non-oropharynx HNSCCs as well as in OPSCC.
A significant correlation was found between the presence of HPV DNA and improved recurrence-free survival in OPSCC, with HPV DNA-negative patients demonstrating an apparent greater risk of recurrence compared with HPV DNA-positive patients. OPSCC patients with HPV mRNA expression (active infection) also displayed improved recurrence-free survival compared with OPSCC patients without HPV mRNA expression (P=0.051), in line with previous studies (6,9,32–35). Although HPV DNA-positive patients with nonoropharyngeal SCCs also showed better recurrence-free and disease-free survival, the prognostic value did not reach statistical significance. Since OPSCC patients accounted for 34% of subjects in our series, the fair prognostic significance of HPV status for non-OPSCC HNSCC patients may be influenced by the generally excellent prognosis of patients with OPSCC. Indeed, Isayeva et al systematically reviewed the published data regarding the prognostic significance of HPV in SCCs of the oral cavity, larynx, sinonasal tract and nasopharynx and found no association between HPV status and treatment outcome (36). Further studies are needed to clarify the influence of HPV infection on prognosis in non-OPSCC cases.
Several studies have suggested that p16INK4a expression can be used as a surrogate marker for HPV infection in OPSCC (14,37). Klaes et al defined p16INK4a staining as negative, sporadic, focal or diffuse and found a significant correlation between the presence of HR-HPV and strong diffuse p16INK4a expression (14). However, there are also several contradictory reports on the value of p16INK4a as a biomarker. Smith et al found no concordance between p16INK4a expression and HPV detection in 20% of head and neck cancers (38), possibly due to transcriptionally inactive infection or an alternate pathway of p16INK4a activation (39). Using the same histological criteria as Klaes et al (14), Hoffmann et al reported that 81.2% of HPV DNA-positive HNSCC patients were also p16INK4a positive, compared with only 48.2% of HPV DNA-negative cases, including 3 cases with strong and diffuse p16INK4a staining. Moreover, no p16INK4a expression could be detected in 3 of 14 HPV DNA+/RNA+ HNSCC lesions in their series. They concluded that p16INK4a expression status alone is inadequate for identifying biological active or inactive HPV infections in HNSCC (40).
In the present study, the sensitivity of general p16INK4a expression for detecting HPV DNA in HNSCC was also low, and there was generally low rate of agreement between p16INK4a-positive status and HR-HPV E6/E7 mRNA expression in both HNSCC (κ=0.56) and OPSCC (κ=0.57). These results indicate that p16INK4a expression alone is not suitable for identifying HPV-related tumors. However, p16INK4a over-expression (p16INK4a expression score ≥3) was a sensitive and specific marker for detecting HR-HPV mRNA expression in both HNSCC and OPSCC. This result also underscores the potential of our scoring system for evaluating p16INK4a expression and determining prognosis.
Recent studies have demonstrated a significant correlation between p16INK4a expression as a surrogate marker of HPV infection and fair prognosis in OPSCC. Lassen et al reported that p16INK4a-positive HNSCC showed a better response to conventional radiotherapy than p16INK4a-negative HNSCC, and ascribed this survival benefit to a better locoregional control rate (13). In a study by Fischer et al (18), p16INK4a-negative OPSCC patients demonstrated a more than 2-fold greater risk of death compared with p16INK4a-positive patients. Although locoregional OPSCC relapse was independent of p16INK4aexpression, multivariate survival analysis indicated that p16INK4a expression was an independent prognostic indicator for OPSCC, but not HNSCC, after adjustment for clinical T classification, clinical N classification, and treatment modality (18). In the present study, the combined evaluation of HPV DNA status and p16INK4a overexpression could predict HPV E6/E7 mRNA expression in OPSCC with both high specificity (100%) and sensitivity (94.4%). Furthermore, the combined evaluation of HPV DNA status and p16INK4a overexpression was an independent prognostic indicator for HNSCC in multivariate analysis. Given the time and expense of E6/E7 mRNA analysis, this combination may be particularly useful for larger scale clinical studies.
The combined evaluation of HPV DNA status and p16INK4a overexpression was strongly correlated with HPV mRNA expression in OPSCC. Since the combination of HPV DNA-positive status and p16INK4a overexpression showed a close relation with fair recurrence-free survival in HNSCC in multivariate analysis, the combination can serve as an accurate surrogate marker for biologically active HPV infection. This combined evaluation appears to be a useful and reliable method for detecting HPV-related HNSCC and determining its prognosis.
Acknowledgments
This study was supported by grant KAKENHI 23592535 (Japan Society for the Promotion of Science) to M.S., grant KAKENHI 22791614 (Japan Society for the Promotion of Science) to M.H. and a grant from the Japan-China Medical Association to Z.D. This study was also supported and conducted in cooperation with the Ryukyu Society for the Promotion of Oto-Rhino-Laryngology.
