Abstract
Early pubertal timing places girls at elevated risk for a breadth of negative outcomes, including involvement in delinquent behavior. While previous developmental research has emphasized the unique social challenges faced by early maturing girls, this relation is complicated by genetic influences for both delinquent behavior and pubertal timing, which are seldom controlled for in existing research. The current study uses genetically informed data on 924 female-female twin and sibling pairs drawn from the National Longitudinal Study of Adolescent Health to (1) disentangle biological versus environmental mechanisms for the effects of early pubertal timing and (2) test for gene-environment interactions. Results indicate that early pubertal timing influences girls’ delinquency through a complex interplay between biological risk and environmental experiences. Genes related to earlier age at menarche and higher perceived development significantly predict increased involvement in both non-violent and violent delinquency. Moreover, after accounting for this genetic association between pubertal timing and delinquency, the impact of non-shared environmental influences on delinquency are significantly moderated by pubertal timing, such that the non-shared environment is most important among early maturing girls. This interaction effect is particularly evident for non-violent delinquency. Overall, results suggest early maturing girls are vulnerable to an interaction between genetic and environmental risks for delinquent behavior.
Adolescent delinquency provokes a multitude of clinical, family, social, and economic concerns. Delinquent behavior problems and comorbid externalizing disorders comprise over 50% of mental health referrals for children and adolescents. In addition, adolescents under the age of 18 account for over 15% of arrests in the United States (Federal Bureau of Investigation, 2004), and a history of juvenile delinquent problems is one of the strongest predictors of criminal behavior in adulthood (e.g., Maughan & Rutter, 2001). The preponderance of research on the causes of antisocial behavior has focused on males, likely because antisocial behavior is, on average, “less common, less serious, and less persistent” in females (Fontaine, Carbonneau, Vitaro, Barker, & Tremblay, 2009, p. 363). However, the incidence of antisocial behavior in females increases markedly in adolescence, and is associated with a breadth of negative developmental outcomes, including low educational attainment, early childbearing, substance use disorders, family violence, and criminal offending (Fontaine et al., 1999; Odgers et al., 2008). In fact, antisocial behavior increases, relative to childhood levels, more dramatically in girls than in boys during adolescence, resulting in the narrowest sex ratio seen across the lifespan (Silverthorn & Frick, 1999). Thus, the transition from childhood to adolescence constitutes a point of heightened vulnerability for the emergence of antisocial behavior problems in girls.
At the core of the transition from childhood to adolescence is puberty. Puberty has long been hypothesized to be a psychological “stumbling block” for girls’ transition into adulthood, and individual differences in the pubertal transition may hold explanatory power for understanding why some girls develop antisocial behavior problems in adolescence. In particular, pubertal timing (an earlier timing of physical maturation relative to same-age peers) reliably predicts heightened risk for involvement in delinquent activity during adolescence (Caspi & Moffitt, 1991; Caspi, Lynam, Moffitt, & Silva, 1993; Flannery, Rowe, & Gulley, 1993; Haynie, 2003; Magnusson, Stattin, & Allen, 1985; for reviews see Mendle, Turkheimer, & Emery, 2007; Negriff & Susman, 2011). This association persists both for petty norm violations, such as shoplifting, vandalism, and truancy (Kaltiala-Heino et al., 2003; Flannery et al., 2003; Storvall & Wichstrom, 2002), and for aggressive behaviors, such as bullying or physically harming another person (Kaltiala-Heino et al., 2003; Lynne, Graber, Nichols, Brooks-Gunn, & Botvin, 2007; Haynie, 2003). Notably, these associations are not limited to adolescence: girls who experienced an earlier timing of puberty are more likely to have records of adult criminal behavior (Stattin & Magnusson, 1990). Early pubertal timing is moreover related to diagnostic antecedents of adolescent delinquency, particularly symptoms of Conduct Disorder and Oppositional Defiant Disorder (Ge, Brody, Conger, & Simons, 2006; Susman, et al., 2007). Thus, results from over two decades of research have converged on a consensus that early pubertal timing increases girls’ risk for delinquent behavior.
Yet despite this large body of evidence, pinpointing the specific mechanisms underlying the association between pubertal timing and delinquency has been difficult. Pubertal development involves a complex suite of changes across biological (e.g., hormonal, somatic, and neural changes), psychological (e.g., cognition, affect, and self-perception), and social (e.g., peer, parent, and romantic relationships) domains. Moreover, the timing of puberty is itself influenced by a host of genetic and environmental factors. Consequently, a major conceptual challenge for research on early pubertal development is identifying which aspect of early pubertal timing is most pathogenic with regards to antisocial behavior. In the current paper, we describe two streams of research on this topic. The first stream focuses on genetic influences on pubertal timing and the implications of these genetically-influenced differences for adolescent brain development; the second focuses on the social and environmental challenges faced by early maturing girls. We then describe how twin and sibling designs can be used to discriminate among competing hypotheses regarding the pathways linking pubertal timing and adolescent delinquency.
Potential Biological Mechanisms for the Effects of Early Pubertal Timing
In order to specify more precisely the pathways by which early pubertal maturation confers risk for antisocial behavior, it is necessary to acknowledge the importance of genetic influences on girls’ pubertal timing. While it is not yet fully known why some girls begin puberty earlier than others, girls’ “innate developmental clock” appears to be under strong genetic control. Behavior genetic studies report correlations for age at menarche are consistently higher among MZ than among DZ twins (Doughty & Rodgers, 2000; Meyer, Eaves, Heath & Martin, 1991; Rowe, 2000; Treloar & Martin, 1990), with approximately 61-68% of the variance in menarche accounted for by genetic effects. Likewise, estimates for the heritability of secondary sexual characteristics, such as breast development or skin changes, range from .50 (Ge, Natsuaki, Neiderhiser, & Reiss, 2007) to .88 (Mustanski, Viken, Kaprio, Pulkkinen, & Rose, 2004). Molecular genetic studies have also identified specific genes involved in pubertal timing. Most notably, the GPR54 gene has been shown to influence the initial secretion of gonadotropin releasing hormone (GnRH), which is necessary for gonadal maturation (Seminara et al., 2003). However, understanding of the genetic basis of pubertal timing is incomplete. As Sisk and Foster (2004) describe, “Identification of master regulatory genes directing the unique maturational components of the first and most important transition to fertility remains an unsolved part of the puberty mystery” (p. 1042).
In addition, it is well-established that antisocial behavior is heritable (e.g., Arsenault et al., 2003; D’Onofrio et al., 2007; Scourfield, Van den Bree, Martin, & McGuffin, 2004; Slutske et al., 1997; Young et al., 2002; for reviews see Miles & Carey, 1997; Raine, 2002; Rhee & Walman, 2002; Rowe, 2001). Although there have been few empirical tests of whether genes related to early pubertal timing overlap with those for delinquency (Mendle et al., 2007), common genetic influences have been implicated in the association between delinquency and fertility-relevant outcomes strongly correlated with pubertal timing (Udry, 1979), including age of first sexual intercourse (Harden, Mendle, Hill, Turkheimer, & Emery, 2008), risky sexual behavior (Verweij, Zeitsch, Bailey, & Martin, 2009), and adolescent childbearing (Harden et al., 2007). In addition, shorter alleles of the X-linked androgen receptor (AR) gene have been associated with aggression and impulsivity in males and with earlier ages of physical maturation in females (Comings et al., 2002).
Emerging research on adolescent brain development has suggested a novel explanation for why genetically-influenced differences in pubertal timing may be important for girls’ delinquency. In addition to a cascade of endocrine events that culminate in reproductive maturity, puberty also involves a cascade of neural changes, a “second period of structural reorganization and plasticity in the brain” (Blakemore, Burnett, & Dahl, 2010). One key change initiated by the hormonal events at puberty is the remodeling of neural circuits involved in reward-motivated behavior, particularly in the striatum, nucleus accumbens and in dopaminergic pathways to the prefrontal cortex (Blakemore et al., 2010; Forbes & Dahl, 2010; Kuhn et al., 2010). Female pubertal hormones (most notably, estradiol and progesterone) have significant effects on the anatomy of dopamine systems relevant forreward-motivated behavior (Kuhn et al., 2010). Moreover, pubertal development, independent of chronological age, has been found to be associated with changes in activity in the striatum and medial and ventrolateral prefrontal cortex in response to monetary rewards and social threats (Forbes, et al., in press; Forbes, et al., 2010). This is congruent with findings that pubertal development and pubertal hormones, independent of age, are linked to changes in the personality trait of sensation-seeking, which has been conceptually linked to responsiveness to rewards and novelty (Martin, Kelly, Rayens, Brogli, Brenzel, Smith, & Omar, 2002; Martin, Logan, Leukefeld, Milich, Omar, & Clayton, 2001; Steinberg, Albert, Cauffman, Banich, Graham, Woolard, 2008; Zuckerman, Buchsbaum, & Murphy, 1980).
Because the timing of pubertal development is strongly influenced by genes, early maturing girls can thus be thought of as having genetic propensities for early neurobiological change. Moreover, for early maturing girls, these puberty-linked neurobiological changes are asynchronouswith age-based neurobiological changes in cortical structures that underlie behavioral inhibition and effortful control. As articulated by the dual systems model of adolescent brain development (Casey, Galvan, & Getz, 2007; Somerville, Jones, & Casey, 2010; Steinberg, 2008), this “maturity gap” between the development of subcortical versus cortical brain regions results in an elevated propensity for adolescents – particularly early maturing adolescents – to engage in delinquent behavior, and in risk-taking behavior more generally.
