Abstract
OBJECTIVE:
To define a quantitative stratification algorithm for the risk of early-onset sepsis (EOS) in newborns ≥34 weeks’ gestation.
METHODS:
We conducted a retrospective nested case-control study that used split validation. Data collected on each infant included sepsis risk at birth based on objective maternal factors, demographics, specific clinical milestones, and vital signs during the first 24 hours after birth. Using a combination of recursive partitioning and logistic regression, we developed a risk classification scheme for EOS on the derivation dataset. This scheme was then applied to the validation dataset.
RESULTS:
Using a base population of 608 014 live births ≥34 weeks’ gestation at 14 hospitals between 1993 and 2007, we identified all 350 EOS cases <72 hours of age and frequency matched them by hospital and year of birth to 1063 controls. Using maternal and neonatal data, we defined a risk stratification scheme that divided the neonatal population into 3 groups: treat empirically (4.1% of all live births, 60.8% of all EOS cases, sepsis incidence of 8.4/1000 live births), observe and evaluate (11.1% of births, 23.4% of cases, 1.2/1000), and continued observation (84.8% of births, 15.7% of cases, incidence 0.11/1000).
CONCLUSIONS:
It is possible to combine objective maternal data with evolving objective neonatal clinical findings to define more efficient strategies for the evaluation and treatment of EOS in term and late preterm infants. Judicious application of our scheme could result in decreased antibiotic treatment in 80 000 to 240 000 US newborns each year.
Keywords: early-onset sepsis, late preterm infant, predictive modeling, term newborn
What’s Known on This Subject:
The management of term and near-term newborns suspected of early-onset sepsis, particularly when they are not clearly symptomatic, remains controversial. Methods for quantifying risk that combine maternal factors with a newborn's evolving clinical examination have been lacking.
What This Study Adds:
This study provides a method for predicting risk of early-onset sepsis. It combines maternal risk factors with objective measures of a newborn's clinical examination and places newborns into 3 risk groups (treat empirically, observe and evaluate, and continued observation).
Evaluation of term and near-term infants for early-onset sepsis (EOS) remains a vexing problem in neonatology. In these infants, current incidence of EOS ranges between 0.5 and 1.2 cases per thousand live births.1,2 This represents a threefold to fivefold decrease over the past 20 years.3–7 Decreases in incidence have been most pronounced in group B Streptococcus (GBS; Streptococcus agalactiae) sepsis, with 2010 national surveillance reporting 0.25 cases per 1000 live births8 in contrast to 1.8 cases per 1000 live births in 1990.3 Decreased EOS incidence is attributed to systematic screening for GBS and increased use of intrapartum antibiotic therapy.
Although the incidence of EOS has fallen, the incidences of evaluation and treatment of EOS remain substantial. Current evaluation algorithms recommended by the Centers for Disease Control and Prevention (CDC) and the Committee on the Fetus and Newborn of the American Academy of Pediatrics do not specify a severity or duration of clinical signs of illness that should lead to EOS evaluation, nor do these algorithms specify how to interpret recommended laboratory tests, such as the complete blood count.9,10 In addition, neither make an assessment of how many infants actually should be or would be evaluated or treated.9,11 Concern for EOS results in the evaluation and empirical antibiotic treatment of hundreds of thousands of uninfected newborns annually,12–15 resulting in maternal/infant separation and significant expenditures. For example, EOS evaluations based on the CDC 2002 guidelines11 resulted in the evaluation of 15% of all well-appearing infants born at ≥35 weeks’ gestation at 1 of our centers.14 After revision of our EOS policies to align with the CDC 2010 guidelines, we still found that 13% of both well-appearing and ill-appearing infants were evaluated for EOS and 11% were treated empirically with antibiotics, although only 0.04% of the cohort of 7004 infants had blood-culture confirmed infection.14
More efficient approaches to EOS evaluation are needed. Our team recently described an efficient predictive model for EOS in newborns ≥34 weeks’ gestation based on information available at the moment of birth.2 This multivariate model uses highest maternal antepartum temperature, gestational age, length of time a mother’s membranes were ruptured, GBS carriage status, and type of intrapartum antibiotic therapy received to provide a preliminary risk estimate for EOS; it is currently available for clinical use.16,17 In this phase of our study, we again use objective data to account for the evolving clinical condition of the infant in the first 12 hours after birth. We combine the probability of EOS based on maternal risk factors with the newborn’s evolving clinical presentation to generate a new, updated, posterior probability (PP). This simple, clinically accessible risk stratification scheme permits clinicians to quickly place newborns into 1 of 3 care pathways (treat empirically with antibiotics, evaluate with treatment conditional on further information, and continued observation) more efficiently than currently recommended approaches.
