Abstract
Objective:
Although there is some evidence that methamphetamine (MA) abuse may play a causative role in the development of schizophrenia, studies directly linking these 2 are rare.
Methods:
In our study, the effect of MA abuse on the development of schizophrenia was investigated in 15 MA abusers who are offspring of patients with schizophrenia and 15 siblings of MA abusers without a history of drug abuse. Cognitive deficits and resting-state brain function were evaluated in all participants. Correlations between cognitive deficits and schizophrenia development were investigated.
Results:
Significantly more cognitive impairments were observed in MA abusers, compared with their siblings without a history of drug use. Significant abnormalities in regional homogeneity (ReHo) signals were observed in resting brain in MA abusers. Decreased ReHo was found to be distributed over the bilateral cingulate gyrus, right Brodmann area 24, and bilateral anterior cingulate cortex. Seven MA abusers were diagnosed with schizophrenia, while 1 control sibling was diagnosed with schizophrenia during the 5-year follow-up. The cognitive scores correlated with the development of schizophrenia in MA abusers.
Conclusion:
Our study provides direct evidence for the causative role of MA use in the etiology of schizophrenia and highlights the role of MA-induced brain abnormalities in cognitive deficiency and development of schizophrenia.
Keywords: resting-state brain function, cognitive impairment, methamphetamine, abuse, schizophrenia, first-degree relative, magnetic resonance imaging
Abstract
Objectif :
Bien que certaines données probantes soutiennent que l’abus de méthamphétamine (MA) puisse jouer un rôle causal dans le développement de la schizophrénie, les études qui lient directement ces deux facteurs sont rares.
Méthodes :
Dans notre étude, l’effet de l’abus de MA sur le développement de la schizophrénie a été investigué chez 15 personnes abusant de MA qui sont des enfants de patients souffrant de schizophrénie, et chez 15 frères et sœurs de personnes abusant de MA sans histoire d’abus de drogues. Les déficits cognitifs et la fonction cérébrale au repos ont été évalués chez tous les participants. Les corrélations entre les déficits cognitifs et le développement de la schizophrénie ont été investiguées.
Résultats :
Un nombre significativement plus élevé de déficits cognitifs a été observé chez les personnes abusant de MA, comparé à leurs frères et sœurs sans antécédents d’abus de drogues. Des anomalies significatives des signaux d’homogénéité régionale (HoRe) ont été observées dans le cerveau au repos des personnes abusant de MA. Il a été constaté qu’une HoRe réduite était distribuée sur le gyrus cingulaire bilatéral, sur l’aire 24 droite de Brodmann, et sur le cortex cingulaire antérieur bilatéral. Sept personnes abusant de MA ont reçu un diagnostic de schizophrénie, tandis qu’un frère ou une sœur témoin a reçu 1 diagnostic de schizophrénie durant le suivi à 5 ans. Les scores cognitifs corrélaient avec le développement de la schizophrénie chez les personnes abusant de MA.
Conclusion :
Notre étude fournit une preuve directe du rôle causal de l’utilisation de MA dans l’étiologie de la schizophrénie et met en évidence le rôle des anomalies cérébrales induites par la MA dans les déficiences cognitives et le développement de la schizophrénie.
Schizophrenia is a highly heterogeneous and usually lifelong psychiatric disorder. The etiology and pathogenesis of schizophrenia are still unclear. Numerous genetic and environmental risk factors have been proposed to be involved in the development of schizophrenia. Although genetics were thought to play a crucial role in the susceptibility to schizophrenia with a general heritability estimate of 80%,1 only about 10% of first-degree relatives of patients with schizophrenia developed schizophrenia,2 which is still 10 times higher than the number of people in the general population who develop schizophrenia.3,4 Interactions between environmental factors and genetics have been hypothesized to be another critical mechanism involved in the development of schizophrenia.5 One of the major environmental factors is the abuse of drugs, such as MA and cannabis.6 However, direct evidence for the interaction between genetics and MA is accumulating slowly.