The Social Challenges of Early Pubertal Timing
In contrast, the prevailing view in the developmental literature emphasizes the unique environmental dilemmas posed by early pubertal timing. The most widely accepted theoretical explanation for the relation between pubertal timing and delinquency, as well as a breadth of other psychosocial problems more common among girls with early pubertal timing, is the maturation disparity hypothesis (reviewed in Ge & Natsuaki, 2009). This theory stresses that a girl’s changing physical appearance during puberty creates new social experiences that may promote delinquent behavior, including increased autonomy from parents, increased romantic and sexual attention from males, and increased affiliation with older peer groups (Caspi et al., 1993; Ge, Brody, Conger, & Simmons, 2002). Early maturers are believed to be particularly vulnerable to the psychosocial risks posed by these new experiences, because they are forced to navigate the changing social landscape with fewer emotional or cognitive resources than girls who reach the same developmental milestones at a later chronological age. Moreover, theories specific to adolescent delinquency, most notably Moffitt's (1993) taxonomy of adolescent-limited delinquency, emphasize the lack of rights and privileges granted by adults to physically mature adolescents. Caught between a mature physical appearance and an immature social status, adolescents are believed to engage in rebellious activities designed to demonstrate independence (i.e., running away from home, lying to parents, truancy), imitate adult actions (sexual activity, drinking), or showcase lack of concern for adult-enforced social rules (larceny, property damage; Moffitt, 1993; Moffitt et al., 1996, 2001).
In addition to experiencing heightened environmental stress during the pubertal transition, early maturing girls are also more likely to have experienced prior environmental stress in early childhood. In particular, early childhood stressors such as father absence (Ellis, 2004); child maltreatment (Bergevin, Bukowski, & Karavasilis, 2003; Turner, Runz, & Galambos, 1999; Wise, Palmer, Rothman, & Rosenberg, 2009); harsh parenting (Belsky et al., 2007), and poverty (Obeidallah et al., 2000); are all correlated with a comparatively precocious timing of development. While these associations have been interpreted in light of Belsky, Steinberg, and Draper (1991)'s evolutionary hypothesis, it is important to note that this line of research has been strongly criticized by behavioral geneticists for interpreting correlations between parent characteristics and child outcomes as due to purely environmental mechanisms, without considering the role of genetic transmission from parent to child (Rowe, 2002). In our previous genetically-informed research on this topic, we have presented evidence that early family structure (father absence and step-fathering) are associated with accelerated pubertal and sexual development via genetic transmission rather than an environmental effect (Mendle et al., 2006, 2009). Nevertheless, regardless of their causal status, adverse home environments are certainly correlated with early pubertal development, and these early environmental stressors may partially account for the elevated rates of antisocial behavior problems seen in early maturing girls.
Integrating Biological and Social Risk: Gene-Environment Interaction
Early pubertal timing is not only associated with increased exposure to environmental risks for delinquency, but early maturing girls may also be more sensitive to environmental risks. In support of this, social context has been shown to be a critical determinant of resiliency versus vulnerability for early developers. For example, Caspi, Lynam, Moffitt, and Silva (1993) found that early maturing girls were at elevated risk for delinquency only if they attended mixed-sex schools versus same-sex schools. They attributed this interaction effect to greater exposure to male peers and male romantic partners. Likewise, Natsuaki, Biehl, & Ge (2009) found that engagement in a romantic relationship was associated with greater psychological distress for early maturers rather than their peers. Finally, pubertal timing has been found to interact with characteristics of the parent-child relationship and with neighborhood characteristics, with early maturing girls showing the highest involvement in delinquent behavior when maternal nurturance and parental monitoring were low (Mrug et al., 2008) and when concentrated neighborhood disadvantage was high (Obeidallah, Brennan, Brooks-Gunn, & Earls, 2004).
Considering the biological and environmental risks faced by early maturing girls, it is possible that early pubertal timing may increase delinquent behavior via a heightened sensitivity to environmental context, aka gene x environment interaction (GxE). Only one previous study has tested this hypothesis. Burt et al. (2007) found compelling evidence for G x E interaction in the association between timing of menarche and conduct disorder symptoms: Among girls with an age of menarche of 11 years or earlier, only 8% of the variance in conduct disorder symptoms could be attributed to genes and the remaining 92% was attributable to environmental influences. In contrast, for girls who experienced an average menarcheal timing (12-13 years), the majority of the variance in conduct disorder symptoms (67%) was attributable to genes and only 33% attributable to the environment. These findings are consistent with more general observations from the behavior genetics literature that heritability is not static; rather, genetic propensities may be suppressed or augmented in certain social circumstances (e.g., Harden, Hill, Emery, & Turkheimer, 2008; Harden, Turkheimer, & Loehlin, 2007).
Sensitivity to the unique environmental challenges of puberty may be particularly pertinent for non-violent, rule-breaking forms of delinquent behavior (such as property crime). In contrast, violent or aggressive delinquency differs behaviorally, developmentally, and etiologically from rule-breaking (Achenbach, 1991, 2001; Barker et al., 2009; Eley, Lichtenstein, & Stevenson, 1999; Loeber, Burke, & Pardini, 2009; Moffitt, 1993; Tackett, Krueger, Sawyer, & Graetz, 2003; Tuvblad, Eley, & Lichtenstein, 2005). Adolescents with histories of violent behavior show poor executive functioning, verbal processing, and neuropsychological impairment (Barker et al., 2007; Déry, Toupin, Pauzé, Mercier, & Fortin 1999), while results from neuroimaging studies confirm that adolescents with high levels of violent behavior display reduced activation in the frontal and temporal cortices, compared to normal controls, when watching pain inflicted on another person (Decety, Michalska, Akitsuki, & Lahey, 2009). Similar decrements have been observed in adults with histories of physical aggression (e.g., Siever, 2008; Volkow et al., 1995). There deficits are believed to be rooted in heritable predispositions (Moffitt,1993), and a broad array of candidate genes have been identified as playing a role in violent behavior (Brunner et al., 1993; Davridge et al., 20004; Flory et al., 2007; Meyer-Lindenburg et al., 2006; Mik et al., 2007). In contrast, non-violent forms of delinquency are developmentally transient (Stanger et al., 1997), show less genetic stability (Eley, Lichtenstein, & Moffitt, 2003; Burt & Neiderhiser, 2009) and are more strongly influenced by environmental factors (Burt, 2009).
Measurement of Pubertal Timing
Lastly, the relative importance of biological versus environmental pathways for the relation between pubertal timing and delinquent behavior may depend, in part, on how the construct of pubertal timing is measured and operationalized. Typically, assessing pubertal stage using a physical examination by a trained physician or nurse has been considered the “gold standard” of objective measurement; however, physical exams are infrequently used, particularly in large-scale epidemiological research. Adolescent self-report of age at menarche is a far more common method for measuring pubertal timing, because of its relative ease of use. Test-retest reliability coefficients for self-report of menarche have been found to be good (.61-.81; Dorn, Dahl, Woodward, & Biro, 2006). Moreover, adults’ retrospective reports of menarche are quite accurate as compared to childhood medical records, even after spans of up to 40 years (Bean, Leeper, Wallace, Sherman, & Jagger, 1979; Casey et al., 1991).
A third strategy, which was developed as an alternative to physical exam, is to query adolescents’ regarding changes in breast size and other secondary sex characteristics (perceived development; e.g., Biehl, Natsuaki, & Ge, 2009; Natsuaki, Biehl, & Ge, 2009; Graber, Lewinsohn, Seeley, & Brooks-Gunn, 1997; Graber, Seeley, Brooks-Gunn, & Lewinsohn, 2004; Wichstrom, 2000). Measures of perceived pubertal development have only modest agreement with more objective measures of pubertal timing, such as menarcheal age or Tanner stages as determined by physical exam (Dorn et al., 2006). Nevertheless, girls’ perceptions of themselves as physically mature, regardless of the accuracy of those perceptions, may be salient for their risk for engaging in delinquent behavior. In particular, girls who perceive themselves as more advanced in their pubertal development may also perceive a wider “maturity gap” until they gain full adult status (Moffitt, 1993), which may provoke additional delinquent behavior. A limited number of studies have evaluated the relative impact of objectively early maturation versus girls’ perceptions of themselves as early maturers (Brooks-Gunn and Warren 1985; Brooks-Gunn, Attie, Burrow, Rosso, & Warren, 1989; Michael & Eccles, 2003; Rierdan et al., 1988). These studies have consistently found that girls who define themselves as early maturers -- even if their development is objectively on-time or even late – are more likely to exhibitadverse developmental outcomes. Thus, both “objective” early pubertal timing (e.g., menarche) and girls’ “subjective” perceptions of early pubertal development may result in increased antisocial behavior, but no previous research has examined both objective and perceived pubertal timing while also attempting to disentangle biological versus social processes.
Goals of the Current Study
Collectively, previous research indicates that, while the relation between pubertal timing and girls’ delinquent behavior is well-established, the underlying mechanisms remain to be elucidated. The present study uses a behavioral genetic approach to this question. We utilize two different measures of pubertal timing, perceived development and menarcheal age, to predict both nonviolent and violent delinquency in a sample of sister dyads from the National Longitudinal Study of Adolescent Health (Add Health). The utility of the behavioral genetic approach is two-fold. First, comparisons between biological relatives allow us to discriminate between genetic and environmental pathways of risk: If Twin A matures earlier than Twin B, does she also show higher levels of delinquent behavior? A significant within-twin pair association, which controls for all genetic factors shared by twins, would indicate that environmental differences (rather than genetic differences) in pubertal timing are important pathways of risk for delinquency. Second, recent developments in statistical methods for twin data allow us to test hypothesis about gene-by-environment interaction: Are environmental or genetic influences on delinquency stronger among early maturing girls?
Method
Participants
Data are drawn from the National Longitudinal Study of Adolescent Health (Add Health, Udry, 2003), a study of health behaviors in a nationally representative study of adolescents. The Add Health study used stratified random sampling of U.S. high schools (79% of targeted schools agreed to participate). From the participating schools, 90,118 students completed a confidential in-school survey during then 1994-1995 academic year. School rosters were then used to randomly selected a sample of over 20,000 adolescents (10,480 females; 10,264 males) to complete an comprehensive, 90-minute, in-home interview between April and December 1995. Participants ranged in age from 11 to 21 years (M = 16 years, 25th percentile = 14 years, 75th = 17 years). There have been three follow-up interviews with the Add Health participants: Wave II in 1996, Wave III in August 2001-2002, and Wave IV in 2007-2008.
