Abstract
Cancer stem cells have tumor-initiation and tumor-maintenance capabilities. Stem-like cells are present in colorectal adenomas, but their relationship to adenoma pathology and patient characteristics, including metachronous development of an additional adenoma (“recurrence”), have not been studied extensively. We evaluated the expression of aldehyde dehydrogenase isoform 1A1 (ALDH1A1), a putative stem cell marker, in baseline adenomas from the placebo arm of chemoprevention trial participants with colonoscopic follow-up. An exploratory set of 20 baseline adenomas was analyzed by ALDH1A1 immunohistochemistry with morphometry, and a replication set of 89 adenomas from 76 high-risk participants was evaluated by computerized image analysis. ALDH1A1 labeling indices (ALIs) were similar across patient characteristics and in advanced and non-advanced adenomas. There was a trend toward higher ALIs in adenomas occurring in the right than left colon (p=0.09). ALIs of synchronous adenomas were correlated (intraclass correlation coefficient 0.67). Participants in both sample sets who developed a metachronous adenoma had significantly higher ALIs in their baseline adenoma than participants who remained adenoma-free. In the replication set, the adjusted odds for metachronous adenoma increased 1.46 for each 10% increase in ALIs (p=0.03). A best-fit algorithm-based cut-point of 22.4% had specificity of 75.0% and positive predictive value of 70.0% for metachronous adenoma development. A larger population of ALDH1A1-expressing cells in an adenoma is associated with a higher risk for metachronous adenoma, independent of adenoma size or histopathology. If confirmed, ALDH1A1 has potential as a novel biomarker in risk assessment and as a potential stem-cell target for chemoprevention.
Keywords: Colorectal adenoma, stem cells, tumor markers, aldehyde dehydrogenase
Introduction
Colorectal adenoma is a well-known precursor to colorectal carcinoma, and removal is associated with a reduced incidence of colorectal cancer (1–6). Among patients who form an adenoma, risk of developing future metachronous colorectal neoplasia in another site in the large bowel, commonly termed “recurrence”, is based on the clinical-pathologic features of the patient and the presenting adenoma. Subsequent risk is higher in patients who present with clinical features of three or more adenomas or at least one adenoma that is large (≥ 1 centimeter) or has histopathologic features of villous architecture or high-grade dysplasia, termed an advanced adenoma (6–8).
While colorectal adenomas are relatively common in the general population, with a prevalence of 20–30% over age 50 years, most are small lesions with low-grade dysplasia, of which it is estimated that only approximately 5–10% would progress to cancer (6). As a result, yields from endoscopy-based short-interval surveillance for three to five years are dominated by the detection and removal of small, low-risk adenomas with little discernible clinical significance. These results challenge the cost-effective delivery of endoscopic follow up and the overall clinical value of screening and surveillance for prevention of colorectal cancer in the average-risk population (9).
While controversial, cancer cells with stem cell-like properties in solid tumors are thought to comprise a subpopulation with self-renewing and multi-potent differentiation properties that are functionally analogous to adult stem cells (10–12). Cancer stem cells are defined functionally by their ability to initiate tumors in xenografts, form spheroids in vitro, have tumor maintenance capabilities after chemotherapy, and express various markers in a broad spectrum of organ-specific tumors including colorectal carcinoma (13–21).
Stem-like cells have been reported among premalignant colorectal cells of patients (21–27) and in animal models of colorectal tumorigenesis (28, 29). However, in contrast to the large number of published studies on subpopulations of cells with tumor-initiating properties in cancers, there is a paucity of information about such cells in benign neoplasms such as colorectal adenoma, including their clinical-pathologic associations and clinical relevance.
Numerous expression biomarkers for identifying stem cells and cells with stem-like properties have been described. (10, 11, 13, 30, 31) These include the product of the aldehyde dehydrogenase isoform 1A1 (ALDH1A1) gene, a member of the ALDH superfamily on chromosome 9q21.13 (32–38). This superfamily of proteins catalyzes the irreversible oxidation of a range of endogenous and exogenous intracellular aldehydes to their corresponding carboxylic acids, and metabolizes aldehyde intermediates for synthesis from retinol toretinoic acid, a modulator of cell proliferation. These enzymes also metabolize folate; a micronutrient that is important in various cellular functions in which deficiency has been associated with risk of colorectal tumors (36). The enzymatic activity and expression of ALDH1A1 have been shown to mark subpopulations of colorectal epithelial cells with stem-like properties (10, 13, 20, 23, 39, 40). Mucosal epithelial cells as well as tumor cells isolated from patients with colitis (32) by use of antibody to ALDH1A1 form spheroids and xenografts, representing properties that are characteristic of stem cells. Thus, ALDH1 immunohistochemistry has been used extensively to evaluate stem-like cells in colorectal tumors (10, 13, 20, 23, 39, 40) as well as numerous other tumor types.
In this study, we hypothesized that stem-like cells in colorectal adenoma could be related to clinical-pathologic characteristics and the development of metachronous adenoma. We therefore characterized the population of ALDH1A1-expressing cells as an indicator of ‘stem-cell burden’ in baseline adenoma from an exploratory subset and a replication subset of placebo-treated participants with available tissues from two completed chemoprevention trials with protocol driven follow-up colonoscopy (41, 42).