There is an estimated 51 million people using MA, worldwide, and about 5% of the adult population in the United States have used MA on at least 1 occasion.7 Although early studies suggested that the abuse of MA can trigger the development of persistent psychotic syndromes, the idea that MA causes schizophrenia is still controversial.8 One study9 in Thailand has found that about 25% of MA users who had been hospitalized due to an acute psychotic episode were diagnosed with schizophrenia during a 5-year follow-up. This is currently the strongest available evidence that MA may increase the risk of schizophrenia. Studies10,11 in Taiwan have shown that familial risk for schizophrenia increases the likelihood of MA users experiencing psychotic episodes. Persistent psychosis was found to be associated with patients who had a 2-times greater familial morbid risk of schizophrenia, compared with those whose symptoms were transient.11 These findings may suggest an interaction between genetics and environmental factors. However, more evidence should be obtained from comparative studies between MA-abusing offspring of patients with schizophrenia and their nonabusing siblings. Unfortunately, there is currently a lack of studies in this area.
Clinical Implications
MA abuse is a risk factor for schizophrenia.
MA-induced brain damage plays a role in the development of schizophrenia.
Abnormalities in the cingulate cortex are associated with cognitive deficiency in patients with schizophrenia.
Limitations
Our study lacks a dynamic observation of brain functional abnormalities.
Only genetic factors were investigated.
In addition, the neurological basis of MA-induced schizophrenia has rarely been reported, although in vivo imaging has been widely employed to find the structural and functional abnormalities in the brain of MA users.12,13 There is an accumulation of evidence showing damage in frontostriatal regions subserving selective attention and memory, such as the striatum, frontal cortex, hippocampus, and amygdala.13–16 For example, MRI demonstrated an increase in cortical grey matter loss with age in MA users.17 In contrast, an increase in regional striatal volume was observed in adolescent MA and cannabis abusers.15 In addition, functional MRI revealed high brain activation in the cingulate and low activation in the frontal lobe when MA abusers watched MA cue pictures.18 Moreover, hypofunction in cortical areas was thought to be associated with the cognitive deficits in MA abusers.14 However, the role of structural and functional damages in the brain on the development of schizophrenia has not been validated. In our study, we used MRI to investigate resting-state brain function of MA abusers who are children of patients with schizophrenia. Our study may further increase our understanding of the role of MA in the interaction between genetics and environment in the development of schizophrenia.
Subjects and Methods
Subjects
Fifteen MA abusers whose parents have previously been diagnosed and treated as patients with schizophrenia were recruited from the Changsha Addiction Treatment Center and Huaihua Addiction Treatment Center from December 2004 to December 2006. Fifteen siblings of the MA abusers were recruited as control subjects. The control siblings had parents with schizophrenia, but had no history of drug use or schizophrenic symptoms. Diagnoses of schizophrenia met the DSM-IV criteria for schizophrenia. The inclusion criteria were as follows:
The MA abusers met DSM-IV criteria for lifetime MA dependence determined from the SCID,19 but were abstinent for a minimum of 2 months, which was confirmed by guardians of the patients.
The MA abusers and the 15 siblings had no history of alcohol, marijuana, or other drug abuse.
No schizophrenia was diagnosed in MA abusers and the 15 siblings when they were recruited for our study.
The MA abusers and the 15 siblings had no history of manic psychosis or other psychotic disorders and nonpsychotic disorders, such as anxiety or depression, neurological diseases or head injury, or other severe medical conditions.
All subjects were followed up for 5 years.
MA abusers consisted of 10 males and 5 females with an average age of 26.8 years (SD 2.8; range 18 to 29 years). The 15 siblings of MA abusers are composed of 8 brothers and 7 sisters, with an average age of 25.0 years (SD 2.5; range 19 to 27 years). They were given a neurologic examination within 1 week of recruitment, which showed no abnormalities in the brain. The subjects were also given a SCID before and 5 years after they were recruited for our study. Our study was approved by the Ethics Review Board of Central South University, and all subjects signed informed consent forms.
Cognition Tests
A set of cognitive measurements were performed within 1 week of recruitment as previously described20: first, verbal memory and visual memory were tested by measuring delayed recall time of 1 story or 4 pictures from a Chinese version of the logical memory and visual reproduction subtest, respectively, from the Wechsler Memory Scale—Revised. Immediate recall (0 minutes) and delayed recall (30 minutes) were tested, and savings were calculated (delayed score/immediate score); and second, attention and vigilance was tested using the computerized visual and auditory versions of the T.O.V.A., a Chinese version of continuous performance tests. In the visual version of the T.O.V.A., geometric shapes were used. The auditory version of the T.O.V.A. used 2 easily recognized tones as the target and nontarget stimuli. The commission error, omission errors, and average reaction time were scored.