The Add Health study deliberately oversampled sibling pairs, even if one member of the pair did not attend a high school in the original probability sample. The current study only uses female-female sibling pairs, thus our sub-sample includes 1848 females from 924 sibling pairs: 145 monozygotic (MZ) twin pairs, 116 dizygotic (DZ) twin pairs, 369 full sibling pairs (FS), 117 half- sibling pairs (HS), 112 pairs of cousins raised in the same household (CO), and 65 non-biologically related pairs (e.g., step-siblings or adoptive siblings; NR). Twin zygosity was determined primarily on the basis of self-report and responses to four questionnaire items concerning similarity of appearance and frequency of being confused for one's twin. Similar questionnaires have been utilized widely in twin research and have been repeatedly cross-validated with zygosity determinations based on DNA (e.g., Loehlin & Nichols, 1976; Spitz et al., 1996). Race/ethnicity were classified as either White (N=984, 53.2%), African-American (N=493, 26.7%), Hispanic (N=245, 13.3%), or Other (including Asian-American and Native-American, N=126, 6.8%). The mean age was 16.12 years (SD=1.67 years).
Measures
Pubertal timing
. In the in-home interview for both waves I and II, participants were asked whether they had ever had a menstrual period, and if so, which month and year it first occurred. At Wave III, participants were asked “how old were you when you got your period for the first time?” From these waves of data, age at menarche was constructed as follows: If the participant reported menarche occurring before Wave I, the Wave I report of age at menarche was used (86.8% of participants). If the participant reported menarche occurring between Waves I and II, the Wave II report of age at menarche was used (8.2% of participants). Lastly, if participants reported getting menarche between Waves II and III, the Wave III report was used (2.7%). Menarche data was missing for 23 individuals (1.2% of the sample). The mean age of menarche in the sample was 12.23 years (SD =1.42, Range: 7.0 years – 19.0 years). African-American girls, on average, experienced menarche at a significantly younger age (M=12.01, SD=1.43, P < .01; based on a mixed effects model that accounted for clustering of girls within sibling pairs) than White girls (M=12.34, SD=1.44). Hispanic girls also trended towards younger mean ages at menarche (M=12.14, SD=1.37), although this difference was not significant (P=.08) in the sibling pairs subsample.
In addition, at Wave 1, participants’ perceptions of their level of pubertal development were assessed using two items about breast size [1=”My breasts are about the same size as when I was in grade school” to 5 = “My breasts are a whole lot bigger than when I was in grade school; they are as developed as a grown woman's breasts”] and body curviness [1 = “My body is about as curvy as when I was in grade school” to 5 = “My body is a whole lot more curvy then when I was in grade school”]. Higher scores on these items thus represent a higher perceived pubertal status; to calculate a measure of subjective pubertal timing, we calculated the deviation of each participant's score from the mean level of development reported by adolescents of the same age and standardized this deviation score (M=0, SD=1). Thus, higher scores reflect more advanced perceived development relative to other adolescents of the same age.
To evaluate the association between pubertal timing as measured by age at menarche and girls' perceived pubertal development, we divided the sample into early (11-14), middle (15-16), and late (17-21) adolescens. For each age group, perceived development was only modestly correlated with age at menarche, and the magnitude of this association was consistent across development (early adolescents: r = -.23, 95% CI = -.31, -.14; middle adolescents: r = -.18 (95% CI = -.25, -.11); late adolescents: r = -.17, 95% CI = -.25, -.09).
Non-violent and violent delinquency
Non-violent delinquency was measured using 11 items from the Wave I interview which asked how often in the last 12 months adolescents had engaged in various forms of theft (e.g., stole something worth more than $50, shoplifted), property crime (e.g., painted graffiti, deliberately damaged property), and rule-breaking (e.g., lied to parents, ran away from home). Responses were on a four-point scale [0=Never, 1=One or two times, 2=Three or four times, 3=Five or more times]. Violent delinquency was measured using 7 items from the Wave I interview which asked how often in the last 12 months adolescents had engaged in various violent or aggressive behaviors (e.g., seriously injured another person, was in a group fight, shot or stabbed someone). Following Guo, Roettger, and Cai (2008), responses to violent delinquency items were also on a 0-3 scale, except for two items (shooting or stabbing someone; pulling a knife or gun on someone). These items were scored as 0=Never and 3=One or more times. Both delinquency scales showed good internal reliability (alpha = .92 for non-violent and .77 for violent). We calculated sum scores for both delinquency scales (M non-violent = 3.27, SD = 4.13; M violent = 2.29, SD = 3.39) and then standardized them (M=0, SD=1) by age in years, so that higher scores can be interpreted as more delinquent behavior relative to other same-aged adolescents. Age at menarche was significantly and negatively correlated with both non-violent delinquency (r = -.09, 95% CI = -.18, -.05) and violent delinquency (r = -.11; 95% CI = -.15, -.02). Perceived development was also significantly correlated with both forms of delinquency (non-violent: r = 0.16; 95% CI = .10, .22; violent: r = .11, 95% CI = .04, .17).
Sibling correlations for perceived development, age at menarche, violent, and non-violent delinquency, by type of sibling pair, are summarized Table 1.
Table 1.
Sibling Pair Correlations and Zero-order Correlations among Variables.
| Non-Violent Delinquency | Violent Delinquency | Age at Menarche | Perceived Development | |
|---|---|---|---|---|
| Sibling Pair Correlations | ||||
| MZ Twins | .46 (N=140) | .54 (N=140) | .61 (N=139) | .38 (N=137) |
| DZ Twins | .44 (N=113) | .38 (N=113) | .31 (N=111) | .29 (N=109) |
| Full Siblings | .30 (N=365) | .46 (N=365) | .29 (N=362) | .17 (N=355) |
| Half Siblings | .35 (N=109) | .30 (N=110) | .18 (N=109) | .08 (N=109) |
| Cousins | .26 (N=65) | .22 (N=65) | .22 (N=65) | .04 (N=64) |
| Non-Biological Siblings | .20 (N=111) | .08 (N=111) | .08 (N=110) | .04 (N=107) |
Note. Sample sizes refer to number of pairs. Significant correlations (P < .05) are in bold font.
Analytic Plan
Data were analyzed using structural equation modeling in Mplus (Muthen & Muthen, 1998-2010), using Full Information Maximum Likelihood (FIML) to account for missing data. Absolute model fit was assessed using the CLI and the RMSEA. Nested models were compared using differences in log-likelihood, which are distributed as chi-square.
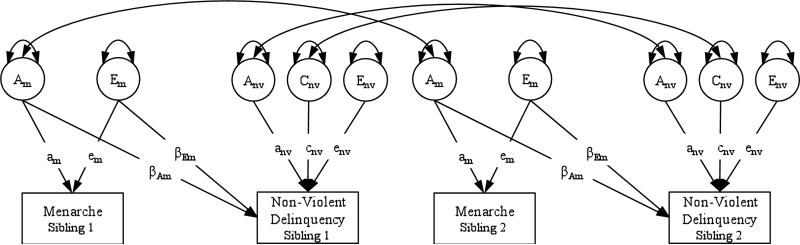
Data were analyzed in two steps. First, we fit bivariate twin models that tested whether the associations between pubertal timing, as measured by age at menarche and perceived development, and violent and non-violent delinquency were due to environmental versus genetic pathways. An example of this model, for non-violent delinquency, is shown in Figure 1. The twin model (see Neale & Cardon, 1992 for full details) typically decomposes variation in a given phenotype into three latent factors: variance due to additive genes (A), variance due to environmental influences that make twins and siblings similar to each other, aka, the shared environment (C), and variance due to environmental influences that make twins and siblings different from each other, aka, the non-shared environment, plus measurement error (E). The correlation between the additive genetic factors for the first and second sibling in each pair are fixed according to genetic theory: 1.0 for MZ twins, 0.5 for DZ twins and full siblings, 0.25 for half siblings, 0.125 for cousins, and 0 for non-biologically related siblings.
Figure 1.
Bivariate twin model of age at menarche and non-violent delinquency.
Previous analyses of the AddHealth data, which combined menarched and perceieved development as indicators of a single construct, found that shared environmental influences on pubertal timing are negligible (Ge et al., 2007). Our re-analysis of the data found that shared environmental influences on menarche were non-significant and could be dropped from the model without loss of fit (ACE model: χ2=25.41 (df = 26; P = .50); AE model: χ2 =26.98 (df=27; P = .47); δχ2 = 1.57, Δdf = 1, P = .21). In contrast, a model without additive genetic influences on menarche did fit significantly worse (CE model: χ2=59.14 (df=27, P<.01); Δχ2 = 33.7, Δdf = 1, P < .01). Similarly, shared environmental influences on perceived development were also non-significant and could be dropped from the model without loss of fit (ACE model: χ2=25.95 (df = 26; P = .47); AE model: χ2 =26.32 (df=27; P = .50); Δχ2 = .37, Δdf = 1, P = .54), while a model without additive genetic influences on menarche did fit significantly worse (CE model: χ2 =35.16 (df=27; P = .13); Δχ2 = 9.21, Δdf = 1, P < .001) Consequently, the models in the current paper only include genetic and non-shared environmental influences on each measure of pubertal timing.
In the first multivariate model, delinquency was regressed on the A and E factors of each measure of pubertal timing (parameters labeled bA and bE). The regression on A tests whether higher involvement in delinquent behavior is predicted by genes related to pubertal timing. In contrast, the regression on E essentially compares within sibling pairs: Does the twin who has earlier pubertal timing than her co-twin also demonstrate higher delinquency?
