Abstract
Objective:
To determine the effects of various therapeutic interventions on increasing voluntary quadriceps muscle activation.
Background:
Decreased voluntary quadriceps activation is commonly associated with knee injury. Recently, research has focused on developing specific disinhibitory interventions to improve voluntary quadriceps activation; yet, it remains unknown which interventions are most effective in promoting this improvement.
Data Sources:
We searched Web of Science from January 1, 1965 through September 27, 2012, using the key words quadriceps activation and transcutaneous electrical nerve stimulation, transcranial magnetic stimulation, cryotherapy, focal joint cooling, joint mobilization, joint mobilisation, joint manipulation, manual therapy, and neuromuscular electrical stimulation.
Study Selection:
Studies evaluating the effect of disinhibitory interventions on volitional quadriceps activation were used in our review. Standardized effect sizes (Cohen d) and 95% confidence intervals (CIs) were calculated from voluntary quadriceps activation means and standard deviations measured at baseline and at all available postintervention time points from each study.
Data Synthesis:
Ten studies were grouped into 5 categories based on intervention type: manual therapy (4 studies), transcutaneous electrical nerve stimulation (2 studies), cryotherapy (2 studies), neuromuscular electrical stimulation (2 studies), and transcranial magnetic stimulation (1 study). Transcutaneous electrical nerve stimulation demonstrated the strongest immediate effects (d = 1.03; 95% CI = 0.06, 1.92) and long-term effects (d = 1.93; 95% CI = 0.91, 2.83). Cryotherapy (d = 0.76; 95% CI = −0.13, 1.59) and transcranial magnetic stimulation (d = 0.54; 95% CI = −0.33, 1.37) had moderate immediate effects in improving voluntary quadriceps activation, whereas manual therapy (d = 0.38; 95% CI = −0.35, 1.09) elicited only weak immediate effects. Neuromuscular electrical stimulation produced weak negative to strong positive effects (range of d values = −0.50 to 1.87) over a period of 3 weeks to 6 months.
Conclusions:
Transcutaneous electrical nerve stimulation demonstrated the strongest and most consistent effects in increasing voluntary quadriceps activation and may be the best disinhibitory intervention for improving the same.
Key Words: arthrogenic muscle inhibition, disinhibitory modalities, knee
Quadriceps function is critical for optimal locomotion and energy attenuation in the lower extremity.1,2 The ability to eccentrically contract the quadriceps is critical for optimal knee range of motion during the weight-acceptance phase of gait.1 It is hypothesized that patients with quadriceps dysfunction lack the ability to eccentrically contract the quadriceps in an effort to obtain optimal knee range of motion, which is critical for attenuating energy and maintaining proper contact forces at the joint surfaces.3 Physical performance, as demonstrated with the get-up-and-go test, is also impaired in patients with knee injuries,4 indicating that concentric quadriceps dysfunction may limit patients' ability to propel themselves during ambulation. After knee injury, patients often display stiffer knee-movement strategies or less knee flexion during the stance phase of gait, which is thought to alter joint contact forces and increase the risk of joint deterioration.3
Quadriceps dysfunction predicts a compound variable of both physical performance and self-reported function5 in patients with knee osteoarthritis as well as mortality6 in patients with chronic obstructive pulmonary disorder. Osteoarthritis,7 total knee arthroplasty,8 anterior knee pain,9 and anterior cruciate ligament deficiency or reconstruction10 are examples of knee injuries that present with quadriceps dysfunction, suggesting that proper functioning of this muscle group is critical for patients to maintain an acceptable quality of life with a broad range of conditions.
Impaired functioning of this crucial muscle group is thought to arise from central nervous system alterations presenting as decreased motor output of the knee extensors.11 These neural alterations after knee injury often manifest as decreased voluntary quadriceps activation,10 which can be modulated by altered excitability of spinal reflexive12,13 and cortical14 motor pathways. Immediately after knee joint injury, a decrease in voluntary quadriceps activation may be a protective mechanism to prevent further injury.15–17 However, if these neural abnormalities are not targeted with specific interventions used to disinhibit an inhibited muscle, quadriceps dysfunction may persist and become a factor limiting successful knee-injury management.11
Conventional rehabilitation strategies typically focus on strength training to reestablish normal quadriceps function without specifically addressing decreased voluntary quadriceps activation.11 Traditional therapeutic exercise has demonstrated minimal improvements in quadriceps strength and voluntary quadriceps activation18 and small effects in decreasing pain (standardized mean difference = 0.40) and improving disability (standardized mean difference = 0.37).19 Because restoring voluntary quadriceps activation predicts the ability to develop quadriceps muscle strength in clinical populations, interventions targeting these activation deficits seem imperative.20 A new rehabilitative paradigm has been proposed that seeks to combine interventions to increase voluntary quadriceps activation with traditional therapeutic exercise to improve clinical outcomes.11,15,17 Techniques specifically used to alter motor excitability after joint injury for the purpose of improving voluntary quadriceps activation and to enhance therapeutic exercise are termed disinhibitory interventions.15 Disinhibitory interventions are intended to alter neuromuscular function by targeting mechanoreceptors locally at the injured joint, targeting the peripheral nervous system at points either proximal or distal to the injured joint, or targeting the central nervous system directly.
To date, a systematic evaluation of viable interventions that can effectively disinhibit the quadriceps, thus increasing voluntary quadriceps activation, has not been performed. Therefore, the purpose of our study was to investigate the effectiveness of documented disinhibitory interventions for increasing voluntary quadriceps activation. It is imperative to understand the preliminary effects of a variety of disinhibitory interventions as a foundation for further research that will guide future rehabilitative science and improve knee-injury management.