MRI Scan
All subjects were imaged using a 1.5T MRI scanner (General Electric Medical Systems, Milwaukee, WI). The scan was completed in 7 to 10 minutes in a quadrature head coil. Froth pads were used to limit head motion and reduce scanner noise. Subjects were not given any cognitive tasks and were asked to remain motionless and think of nothing while in the machine.
Every subject received a whole brain scan. The baseline of axial link line paralleled the anterior–posterior link line. A series of 20 axial slices were collected using fast echo planar image acquisition parameters as follows: T1-weighted FLAIR: TR/TE/TI = 2000 ms/24 ms/750 ms; slice thickness = 5 mm; interval = 1 mm; scan FOV = 24 cm × 24 cm, and matrix = 256 hus × 256 hus. Resting-state brain function was evaluated, and images were acquired using T1 FLAIR. A series of 3620 axial slices were collected using fast echo planar image acquisition parameters as follows: TR = 2000 ms; TE = 40 ms; 90-degree flip angle; FOV = 24 cm × 24 cm; matrix = 64 hus × 64 hus; slice thickness = 5 mm; and interval = 1.0 mm.
A series of 172 sagittal axial slices were collected using the following parameters: TR = 12 ms; TE = 4.2 ms; 15-degree flip angle; FOV = 24 cm × 24 cm, matrix = 512 hus × 512 hus; slice thickness = 5 mm; interval = 0 mm. The K-space, real-time data were collected using echo planar imaging (blood oxygen level dependent program) and images were reconstructed using Sun UltraSparc AW4.0 (Advantage Workstation, GE Healthcare, Milwaukee, WI). Structural MRI data were pre-processed using statistical parametric mapping 2 in Matlab 6.5 and brain functional imaging analysis software (Analysis of Functional Neuro Images).21
Statistical Analysis
To compare the differences of resting-state brain function between MA abusers and siblings of MA abusers, KCC was used to measure the ReHo.22 The KCC values at the corresponding positions were analyzed by a Student t test. A P value of less than 0.005 in each voxel plus the area of more than 5 voxels was considered statistically significant. A Student t test was used to compare the differences in behaviours between MA abusers and their siblings. The prevalence was analyzed using a chi-square test. A P value of less than 0.05 was considered statistically different.
Results
Demographic Study
The 15 MA abusers were Han Chinese, with an average age of 26.8 years (SD 2.8) and an education of 9.53 years (SD 2.67). The 15 sibling control subjects were also Han Chinese, with an average age of 25.0 years (SD 2.5) and an education of 10.30 years (SD 2.84). Among the 15 MA abusers, 3 had a history of smoking cigarettes (12.5 packs per year [SD 1.5]). Among the 15 siblings, 4 had a history of smoking cigarettes (11.6 packs per year [SD 1.7]). The paired Student t test showed that there were no significant differences in age (P > 0.05), sex, educational background, or smoking amount (P > 0.05) between the 15 MA abusers and the 15 sibling control subjects. Neither the MA abusers nor the nondrug using siblings met the diagnosis of schizophrenia, according to DSM-IV criteria, when they were recruited for our study.
Cognition Ability Assessments
As shown in Table 1, MA abusers had significantly more visual omission errors (P < 0.001), and longer average reaction times (P < 0.05), as well as significantly slower visual reproduction immediate recall and visual reproduction delayed recall times (P < 0.01 and P < 0.05, respectively). MA abusers showed significantly more audio commission errors (P < 0.001), but there were no differences in audio omission errors and average reaction time (P > 0.05), compared with the control subjects. These results suggest that there were more cognitive deficits in MA abusers than in sibling control subjects.
Table 1.