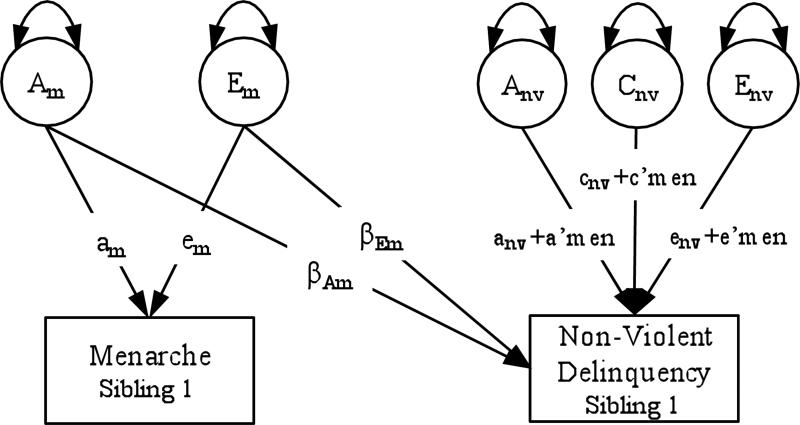
Second, we fit bivariate interaction models that tested whether, after controlling for the genetic and environmental associations between pubertal timing and delinquency, early pubertal timing significantly moderated the residual genetic and environmental variance in delinquency. An example of this model, for menarche and non-violent delinquency, is illustrated in Figure 2 (only one twin per pair shown). This model is identical in form to the bivariate twin model, described previously, except that the paths from the A, C, and E components of delinquency are moderated by pubertal timing (either age at menarche or perceived development). A significant interaction effect on path from the E component of delinquency would indicate that girls with early pubertal timing are more sensitive to environmental influences.
Figure 2.
Bivariate interaction model of age at menarche and non-violent delinquency.
Results
Is the Association between Early Pubertal Timing and Higher Delinquency Due to Genetic or Environmental Pathways?
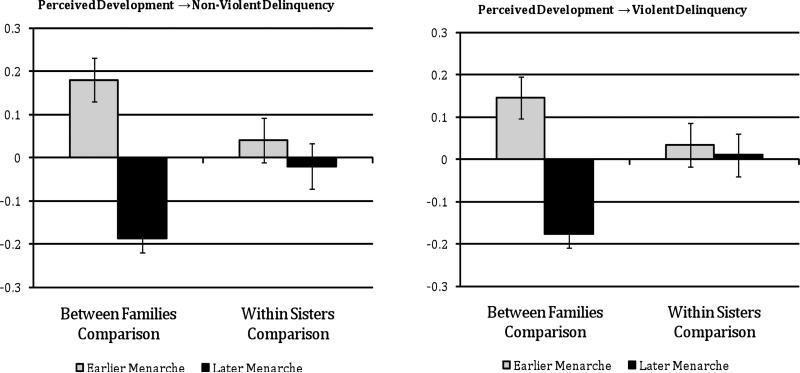
Means comparisons
In order to illustrate the association between perceived development and delinquency, we first divided the sample into “Higher” versus “Lower” perceived pubertal development (based on a mean split), and calculated the mean levels of non-violent and violent delinquency for each group. These mean comparisons are shown in Figure 3. The first set of bars in each plot (labeled ‘Between Families Comparison’) shows the mean levels of delinquency for adolescents from sibling pairs where both adolescents had higher perceived development versus adolescents from sibling pairs where both adolescents had lower perceived development. These results are consistent with the extant literature on pubertal timing: On average, adolescents who perceive themselves to be more mature, relative to their peers’ self-perceptions, show higher levels of both violent and non-violent delinquency. The second set of bars in each plot (labeled “Within Family Comparison”) shows the mean levels of delinquency for siblings who are discordant for perceived development. In contrast to the mean difference evident in the between-families comparison, siblings who differ in their perceived pubertal development do not appreciably differ in their involvement in violent or non-violent delinquency. Because there is no shared environmental variance, this suggests that genetic factors shared by siblings may account for the association between perceived development and delinquency.
Figure 3.
Between- and within-family means comparisons for girls with higher versus lower perceived development.
Note. “Higher” versus “lower” perceived development categorized using a mean split.
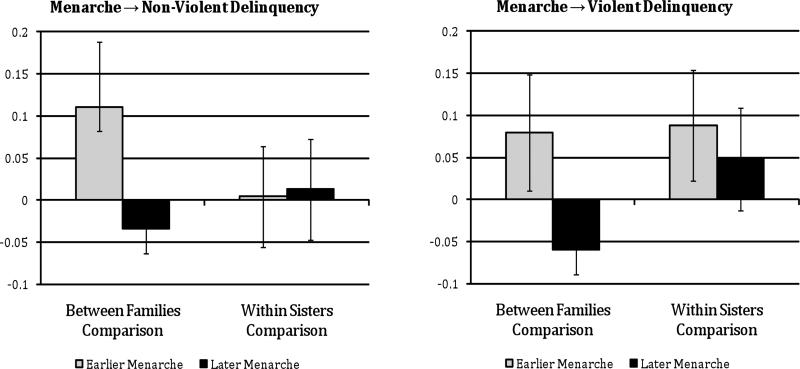
Next, we divided the sample into “Earlier Age at Menarche” (less than 12 years old) and “Later Age at Menarche” (12 years old or older) groups1, and calculated a similar set of between-family and within-family comparisons (illustrated in Figure 4). Again, results from the between-family comparison were consistent with previous research on pubertal timing and delinquency: Girls with an earlier age at menarche demonstrated, on average, higher levels of delinquency. However, this association was sharply attenuated when comparing within sibling pairs who were discordant for earlier versus later age at menarche.
Figure 4.
Between-and within-family means comparisons for girls with earlier versus later age at menarche.
Note. “Earlier” age at menarche < 12 years old; “Later” age at menarche ≥ 12 years old.
Twin models
Results from the bivariate twin models are summarized in Table 2. Overall, the fit of the bivariate models was fair to good (RMSEAs < 0.07). Genetic influences accounted for approximately 41% of the variance in perceived development. Genetic influences for more advanced perceived development, in turn, significantly predicted higher levels of both non-violent (bA = .237) and violent delinquency (bA = .174). Similarly, genetic influences accounted for 61% of the variance in menarcheal age, and genes related to earlier age at menarche significantly predicted higher levels of non-violent delinquency (bA = -.109), but not violent delinquency. After controlling for these genetic associations, the environmental paths between perceived development and delinquency, and between age at menarche and delinquency, were not significant. Overall, results from the bivariate twin models suggest that biological differences in pubertal timing – rather than environmental mechanisms – are responsible for the main effects of pubertal timing on delinquency.
Table 2.
Parameters from Bivariate Twin Models of Pubertal Timing and Delinquency.
| Model | ||||
|---|---|---|---|---|
| Perceived → Non-Violent | Menarche → Non-Violent | Perceived → Violent | Menarche → Violent | |
| Indices of Model Fit | ||||
| χ2(df P) | 111.73 (73, .002) | 105.80 (73,.007) | 118.78 (73, <.001) | 125.04 (73,.001) |
| RMSEA | .059 | .054 | .064 | .068 |
| Variance in Pubertal Timing | ||||
| Var (A) | 1.33 (.20)* | 1.24 (.10)* | 1.33 (.20)* | 1.24 (.10)* |
| Var (E) | 1.93 (.18)* | 0.78 (.08)* | 1.93 (.18)* | 0.78 (.08)* |
| Regression Parameters | ||||
| Genetic path (bA) | .237 (.063)* | −.109 (.043)* | .174 (.059)* | −.036 (.041) |
| Environmental path (bE) | −.037 (.033) | .049 (.054) | −.045 (.030) | −.017 (.048) |
| Variance in Delinquency | ||||
| Var (A) | .278 (.098)* | .323 (.091)* | .373 (.076)* | .409 (.071)* |
| Var (C) | .164 (.053)* | .181 (.053)* | .188 (.048)* | .193 (.048)* |
| Var (E) | .472 (.051)* | .473 (.052)* | .386 (.039)* | .389 (.039)* |
Note. Standard errors are in parentheses.
Parameter significantly different from zero at P < .05.
Does Early Pubertal Timing Result in Increased Sensitivity to Environmental Risks?
Results from the best-fitting interaction models are summarized in Table 3. Across all models, a consistent pattern emerged for non-shared environmental influences to be stronger for girls with earlier pubertal timing. For models using perceived development, the e’ parameters were significant and positive (.110 for non-violent delinquency and .065 for violent delinquency), indicating that girls who rated their pubertal development as advanced relative to their peers were more sensitive to the non-shared environment. Similarly, for models using age at menarche, the e’ parameters were significant and negative (-.093 for non-violent delinquency and -.100 for violent delinquency), indicating that girls with earlier menarche were more sensitive to the non-shared environment.
Table 3.
Unstandardized Parameter Estimates from Best-Fitting Interaction Models of Pubertal Timing and Delinquency.
| Perceived → Non-Violent | Menarche → Non-Violent | Perceived → Violent | Menarche → Violent | |
|---|---|---|---|---|
| Full Model Fit (-2LL) | 11734.15 | 11181.84 | 11693.26 | 11138.5 |
| Regression Parameters | ||||
| Genetic path (bA) | .232 (.065)* | −.093 (.043) | .187 (.061)* | −.018 (.039) |
| Environmental path (bE) | −.041 (.034) | .036 (.054) | −.055 (.031) | −.026 (.044) |
| Variance in Delinauencv | ||||
| Main effect of genes (a0) | .380 (.160)* | −1.140 (.864)† | .565 (.071)* | 1.352 (.382)* |
| Gene x puberty interaction (a') | −.082 (.030)* | 0.124 (.056)* | .024 (.026) | −.063 (.030)* |
| Main effect of shared environment (c0) | .455 (.074)* | 1.877 (.600)* | .443 (.055)* | −.946 (.334)* |
| Shared env. x puberty interaction (c') | .057 (.030) | −.115 (.054)* | [0] ‡ | .115 (.025)* |
| Main effect of non-shared environment (e0) | .713 (.036) | 1.863 (.226)* | .636 (.032)* | 1.846 (.172)* |
| Non-shared env. x puberty interaction (e') | .110 (.016)* | − .093 (.016)* | .065 (.019)* | − .100 (.013)* |
Parameter significantly different from zero at P < .05.
Variance in delinquency due to genes is calculated as (a0 + a'menarche)2, thus within the observed range of menarcheal age (±2 SDs from mean ~9 to 14 years), the genetic variance is positive.
Nested model comparisons indicated that this interaction parameter could be fixed to zero without significant loss of model fit.