METHODS
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist21 as a guide for developing this systematic review. We performed a search of all databases within Web of Science from January 1, 1965 to September 27, 2012, pairing the terms quadriceps activation with transcutaneous electrical nerve stimulation (TENS), transcranial magnetic stimulation (TMS), cryotherapy, focal joint cooling, joint mobilization, joint mobilisation, joint manipulation, manual therapy, and neuromuscular electrical stimulation (NMES). Inclusion criteria were studies evaluating maximal voluntary quadriceps activation (ie, maximal motor-unit recruitment and firing frequency); using either the superimposed-burst or interpolated-twitch technique (which both use maximal voluntary force and supermaximal force elicited with an exogenous electrical stimulus to evaluate voluntary quadriceps activation); involving healthy or injured samples; written in English and investigating the effect of therapeutic interventions on voluntary quadriceps activation measured using an exogenous stimulus; and reporting means and standard deviations, thereby allowing standardized effect sizes to be calculated. If the means and standard deviations of the voluntary quadriceps activation were not provided in tabular form in the publication,22–25 we attempted to contact the authors by e-mail to acquire this information. We also cross-referenced the bibliographies of all relevant studies to locate any additional pertinent articles not found in the online search.26 We excluded all studies with single-patient case-report designs.27,28
Methodologic Quality Assessment
Two investigators (M.S.H. and B.G.P.) independently evaluated and scored the methodologic quality of each of the 10 included studies using the Physiotherapy Evidence Database (PEDro)23 and Oxford level of evidence29 scoring systems (Table 1). If there was a disagreement in the scores, investigators met and came to an agreement on the scores.
Table 1.
Demographic Variables and Quality Assessment of Included Studies
| Intervention |
Authors (Year) |
Study Design |
Groups |
Participants, No. |
Sex (Men/ Women) |
Condition |
Age, y (Mean ± SD) |
PEDro Score (Range, 0–10)a |
Oxford Level of Evidence (Range, 1a–5) |
| Manual therapy (average PEDro score = 5) | Grindstaff et al (2009)31 | Randomized control trial | Lumbopelvic manipulation | 15 | NA | Healthy | 24.6 ± 6.2 | 6 | 1b– |
| Passive range of motion | 13 | NA | 28.6 ± 8.2 | ||||||
| Prone extension | 13 | NA | 27 ± 5.9 | ||||||
| Grindstaff et al (2012)29 | Randomized control trial | Lumbopelvic manipulation | 13 | NA | Patellofemoral pain syndrome | 25.4 ± 7.7 | 6 | 1b– | |
| Passive range of motion | 15 | NA | 25.1 ± 9.6 | ||||||
| Prone extension | 13 | NA | 24.6 ± 7.4 | ||||||
| Drover et al (2004)26 | Case series | Active release therapy | 9 | 4/5 | Unilateral knee pain | 25.7 ± 3.5 | 4 | 4 | |
| Contralateral limb | |||||||||
| Suter et al (1999)35 | Case series | Lumbopelvic manipulation | 18 | 1/17 | Unilateral/bilateral knee pain | 30.5 ± 13 | 4 | 4 | |
| Contralateral limb | |||||||||
| Cryotherapy (average PEDro score = 5) | Pietrosimone et al (2009)32 | Crossover | Cryotherapy | 11 | 6/5 | Healthy | 25 ± 5 | 4 | 3– |
| Control | |||||||||
| Pietrosimone et al (2009)32 | Randomized control trial | Cryotherapy | 11 | 6/5 | Tibiofemoral osteoarthritis | 58 ± 8.4 | 6 | 1b– | |
| Control | 12 | 5/7 | 54 ± 9.9 | ||||||
| TENS (average PEDro score = 6.5) | Pietrosimone et al (2009)32 | Randomized control trial | TENS | 10 | 6/4 | Tibiofemoral osteoarthritis | 56 ± 10.1 | 6 | 1b– |
| Control | 12 | 5/7 | 54 ± 9.9 | ||||||
| Pietrosimone et al (2011)18 | Randomized control trial | TENS | 12 | 6/6 | Tibiofemoral osteoarthritis | NA | 7 | 1b– | |
| Placebo TENS | 12 | 4/8 | |||||||
| Control | 12 | 5/7 | |||||||
| NMES (average PEDro score = 5.5) | Palmieri-Smith et al (2010)30 | Randomized control trial | NMES | 16 | 0/16 | Knee osteoarthritis | 58 ± 2.7 | 7 | 1b– |
| Control | 14 | 0/14 | 56.8 ± 2.9 | ||||||
| Stevens et al (2004)34 | Case series | NMES | 5 | 5/0 | Bilateral total knee arthroplasty | 66.6 ± 4.9 | 4 | 4 | |
| Control | 3 | 2/1 | 68.7 ± 7.5 | ||||||
| TMS (average PEDro score = 8) | Gibbons et al (2010)16 | Randomized control trial | TMS | 11 | 7/4 | Partial meniscectomy | 38.1 ± 16.2 | 8 | 1b– |
| Control | 9 | 7/2 | 38.2 ± 17.5 |
Abbreviations: NA indicates not available; NMES, neuromuscular electrical stimulation; PEDro, Physiotherapy Evidence Database; TENS, transcutaneous electrical nerve stimulation; TMS, transcranial magnetic stimulation.
The average PEDro score was 5.6.
Data Extraction and Analysis
Study sample sizes, means, and standard deviations of voluntary quadriceps activation (Table 2) were collected or calculated from each of the studies. The Cohen d effect sizes with corresponding 95% confidence intervals (CIs) were calculated comparing the postintervention time values to the baseline value for each participant (Cohen d = [meanpost – meanpre]/pooled standard deviation). Studies were further stratified into 2 operationally defined time ranges: immediate (posttesting data collected during a single intervention session, range = 20–60 minutes after the intervention) and long-term (posttesting data completed over multiple sessions, range = 2 weeks–6 months after the intervention) effects. One group30 did not publish posttest means and standard deviations, but we were able to extrapolate posttest means from change scores and pretest means and use the pretest standard deviation to calculate effect sizes. Another group18 did not publish posttest standard deviations; however, these data were obtained from the authors, allowing us to calculate effect sizes.
Table 2.
Voluntary Quadriceps Activation Data From the Included Studies
Abbreviations: NA indicates not applicable; NMES, neuromuscular electrical stimulation; TENS, transcutaneous electrical nerve stimulation; TMS, transcranial magnetic stimulation.