Materials and Methods
Exploratory and replication study groups
Two sets of specimens were obtained from participants randomized to the placebo arms of the Wheat Bran Fiber Trial (42) or Ursodeoxycholic Acid Trial (41) at The University of Arizona for the prevention of metachronous colorectal adenomas. Characteristics of these exploratory and replication sample subsets are presented in Table 1. Age, gender, and adenoma clinical characteristics (size and location) were obtained by review of colonoscopy and pathology reports in medical records. Tubular, tubulovillous, or villous architecture and grade of dysplasia in routine histopathologic slides prepared from tissue blocks were determined by central review by gastrointestinal pathologists participating in the conduct of the trials.
Table 1.
Baseline characteristics of study participants with baseline adenoma specimens in the exploratory and replication sets
| Exploratory set (n = 20 subjects)1 | Replication set (n = 76 subjects)2 | |
|---|---|---|
| Age in years, mean ± SD | 66.0 ± 9.7 | 64.9 ± 9.3 |
| Male sex3 | 16 (80.0) | 48 (63.2) |
| Family history of colorectal cancer3 | 4 (20.0) | 15 (20.6) |
| Current smoker3 | 1 (5.00) | 6 (7.89) |
| Advanced adenoma3 | 12 (60.0) | 76 (100) |
| Large adenoma (≥ 1 cm) | 11 (55.0) | 66 (86.8) |
| High-grade dysplasia | 1 (5.00) | 10 (13.2) |
| Villous histology | 7 (35.0) | 51 (67.1) |
| Any proximal adenoma3 | 6 (30.0) | 34 (47.2) |
| Multiple (≥ 3) adenomas3 | 2 (10.0) | 24 (31.6) |
| Adenoma at follow-up3 | 10 (50.0) | 40 (52.6) |
Includes 20 subjects with colorectal adenomas
Includes only participants at high risk of metachronous adenoma on the basis of presentation with ≥ 3 adenomas and/or an advanced adenoma (≥ 1.0 cm or with villous histology or high-grade dysplasia).
Number and (%)
For the initial exploratory study sample, we identified 20 baseline tubular adenomas with low-grade dysplasia from a convenience sample of trial participants who had residual banked tissue blocks and known metachronous adenoma status at follow-up colonoscopy. Ten (50.0%) of these subjects with a baseline adenoma in the exploratory set had a metachronous adenoma at follow up. Eleven mucosal biopsy specimens from other trial participants were used as positive controls for ALDH1A1 immunohistochemistry (Figures 1A and 1B).
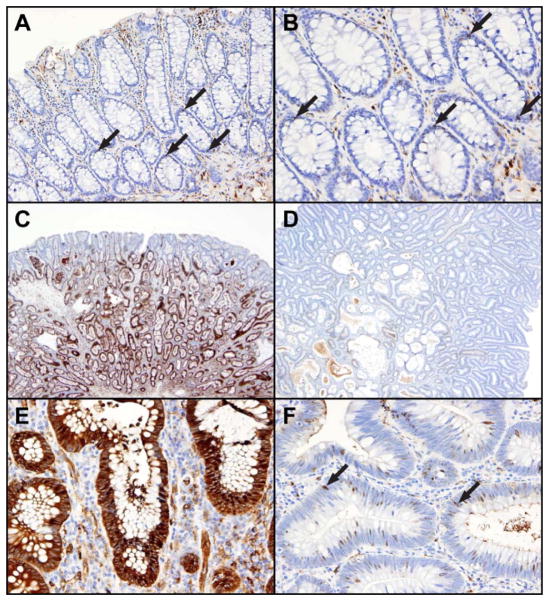
Figure 1. Examples of ALDH1A1 stem-like cell marker expression by immunohistochemistry in control colorectal mucosa and in baseline adenomas in relation to metachronous adenoma development (“recurrence”).
In non-neoplastic mucosa, the epithelial stem-like cells (arrows) are located near the bases of crypts (Panel A, with higher magnification in Panel B). In contrast, the stem-like cells in adenomas (Panels C through F) are distributed throughout the neoplastic glands, with smaller numbers near the luminal surface than in the deeper glands. An advanced adenoma with low-grade dysplasia but size greater than 1 centimeter in a participant who developed a metachronous adenoma (Panels C and E) has numerous ALDH1A1-positive stem-like cells, in contrast to an advanced adenoma with low-grade dysplasia that has only scattered stem-like cells (arrows) in a participant who was adenoma-free at follow-up (Panels D and F).
After completion of the analysis of the exploratory subset, 98 additional participants with at least one available inventoried baseline adenoma tissue block and recorded follow-up colonoscopy results were identified from the tissue database of the placebo-control arms of the trials. This second subset of participant samples was designated as the replication set. Immunohistochemistry for ALDH1A1 could not be performed on 28/124 (22.6%) inventoried tissue blocks from 22 participants (22.4%) because of inadequate residual adenoma tissue in their archived specimen. The resulting study set of 89 individual adenomas was derived from 76 subjects who presented with ≥ three adenomas and/or histopathological features of advanced adenoma (i.e. ≥ 1.0 cm or with villous histology or high-grade dysplasia) (38,40,41,43).
In total, 65 participants (85.5%) in the replication set had one baseline adenoma, and the other 11 (14.5%) had at least two synchronous baseline adenomas, with two of these 11 participants having a third baseline adenoma. These subjects with multiple adenomas permitted within-participant comparisons of adenoma ALDH1A1 levels. In addition, among the 76 subjects, four participants had two blocks available from the same adenoma, permitting a limited intra-adenoma comparison.
The clinical trial protocols, including retrieval and subsequent laboratory evaluation of specimens obtained at colonoscopy, were reviewed and approved by The University of Arizona Institutional Review Board. The Institutional Review Board at The University of Texas MD Anderson Cancer Center approved the laboratory studies protocol.