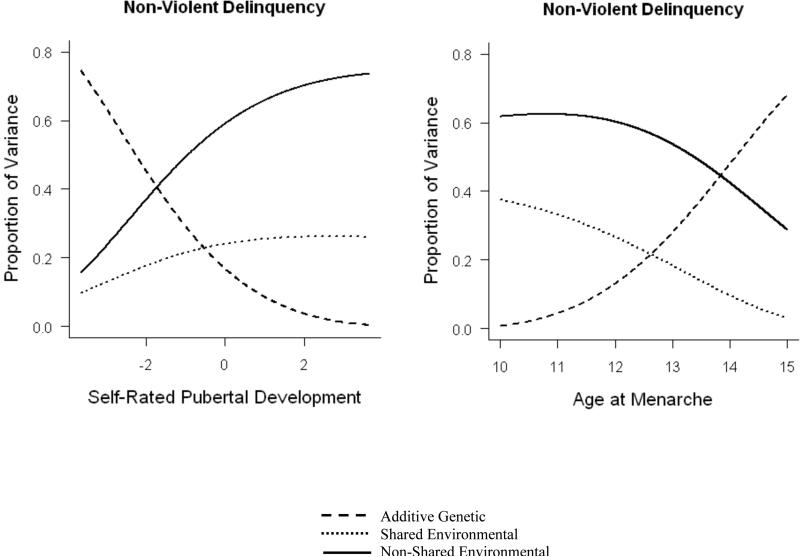
For models of non-violent delinquency, there were significant interactions between pubertal timing and genes, such that genetic effects were less influential for girls with higher perceived development (a’ = -.082) and earlier ages at menarche (a’ = .124). In addition, there was a significant interaction between age at menarche and the shared environmental component of non-violent delinquency, with higher shared environmental influences in girls with an early age at menarche (c’ = -.115). A similar interaction with the shared environment was evident for perceived development (c’ = .057); although this parameter was not significantly different than zero, nested model comparisons indicated that this interaction could not be dropped from the mode without a significant decrease in model fit (Δχ2 = 251.96, P < .001). Overall, interaction models of non-violent delinquency indicated that early maturing girls were more sensitive to shared and environmental influences, and less influenced by genes. Results from the full interaction models for non-violent delinquency are illustrated in Figure 5.
Figure 5.
Additive genetic, shared environmental, and non-shared environmental influences on non-violent delinquency are moderated by perceived development and age at menarche.
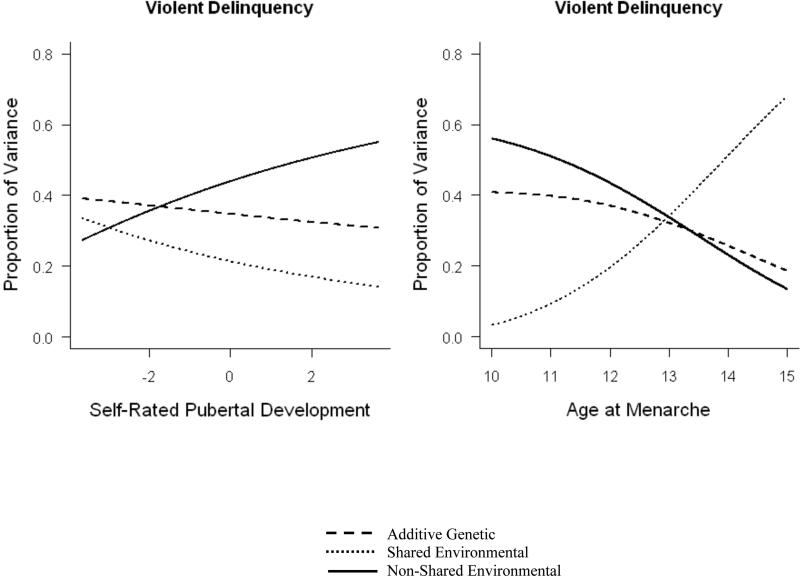
For models of violent delinquency, the pattern of results for the genetic and shared environmental interactions was less clear. The interaction between perceived development and genes was not significantly different than zero (a’ = .024), although this parameter could not be dropped from the model without a significant loss in model fit (Δχ2 = 208.38, P < .001). The interaction between perceived development and shared environment was also not significant, and nested model comparisons indicated that the c’ parameter could be dropped from the model (Δχ2 = 0.12, P = .73). The only clear result from the interaction model of perceived development and violent delinquency, then, was the significant e’ parameter, indicating that early maturing girls were more sensitive to non-shared environmental influences. Results from the best-fitting interaction model (with c’ fixed to zero) for perceived development and violent delinquency are illustrated in the left side of Figure 6. In contrast, for menarche and violent delinquency, both the genetic (a’ = -.063) and shared environmental (c’ = .115) interactions were significant, but in the opposite direction as observed for non-violent delinquency. That is, genetic influences were higher and shared environmental influences were lower for early maturing girls. Results from the full interaction model for menarche and violent delinquency are illustrated in the right side of Figure 6.
Figure 6.
Additive genetic, shared environmental, and non-shared environmental influences on violent delinquency are moderated by perceived development and age at menarche.
Discussion
Early pubertal timing puts girls at risk for a breadth of emotional and behavioral problems, including heightened involvement in delinquent behavior during adolescence and criminal behavior in adulthood. Because pubertal development involves an orchestrated set of changes across physiological, cognitive, and social domains, it has been challenging for research to identify the specific aspects of early pubertal timing that are most salient for delinquent behavior. Specifically, this study addressed the question of whether genetic differences accounted for the increased delinquent behavior seen in early maturing girls. Using nationally representative data from over 900 adolescent sister pairs of varying degrees of genetic relatedness, our behavioral genetic analyses indicated that a common set of genes predisposed girls toward early pubertal maturation and resulted in elevated involvement in both non-violent and violent forms of delinquency. This result was consistent across measures of pubertal timing, including both objective reports of menarcheal age and girls’ self-reports of pubertal development. After accounting for these common genetic influences, there were no significant environmentally-mediated paths between pubertal timing and delinquency. That is, sisters who differed in their pubertal timing did not significantly differ in their delinquency. Overall, our results challenge a purely socioenvironmental account of why early maturing girls show increased involvement in delinquent behavior, and suggests that the genetic differences between early and late maturing girls hold more salience for their behavioral problems than environmental differences.
At the same time, results from our interaction models indicated that early pubertal timing moderated the residual genetic and environmental influences on delinquency. For both measures of pubertal timing, and for both violent and non-violent forms of delinquency, the magnitude of non-shared environmental influence on delinquency was highest for early maturing girls. That is, after accounting for the main effects of pubertal timing on delinquency, there was greater within-family environmental variability in early maturing sister pairs than in later maturers. Thus there are two pathways of influence between early pubertal timing and delinquency: Genetic factors related to early maturation directly influence delinquent behavior, and early maturation also increases sensitivity to other environmental influences on delinquency. The interaction between pubertal timing and non-shared environmental influence was more marked for non-violent than for violent delinquent behavior. This is consistent with previous theoretical and empirical reports indicating that non-violent forms of delinquent behavior are more substantially influenced by environmental risks, whereas violent delinquency is more consistent across contexts and across the life course (Burt, 2009; Burt & Neiderhiser, 2009; Eley et al., 1999, 2003; Loeber, et al., 2009; Moffitt, 1993; Tackett, et al., 2003; Tuvblad, et al., 2005).
Although the behavior genetics models used in the current analyses are capable of discriminating between genetic and environmental pathways, identifying the specific genes responsible for the observed association remains an important question for future research. One possibility is the androgen receptor gene (AR). Comings et al. (2002) found that the short allele of the GGC repeat polymorphism was associated with both earlier menarche in girls and higher aggression and impulsivity in males. (Jorm, Christensen, Rodgers, Jacomb, & Easteal (2004) failed to replicate the association between AR and fathers’ parenting behaviors, but did not present data regarding the association between AR and menarche.) It is important to note, however, that the effect sizes associated with individual genetic polymorphisms are typically very small, and no single gene is likely to account fully for the association between early pubertal timing and delinquency. Moreover, it is difficult to speculate about specific risk genotypes when the genetic origins of individual differences in pubertal timing remain largely unknown.
In addition to genetic main effects, we also found that early maturing girls were more sensitive to environmental influences. This may reflect the impact of early pubertal maturation on brain development. In particular, pubertal maturation is linked to changes in dopaminergic neural circuits involved in responsiveness to emotional and motivational stimuli (Blakemore et al., 2010; Forbes & Dahl, 2010; Kuhn et al., 2010), and pubertal timing has been shown to predict neural activity in the striatum and prefrontal cortex in response to rewards and social threats (Forbes, et al., in press; Forbes, et al., 2010). These changes in responsiveness to rewards and emotions precede age-based neural changes in cortical structures that underlie behavioral inhibition and effortful control (Casey et al., 2007; Somerville et al., 2010; Steinberg, 2008). Thus, from developmental neuroscience perspective, early maturing girls are expected to be particularly attuned to the rewards inherent in risky environmental situations, and this process may underlie the greater non-shared environmental influence evident in the current results.
Moreover, for non-violent delinquent behavior, genetic factors independent of pubertal timing were less influential for early maturing girls. This result is consistent with previous research by Burt et al. (2006), which showed substantial heritability (>60%) for conduct disorder symptoms in girls with on-time menarche, and very weak genetic influence (<10%) for girls with menarche prior to age 11. More broadly, the amplification of genetic variance among later maturing girls can be conceptualized in term of the “social push” hypothesis (Raine and Venables, 1981): Biological vulnerabilities are more important when the environment is relatively benign and less important in the presence of social stressors that predispose to antisocial behavior. For example, previous research has found that psychophysiological variables, such as low resting heart rate and reduced electrodermal conditioning, are stronger predictors of antisocial behavior among children with more advantaged and harmonious home environments (reviewed in Raine, 2002). Thus, among later maturing girls who do not experience the social challenges of early pubertal timing, genetic factors may be more important for differentiating who goes on to show delinquent behavior and who does not.