RESULTS
Included Studies
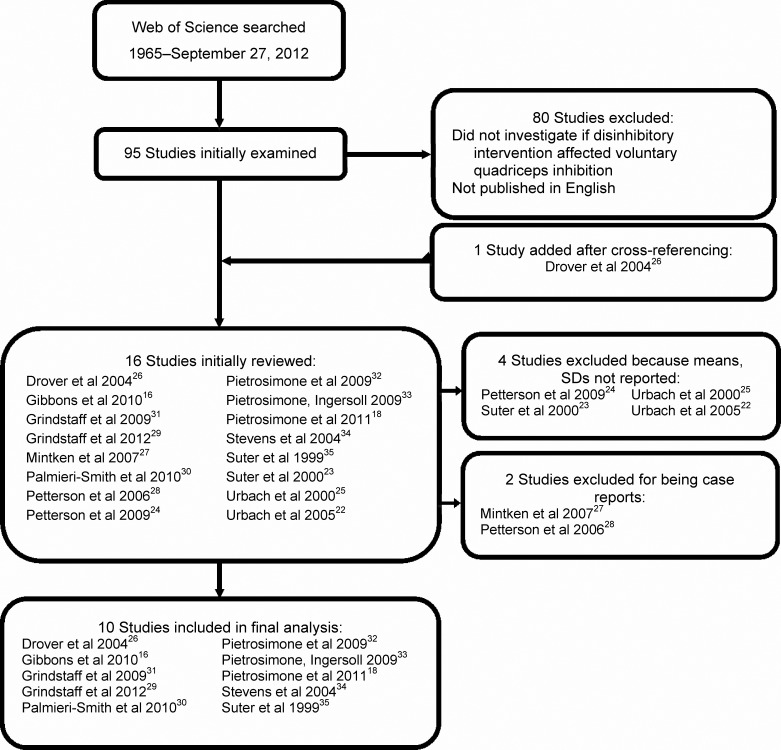
The initial online search identified 95 potential studies (Figure 1). Upon further assessment, 80 of these studies were excluded because they did not investigate if a disinhibitory intervention affected voluntary quadriceps activation or they were not published in English. One additional investigation26 was included after cross-referencing. Four studies22–25 were excluded because means and standard deviations of voluntary quadriceps activation data were not reported, and 2 studies27,28 were excluded because they were case reports. Ten studies matched our inclusion criteria and contained the appropriate statistics.16,18,26,29–35 Four of the investigations used manual therapy techniques: an active release technique26 and a lumbopelvic joint manipulation.29,31,35 Two studies32,33 addressed the effect of cryotherapy on voluntary quadriceps activation. Two groups explored the effect of TENS18,32 (Pietrosimone et al32 also included cryotherapy), 2 investigated NMES,30,34 and 1 evaluated TMS.16
Figure 1.
Search method for articles included in systematic review.
Methodologic Quality Assessment
The average PEDro score for the included studies was 5.6 (Table 1). After we stratified by therapeutic intervention, the TMS study16 had the highest methodologic quality with a PEDro score of 8.0, whereas the studies using manual therapy26,29,31,35 and cryotherapy32,33 had the lowest collective PEDro scores (5.0). The remainder of the interventions presented with average scores of 5.5 for NMES30,34 and 6.5 for TENS.18,32
Effects of Disinhibitory Interventions
The effect sizes for each intervention are displayed with forest plots in Figures 2 through 6 and are categorized by the specific time points at which the postintervention data were collected.
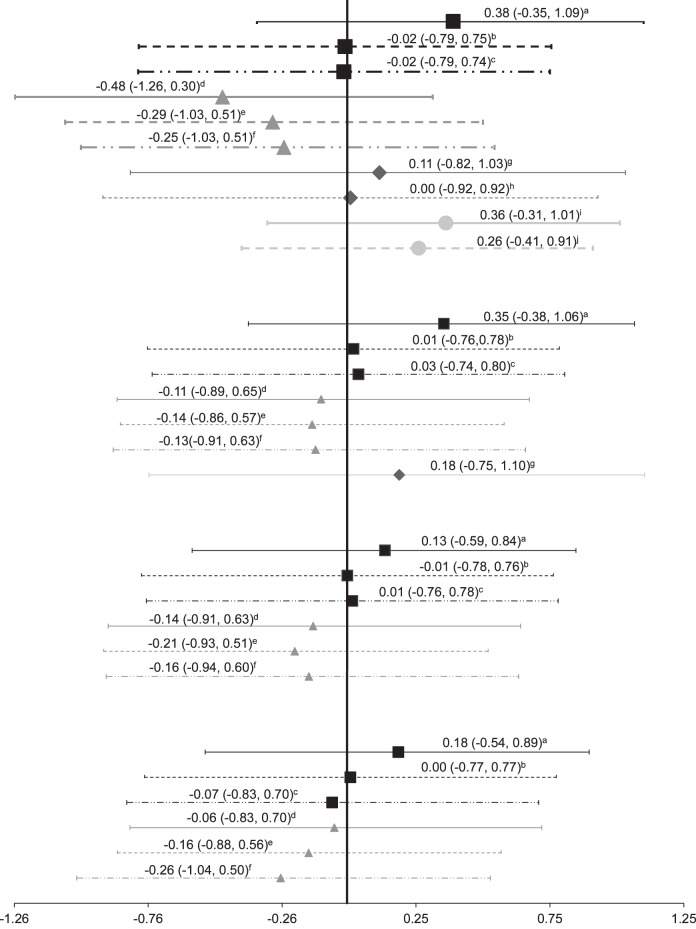
Figure 2.
Manual therapy effect sizes. ROM indicates range of motion; a Grindstaff et al (2009)31: lumbopelvic manipulation; b Grindstaff et al (2009)31: passive range of motion; c Grindstaff et al (2009)31: prone extension; d Grindstaff et al (2012)29: lumbopelvic manipulation; e Grindstaff et al (2012)29: passive range of motion; f Grindstaff et al (2012)29: prone extension; g Drover et al (2004)26: active-release technique; h Drover et al (2004)26: contralateral limb; i Suter et al (1999)35: lumbopelvic manipulation; j Suter et al (1999)35: contralateral limb.
Figure 6.