Abbreviations:
- CCRT
concurrent chemoradiotherapy;
- CI
confidence interval;
- CRT
chemoradiotherapy;
- FFPE
formalin-fixed, paraffin-embedded;
- HNSCC
head and neck squamous cell carcinoma;
- HPV
human papillomavirus;
- HR
hazard ratio;
- HR-HPVs
high-risk HPV variants;
- OPSCC
oropharyngeal squamous cell carcinoma;
- PCR
polymerase chain reaction;
- RT
radiation therapy
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez L, Wunsch-Filho V, Franceschi S, Hayes RB, Herrero R, Kelsey K, Koifman S, La Vecchia C, Lazarus P, Levi F, Lence JJ, Mates D, Matos E, Menezes A, McClean MD, Muscat J, Eluf-Neto J, Olshan AF, Purdue M, Rudnai P, Schwartz SM, Smith E, Sturgis EM, Szeszenia-Dabrowska N, Talamini R, Wei Q, Winn DM, Shangina O, Pilarska A, Zhang ZF, Ferro G, Berthiller J, Boffetta P. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 4.Näsman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, Ahrlund-Richter S, Marklund L, Romanitan M, Lindquist D, Ramqvist T, Lindholm J, Sparén P, Ye W, Dahlstrand H, Munck-Wikland E, Dalianis T. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 5.Deng Z, Hasegawa M, Matayoshi S, Kiyuna A, Yamashita Y, Maeda H, Suzuki M. Prevalence and clinical features of human papillomavirus in head and neck squamous cell carcinoma in Okinawa, southern Japan. Eur Arch Otorhinolaryngol. 2011;268:1625–1631. doi: 10.1007/s00405-011-1515-0. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 8.Deng Z, Hasegawa M, Yamashita Y, Matayoshi S, Kiyuna A, Agena S, Uehara T, Maeda H, Suzuki M. Prognostic value of human papillomavirus and squamous cell carcinoma antigen in head and neck squamous cell carcinoma. Cancer Sci. 2012;103:2127–2134. doi: 10.1111/cas.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 10.Heath S, Willis V, Allan K, Purdie K, Harwood C, Shields P, Simcock R, Williams T, Gilbert DC. Clinically significant human papilloma virus in squamous cell carcinoma of the head and neck in UK practice. Clin Oncol (R Coll Radiol) 2012;24:e18–23. doi: 10.1016/j.clon.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, Meijer CJ, Braakhuis BJ, Leemans CR, Brakenhoff RH. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann M, Ihloff AS, Gorogh T, Weise JB, Fazel A, Krams M, Rittgen W, Schwarz E, Kahn T. p16(INK4a) overexpression predicts translational active human papillomavirus infection in tonsillar cancer. Int J Cancer. 2010;127:1595–1602. doi: 10.1002/ijc.25174. [DOI] [PubMed] [Google Scholar]
- 13.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4Aexpression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 14.Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, Dallenbach-Hellweg G, Schmidt D, von Knebel Doeberitz M. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 15.Singhi AD, Westra WH. Comparison of human papilloma-virus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 16.Schache AG, Liloglou T, Risk JM, Jones TM, Ma XJ, Wang H, Bui S, Luo Y, Sloan P, Shaw RJ, Robinson M. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br J Cancer. 2013;108:1332–1339. doi: 10.1038/bjc.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bussu F, Sali M, Gallus R, Vellone VG, Zannoni GF, Autorino R, Dinapoli N, Santangelo R, Martucci R, Graziani C, Micciche F, Almadori G, Galli J, Delogu G, Sanguinetti M, Rindi G, Valentini V, Paludetti G. HPV infection in squamous cell carcinomas arising from different mucosal sites of the head and neck region. Is p16 immunohistochemistry a reliable surrogate marker? Br J Cancer. 2013;108:1157–1162. doi: 10.1038/bjc.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer CA, Zlobec I, Green E, Probst S, Storck C, Lugli A, Tornillo L, Wolfensberger M, Terracciano LM. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010;126:1256–1262. doi: 10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 19.Klingenberg B, Hafkamp HC, Haesevoets A, Manni JJ, Slootweg PJ, Weissenborn SJ, Klussmann JP, Speel EJ. p16 INK4A overexpression is frequently detected in tumour-free tonsil tissue without association with HPV. Histopathology. 2010;56:957–967. doi: 10.1111/j.1365-2559.2010.03576.x. [DOI] [PubMed] [Google Scholar]
- 20.Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 21.de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3’ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76:1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 22.Manos MM, Ting Y, Wright DK, Lewis AJ, Broker TR, Wolinsky SM. Use of polymerase chain reaction amplification for the detection of genital human papillomaviruses. Cancer Cells. 1989;7:209–214. [Google Scholar]