Limitations and Future Directions
The current study is limited by several features of the Add Health data, and it is worth considering the impact of those limitations on our results. First, Add Health participants were drawn from a broad age range at the initial assessment wave. To correct for this age heterogeneity, we have standardized all variables by age, consistent with previous analyses of pubertal timing variables in Add Health (Ge et al., 2007). Moreover, the mean age of participants (age 16 years) is older than is typical for studies of pubertal timing; indeed, given the mean menarcheal age is 12.2 years, the average participant was likely to be post-pubertal. Thus, the current analyses may have failed to detect environmentally-mediated effects of pubertal timing that were specific to the early adolescent transition and that did not persist into mid-adolescence.
Second, the Add Health survey used an abbreviated self-report measure of pubertal development. Dorn and colleagues (2006) have noted that agreement between different measurement modalities for pubertal timing (e.g., physician exam versus self-report) is low to moderate, thus a different pattern of results may have been evident if pubertal timing had been measured at an earlier chronological age or by a measurement strategy other than self-report. In addition, menarche occurs relatively late in the process of puberty; given that children also differ in their rate of pubertal change (pubertal tempo), menarcheal age may misrepresent the pubertal experiences of girls who begin puberty relatively early but mature relatively slowly (Mendle, Harden, Graber, & Brooks-Gunn, 2010). To our knowledge, no previous study has combined a genetically informative sample with repeated assessments of pubertal development by multiple modalities (e.g., self-report and physical exam). While such a design would, of course, be quite resource-intensive, it also holds potential to shed new light on the mechanisms by which early pubertal timing impacts girls’ psychosocial development.
Third, the current data are silent regarding girls’ history of behavior problems in childhood, thus it remains unclear whether the impact of pubertal timing is consistent across childhood- versus adolescent-onset trajectories of antisocial behavior. According to Moffitt's (1993, 2003) taxonomy of “adolescent-limited” versus “life-course persistent” antisocial behavior, individuals with a history of childhood behavioral problems that persist into adolescence (and subsequently into adulthood) are more likely to be characterized by a constellation of severe risk factors, including adverse family environments, genetic vulnerabilities, and neuropsychological deficits. While some authors have argued that this trajectory is not applicable for females, given the low prevalence of female externalizing problems in childhood (e.g., D'Unger, Land, & McCall, 2002; Silverthorn & Frick, 1999; Silverthorn, Frick, & Reynolds, 2001), a review of longitudinal research on female antisocial behavior by Fontaine et al. (2009) concluded that a very small proportion of females (1-2% of the population) do show an “early-starter” or “childhood-onset” trajectory of antisocial behavior. Given the rarity of this trajectory, and the community nature of the AddHealth sample, the current results are probably descriptive of the more common, adolescent-limited trajectory of delinquency, which has been estimated to characterize up to 25% of adolescent girls (Fontaine et al., 2009). To our knowledge, however, no study has specifically examined whether the sequelae of early pubertal timing are moderated by history of childhood behavior problems; this is a fruitful question for future research.
Fourth, the AddHealth study is characterized by a high degree of racial and ethnic diversity within a genetically informed sample. In contrast, virtually all of the previous genetically informed research on pubertal timing and its effects on psychopathology have been conducted using highly homogenous samples of Northern Europeans (e.g., Dick, Rose, Viken, & Kaprio, 2000) or European-Americans (e.g., Burt et al., 2009; Culbert, Burt, McGue, Iacono, & Klump, 2009). Thus one major contribution of the current study is its representation of African-American, Hispanic/Latina, and Asian-American girls in behavioral genetic research. At the same time, the number of sibling pairs in AddHealth was too small to conduct analyses (particularly tests of GxE interaction) separately by race/ethnicity and still maintain adequate power. Although some authors have found that the developmental correlates of early pubertal timing differ across race/ethnic groups (e.g., Cavanagh, 2009), Lynne et al. (2007) reported that early maturation predicts delinquent and aggressive behavior in Hispanic/Latina and African-American adolescents as well as in White adolescents. Currently, it remains unknown whether environmental mechanisms are more or less important in racial and ethnic minorities.
Finally, the AddHealth study does not include measures of relational aggression (i.e., behavior that is intended to harm others through damaging their peer relationships or social standing; Crick & Grotpeter, 1995), but instead focuses on only overt or physical manifestations of aggressive behavior. Girls are increasingly more likely to engage in relational aggression, relative to boys, as they transition from childhood to adolescence (Archer, 2004), and this increase may be linked to pubertal development. Very few studies have examined whether relational forms of aggression are also more prevalent in early maturing girls. However, the little extant research on this topic has found that both pubertal stage and early pubertal timing are associated with higher relational aggression, particularly for girls who experienced less positive parenting (Hemphill et al., 2010; Mrug et al., 2008). The only behavior genetic study of relational aggression in adolescents found that, like physical aggression, it is substantially heritable (Tackett, Waldman, & Lahey, 2009), but it remains unclear whether the mechanisms underlying the association between pubertal timing and relational aggression are the same as other forms of antisocial behavior.
Conclusions
Overall, this study provides evidence for a complex interplay between genetic and environmental risk in the association between early pubertal timing and adolescent girls’ involvement in violent and non-violent delinquent behavior. Genes predisposing girls to earlier pubertal timing also increased vulnerability to delinquent behavior, and this common set of genetic influences entirely accounted for the main effect of pubertal timing. In addition, non-shared environmental influences were stronger for early maturing girls, whereas genetic influences were weaker (for non-violent delinquency). Effects were generally consistent across measures of pubertal timing (menarcheal age versus self-reported development). Future research is necessary to identify specific risk genotypes that underlie the common genetic influences, and to determine the mechanisms underlying early maturing girls’ greater sensitivity to environmental influences on delinquency.
Acknowledgements
Dr. Paige Harden is a Faculty Research Associate of the Population Research Center at the University of Texas at Austin, which is supported by a center grant from the National Institute of Child Health and Human Development (5-R24-HD042849).
This research uses data from Add Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, and funded by a grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 17 other agencies. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Persons interested in obtaining data files from Add Health should contact Add Health, Carolina Population Center, 123 W. Franklin Street, Chapel Hill, NC 27516-2524 (addhealth@unc.edu). No direct support was received from grant P01-HD31921 for this analysis.
Footnotes
Previous research has used various cut-offs to define “early” pubertal maturation; we chose 12 years as the cut-off because it was close to the mean menarcheal age in the full sample, thus yielding groups of approximately equal size. This choice is consistent with classifications used by numerous previous studies (e.g., Caspi & Moffitt, 1991; Deardorff, Gonzales, Christopher, Roosa, & Millsap, 2005; Stattin & Magnusson, 1990). Ultimately, any dichotomization of menarche into “early” versus “late” is to some degree arbitrary, and our subsequent, more rigorous behavioral genetic analyses therefore examine both menarche and perceived development as continuous variables.
References
- Achenbach TM. Manual for the Child Behavior Checklist and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. University of Vermont, Research Center for Children, Youth and Families; Burlington, VT: 2001. [Google Scholar]
- Archer J. Sex differences in aggression in real-world settings: A meta-analytic review. Review of General Psychology. 2004;8:291–322. [Google Scholar]
- Arsenault L, Moffitt TE, Caspi A, Taylor A, Rijsdijk FV, Jaffee SR, Ablow JC, Measelle JR. Strong genetic effects on cross-situational antisocial behaviour among 5-year old children according to mothers, teachers, examiner-observers, and twins' self-reports. Journal of Child Psychology and Psychiatry. 2003;44:832–848. doi: 10.1111/1469-7610.00168. [DOI] [PubMed] [Google Scholar]
- Barker ED, Larsson H, Viding E, Maughan B, Rijsdijk F, Fontaine N, Plomin R. Common genetic but specific environmental influences for aggressive and deceitful behaviors in preadolescent males. Journal of Psychopathology & Behavioral Assessment. 2009;31:299–308. [Google Scholar]
- Barker ED, Seguin JR, White HR, Bates ME, Lacourse E, Carbonneau R, Tremblay RE. Developmental trajectories of male physical violence and theft. Archives of General Psychiatry. 2007;64:592–599. doi: 10.1001/archpsyc.64.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean JA, Leeper JD, Wallace RB, Sherman BM, Jagger H. Variations in the reporting of menstrual histories. American Journal of Epidemiology. 1979;109:181–185. doi: 10.1093/oxfordjournals.aje.a112673. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Development. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg LS, Houts RM, Friedman SF, DeHart G, Roisman GI, the NICHD Early Child Care Research Network Family Rearing Antecedents of Pubertal Timing. Child Development. 2007;78:1302–1321. doi: 10.1111/j.1467-8624.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- Bergevin TA, Bukowski WM, Karavasilis L. Child sexual abuse and pubertal timing: implications for long-term psychosocial adjustment. In: Hayward C, editor. Gender Differences at Puberty. Cambridge University Press; Cambridge, England: 2003. pp. 187–216. [Google Scholar]