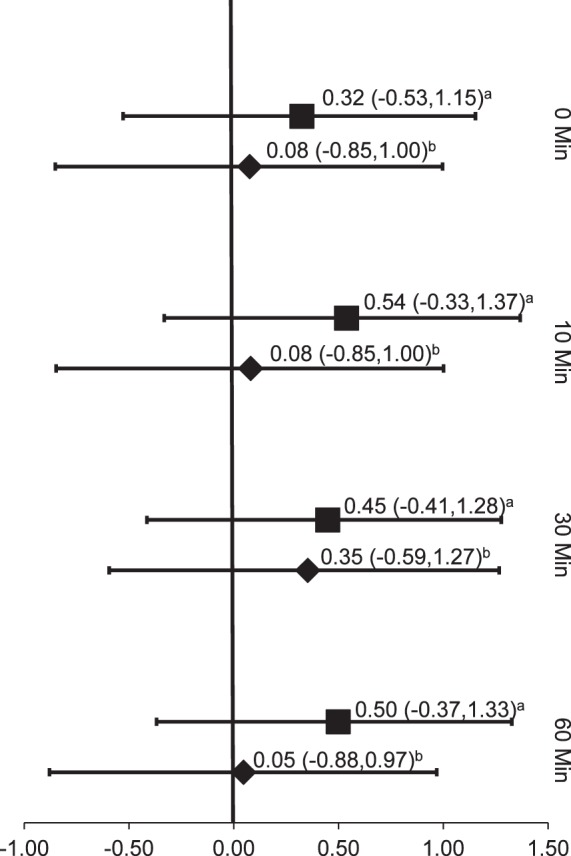
Transcranial magnetic electrical stimulation effect sizes. a Gibbons et al (2010)16: transcranial magnetic electrical stimulation; b Gibbons et al (2010)16: controls.
Manual Therapy
The Oxford level of evidence for each of the manual therapy studies ranged from 1b–29,31 to 4,26,35 with the highest level of evidence in the studies with healthy31 patients and people with patellofemoral pain syndrome29 (Table 1). The effect sizes of the manual therapy interventions, indicating an increase in voluntary quadriceps activation, were weak during all of the postintervention time points measured. The highest effect size was immediately (0 minutes) after a lumbopelvic manipulation31 (Cohen d = 0.38, 95% CI = −0.35, 1.09). The manual therapy studies26,29,31,35 focused on immediate effects, with the postintervention time points ranging from 0 to 60 minutes. The forest plot for manual therapy (Figure 2) shows that the effect sizes tended to decrease as more time elapsed after the intervention. The effect size of 0.38 at 0 minutes after the intervention31 decreased to 0.18 (95% CI = −0.54, 0.89) at 60 minutes. One group29 found weak negative effect sizes with a lumbopelvic manipulation at all of the time points tested (0, 20, 40, and 60 minutes after the intervention). Two investigations29,31 included comparison groups that received lumbar passive range of motion, which resulted in negligible effect sizes. The one study26 that used an active release technique noted small effect sizes at both 0 and 20 minutes after the intervention. All of the 95% CIs were wide and crossed zero (Figure 2).
Cryotherapy
The investigations of cryotherapy presented with Oxford level of evidence scores ranging from 1b–32 to 3–,33 with the highest level of evidence seen in patients with knee osteoarthritis32 (Table 1). These groups32,33 focused on the immediate effects after the intervention, with posttesting measurements occurring at 20, 30, and 45 minutes. Cryotherapy had a moderate effect in increasing voluntary quadriceps activation, with a mean effect size of 0.52, whereas the control group had a mean effect size of −0.16. Cryotherapy produced an increase in voluntary quadriceps activation when it lasted for at least 45 minutes. A large increase in effect size (0.46 to 0.76) was seen between the 30-minute and 45-minute time points after cryotherapy to osteoarthritic knees.32 All the intervention-group effect sizes were higher than those for their control counterparts at every time point, yet all associated CIs were wide and crossed zero (Figure 3).
Figure 3.
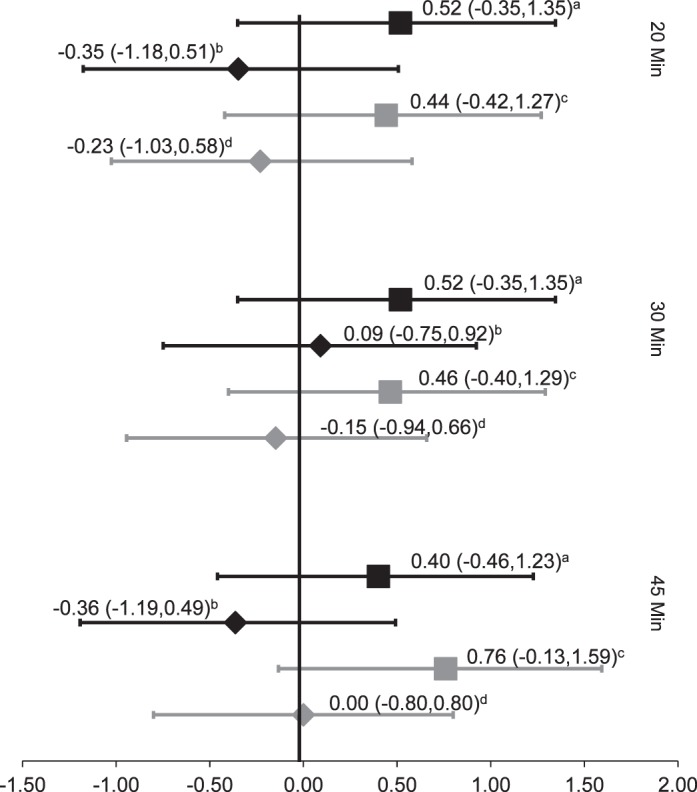
Cryotherapy effect sizes. a Pietrosimone et al (2009)32: healthy controls, ice; b Pietrosimone et al (2009)32: healthy controls; c Pietrosimone et al (2009)32: patients with osteoarthritis, ice; d Pietrosimone et al (2009)32: patients with osteoarthritis.