Cognition tests
| Variable | Sibling control subjects (n = 15) Mean (SD) | MA abusers (n = 15) Mean (SD) | P |
|---|---|---|---|
| Visual commission error RS | 7.73 (1.06) | 9.04 (2.22) | 0.05 |
| Visual omission errors RS | 2.20 (0.63) | 8.31 (2.18) | <0.001a |
| Visual average reaction time RS | 764.7 (108.8) | 899.8 (192.2) | 0.03b |
| Audio commission errors RS | 10.12 (1.67) | 14.36 (1.84) | <0.001a |
| Audio omission errors RS | 6.35 (1.54) | 7.29 (1.67) | 0.12 |
| Audio average reaction time (ms) RS | 783.1 (126.8) | 850.3 (139.2) | 0.18 |
| Visual reproduction immediate recall | 10.19 (2.01) | 7.19 (1.34) | 0.002c |
| Visual reproduction delayed recall | 10.36 (2.90) | 8.13 (1.94) | 0.02b |
| Visual reproduction savings, % | 1.15 (0.27) | 1.15 (0.25) | 0.98 |
RS = raw score
P < 0.001 compared with control subjects
P < 0.05
P < 0.01
Changes in Resting-State Brain Function
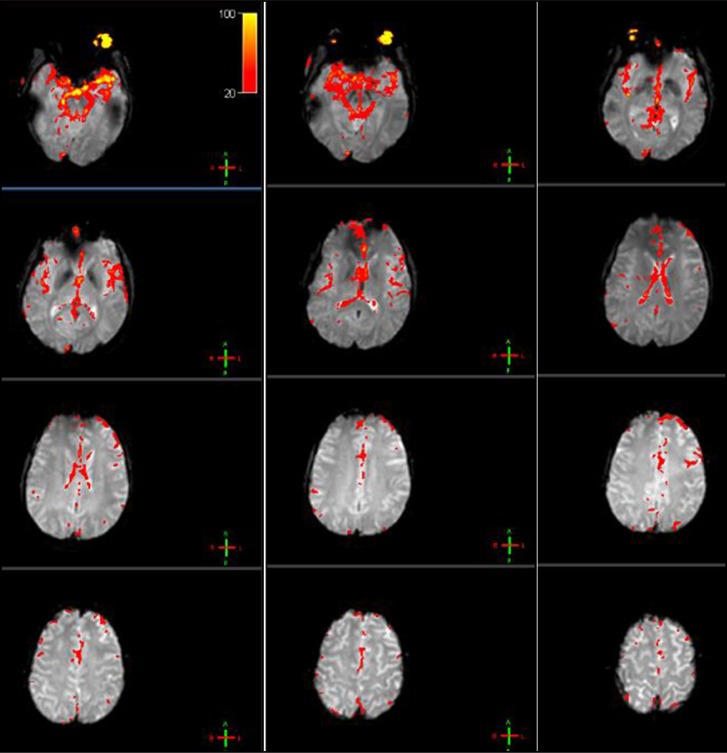
The whole brain was scanned in both MA abusers and sibling control subjects. Compared with sibling control subjects, the ReHo signals were significantly decreased in MA abusers in the bilateral cingulate gyrus, right Brodmann area 24, and bilateral ACC. No increased signals were observed (Table 2, Figure 1).
Table 2.
The areas with lowered resting-state ReHo in MA abusers
| Anatomy position | Hemisphere | Z | Pa | MNI (XYZ) |
|---|---|---|---|---|
| Anterior cingulate | left | 2.98 | 0.001 | −8 39 18 |
| Anterior cingulate | right | 2.91 | 0.002 | 12 17 29 |
| Brodmann area 24 | right | 2.94 | 0.001 | −12 12 36 |
| Cingulate gyrus | left | 3.44 | <0.001 | −16 −39 39 |
| Cingulate gyrus | right | 2.65 | 0.003 | −8 39 17 |
MNI = Montreal Neurological Institute. X = left–right direction. Its value goes from negative to positive and refers to the most far left to right side of the brain. Y = the front–rear direction. Its value goes from negative to positive and corresponds to the most front to the most back of the brain. Z: the up–down direction. Its value from negative to positive corresponds to the brain from the top to the bottom.
false discovery rate corrected
Figure 1.
The ReHo changed area in the MA abusers. Red indicated the area with significantly reduced local consistency. (Image is available in colour online.)
The Prevalence of Schizophrenia
The 15 MA abusers and 15 sibling control subjects were followed up for 5 years. These participants were given a SCID within 1 week of recruitment for our study. No schizophrenia was diagnosed in both groups. During the 5-year follow-up, 1 subject in the sibling control group was diagnosed with schizophrenia, whereas 7 MA abusers were diagnosed with schizophrenia, suggesting a 7-fold increase in the prevalence of schizophrenia in MA abusers (Table 3, P < 0.05). Among the 7 MA abusers, standard toxicology tests confirmed that 4 MA abusers relapsed and retook MA and 3 remained totally abstinent.