- Biehl MC, Natsuaki MN, Ge X. The influence of pubertal timing on alcohol use and heavy drinking trajectories. Journal of Youth and Adolescence. 2007;36:153–167. [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Human Brain Mapping. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP. The effects of delayed menarche in different contexts: dance and non-dance students. Journal of Youth and Adolescence. 1985b;14:285–300. doi: 10.1007/BF02089235. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Attie I, Burrow C, Rosso J, Warren M. The impact of puberty on eating concerns in athletic and non-athletic contexts. Journal of Early Adolescence. Special issue: Early adolescent transitions: longitudinal studies of biological, psychological, and social interactions. 1989;9:269–290. [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Burt SA. Are there meaningful etiological differences within antisocial behavior? Clinical Psychology Review. 2009;29:163–178. doi: 10.1016/j.cpr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Burt SA, McGue M, DeMarte JA, Krueger RF, Iacono WG. Timing of menarche and the origins of conduct disorder. Archives of General Psychiatry. 2006;63:890–896. doi: 10.1001/archpsyc.63.8.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Neiderhiser JM. Aggressive versus nonaggressive antisocial behavior: Distinctive etiological moderation by age. Developmental Psychology. 2009;45:1164–1176. doi: 10.1037/a0016130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey VA, Dwyer J, Colman KA, Krall EA, Gardner J, Valadian I. Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Annals of Human Biology. 1991;18:155–166. doi: 10.1080/03014469100001492. [DOI] [PubMed] [Google Scholar]
- Caspi A, Lynam D, Moffitt TE, Silva PA. Unraveling girls' delinquency: Biological, dispositional, and contextual contributions to adolescent misbehavior. Developmental Psychology. 1993;32:631–635. [Google Scholar]
- Caspi A, Moffitt TE. Individual differences are accentuated during periods of social change: the sample case of girls at puberty. Journal of Personality and Social Psychology. 1991;61:157–168. doi: 10.1037//0022-3514.61.1.157. [DOI] [PubMed] [Google Scholar]
- Cavanagh SE. Sexual debut of girls in early adolescence: The intersection of race, pubertal timing, and friendship group characteristics. Journal of Research on Adolescence. 2004;14:285–312. [Google Scholar]
- Comings DE, Muhleman D, Johnson JP, MacMurray JP. Parent-daughter transmission of the androgen receptor gene as an explanation of the effect of father absence on age at menarche. Child Development. 2002;73:1046–1051. doi: 10.1111/1467-8624.00456. [DOI] [PubMed] [Google Scholar]
- Crick NR, Grotpeter JK. Relational aggression, gender, and social-psychological adjustment. Child Development. 1995;66:710–722. doi: 10.1111/j.1467-8624.1995.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Davridge KM, Atkinson L, Douglas L, Lee V, Shapiro S, Kennedy JL, Beitchman JH. Association of the serotonin transporter and 5-HT1Dbeta receptor genes with extreme, persistent and pervasive aggressive behaviour in children. Psychiatric Genetics. 2004;14:143–146. doi: 10.1097/00041444-200409000-00004. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y, Lahey BB. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biological Psychology. 2009;80:773–834. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deerdorff J, Gonzales NA, Christopher FS, Roosa MW, Millsap RE. Early puberty and adolescent pregnancy: The influence of alcohol use. Pediatrics. 2005;116:1451–1456. doi: 10.1542/peds.2005-0542. [DOI] [PubMed] [Google Scholar]
- Déry M, Toupin J, Pauzé R, Mercier H, Fortin L. Neuropsychological characteristics of adolescents with conduct disorder: Association with attention-deficit-hyperactivity and aggression. Journal of Abnormal Child Psychology. 1999;27:225–236. doi: 10.1023/a:1021904523912. [DOI] [PubMed] [Google Scholar]
- Dick DM, Rose RJ, Viken RJ, Kaprio J. Pubertal timing and substance use: association between and within families across late adolescence. Developmental Psychology. 2000;36:180–189. [PubMed] [Google Scholar]
- D'Onofrio BM, Slutske WS, Turkheimer E, Emery RE, Harden KP, Heath AC, Madden PAF, Martin NG. The intergenerational transmission of childhood conduct problems: A children of twins study. Archives of General Psychiatry. 2007;64:820–829. doi: 10.1001/archpsyc.64.7.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early Adolescence: a user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10:30–56. [Google Scholar]
- Doughty D, Rodgers JL. Behavior genetic modeling of menarche in U.S. females. In: Rodgers JL, Rowe DC, Miller WB, editors. Genetic influences on human fertility and sexuality: theoretical and empirical contributions from the biological and behavioral sciences. Kluwer; Boston: 2000. [Google Scholar]
- D'Unger AV, Land KC, McCall PL. Sex differences in age patterns of delinquent/criminal careers: Results from Poisson latent class analyses of the Philadelphia cohort study. Journal of Quantitative Criminology. 2002;18:349–375. [Google Scholar]
- Eley TC, Lichtenstein P, Moffitt TE. A longitudinal behavioral genetic analysis of the etiology of aggressive and nonaggressive antisocial behavior. Development and Psychopathology. 2003;15:383–402. doi: 10.1017/s095457940300021x. [DOI] [PubMed] [Google Scholar]
- Eley TC, Lichtenstein P, Stevenson J. Sex differences in the etiology of aggressive and nonaggressive antisocial behavior: Results from two twin studies. Child Development. 1999;70:155–168. doi: 10.1111/1467-8624.00012. [DOI] [PubMed] [Google Scholar]
- Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychological Bulletin. 2004;130:920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Federal Bureau of Investigation . Crime in the United States 2004. Author; Washington, DC: 2004. Available from: http://www2.fbi.gov/ucr/cius_04/documents/CIUS2004.pdf. [Google Scholar]
- Flannery DJ, Rowe DC, Gulley BL. Impact of pubertal status, timing, and age on adolescent sexual experience and delinquency. Journal of Adolescent Research. 1993;8:21–40. [Google Scholar]
- Flory JD, Xu K, New AS, Finch T, Goldman D, Siever LJ. Irritable assault and variation in the COMT gene. Psychiatric Genetics. 2007;17:344–346. doi: 10.1097/YPG.0b013e3281c8f126. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain and Cognition. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Ryan ND, Dahl RE. Neural systems of social threat processing in adolescents: Influence of pubertal maturaion and relation to real-world negative affect and depressive symptoms. Developmental Neuropsychology. doi: 10.1080/87565641.2010.550178. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarillo SR, Dahl RE. Puberty, neural response to reward, and health adolescents' positive affect in natural environments. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:162–172. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Brody GH, Conger RD, Simons RL. Pubertal maturation and African American children's internalizing and externalizing symptoms. Journal of Youth and Adolescence. 2006;35:531–540. [Google Scholar]
- Foster H, Hagan J, Brooks-Gunn J. Growing up fast: stress exposure and subjective weathering in emerging adulthood. Journal of Health and Social Behavior. 2008;49:162–177. doi: 10.1177/002214650804900204. [DOI] [PubMed] [Google Scholar]
- Ge X, Brody GH, Conger RD, Simons RL, Murry VM. Contextual amplification of pubertal transition effects on deviant peer affiliation and externalizing behavior among African American children. Developmental Psychology. 2002;38:42–54. doi: 10.1037//0012-1649.38.1.42. [DOI] [PubMed] [Google Scholar]
- Ge X, Natsuaki MN. In search of explanations of early pubertal timing effects on developmental psychopathology. Current Directions in Psychological Science. 2009;18:327–331. [Google Scholar]
- Ge X, Natsuaki MN, Neiderhiser JM, Reiss D. Genetic and environmental influences on pubertal timing: results from two national sibling studies. Journal of Research on Adolescence. 2007;17:767–788. [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood? Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:718–726. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- Harden KP, Hill JE, Emery RE, Turkheimer E. Gene-environment correlation and interaction in peer effects on adolescent alcohol and tobacco use. Behavior Genetics. 2008;38:339–347. doi: 10.1007/s10519-008-9202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Lynch SK, Turkheimer E, Emery RE, D'Onofrio BM, Slutske W, Waldron M, Heath AC, Statham DJ, Martin NG. A behavior genetic investigation of adolescent motherhood and offspring mental health problems. Journal of Abnormal Psychology. 2007;116:667–683. doi: 10.1037/0021-843X.116.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Mendle JE, Hill JE, Turkheimer E, Emery RE. Rethinking timing of first sex and delinquency. Journal of Youth and Adolescence. 2008;37:373–385. doi: 10.1007/s10964-007-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Turkheimer E, Loehlin JC. Genotype by environment interaction in adolescents' cognitive aptitude. Behavior Genetics. 2007;37:273–283. doi: 10.1007/s10519-006-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynie D. Context of risk? Explaining the link between girls' pubertal development and their delinquency involvement. Social Forces. 2003;82:355–397. [Google Scholar]
- Hemphill SA, Kotevski A, Herrenkohl TI, Toumbourou JW, Carlin JB, Catalano RF, Patton GC. Pubertal stage and the prevalence of violence and social/relational aggression. Pediatrics. 2010;126:298–305. doi: 10.1542/peds.2009-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Rodgers B, Jacomb PA, Easteal S. Association of adverse childhood experiences, age of menarche, and adult reproductive behavior: Does the androgen receptor gene play a role? American Journal of Medical Genetics. 2004;125B:105–11. doi: 10.1002/ajmg.b.20114. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Marttunen M, Rantanen P, Rimpela M. Early puberty is associated with mental health problems in middle adolescence. Social Science and Medicine. 2003;57:1055–1064. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, Walker QD. The emergence of gonadal hormone influence on dopaminergic function during puberty. Hormones and Behavior. 2010;58:122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R, Burke JD, Pardini DA. Development and etiology of disruptive and delinquent behavior. Annual Review of Clinical Psychology. 2009;5:291–310. doi: 10.1146/annurev.clinpsy.032408.153631. [DOI] [PubMed] [Google Scholar]
- Lynne SD, Graber JA, Nichols TR, Brooks-Gunn J, Bolvin GJ. Links between pubertal timing, peer influences, and externalizing behaviors among urban students followed through middle school. Journal of Adolescent Health. 2007;40:181.e7–181.e13.. doi: 10.1016/j.jadohealth.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson D, Stattin H, Allen VL. Biological maturation and social development: a longitudinal study of some adjustment processes from midadolescence to adulthood. Journal of Youth and Adolescence. 1985;14:267–283. doi: 10.1007/BF02089234. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, et al. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Martin CA, Logan TK, Leukefeld C, Milich R, Omar H, Clayton R. Adolescent and young adult substance use: Associations with sensation-seeking, self-esteem, and retrospective report of early pubertal onset. A preliminary examination. International Journal of Adolescent Medicine and Health. 2001;13:211–219. [Google Scholar]