Transcutaneous Electrical Nerve Stimulation
The TENS studies18,32 in patients with knee osteoarthritis both had an Oxford level of evidence of 1b–. These authors examined immediate and long-term postintervention effects. One group32 reported at immediate time points of 20, 30, and 45 minutes. The TENS produced a weak effect size (0.38, 95% CI = −0.52, 1.25) at 20 minutes, which greatly increased by 30 minutes (0.63, 95% CI = −0.30, 1.50). The effect size remained strong at the final time point of 45 minutes (1.03; 95% CI = 0.06, 1.92), and the CIs did not cross zero. The effect sizes of the control group were weak and negative at all time points.32
Long-term effects of TENS were stronger than the immediate effects at 2 (1.93; 95% CI = 0.91, 2.83) and 4 weeks (1.81, 95% CI = 0.80, 2.68). For both time intervals, the CIs were relatively narrow and did not cross zero. This group18 also used a placebo TENS group, which showed weaker effect sizes than the true TENS groups at all time points (0.88 at 2 weeks, 0.33 at 4 weeks; Figure 4).18
Figure 4.
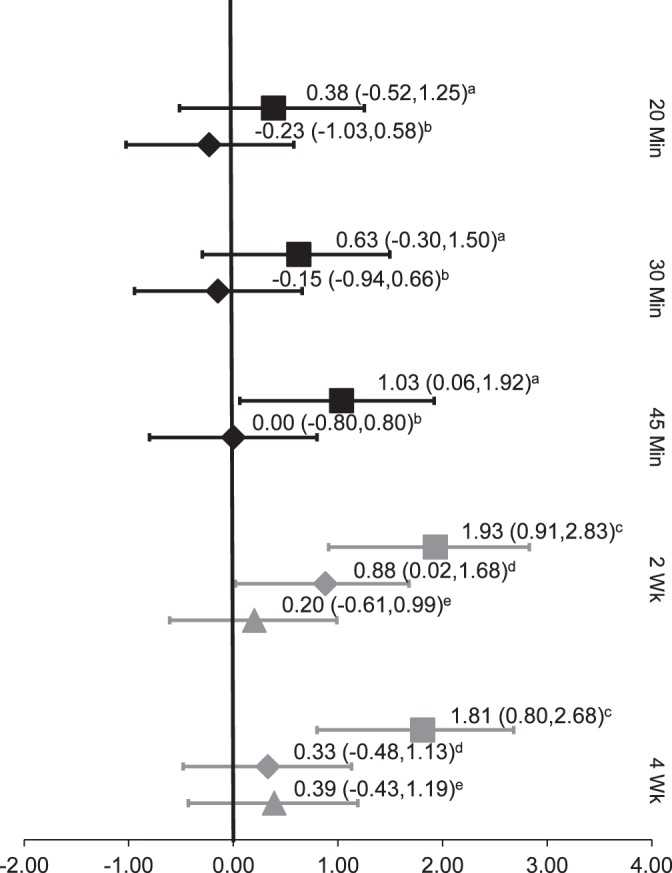
Transcutaneous electrical nerve stimulation (TENS) effect sizes. a Pietrosimone et al (2009)32: TENS; b Pietrosimone et al (2009)32: controls; c Pietrosimone et al (2011)18: TENS; d Pietrosimone et al (2011)18: placebo; e Pietrosimone et al (2011)18: exercise.
Neuromuscular Electrical Stimulation
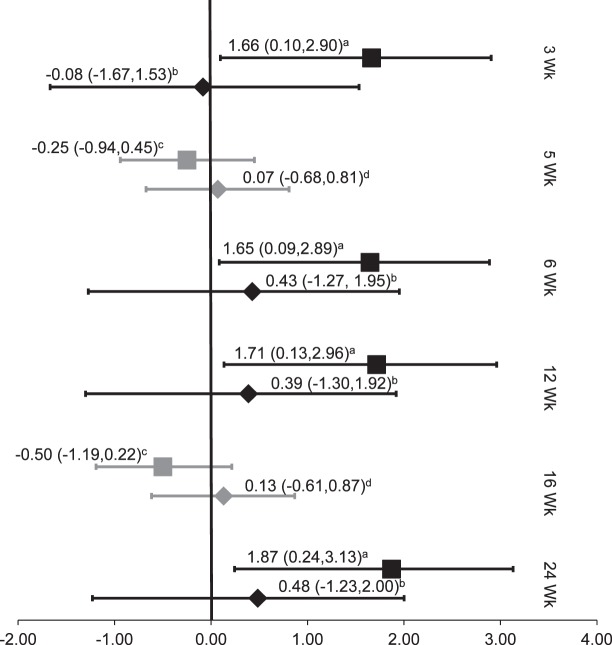
For the studies of NMES, the Oxford level of evidence ranged from 1b–30 to 4,34 with the highest level of evidence in patients with knee osteoarthritis.30 These groups focused on long-term effects on voluntary quadriceps activation. One investigation34 revealed a strong effect size (1.66, 95% CI = 0.10, 2.90) at the 3-week time point. The effect size remained strong at 6 weeks (1.65, 95% CI = 0.09, 2.89) and increased at both 3 months (1.71, 95% CI = 0.13, 2.96) and 6 months (1.87; 95% CI = 0.24, 3.13). None of the CIs crossed zero at any time points, and the effects were much stronger than for any of the control groups (mean Cohen d = 0.24). Palmieri-Smith et al30 found conflicting results while administering a similar NMES protocol. At 5 weeks, the participants experienced a weak negative effect size after NMES (−0.25, 95% CI = −0.94, 0.45). At 16 weeks, the experimental group continued to decline and showed a moderate negative effect size (−0.50, 95% CI = −1.19, 0.22); the CIs at both time points were wide and encompassed zero (Figure 5).
Figure 5.
Neuromuscular electrical stimulation (NMES) effect sizes. a Stevens et al (2004)34: NMES; b Stevens et al (2004)34: exercise; c Palmieri-Smith et al (2010)30: NMES; d Palmieri-Smith et al (2010)30: controls.