Immunohistochemistry for ALDH1A1 expression
Expression of the stem cell marker ALDH1A1 was evaluated by immunohistochemistry, as reported previously (43). The mouse monoclonal antibody (Catalog #611195, BD Biosciences, San Diego, CA) identified cells that formed spheroids in vitro and successful xenografts in vivo in a previous study (40). Manual immunohistochemistry was used for the exploratory set of cases. For evaluation of the larger replication set, the immunohistochemistry procedure was automated on an IntelliPATH FLX slide stainer (Biocare Medical, Concord, CA). Reagents, steps and conditions were identical to those used for the exploratory set.
Morphometric and image analysis of ALDH1A1 expression
Morphometry was used to enumerate ALDH1A1-positive stem-like cells in neoplastic epithelium of the adenomas of the exploratory set and in the crypt columns of control mucosa. Quantitation of ALDH1A1 expression based upon predominantly nuclear staining was performed in coded slides by a gastrointestinal pathologist, (A.N.B.) without access to information about the participant’s metachronous adenoma status. The topographical distributions of ALDH1A1-expressing epithelial cells in the neoplastic glands of the adenomas and in the control mucosal crypt axes of the immunohistochemistry controls were evaluated by counting cells in four specified regions: surface epithelium and the calculated lower, middle, and upper thirds of the adenoma glands and crypt columns. The total number of cells and the number with nuclear staining were enumerated in twenty microscopic fields at 40X magnification in each of the four topographic sites for each slide, and the ALDH1A1 labeling indices (ALIs) were calculated as percentage.
For the larger replication set of adenomas, ALIs were quantitated by computerized image analysis with the Aperio ScanScope XT automated image analyzer (Aperio Technologies, Inc., Vista, CA). Aperio Genie Pattern Recognition software was used to identify adenoma glands for evaluation, and the Aperio Genie nuclear v9.1 algorithm was used to create a custom expression classifier. Multiple sampled areas of each adenoma were digitally extracted to create a montage consisting of all acquired representative areas. The same steps were then repeated to identify stroma and other non-evaluated tissue areas with histologic defects that interfered with adenoma epithelial image analysis. The montage for each individual adenoma was saved in a custom Genie Training Macro that was tested to assess the accuracy of separating tumor from stroma and unwanted areas by comparison to histopathology. The results were deemed satisfactory when >90% mean sensitivity and specificity were obtained to represent the adenoma epithelium and unwanted areas.
Because ALDH1A1 is predominantly expressed in nuclei (44), the Aperio Genie nuclear v9.1 algorithm was used to create a custom ALDH1A1 classifier with color computer graphics (Supplementary Figure 1). A curvature threshold adjustment was made to de-cluster groups of closely apposed or overlapping nuclei. The original factory algorithm was also adjusted to increase the upper threshold setting from 230 to 250 in order to assure accurate detection of positive and negative regions representative of the light microscopic appearances. The final custom algorithm combined with the training macro was then used on all slides. The results were presented as a separate analysis of the adenoma in each slide with the percentage of positive regions in three levels of expression intensity. Because of the frequent random orientation of adenoma glands and the finding in the exploratory set that differences were apparent in all gland compartments, ALDH1A1-expressing regions across the glands in the entire immunohistochemistry tissue sections were quantitated.
Topographical evaluation of the upper regions of adenoma glands in the replication set was done in an exploratory analysis to provide ALDH1A1 labeling indices for the upper third of glands that was included in the evaluation of the exploratory set of adenomas. The oriented areas, when present, of each specimen that included both the surface epithelium and the muscularis mucosae and/or deep lamina propria visible in its whole slide image were demarcated for image analysis of the upper thirds of the glands.
Statistical analysis
Demographic and clinical characteristics of participants and pathologic characteristics of adenomas were analyzed. In the exploratory set, comparison of ALDH1A1 labeling indices (ALIs) across compartments within the same participant was performed using Wilcoxon signed rank tests. Comparisons in specimens from participants with and without a subsequent metachronous adenoma were performed using Wilcoxon rank sum tests. In the replication set, logistic regression with a likelihood ratio test was used to assess the statistical significance of the relationship between ALIs and subsequent adenoma detection at follow-up colonoscopy. Therefore, all participant-level analyses were performed using both the mean and the maximum ALI value for all adenomas of each participant. We also evaluated the relationship between ALIs and clinical-pathologic covariates using logistic regression, including characteristics of the advanced or non-advanced adenomas at baseline. In addition, we used a classification and regression tree (CART) analysis based on binary recursive partitioning to explore an optimal cutpoint for ALIs to maximize the reduction in impurity and best classify the subject for metachronous adenoma status at follow-up.
Results
Exploratory set: ALDH1A1 expression in baseline adenomas and subsequent metachronous adenoma status
For adenomas in the exploratory set (Figure 1C–F), ALDH1A labeling indices (ALIs) were higher overall (mean 31.7% for deep gland epithelium and 5.0% for surface epithelium) compared to non-neoplastic control mucosa (6.3% for lower crypt epithelium and 1.8% for surface epithelium; p=0.02). Independent of whether or not the baseline adenoma was advanced, the participants who developed a metachronous adenoma had significantly higher ALIs in their baseline adenoma when compared to participants who did not have an adenoma at follow-up (53.8% vs. 11.9%, p=.002, for deep glands; 34.5% vs. 6.7%, p=.002, for middle glands; 16.3% vs. 2.4%, p=.002, for upper glands; 8.2% vs. 2.1%, p=.03, for surface epithelium, Figure 2).