Table 3.
Prevalence of schizophrenia
| Group | Schizophrenia
|
P | |
|---|---|---|---|
| Sibling control subjects | 1 | 14 | 0.01 |
| MA abusers | 7 | 8 | |
Correlation Analysis
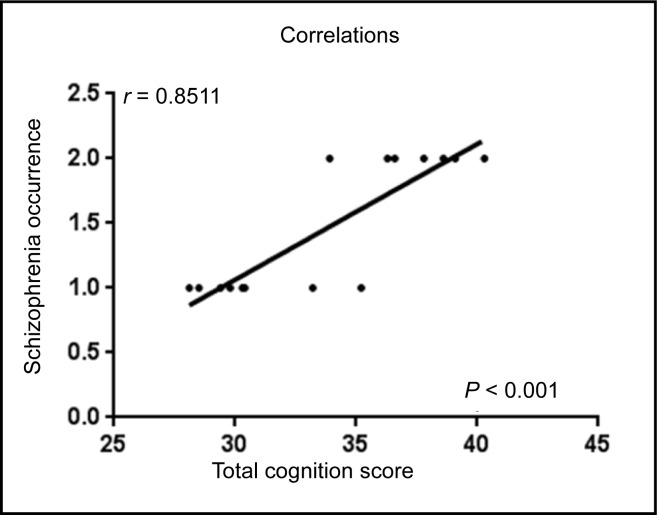
To perform correlation analysis between cognitive functions and occurrence of schizophrenia, a total cognition score for each MA abuser was calculated. The total cognition score of each MA abuser was the sum of the scores for all variables of cognitive tests. The score for each variable in MA abusers, such as visual omission errors, visual average reaction times, visual reproduction immediate recall, visual reproduction delayed recall times, and audio commission errors, was calculated by the percentage of increase or decrease in the score of each variable by comparing it with its average score in the sibling control subjects. Each 10% increase or decrease in percentage was scored 1 point. The total scores of each MA abuser were used for correlation analysis with the occurrence of schizophrenia (2 for occurrence and 1 for no occurrence). A significant correlation between total cognition score and schizophrenia occurrence was observed in MA abusers (Figure 2, P < 0.001). This finding suggested a significant association between cognitive deficits and the occurrence of schizophrenia.
Figure 2.
Correlation analysis between cognition functions and occurrence of schizophrenia in MA abusers (total cognition score for each MA abuser was calculated).
The score 2 in y-axis represented the occurrence of schizophrenia, while score 1 represented no occurrence of schizophrenia.
Discussion
Although a few studies provided evidence for the causative role of MA use in the development of schizophrenia in the general population and an implication for the role of the interaction between MA and genetics in the etiology of schizophrenia,9,11 no studies have investigated these critical issues in at-risk families with a history of schizophrenia. Also, few studies reported the correlation between MA-induced cognitive deficits, brain functional abnormalities, and the development of schizophrenia. In our study, 15 MA abusers who are offspring of patients with schizophrenia and 15 siblings of MA abusers who have parents with schizophrenia, but did not have a history of drug use, were recruited for the analysis of cognitive deficits, brain functional abnormalities, and prevalence of schizophrenia during a 5-year follow-up. This made it possible to investigate the causative role of MA in the development of schizophrenia and the interaction between environmental factors and genetics in the etiology of schizophrenia. Interestingly, our study demonstrated for the first time that MA users in at-risk families of patients with schizophrenia have functional brain abnormalities and cognitive deficits. MA abusers exhibited a 7-fold higher prevalence of schizophrenia than non-MA using offspring of patients with schizophrenia. Our study provided evidence for the causative role of MA in the etiology of schizophrenia and highlighted the interaction between MA and genetics in the development of schizophrenia.