Transcranial Magnetic Stimulation
The sole TMS study16 investigated the effects of the disinhibitory intervention on voluntary quadriceps activation in patients with a partial meniscectomy and had an Oxford level of evidence of 1b–. This group evaluated the immediate (0, 10, 30, and 60 minutes after treatment) effect sizes of TMS on voluntary quadriceps activation. Weak effect sizes were noted immediately after the TMS treatment (0.34, 95% CI = −0.53, 1.15). Moderate effect sizes were found at 10 minutes (0.54, 95% CI = –0.33, 1.37), 30 minutes (0.45, 95% CI = −0.41, 1.28), and 60 minutes (0.50, 95% CI = −0.37, 1.33). The effect sizes for the intervention groups were greater than those for the control groups; however, all effect sizes had very wide CIs that crossed zero (Figure 6).
DISCUSSION
Currently, only 10 studies have evaluated the efficacy of disinhibitory interventions in increasing voluntary quadriceps activation. The PEDro scores of the studies range from 4 to 8, with an average of 5.6, demonstrating a moderate level of methodologic quality. This average PEDro score may reflect the difficulty of blinding participants and the therapists administering the intervention.
After separating these studies into 5 intervention categories, we found that TENS had the strongest effect in increasing voluntary quadriceps activation and manual therapy the weakest. However, this research area to date lacks the large clinical trials that may best evaluate the effectiveness of these interventions in increasing voluntary quadriceps activation.
Many of the studies demonstrated moderate to strong effect sizes, but the CIs were wide and crossed zero.16,26,29,31–33,35 Therefore, the efficacy of these interventions is questionable; it is not possible to claim a definitive positive effect from the treatment. The only interventions that demonstrated strong effect sizes with 95% CIs that did not cross zero were TENS18,32 and NMES.34 Thus, TENS and NMES are the only 2 interventions that we can say elicit increases in voluntary quadriceps activation.
The current literature finds TENS to be the most effective intervention in increasing voluntary quadriceps activation because it produced positive homogeneous findings and CIs that did not cross zero. The efficacy of TENS could be attributed to the specific mechanism by which it is hypothesized to target neuromuscular dysfunction: the altered afferent activity caused by the exogenous electrical stimuli of TENS applied over the injured joint may target presynaptic reflex inhibitory mechanisms36 that lead to quadriceps dysfunction.12 One benefit of TENS therapy is that it can be administered while the patient is completing a variety of exercises and activities of daily living, allowing for a larger dosage of this disinhibitory intervention. This unique combination of TENS in conjunction with therapeutic exercise may explain the cumulative increase in voluntary quadriceps activation after this disinhibitory intervention (Figure 4).18,32 Although TENS is traditionally used as a pain-management intervention, changes in voluntary quadriceps activation did not correlate with changes in pain, suggesting that the pain relief may occur through neurologic mechanisms that are independent of voluntary activation.32
Manual therapy and cryotherapy are also thought to potentially alter afferent stimuli arising from the injured joint, yet their current protocols have not been as effective as TENS for increasing voluntary quadriceps activation. Grindstaff et al29 incorporated a running intervention with the lumbopelvic manipulation. The running may have confounded the effect of the lumbopelvic manipulation; unfortunately, the study design limits our ability to determine if or how running modified effects from the manipulation.
Transcranial magnetic stimulation involves an exogenous stimulus applied to a region of the motor cortex during maximal voluntary quadriceps contraction that may target descending corticospinal pathways by posttetanic potentiation.16 Posttetanic potentiation refers to transient accumulation of calcium in presynaptic terminals after the TMS stimulus is administered, thus allowing more neurotransmitter to be released.37 Greater neurotransmitter secretion increases synaptic efficacy, which may excite the previously inhibited α motor neurons and increase voluntary quadriceps activation after a single TMS treatment.37 Long-term potentiation explained by the Hebbian postulate37 (repeated, coordinated neural activity across a synapse will strengthen that neural transmission) may illustrate a potential mechanism for long-term increases in voluntary quadriceps activation after multiple TMS treatments.
The NMES studies30,34 used similar protocols but produced contradicting results, raising questions about the true efficacy of this intervention. The NMES intensity was set as maximally tolerated by each patient (Palmieri-Smith and Thomas30: >35% of maximal voluntary isometric contraction; Stevens et al34: 29%–53% maximal voluntary isometric contraction), which may not have stimulated enough muscle to increase voluntary activation. Previous researchers38 investigating NMES demonstrated an increase in strength by using an intensity that produced 60% of the patient's maximal voluntary isometric contraction. The conflicting results produced by the similar NMES protocols may be attributed to the difference in the voluntary quadriceps activation deficits demonstrated between the study populations at baseline (baseline voluntary quadriceps activation for NMES group: Palmieri-Smith et al30 = 0.90, Stevens et al34 = 0.59).
Although TENS and NMES both involve an exogenous electrical stimulus that depolarizes nerve fibers in the peripheral nervous system, the interventions have different goals. The therapeutic goals of TENS and NMES dictate the settings and placement of the modality. Whereas TENS is administered to the injured joint with a low-intensity stimulus to specifically target sensory nerve fibers, NMES applies a higher-intensity and longer-phase duration directly to the target muscle that depolarizes α motor neurons, producing an involuntary contraction of the muscle.39–41 No specific theory addresses how NMES affects the central nervous system, where muscle inhibition is thought to originate; however, NMES likely increases quadriceps strength by decreasing atrophy within the affected muscle.30 These different interventions probably affect the central nervous system through a variety of mechanisms, which suggests that individual variations in patient physiology or condition may predict the best intervention to use.
These results, plus the inadequacy of traditional rehabilitation outcomes for restoring full function after joint injury,19,42 illustrate the need for a paradigm shift in the nonsurgical management of joint injury. This shift should reflect the addition of disinhibitory interventions before traditional strength training to improve neuromuscular capabilities. Pietrosimone and Saliba20 demonstrated that changes in voluntary quadriceps activation predicted 47% of the variance in the change in quadriceps strength after a 4-week therapeutic exercise intervention. Also, the magnitude of change in voluntary activation did not always parallel the magnitude of change in strength: a seemingly small increase in voluntary quadriceps activation could create a large change in strength.20
It should be noted that many of these interventions demonstrate an immediate ability to increase voluntary activation, suggesting that they may improve patient performance during single bouts of therapeutic exercise. Currently, it remains unknown how much of an increase in voluntary activation is necessary to see benefits in strength development and physical function. Therefore, small increases in voluntary quadriceps activation may be enough to enhance overall therapeutic outcomes.