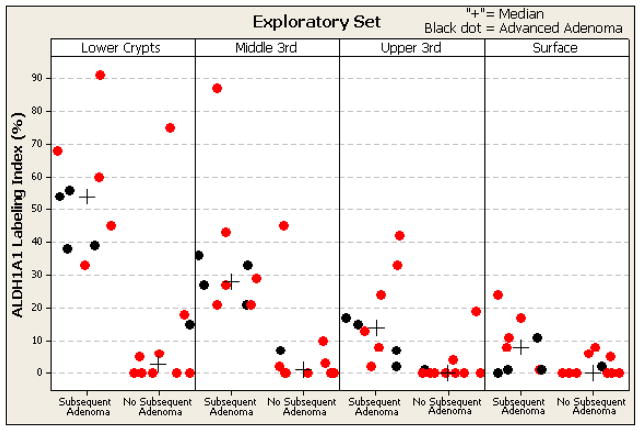
Figure 2. Topographical Expression of ALDH1A1 in 19 polyps in relation to metachronous adenoma development.
ALDH1A1 labeling index by manual immunohistochemistry and morphometric analysis is shown for each subject. The ALDH1A1 labeling index for each of the four topographic sites (lower third, middle third and upper third of crypts and surface epithelium) is shown for non-advanced adenomas (red dots) and advanced adenoma (black dots) grouped by development of a subsequent adenoma. The subjects with a metachronous adenoma have significantly higher median ALDH1 values (indicated by +): 53.8% vs. 11.9%, p=.002, for deep glands; 34.5% vs. 6.7%, p=.002, for middle glands; 16.3% vs. 2.4%, p=.002, for upper glands; 8.2% vs. 2.1%, p=.03, for surface epithelium.
Replication set: ALDH1A1 expression in baseline adenomas and characteristics of adenomas and participants
To assess the general reproducibility of the results obtained in the exploratory set, we evaluated ALIs in a second, larger set of specimens that were from a high-risk cohort (i.e. patients presenting with 3 or more adenomas or an adenoma with advanced features). ALIs were obtained in a final sample size of 89 individual baseline adenomas from 76 participants. ALDH1A1 expression was determined with automated immunohistochemistry and quantitated with computerized image analysis across each adenoma as contrasted with evaluation of topographical glandular regions, due to variability in the orientation of adenomas in the second set of tissue blocks. At the polyp level, 33 of the baseline adenomas were advanced, and 56 were non-advanced small tubular adenoma.
There were no significant associations between ALIs of the adenomas and participant age or gender (data not shown). We observed a non-significantly higher mean ALI among proximal adenomas compared to distal adenomas in the left colon and rectum (22.1% versus 16.0%, p=0.09). Of note, at the individual adenoma level there were no differences in ALIs between non-advanced and advanced adenomas (mean ALDH1A1 of 16.3% for non-advanced versus 18.5% for advanced, p = 0.45, Figure 3). High and low ALIs were found in both advanced and non-advanced adenoma. The ALDH1A1 LIs in the 11 participants with more than one adenoma (i.e. synchronous baseline adenomas) were similar in 9 participants and discordant in 2 (Figures 3 and 4 and Supplemental Figure 2), with an overall intraclass correlation coefficient of 0.67.
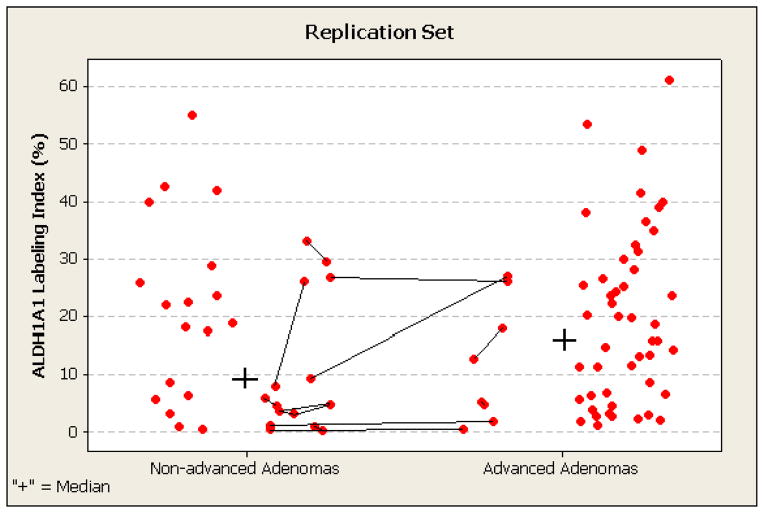
Figure 3. Percentage of ALDH1A1-expressing stem-like cells in 89 baseline non-advanced and advanced adenomas in replication set.
ALDH1A1 labeling index by automated immunohistochemistry and computerized image analysis is shown for each adenoma (lesion-level data). Multiple adenomas in individual participants are connected by lines and have similar intra-subject indices in 9 of 11 subjects (intra-class correlation coefficient 0.67). The median ALDH1A1 labeling indices for the non-advanced and advanced adenoma subgroups are indicated by the plus signs, and are not statistically significantly different: 9.3% for non-advanced adenomas and 15.9% for advanced adenomas.