Previous studies have demonstrated that chronic MA abusers show prolonged psychiatric signs under conditions of intoxication, withdrawal, or both. The prolongation of psychosis in chronic MA users has often been regarded as manifestations of preexistent schizophrenia.23 Cognitive deficits have been suggested to be an endophenotype of schizophrenia.24,25 In our study, cognitive deficits were observed more frequently in MA abusers than in siblings of MA abusers who did not have a history of drug use, suggesting that MA causes impairments in cognition. Consistent with our previous study (see Zang et al22) that there are mild cognitive abnormalities in first-degree relatives of schizophrenia without a history of drug use, the nondrug using siblings also exhibited mild cognitive abnormalities, compared with healthy control subjects, but neither the MA abusers nor the nondrug using siblings met the diagnosis of schizophrenia, according to DSMIV criteria, when they were first recruited for our study. However, 7 out of the 15 MA abusers were diagnosed with schizophrenia during the 5-year follow-up. In contrast, only 1 of the 15 siblings who did not use drugs was diagnosed with schizophrenia. This finding suggests a causative role of MA abuse in the development of schizophrenia.
A previous study26 revealed that MA-dependent participants had less DLPFC activation and did not show ventromedial cortex activation during the 2-choice prediction task, suggesting a functional abnormality in the prefrontal cortex of MA abusers. A family study of schizophrenia demonstrated inefficient activation of DLPFC in patients with schizophrenia27 and decreased activation of the ACC in high-risk siblings whose 2 relatives were diagnosed with schizophrenia.28 In our study, both the cognitive deficits and brain functional abnormalities in the cingulate were observed in MA abusers. The cingulate has been widely reported to be associated with cognitive performance.29 Importantly, cognitive deficits significantly correlated with the occurrence of schizophrenia. Therefore, our findings strongly suggest that MA-induced brain functional abnormalities in the cingulate cortex leads to cognitive deficits, which enhances the development of schizophrenia.
We acknowledge that there were 3 limitations to our study. First, the sample size of participants was relatively small. However, MA abusers who are offspring of patients with schizophrenia are relatively rare. Even so, the sample size is comparable with most MRI studies in at-risk siblings of patients with schizophrenia to date. Of course, a large sample study would be more desirable and can better confirm the findings in our study. Second, brain functional abnormalities were not dynamically observed.
It is also unclear whether brain damage exists, which is stronger evidence for the relation between brain damage and the development of schizophrenia. Third, the effects of abstinence, and social and psychological factors on the development of schizophrenia in MA abusers were not evaluated. Despite these limitations, our study first provided direct evidence for the causative role of MA abuse in the development of schizophrenia and associated brain functional abnormalities.
Acknowledgments
No funding was provided for this research. All authors declare no conflict of interest.
Abbreviations
- ACC
anterior cingulate cortex
- DLPFC
dorsolateral prefrontal cortex
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- FLAIR
fluid-attenuated inversion recovery
- FOV
field of view
- KCC
Kendall’s coefficient of concordance
- MA
methamphetamine
- MRI
magnetic resonance imaging
- ReHo
regional homogeneity
- SCID
Structured Clinical Interview for DSM-IV
- TE
echo time
- TI
inversion time
- T.O.V.A.
Test of Variables of Attention
- TR
repetition time
References
- 1.Owen MJ. Genomic approaches to schizophrenia. Clin Ther. 2005;27(Suppl A):S2–S7. doi: 10.1016/j.clinthera.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Lim LC, Sim LP. The prevalence of schizophrenia in relatives of schizophrenic patients. Singapore Med J. 1992;33(6):645–647. [PubMed] [Google Scholar]
- 3.Cardno AG, Gottesman II. Twin studies of schizophrenia: from bowand-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000;97(1):12–17. [PubMed] [Google Scholar]
- 4.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 5.Pelayo-Terán JM, Suárez-Pinilla P, Chadi N, et al. Gene– environment interactions underlying the effect of cannabis in first episode psychosis. Curr Pharm Des. 2012;18(32):5024–5035. doi: 10.2174/138161212802884609. [DOI] [PubMed] [Google Scholar]
- 6.Gururajan A, Manning EE, Klug M, et al. Drugs of abuse and increased risk of psychosis development. Aust N Z J Psychiatry. 2012;46(12):1120–1135. doi: 10.1177/0004867412455232. [DOI] [PubMed] [Google Scholar]