This is the first systematic review to investigate the disinhibitory effects of a battery of interventions. However, the current research does have limitations. Voluntary muscle activation, as defined by Kent-Braun and Le Blanc,43 is dictated by the participant's ability to maximally recruit motor units, which can be measured by the superimposed-burst technique or the interpolated twitch technique. Both use an exogenous electrical stimulus applied to the quadriceps musculature during a voluntary contraction, but because of inherent differences between the techniques, the resultant values differ. Yet given the paucity of data regarding this topic, we believed it was important to include all measures of voluntary quadriceps activation. Another limitation is whether the dosage of each intervention was correct to effect voluntary quadriceps activation. Most of the studies focused only on the immediate response after intervention, which is imperative to advancing this research, but more expansive time intervals will provide a more thorough understanding of disinhibitory intervention capabilities. Although individual studies had homogeneous populations, the different studies had different populations, permitting comparisons among a vast array of joint injuries but creating difficulty in generalization over the entire population.
The current review provides a basic framework for research on the effectiveness of disinhibitory interventions. However, future authors should determine the interventions that provide the largest increases in voluntary quadriceps activation. Even though each intervention resulted in increased effect sizes for voluntary quadriceps activation, investigations are needed to identify the optimal dosages of each intervention. Disinhibitory interventions offer promising results for increasing voluntary quadriceps activation, yet a relationship between increased function and quality of life needs to be established. Disinhibitory interventions are not intended to substitute for traditional therapeutic exercise but rather to act as a supplement that promotes substantially greater rehabilitation gains. Although the data are limited, at this time, TENS appears to be the most effective disinhibitory intervention for immediate and sustained gains in voluntary quadriceps activation.
CONCLUSIONS
Various interventions have a wide range of effects on voluntary quadriceps activation. Currently, TENS demonstrates the strongest effects for increasing quadriceps activation. Large-scale studies investigating long-term effects are needed to determine the overall effects of these disinhibitory interventions.
REFERENCES
- 1.Torry MR, Decker MJ, Viola RW, O'Connor DD, Steadman JR. Intra-articular knee joint effusion induces quadriceps avoidance gait patterns. Clin Biomech (Bristol, Avon) 2000;15(3):147–159. doi: 10.1016/s0268-0033(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 2.Hurley MV. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am. 1999;25(2):283–298. doi: 10.1016/s0889-857x(05)70068-5. [DOI] [PubMed] [Google Scholar]
- 3.Palmieri-Smith RM, Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009;37(3):147–153. doi: 10.1097/JES.0b013e3181aa6669. [DOI] [PubMed] [Google Scholar]
- 4.Piva SR, Fitzgerald GK, Irrgang JJ, Bouzubar F, Starz TW. Get up and go test in patients with knee osteoarthritis. Arch Phys Med Rehabil. 2004;85(2):284–289. doi: 10.1016/j.apmr.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald GK, Piva SR, Irrgang JJ, Bouzubar F, Starz TW. Quadriceps activation failure as a moderator of the relationship between quadriceps strength and physical function in individuals with knee osteoarthritis. Arthritis Rheum. 2004;51(1):40–48. doi: 10.1002/art.20084. [DOI] [PubMed] [Google Scholar]
- 6.Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62(2):115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22(1):110–115. doi: 10.1016/S0736-0266(03)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty: the contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am. 2005;87(5):1047–1053. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suter E, Herzog W, Bray RC. Quadriceps inhibition following arthroscopy in patients with anterior knee pain. Clin Biomech (Bristol, Avon) 1998;13((4–5)):314–319. doi: 10.1016/s0268-0033(98)00098-9. [DOI] [PubMed] [Google Scholar]
- 10.Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45(1):87–97. doi: 10.4085/1062-6050-45.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopkins JT, Ingersoll CD. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9(2):135–159. [Google Scholar]
- 12.Palmieri RM, Tom JA, Edwards JE, et al. Arthrogenic muscle response induced by an experimental knee joint effusion is mediated by pre- and post-synaptic spinal mechanisms. J Electromyogr Kinesiol. 2004;14(6):631–640. doi: 10.1016/j.jelekin.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Palmieri RM, Weltman A, Edwards JE, et al. Pre-synaptic modulation of quadriceps arthrogenic muscle inhibition. Knee Surg Sports Traumatol Arthrosc. 2005;13(5):370–376. doi: 10.1007/s00167-004-0547-z. [DOI] [PubMed] [Google Scholar]
- 14.Heroux ME, Trenblay F. Corticomotor excitability associated with unilateral knee dysfunction secondary to anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):823–833. doi: 10.1007/s00167-006-0063-4. [DOI] [PubMed] [Google Scholar]
- 15.Pietrosimone BG, Hopkins JT, Ingersoll CD. The role of disinhibitory modalities in joint injury rehabilitation. Athl Ther Today. 2008;13(6):2–5. [Google Scholar]
- 16.Gibbons CE, Pietrosimone BG, Hart JM, Saliba SA, Ingersoll CD. Transcranial magnetic stimulation and volitional quadriceps activation. J Athl Train. 2010;45(6):570–579. doi: 10.4085/1062-6050-45.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum. 2010;40(3):250–266. doi: 10.1016/j.semarthrit.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Pietrosimone BG, Saliba SA, Hart JM, Hertel J, Kerrigan DC, Ingersoll CD. Effects of transcutaneous electrical nerve stimulation and therapeutic exercise on quadriceps activation in people with tibiofemoral osteoarthritis. J Orthop Sports Phys Ther. 2011;41(1):4–12. doi: 10.2519/jospt.2011.3447. [DOI] [PubMed] [Google Scholar]