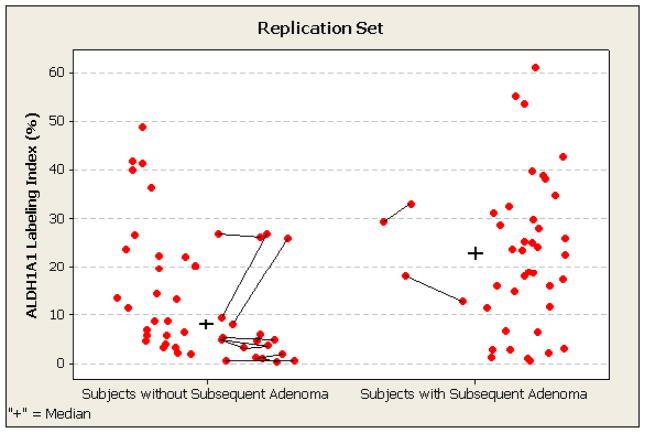
Figure 4. Percentage of ALDH1A1-expressing stem-like cells in 89 baseline adenomas of subjects without and with metatchronous adenoma at follow up colonoscopy (recurrence) in replication set.
ALDH1A1 labeling index by automated immunohistochemistry and computerized image analysis is shown for each adenoma (lesion-level data). Multiple adenomas in individual participants are connected by lines and have similar indices in 9 of 11 subjects (intra-class correlation coefficient 0.67). The median ALDH1A1 labeling indices for the non-advanced and advanced adenoma subgroups (group level data) are indicated by the plus signs. The subjects with a metachronous adenoma have significantly higher values: 23.0% as compared to 8.6% for subjects who remained adenoma-free (p <0.05).
Replication set: ALDH1A1 expression in baseline adenomas and subsequent metachronous adenoma status
As was found in the exploratory set using topographical determination of ALIs, subjects who developed an adenoma at follow-up had a significantly higher mean ALI (Figure 4) than those who did not develop an adenoma at follow-up (22.5% versus 15.0%, p=0.03). When ALDH1A1 expression was treated as a continuous variable, the odds ratio for metachronous adenoma development was 1.44 [95% confidence interval (CI) 1.03–2.02; p=0.03] for a 10% increase in ALI. The increased odds for adenoma development with ALDH1A1 levels remained significant even after adjustment for age and sex and inclusion of an indicator variable for advanced status of the adenoma at baseline (adjusted OR=1.46, 95% CI 1.04–2.06, p=0.03). When we restricted our analyses to those subjects in whom the adenoma could be confidently oriented to assess the upper third of the glands (n=50 of 76), we found that the ALDH1A1 labeling across the whole adenomas was highly correlated with the upper glands. The correlation at the participant-level between [mean] whole and oriented ALDH1A1 values was 0.88. For metachronous adenoma risk, the magnitude and direction of the adjusted association remained (adjusted OR= 1.95, p=0.26).
Replication set: ALDH1A1 expression in baseline adenomas as a predictor of metachronous adenoma development
In an effort to identify a potentially useful ALI cut-point that could be used for further investigation as a tissue biomarker for predicting metachronous adenoma risk in high-risk adenoma patients, we performed a data-driven classification and regression tree (CART) analysis. The splitting algorithm identified an ALI cutoff point of ≥22.4% ALDH1A1-expressing cells as an optimal threshold for classifying risk of metachronous adenoma in our sample (Table 2). The frequency of metachronous adenomas was 75.0% for ALIs above this CART cut-point, and 39.6% for ALIs below the cut-point (OR = 4.58, 95% CI 1.63–12.86; p value=0.004). By contrast, the metachronous adenoma rate was 63.6% for ALIs at or above the mean ALI (18.9%), and 44.2% for expression below the mean (OR = 2.21, 95% CI 0.87–5.60; p value=0.11). Results similar to these with use of the means were obtained for the median ALI values.
Table 2.
ALDH1A1 labeling index and odds of metachronous adenoma in replication set
| ALDH1A1 LI Cutpoint % | Metachronous rate ≥ cutpoint % | Metachronous rate < cutpoint % | * Odds ratio (95% CI) | p value | |
|---|---|---|---|---|---|
| Mean | 18.9 | 63.6 | 44.2 | 2.21 (0.87–5.60) | 0.11 |
| Median | 16.5 | 63.2 | 42.1 | 2.36 (0.94–5.92) | 0.11 |
| CART | 22.4 | 75 | 39.6 | 4.58 (1.63–12.86) | 0.004 |
referent group is metachronous rate < cut-point
We also assessed the sensitivity and specificity of ALI for the development of a metachronous adenoma using a cutoff of 0.5 for the predicted probability. Incorporating ALI increased both the sensitivity and specificity over age, male gender and advanced status of adenomas, especially when ALDH1A1 expression was treated as a binary variable based upon the CART cut-point of ≥22.4% or <22.4% (Table 3). For the final model with the binary ALI cut-point for ALDH1A1 expression, sensitivity for development of an adenoma at follow-up among the high-risk cohort was 52.5% with specificity of 75.0%, and positive and negative predictive values of 70.0% and 58.7%, respectively (Table 3).
Table 3.