- 7.Salo R, Fassbender C, Buonocore MH, et al. Behavioral regulation in methamphetamine abusers: an fMRI study. Psychiatry Res. 2013;211(3):234–238. doi: 10.1016/j.pscychresns.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zorick TS, Rad D, Rim C, et al. An overview of methamphetamine-induced psychotic syndromes. Addict Disord Their Treat. 2008;7(3):143–156. [Google Scholar]
- 9.Kittirattanapaiboon P, Mahatnirunkul S, Booncharoen H, et al. Long-term outcomes in methamphetamine psychosis patients after first hospitalisation. Drug Alcohol Rev. 2010;29(4):456–461. doi: 10.1111/j.1465-3362.2010.00196.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen CK, Lin SK, Sham PC, et al. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychol Med. 2003;33(8):1407–1414. doi: 10.1017/s0033291703008353. [DOI] [PubMed] [Google Scholar]
- 11.Chen CK, Lin SK, Sham PC, et al. Morbid risk for psychiatric disorder among the relatives of methamphetamine users with and without psychosis. Am J Med Genetics. 2005;136B(1):87–91. doi: 10.1002/ajmg.b.30187. [DOI] [PubMed] [Google Scholar]
- 12.Salo R, Fassbender C. Structural, functional and spectroscopic MRI studies of methamphetamine addiction. Curr Top Behav Neurosci. 2012;11:321–364. doi: 10.1007/7854_2011_172. [DOI] [PubMed] [Google Scholar]
- 13.Fatovich DM, McCoubrie DL, Song SJ, et al. Brain abnormalities detected on magnetic resonance imaging of amphetamine users presenting to an emergency department: a pilot study. Med J Aust. 2010;193(5):266–268. doi: 10.5694/j.1326-5377.2010.tb03904.x. [DOI] [PubMed] [Google Scholar]
- 14.Nestor LJ, Ghahremani DG, Monterosso J, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194(3):287–295. doi: 10.1016/j.pscychresns.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Churchwell JC, Carey PD, Ferrett HL, et al. Abnormal striatal circuitry and intensified novelty seeking among adolescents who abuse methamphetamine and cannabis. Dev Neurosci. 2012;34(4):310–317. doi: 10.1159/000337724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orikabe L, Yamasue H, Inoue H, et al. Reduced amygdala and hippocampal volumes in patients with methamphetamine psychosis. Schizophr Res. 2011;132(2–3):183–189. doi: 10.1016/j.schres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Nakama H, Chang L, Fein G, et al. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction. 2011;106(8):1474–1483. doi: 10.1111/j.1360-0443.2011.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin JJ, Ma SH, Xu K, et al. Functional magnetic resonance imaging of methamphetamine craving. Clin Imaging. 2012;36(6):695–701. doi: 10.1016/j.clinimag.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer RL, First MB, Williams JB, et al. Now is the time to retire the term organic mental disorders. Am J Psychiatry. 1992;149(2):240–244. doi: 10.1176/ajp.149.2.240. [DOI] [PubMed] [Google Scholar]
- 20.Liao H, Wang L, Zhou B, et al. A resting-state functional magnetic resonance imaging study on the first-degree relatives of persons with schizophrenia. Brain Imaging Behav. 2012;6(3):397–403. doi: 10.1007/s11682-012-9154-7. [DOI] [PubMed] [Google Scholar]
- 21.Cox RW. Analysis of Functioning Neuro Images (AFNI): software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 22.Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 23.Sekine Y, Iyo M, Ouchi Y, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158(8):1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- 24.Nam HJ, Kim N, Park T, et al. Cognitive profiles of healthy siblings of schizophrenia patients: application of the cognitive domains of the MATRICS consensus battery. World J Biol Psychiatry. 2009;10(4 Pt 2):452–460. doi: 10.1080/15622970802314815. [DOI] [PubMed] [Google Scholar]
- 25.Sevik AE, Yağcıoğlu AE, Yağcıoğlu S, et al. Neuropsychological performance and auditory event related potentials in schizophrenia patients and their siblings: a family study. Schizophr Res. 2011;130(1–3):195–202. doi: 10.1016/j.schres.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacol. 2002;26(1):53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- 27.Callicott JH, Mattay VS, Verchinski BA, et al. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160(12):2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 28.Whalley HC, Simonotto E, Moorhead W, et al. Functional imaging as a predictor of schizophrenia. Biol Psychiatry. 2006;60(5):454–462. doi: 10.1016/j.biopsych.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Deco G, Rolls ET. Attention, short-term memory, and action selection: a unifying theory. Prog Neurobiol. 2005;76(4):236–256. doi: 10.1016/j.pneurobio.2005.08.004. [DOI] [PubMed] [Google Scholar]