- 23.Remmerbach TW, Brinckmann UG, Hemprich A, Chekol M, Kuhndel K, Liebert UG. PCR detection of human papilloma-virus of the mucosa: comparison between MY09/11 and GP5+/6+primer sets. J Clin Virol. 2004;30:302–308. doi: 10.1016/j.jcv.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene. 2002;21:1510–1517. doi: 10.1038/sj.onc.1205214. [DOI] [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 26.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360–363. [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 28.Charfi L, Jouffroy T, de Cremoux P, Le Peltier N, Thioux M, Freneaux P, Point D, Girod A, Rodriguez J, Sastre-Garau X. Two types of squamous cell carcinoma of the palatine tonsil characterized by distinct etiology, molecular features and outcome. Cancer Lett. 2008;260:72–78. doi: 10.1016/j.canlet.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Hafkamp HC, Manni JJ, Haesevoets A, Voogd AC, Schepers M, Bot FJ, Hopman AH, Ramaekers FC, Speel EJ. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008;122:2656–2664. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 30.Hannisdal K, Schjolberg A, De Angelis PM, Boysen M, Clausen OP. Human papillomavirus (HPV)-positive tonsillar carcinomas are frequent and have a favourable prognosis in males in Norway. Acta Otolaryngol. 2010;130:293–299. doi: 10.3109/00016480903071377. [DOI] [PubMed] [Google Scholar]
- 31.Klussmann JP, Mooren JJ, Lehnen M, Claessen SM, Stenner M, Huebbers CU, Weissenborn SJ, Wedemeyer I, Preuss SF, Straetmans JM, Manni JJ, Hopman AH, Speel EJ. Genetic signatures of HPV-related and unrelated oropharyngeal carcinoma and their prognostic implications. Clin Cancer Res. 2009;15:1779–1786. doi: 10.1158/1078-0432.CCR-08-1463. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz SR, Yueh B, McDougall JK, Daling JR, Schwartz SM. Human papillomavirus infection and survival in oral squamous cell cancer: a population-based study. Otolaryngol Head Neck Surg. 2001;125:1–9. doi: 10.1067/mhn.2001.116979. [DOI] [PubMed] [Google Scholar]
- 33.Evans M, Newcombe R, Fiander A, Powell J, Rolles M, Thavaraj S, Robinson M, Powell N. Human papillomavirus-associated oropharyngeal cancer: an observational study of diagnosis, prevalence and prognosis in a UK population. BMC Cancer. 2013;13:220. doi: 10.1186/1471-2407-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung AC, Briolat J, Millon R, de Reynies A, Rickman D, Thomas E, Abecassis J, Clavel C, Wasylyk B. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int J Cancer. 2010;126:1882–1894. doi: 10.1002/ijc.24911. [DOI] [PubMed] [Google Scholar]
- 35.Lindquist D, Romanitan M, Hammarstedt L, Nasman A, Dahlstrand H, Lindholm J, Onelov L, Ramqvist T, Ye W, Munck-Wikland E, Dalianis T. Human papillomavirus is a favourable prognostic factor in tonsillar cancer and its oncogenic role is supported by the expression of E6 and E7. Mol Oncol. 2007;1:350–355. doi: 10.1016/j.molonc.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6(Suppl 1):S104–S120. doi: 10.1007/s12105-012-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang SS, Trunk M, Schiffman M, Herrero R, Sherman ME, Burk RD, Hildesheim A, Bratti MC, Wright T, Rodriguez AC, Chen S, Reichert A, von Knebel Doeberitz C, Ridder R, von Knebel Doeberitz M. Validation of p16INK4aas a marker of oncogenic human papillomavirus infection in cervical biopsies from a population-based cohort in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13:1355–1360. [PubMed] [Google Scholar]
- 38.Smith EM, Wang D, Kim Y, Rubenstein LM, Lee JH, Haugen TH, Turek LP. p16INK4aexpression, human papilloma virus, and survival in head and neck cancer. Oral Oncol. 2008;44:133–142. doi: 10.1016/j.oraloncology.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Thomas J, Primeaux T. Is p16 immunohistochemistry a more cost-effective method for identification of human papilloma virus-associated head and neck squamous cell carcinoma? Ann Diagn Pathol. 2012;16:91–99. doi: 10.1016/j.anndiagpath.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann M, Tribius S, Quabius ES, Henry H, Pfannenschmidt S, Burkhardt C, Gorogh T, Halec G, Hoffmann AS, Kahn T, Rocken C, Haag J, Waterboer T, Schmitt M. HPV DNA, E6*I-mRNA expression and p16INK4Aimmunohistochemistry in head and neck cancer - how valid is p16INK4Aas surrogate marker? Cancer Lett. 2012;323:88–96. doi: 10.1016/j.canlet.2012.03.033. [DOI] [PubMed] [Google Scholar]