Sensitivity and specificity of ALDH1A1 labeling index and clinicopathologic risk factors for metachronous adenoma in replication set
| Model | % correct | Sensitivity | Specificity | False positive | False negative |
|---|---|---|---|---|---|
| Age, gender, any advanced lesion | 22.4 | 42.5 | 0 | 67.9 | 100 |
| plus ALDH1A1 (continuous) | 56.6 | 57.5 | 55.6 | 41 | 45.9 |
| plus ALDH1A1 (≥ median) | 57.9 | 60 | 55.6 | 40.0 | 44.4 |
| plus ALDH1A1 (≥ mean) | 50 | 52.5 | 47.2 | 47.5 | 52.8 |
| plus ALDH1A1 (≥22.4%) | 63.2 | 52.5 | 75.0 | 30 | 41.3 |
Discussion
We report for the first time that the fraction of ALDH1A1-expressing putative stem-like cells in colorectal adenoma epithelium is correlated among synchronous adenomas in individual patients, and that the presence of a larger subpopulation of these adenoma stem-like cells is associated with increased risk for the patient to have a metachronous adenoma at another location in the large bowel at follow-up. Of note, the association between percentage of ALDH1A1-expressing adenoma cells and risk of a metachronous adenoma is independent of both the adenoma and clinical characteristics previously reported to be associated with increased risk (i.e. villous architecture, high-grade dysplasia, and size of the baseline adenoma, and age and male sex of the study subjects). Our findings raise the possibility that a larger stem-like cell subpopulation in a baseline adenoma may serve as a quantifiable biomarker for the propensity of a patient to develop a subsequent colorectal neoplasm. If corroborated, this biomarker could aid in modifying follow-up recommendations for patients who have an adenoma found and removed.
We confirmed previous reports of approximately 6.0% ALDH1-positive cells in non-neoplastic crypts and approximately 30% in adenomatous glands, representing a 5- to 6-fold increase in ALDH1 expression with progression to premalignancy (23). We found similar mean ALDH1A1 labeling percentages: 6.3% in non-neoplastic crypts of participants who all had at least one adenoma, and 31.7% in adenomatous glands. Although our sample size is small, we found for the first time the presence of highly correlated intra-adenoma, intra-participant ALDH1A1 labeling indices in four participants who had at least two blocks from the same adenoma, indicating intra-tumoral homogeneity within different regions of each tumor. Of even greater interest, we also observed strong inter-adenoma/intra-participant correlation in 11 patients with multiple adenomas (Figure 3 and 4 and Supplementary Figure 2). These results support previous findings suggesting that cancer stem cells and adenoma stem-like cells reside in a niche microenvironment (30, 45, 46). The mechanisms leading to the observed correlated deregulation of the stem cell subpopulations and their overgrowth in synchronous adenomas remain to be explained. This concept of a niche effect is consistent with previous work on the concept of field defects in carcinogenesis and supports the occurrence of a generalized intra-patient predisposition to a stem-like cell niche that participates in adenoma development.
The fraction of ALDH1A1-expressing adenoma cells was variable among subjects, with some having large fractions and others small fractions. This observation challenges the role of well-established genetic pathways such as the APC/Wnt pathway that is almost universally involved in colorectal tumorigenesis (54) in neoplastic progression of the non-advanced tubular adenoma to the advanced adenoma via the stem-like cell subpopulation. Regulation of stem cells by several pathways in the niche have been demonstrated, including Wnt, bone morphogenetic protein (BMP), Ets2, Notch, Twist, and Snail signaling that involve stromal-epithelial interactions (13, 25, 47–51). Further, work on the genetic and epigenetic factors regulating the stem cell compartment of adenomas, and the lifestyle and exposures that influence them (62), may offer new opportunities for preventive intervention.
Our study has a number of strengths, including the use of two independent sample sets (an exploratory and replication set) as well as the availability of baseline adenomas from patients from our placebo arms of trials with follow-up colonoscopy at similar intervals (average 3 years). Extensive participant- and adenoma-level characterization with central pathology review and adjudication were done and are additional strengths. We used an established immunohistochemistry method to evaluate the ALDH1A1-positive stem-like cells in their topographical tissue setting. The commercially available ALDH1A1 antibody used in this study has been extensively evaluated and shown in a previous study to identify a colorectal stem-like cell population that had the capacity to form spheroids in vitro and to successfully form tumor xenografts in vivo (40). The topographical distribution and numbers of ALDH1A1-positive stem-like cells in non-neoplastic colorectal mucosa were similar to those in previous reports (23, 34, 39), in contrast to results found with other putative stem cell markers such as CD44 and CD133 (23).
Limitations include our relatively small sample sets and the focus in the replication sample set on high-risk participants for whom adequate pathology material was available as convenience samples in the specimen repository. Further, while our data are suggestive of higher ALDH1A1 LIs in proximal adenomas, which have a higher propensity for ‘recurrence’, proximal adenomas commonly occur along with distal adenomas. These were often collected in the same specimen vial after polypectomy, thus limiting our ability to identify a larger, purely proximal population of adenomas.
In conclusion, our study shows for the first time that ALDH1A1 expression in a baseline adenoma is an independent risk factor for development of a metachronous adenoma. Our findings suggest that the assessment of ALDH1A1 expression is sufficiently promising to warrant further investigation as a biomarker to aid in risk assessment. Efforts in low-risk populations, where surveillance recommendations remain controversial (i.e. patients with one or two small tubular adenomas), are particularly warranted. Our observation of strong intra- and inter-adenoma correlation of ALDH1A1 expression at the patient level adds support to the concept of an adenoma stem cell ‘niche’ as a putative risk site for neoplasia. Corroboration of our findings in a larger sample to obtain precise estimates of the association for clinical utility combined with mechanistic studies may offer new areas for development of colorectal cancer prevention efforts.
Supplementary Material
Acknowledgments
We thank Sendurai Mani, PhD, for helpful discussions and advice, Sherita Meyer-Gauen for developing the automated ALDH1A1 immunohistochemistry, and Betsy Wertheim for generation of the summary data for Table 1.