- Maughan B, Rutter M. Antisocial children grown up. In: Hill J, Maughan J&B, editors. Conduct disorders in childhood and adolescence. Cambridge University Press; Cambridge, England: 2001. [Google Scholar]
- Mendle J, Harden KP, Brooks-Gunn J, Graber JA. Development's tortoise and hare: Pubertal timing, pubertal tempo, and depressive symptoms in boys and girls. Developmental Psychology. 2010;46:1341–1353. doi: 10.1037/a0020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Harden KP, Turkheimer E, Van Hulle CA, D'Onofrio BM, Brooks-Gunn J, Rodgers JL, Emery RE, Lahey BB. Associations between father absence and age at first sexual intercourse. Child Development. 2009;80:1463–1480. doi: 10.1111/j.1467-8624.2009.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Turkheimer E, D'Onofrio BM, Lynch SK, Emery RE, Slutske WS, Martin NG. Family structure and age at menarche: A children-of-twins approach. Developmental Psychology. 2006;42:533–542. doi: 10.1037/0012-1649.42.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Turkheimer E, Emery RE. Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Developmental Review. 2007;27:151–171. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Eaves LJ, Heath AC, Martin NG. Estimating genetic influences on the age-at-menarche: a survival analysis approach. American Journal of Medical Genetics. 1991;39:148–154. doi: 10.1002/ajmg.1320390207. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana BR, Hariri A, Pezawas L, Blasi G, Wabnitz A, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Science. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A, Eccles J. When coming of age means coming undone: links between puberty and psychosocial adjustment in European American and African American girls. In: Hayward C, editor. Gender Differences at Puberty. Cambridge University Press; Cambridge, England: 2003. [Google Scholar]
- Mik HM, Ehtesham S, Baldassarra L, De Luca V, Davidge K, Bender D, Tharmalingam S, et al. Serotonin system genes and childhood-onset aggression. Psychiatric Genetics. 2007;17:11. doi: 10.1097/YPG.0b013e3280114103. [DOI] [PubMed] [Google Scholar]
- Miles DR, Carey G. Genetic and environmental architecture of human aggression. Journal of Personality and Social Psychology. 1997;72:207–217. doi: 10.1037//0022-3514.72.1.207. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. “Life-course-persistent” and “adolescent-limited” antisocial behavior: a developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE. Life-course-persistent and adolescent-limited antisocial behavior: A 10-year research review and a research agenda. In: Lahey BB, Moffitt TE, Caspi A, editors. Causes of conduct disorder and juvenile delinquency. Guilford Press; New York: 2003. pp. 49–75. [Google Scholar]
- Moffitt TE, Caspi A, Dickson N, Silva PA, Stanton W. Childhood-onset versus adolescent-onset antisocial conduct in males: Natural histories from 3–18. Development and Psychopathology. 1996;8:394–424. [Google Scholar]
- Moffitt TE, Caspi A, Rutter M, Silva P. Sex differences in antisocial behavior. Cambridge University Press; Cambridge, UK: 2001. [Google Scholar]
- Mrug S, Elliott M, Gililand J, Grunbaum JA, Tortolero SR, Cuccaro P, Schuster M. Positive parenting and early puberty in girls: Protective effects against aggressive behavior. Archives of Pediatrics and Adolescent Medicine. 2008;162:781–786. doi: 10.1001/archpedi.162.8.781. [DOI] [PubMed] [Google Scholar]
- Mustanski BS, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental influences on pubertal development: longitudinal data from Finnish twins at ages 11 and 14. Developmental Psychologyt. 2004;40:1188–1998. doi: 10.1037/0012-1649.40.6.1188. [DOI] [PubMed] [Google Scholar]
- Natsuaki MN, Biehl MC, Ge X. Trajectories of depressed moood from early adolescent to young adulthood: The effects of pubertal timing and adolescent dating. Journal of Research on Adolescence. 2009;19:47–74. [Google Scholar]
- Negriff S, Susman EJ. Pubertal timing, depression, and externalizing problems: A framework, review, and examination of gender differences. Journal of Research on Adolescence. 2011 Epub ahead of print, doi: 10.1111/j.1532-7795.2010.00708. [Google Scholar]
- Newcomb MD, McGee L. Influence of sensation seeking on general deviance and specific problem behaviors from adolescence to young adulthood. Journal of Personality and Social Psychology. 1991;61:614–628. doi: 10.1037//0022-3514.61.4.614. [DOI] [PubMed] [Google Scholar]
- Obeidallah DA, Brennan RT, Brooks-Gunn J, Kindlon D, Earls F. Socioeconomic status, race, and girls' pubertal maturation: results from the Project on Human Development in Chicago neighborhoods. Journal of Research on Adolescence. 2000;10:443–464. [Google Scholar]; Obeidallah D, Brennan RT, Brooks-Gunn J, Earls F. Links between pubertal timing and neighborhood contexts: Implications for girls' violent behavior. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1460–1468. doi: 10.1097/01.chi.0000142667.52062.1e. [DOI] [PubMed] [Google Scholar]
- Odgers CL, Moffitt TE, Broadbent JM, Dickson N, Hancox RJ, Harrington H, Poulton R, Sears MR, Thomson WM, Caspi A. Female and male antisocial trajectories: From childhood origins to adult outcomes. Development and Psychopathology. 2008;20:673–716. doi: 10.1017/S0954579408000333. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH. Classical conditioning and socialization – a biosocial interaction. Personality and Individual Differences. 1981;2:273–283. [Google Scholar]
- Rhee SI, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128:490–529. [PubMed] [Google Scholar]
- Rierdan J, Koff E, Stubbs ML. Timing of menarche, preparation, and initial menstrual experience: replication and further analyses in a prospective study. Journal of Youth and Adolescence. 1988;18:413–426. doi: 10.1007/BF02132777. [DOI] [PubMed] [Google Scholar]
- Roberti JW. A review of behavioral and biological correlates of sensation seeking. Journal of Research in Personality. 2004;38:256–279. [Google Scholar]
- Rowe DC. Environmental and genetic influences on pubertal development: evolutionary life history traits? In: Rodgers JL, Rowe DC, Miller WB, editors. Genetic influences on human fertility and sexuality: theoretical and empirical contributions from the biological and behavioral sciences. Kluwer; Boston: 2000. [Google Scholar]
- Rowe DC. Biology and crime. Roxbury; Los Angeles: 2001. [Google Scholar]
- Rowe DC. On genetic variation in menarche and age at first sexual intercourse: A critique of the Belsky-Draper hypothesis. Evolution and Human Behavior. 2002;23:365–372. [Google Scholar]
- Scourfield J, Van den Bree M, Martin N, McGuffin P. Conduct problems in children and adolescents: A twin study. Archives of General Psychiatry. 2004;61:489–496. doi: 10.1001/archpsyc.61.5.489. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. New England Journal of Medicine. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Siever LJ. Neurobiology of aggression and violence. American Journal of Psychiatry. 2008;165:429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverthorn P, Frick PJ. Developmental pathways to antisocial behavior: The delayed-onset pathway in girls. Development and Psychopathology. 1999;11:101–126. doi: 10.1017/s0954579499001972. [DOI] [PubMed] [Google Scholar]
- Silverthorn P, Frick PJ, Reynolds R. Timing of onset and correlates of severe conduct disorder in adjudicated girls and boys. Journal of Psychopathology and Behavioral Assessment. 2001;23:171–181. [Google Scholar]
- Sisk C, Foster D. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PAF, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Modeling genetic and environmental influences in the etiology of conduct disorder: A study of 2,682 adult twin pairs. Journal of Abnormal Psychology. 1997;106:266–279. doi: 10.1037//0021-843x.106.2.266. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Achenbach TM, Verhulst FC. Accelerated longitudinal comparisons of aggressive versus delinquent syndromes. Development and Psychopathology. 1997;9:43–58. doi: 10.1017/s0954579497001053. [DOI] [PubMed] [Google Scholar]
- Stattin H, Magnusson D. Paths through life. Vol. 2. Erlbaum; Hillsdale, NJ: 1990. Pubertal maturation in female development. [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Storvall EE, Wichstrom L. Do the risk factors associated with conduct problems in adolescents vary according to gender? Journal of Adolescence. 2002;25:183–202. doi: 10.1006/jado.2002.0460. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, Dorn LD. Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Developmental Psychology. 2007;43:811–822. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- Tackett JL, Krueger RF, Iacono WG, McGue M. Symptom-based subfactors of DSM-defined conduct disorder: Evidence for etiologic distinctions. Journal of Abnormal Psychology. 2005;114:483–487. doi: 10.1037/0021-843X.114.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett JL, Waldman ID, Lahey BB. Etiology and measurement of relational aggression: A multi-informant behavior genetic investigation. Journal of Abnormal Psychology. 2009;118:722–733. doi: 10.1037/a0016949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar SA, Martin NG. Age at menarche as a fitness trait: nonadditive genetic variance detected in a large twin sample. American Journal of Human Genetics. 1990;47:137–148. [PMC free article] [PubMed] [Google Scholar]
- Turner PK, Runtz MG, Galambos NL. Sexual abuse, pubertal timing, and subjective age in adolescent girls : a research note. Journal of Reproductive and Infant Psychology. 1999;17:111–118. [Google Scholar]
- Tuvblad C, Eley TC, Lichtenstein P. The development of antisocial behavior from childhood to adolescence: A longitudinal twin study. European Journal of Child and Adolescent Psychiatry. 2005;14:216–225. doi: 10.1007/s00787-005-0458-7. [DOI] [PubMed] [Google Scholar]
- Udry JR. Age at menarche, at first intercourse, and at first pregnancy. Journal of Biosocial Science. 1979;11:433–441. doi: 10.1017/s0021932000012517. [DOI] [PubMed] [Google Scholar]
- Verweij KJH, Zietsch BP, Bailey JM, Martin NG. Shared aetiology of risky sexual behavior and adolescent misconduct: genetic and environmental influences. Genes, Brains, and Behavior. 2009;8:1–7-113. doi: 10.1111/j.1601-183X.2008.00456.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tancredib LR, Grant C, Gillespie H, Valentine A, Mullani N, Wang GJ, Hollister L. Brain glucose metabolism in violent psychiatric patients: A preliminary study. Psychiatry Research: Neuroimaging. 1995;61:243–253. doi: 10.1016/0925-4927(95)02671-j. [DOI] [PubMed] [Google Scholar]
- Wichstrom L. Predictors of adolescent suicide attempts: a nationally representative longitudinal study of Norwegian adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:603–610. doi: 10.1097/00004583-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Young SE, Smolen A, Corley RP, Krauter KS, DeFries JC, Crowley TC, Hewitt JK. Dopamine transporter polymorphism is associated with childhood externalizing behavior. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2002;114:144–149. doi: 10.1002/ajmg.10155. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Buchsbaum MS, Murphy DL. Sensation seeking and its biological correlates. Psychological Bulletin. 1980;88:187–214. [Google Scholar]