- 19.Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2008;4(CD004376) doi: 10.1002/14651858.CD004376.pub2. doi: 10.1002/14651858.CD004376. [DOI] [PubMed] [Google Scholar]
- 20.Pietrosimone BG, Saliba SA. Changes in voluntary quadriceps activation predict changes in quadriceps strength after therapeutic exercise in patients with knee osteoarthritis. Knee. 2012;19(6):939–943. doi: 10.1016/j.knee.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Urbach D, Berth A, Awiszus F. Effect of transcranial magnetic stimulation on voluntary activation in patients with quadriceps weakness. Muscle Nerve. 2005;32(2):164–169. doi: 10.1002/mus.20353. [DOI] [PubMed] [Google Scholar]
- 23.Suter E, McMorland G, Herzog W, Bray R. Conservative lower back treatment reduces inhibition in knee extensor muscles: a randomized controlled trial. J Manipulative Physiol Ther. 2000;23(2):76–80. [PubMed] [Google Scholar]
- 24.Petterson SC, Mizner RL, Stevens JE, et al. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum. 2009;61(2):174–183. doi: 10.1002/art.24167. [DOI] [PubMed] [Google Scholar]
- 25.Urbach D, Awiszus F. Effects of transcranial magnetic stimulation on results of the twitch interpolation technique. Muscle Nerve. 2000;23(7):1125–1128. doi: 10.1002/1097-4598(200007)23:7<1125::aid-mus18>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 26.Drover JM, Forand DR, Herzog W. Influence of active release technique on quadriceps inhibition and strength: a pilot study. J Manipulative Physiol Ther. 2004;27(6):408–413. doi: 10.1016/j.jmpt.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Mintken PE, Carpenter KJ, Eckhoff D, Kohrt WM, Stevens JE. Early neuromuscular electrical stimulation to optimize quadriceps muscle function following total knee arthroplasty: a case report. J Orthop Sports Phys Ther. 2007;37(7):364–371. doi: 10.2519/jospt.2007.2541. [DOI] [PubMed] [Google Scholar]
- 28.Petterson S, Snyder-Mackler L. The use of neuromuscular electrical stimulation to improve activation deficits in a patient with chronic quadriceps strength impairments following total knee arthroplasty. J Orthop Sports Phys Ther. 2006;36(9):678–685. doi: 10.2519/jospt.2006.2305. [DOI] [PubMed] [Google Scholar]
- 29.Grindstaff TL, Hertel J, Beazell JR, et al. Lumbopelvic joint manipulation and quadriceps activation of people with patellofemoral pain syndrome. J Athl Train. 2012;47(1):24–31. doi: 10.4085/1062-6050-47.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmieri-Smith RM, Thomas AC, Karvonen-Gutierrez C, Sowers M. A clinical trial of neuromuscular electrical stimulation in improving quadriceps muscle strength and activation among women with mild and moderate osteoarthritis. Phys Ther. 2010;90(10):1441–1452. doi: 10.2522/ptj.20090330. [DOI] [PubMed] [Google Scholar]
- 31.Grindstaff TL, Hertel J, Beazell JR, Magrum EM, Ingersoll CD. Effects of lumbopelvic joint manipulation on quadriceps activation and strength in healthy individuals. Man Ther. 2009;14(4):415–420. doi: 10.1016/j.math.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Pietrosimone BG, Hart JM, Saliba SA, Hertel J, Ingersoll CD. Immediate effects of transcutaneous electrical nerve stimulation and focal knee joint cooling on quadriceps activation. Med Sci Sports Exerc. 2009;41(6):1175–1181. doi: 10.1249/MSS.0b013e3181982557. [DOI] [PubMed] [Google Scholar]
- 33.Pietrosimone BG, Ingersoll CD. Focal knee joint cooling increases the quadriceps central activation ratio. J Sports Sci. 2009;27(8):873–879. doi: 10.1080/02640410902929374. [DOI] [PubMed] [Google Scholar]
- 34.Stevens JE, Mizner RL, Snyder-Mackler L. Neuromuscular electrical stimulation for quadriceps muscle strengthening after bilateral total knee arthroplasty: a case series. J Orthop Sports Phys Ther. 2004;34(1):21–29. doi: 10.2519/jospt.2004.34.1.21. [DOI] [PubMed] [Google Scholar]
- 35.Suter E, McMorland G, Herzog W, Bray R. Decrease in quadriceps inhibition after sacroiliac joint manipulation in patients with anterior knee pain. J Manipulative Physiol Ther. 1999;22(3):149–153. doi: 10.1016/S0161-4754(99)70128-4. [DOI] [PubMed] [Google Scholar]
- 36.Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol. 1996;491((pt 1)):197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purves D, Augustine G, Fitzpatrick D, et al. Neuroscience. 2nd ed. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- 38.Currier DP, Mann R. Muscular strength development by electrical-stimulation in healthy-individuals. Phys Ther. 1983;63(6):915–921. doi: 10.1093/ptj/63.6.915. [DOI] [PubMed] [Google Scholar]
- 39.Snyder-Mackler L, Ladin Z, Schepsis AA, Young JC. Electrical-stimulation of the thigh muscles after reconstruction of the anterior cruciate ligament: effects of electrically elicited contraction of the quadriceps femoris and hamstring muscles on gait and on strength of the thigh muscles. J Bone Joint Surg Am. 1991;73(7):1025–1036. [PubMed] [Google Scholar]
- 40.Snyder-Mackler L, Delitto A, Bailey SL, Stralka SW. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament: a prospective, randomized clinical-trial of electrical-stimulation. J Bone Joint Surg Am. 1995;77(8):1166–1173. doi: 10.2106/00004623-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald GK, Piva SR, Irrgang JJ. A modified neuromuscular electrical stimulation protocol for quadriceps strength training following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2003;33(9):492–501. doi: 10.2519/jospt.2003.33.9.492. [DOI] [PubMed] [Google Scholar]
- 42.Hurley MV, Jones DW, Newham DJ. Arthrogenic quadriceps inhibition and rehabilitation of patients with extensive traumatic knee injuries. Clin Sci (Lond) 1994;86(3):305–310. doi: 10.1042/cs0860305. [DOI] [PubMed] [Google Scholar]
- 43.Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19(7):861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]