Grant Support
This work was supported by the Colon Cancer Prevention Program Project Grant (National Cancer Institute/National institute of Health PO1 CA41108) and by the shared resources of The University of Texas MD Anderson Cancer Center Support Grant (P30 CA016672) and the Arizona Cancer Center Support Grant (P30 CA023074). SRH was supported by the Frederick F. Becker Distinguished University Chair in Cancer Research from The University of Texas.
Footnotes
There are no conflicts of interest the authors wish to disclose.
References
- 1.Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner H, Chang-Claude J, Rickert A, Seiler CM, Hoffmeister M. Risk of colorectal cancer after detection and removal of adenomas at colonoscopy: population-based case-control study. J Clin Oncol. 2012;30:2969–76. doi: 10.1200/JCO.2011.41.3377. [DOI] [PubMed] [Google Scholar]
- 3.Manser CN, Bachmann LM, Brunner J, Hunold F, Bauerfeind P, Marbet UA. Colonoscopy screening markedly reduces the occurrence of colon carcinomas and carcinoma-related death: a closed cohort study. Gastrointest Endosc. 2012;76:110–7. doi: 10.1016/j.gie.2012.02.040. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–85. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Winawer SJ, Zauber AG, O’Brien MJ, Ho MN, Gottlieb L, Sternberg SS, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993;328:901–6. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 6.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326:658–62. doi: 10.1056/NEJM199203053261002. [DOI] [PubMed] [Google Scholar]
- 7.Martinez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–41. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imperiale TF, Glowinski EA, Lin-Cooper C, Larkin GN, Rogge JD, Ransohoff DF. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med. 2008;359:1218–24. doi: 10.1056/NEJMoa0803597. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Obata D, Chinzei R, Yoshida S, Sanuki T, Morita Y, et al. Surveillance after colorectal polypectomy; comparison between Japan and U.S. Kobe J Med Sci. 2011;56:E204–13. [PubMed] [Google Scholar]
- 10.Alison MR, Lim SM, Nicholson LJ. Cancer stem cells: problems for therapy? J Pathol. 2011;223:147–61. doi: 10.1002/path.2793. [DOI] [PubMed] [Google Scholar]
- 11.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–9. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 12.Pietras A. Cancer stem cells in tumor heterogeneity. Adv Cancer Res. 2011;112:255–81. doi: 10.1016/B978-0-12-387688-1.00009-0. [DOI] [PubMed] [Google Scholar]
- 13.Vaiopoulos AG, Kostakis ID, Koutsilieris M, Papavassiliou AG. Colorectal cancer stem cells. Stem Cells. 2012;30:363–71. doi: 10.1002/stem.1031. [DOI] [PubMed] [Google Scholar]
- 14.Anderson EC, Hessman C, Levin TG, Monroe MM, Wong MH. The Role of Colorectal Cancer Stem Cells in Metastatic Disease and Therapeutic Response. Cancers (Basel) 2011;3:319–39. doi: 10.3390/cancers3010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewi DL, Ishii H, Kano Y, Nishikawa S, Haraguchi N, Sakai D, et al. Cancer stem cell theory in gastrointestinal malignancies: recent progress and upcoming challenges. J Gastroenterol. 2011;46:1145–57. doi: 10.1007/s00535-011-0442-6. [DOI] [PubMed] [Google Scholar]
- 16.Wilson BJ, Schatton T, Frank MH, Frank NY. Colorectal Cancer Stem Cells: Biology and Therapeutic Implications. Curr Colorectal Cancer Rep. 2011;7:128–35. doi: 10.1007/s11888-011-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhawan P, Ahmad R, Srivastava AS, Singh AB. Cancer stem cells and colorectal cancer: an overview. Curr Top Med Chem. 2011;11:1592–8. doi: 10.2174/156802611796117694. [DOI] [PubMed] [Google Scholar]
- 18.Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138:2151–62. doi: 10.1053/j.gastro.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 19.Ricci-Vitiani L, Fabrizi E, Palio E, De Maria R. Colon cancer stem cells. J Mol Med (Berl) 2009;87:1097–104. doi: 10.1007/s00109-009-0518-4. [DOI] [PubMed] [Google Scholar]
- 20.Huang EH, Wicha MS. Colon cancer stem cells: implications for prevention and therapy. Trends Mol Med. 2008;14:503–9. doi: 10.1016/j.molmed.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel BB, Yu Y, Du J, Levi E, Phillip PA, Majumdar AP. Age-related increase in colorectal cancer stem cells in macroscopically normal mucosa of patients with adenomas: a risk factor for colon cancer. Biochem Biophys Res Commun. 2009;378:344–7. doi: 10.1016/j.bbrc.2008.10.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker L, Huang Q, Mashimo H. Immunostaining of Lgr5, an intestinal stem cell marker, in normal and premalignant human gastrointestinal tissue. Scientific World Journal. 2008;8:1168–76. doi: 10.1100/tsw.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–9. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 25.Fan XS, Wu HY, Yu HP, Zhou Q, Zhang YF, Huang Q. Expression of Lgr5 in human colorectal carcinogenesis and its potential correlation with beta-catenin. Int J Colorectal Dis. 2010;25:583–90. doi: 10.1007/s00384-010-0903-z. [DOI] [PubMed] [Google Scholar]
- 26.Fan LF, Dong WG, Jiang CQ, Xia D, Liao F, Yu QF. Expression of putative stem cell genes Musashi-1 and beta1-integrin in human colorectal adenomas and adenocarcinomas. Int J Colorectal Dis. 2010;25:17–23. doi: 10.1007/s00384-009-0791-2. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi H, Ishii H, Nishida N, Takemasa I, Mizushima T, Ikeda M, et al. Significance of Lgr5(+ve) cancer stem cells in the colon and rectum. Ann Surg Oncol. 2011;18:1166–74. doi: 10.1245/s10434-010-1373-9. [DOI] [PubMed] [Google Scholar]
- 28.Lewis A, Segditsas S, Deheragoda M, Pollard P, Jeffery R, Nye E, et al. Severe polyposis in Apc(1322T) mice is associated with submaximal Wnt signalling and increased expression of the stem cell marker Lgr5. Gut. 2010;59:1680–6. doi: 10.1136/gut.2009.193680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arena V, Caredda E, Cufino V, Stigliano E, Scaldaferri F, Gasbarrini A, et al. Differential CD133 expression pattern during mouse colon tumorigenesis. Anticancer Res. 2011;31:4273–5. [PubMed] [Google Scholar]
- 30.Gires O. Lessons from common markers of tumor-initiating cells in solid cancers. Cell Mol Life Sci. 2011;68:4009–22. doi: 10.1007/s00018-011-0772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders MA, Majumdar AP. Colon cancer stem cells: implications in carcinogenesis. Front Biosci. 2011;16:1651–62. doi: 10.2741/3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindahl R. Aldehyde dehydrogenases and their role in carcinogenesis. Crit Rev Biochem Mol Biol. 1992;27:283–335. doi: 10.3109/10409239209082565. [DOI] [PubMed] [Google Scholar]
- 33.Douville J, Beaulieu R, Balicki D. ALDH1 as a functional marker of cancer stem and progenitor cells. Stem Cells Dev. 2009;18:17–25. doi: 10.1089/scd.2008.0055. [DOI] [PubMed] [Google Scholar]
- 34.Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010;5:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alison MR, Guppy NJ, Lim SM, Nicholson LJ. Finding cancer stem cells: are aldehyde dehydrogenases fit for purpose? J Pathol. 2010;222:335–44. doi: 10.1002/path.2772. [DOI] [PubMed] [Google Scholar]
- 36.Jackson B, Brocker C, Thompson DC, Black W, Vasiliou K, Nebert DW, et al. Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Hum Genomics. 2011;5:283–303. doi: 10.1186/1479-7364-5-4-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcato P, Dean CA, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 2011;10:1378–84. doi: 10.4161/cc.10.9.15486. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Orlicky DJ, Matsumoto A, Singh S, Thompson DC, Vasiliou V. Aldehyde dehydrogenase 1B1 (ALDH1B1) is a potential biomarker for human colon cancer. Biochem Biophys Res Commun. 2011;405:173–9. doi: 10.1016/j.bbrc.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boman BM, Fields JZ, Bonham-Carter O, Runquist OA. Computer modeling implicates stem cell overproduction in colon cancer initiation. Cancer Res. 2001;61:8408–11. [PubMed] [Google Scholar]
- 40.Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, Scott EW, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69:8208–15. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alberts DS, Martinez ME, Hess LM, Einspahr JG, Green SB, Bhattacharyya AK, et al. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst. 2005;97:846–53. doi: 10.1093/jnci/dji144. [DOI] [PubMed] [Google Scholar]
- 42.Alberts DS, Martinez ME, Roe DJ, Guillen-Rodriguez JM, Marshall JR, van Leeuwen JB, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med. 2000;342:1156–62. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 43.Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, et al. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. 2011;6:e20636. doi: 10.1371/journal.pone.0020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcato P, Dean CA, Pan D, Araslanova R, Gillis M, Joshi M, et al. Aldehyde Dehydrogenase Activity of Breast Cancer Stem Cells Is Primarily Due To Isoform ALDH1A3 and Its Expression Is Predictive of Metastasis. STEM CELLS. 2011;29:32–45. doi: 10.1002/stem.563. [DOI] [PubMed] [Google Scholar]
- 45.Cabarcas SM, Mathews LA, Farrar WL. The cancer stem cell niche--there goes the neighborhood? Int J Cancer. 2011;129:2315–27. doi: 10.1002/ijc.26312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borovski T, De Sousa EMF, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–9. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 47.Farrall AL, Riemer P, Leushacke M, Sreekumar A, Grimm C, Herrmann BG, et al. Wnt and BMP signals control intestinal adenoma cell fates. Int J Cancer. 2012 doi: 10.1002/ijc.27500. [DOI] [PubMed] [Google Scholar]
- 48.Munera J, Cecena G, Jedlicka P, Wankell M, Oshima RG. Ets2 regulates colonic stem cells and sensitivity to tumorigenesis. Stem Cells. 2011;29:430–9. doi: 10.1002/stem.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valdes-Mora F, Gomez del Pulgar T, Bandres E, Cejas P, Ramirez de Molina A, Perez-Palacios R, et al. TWIST1 overexpression is associated with nodal invasion and male sex in primary colorectal cancer. Ann Surg Oncol. 2009;16:78–87. doi: 10.1245/s10434-008-0166-x. [DOI] [PubMed] [Google Scholar]
- 50.Hwang WL, Yang MH, Tsai ML, Lan HY, Su SH, Chang SC, et al. SNAIL regulates interleukin-8 expression, stem cell-like activity, and tumorigenicity of human colorectal carcinoma cells. Gastroenterology. 2011;141:279–91. 91e1–5. doi: 10.1053/j.gastro.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Roy S, Majumdar AP. Signaling in colon cancer stem cells. J Mol Signal. 2012;7:11. doi: 10.1186/1750-2187-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.