Methods
The institutional review boards for the protection of human subjects (ethics committees) at all participating institutions approved this nested case-control study. The base population (n = 608 014 live births) consisted of all newborns ≥34 weeks’ gestation born at 12 Kaiser Permanente Northern California (KPNC) hospitals and Boston’s Brigham and Women’s Hospital and Beth Israel-Deaconess Medical Center between 1993 and 2007. The study was restricted to inborn infants without major anomalies as defined by the Vermont-Oxford Neonatal Network (www.vtoxford.org); cases had culture-confirmed EOS within the first 72 hours after birth. Details of the case identification, matching, and other aspects of data collection details have been reported,2 so we focus here on description of neonatal data collection for the 350 cases and 1063 controls who were frequency matched by hospital and year of birth.
Using a structured protocol based on previous work13,18,19available to interested readers on request, trained medical record analysts abstracted the following information for study infants from paper charts, as inpatient electronic records had not yet been deployed at these centers: race, exact date and time of birth, birth weight, gestational age in weeks and days, Apgar scores, mode of delivery, whether the infant required resuscitation as well as the type of resuscitation required, presence of meconium staining, clinical milestones (eg, occurrence and exact timing of seizures or apnea episodes and whether these were documented as definite or possible events), and treatment milestones (eg, exact timing of the duration of use of supplemental oxygen, nasal continuous positive airway pressure, intermittent mandatory ventilation, and/or treatment with systemic antibiotics). We made an a priori subdivision of infants into 2 groups based on a 5-minute Apgar of <5. For infants whose 5-minute score was <5, data collection was limited to the previously mentioned items because these infants clearly met our criteria for unequivocal clinical illness. For infants whose 5-minute Apgar was ≥5, we also abstracted the exact date and time for the following items for the first 24 hours of age to assess evolving clinical status: vital signs (temperature, heart rate, respiratory rate, blood pressure), presence of respiratory distress (nasal flaring, grunting, retractions), and additional details regarding respiratory support.
In addition to multiple audits conducted during the data collection process, 3 of us (GJE, KMP, EMW) conducted a second manual review of the records of all patients with sepsis who were well appearing and had a sepsis risk at birth of <0.65/1000 live births.
Analytic Approach
We developed a risk stratification strategy on a randomly selected subset consisting of 167 cases and 494 controls (derivation dataset). This strategy, described in greater detail in the Supplemental Information, included the use of both sepsis risk at birth as well as newborns’ clinical examination. Because the goal of this study was to stratify infants, we analyzed data in a hierarchical manner beginning with the highest level of risk: once an infant was placed in a defined risk category, she or he was removed from subsequent steps in the analysis. After arriving at a final classification strategy, we then applied it to the remaining 183 patients and 569 controls (validation dataset). Because results for both derivation and validation datasets were so similar, we report only the results of the risk stratification strategy in the entire dataset (additional data are available in the Supplemental Information). We did not find that neonatal treatment with antibiotics decreased vital signs abnormalities in our data collection time frame, so we did not include it in our risk stratification.
Table 1 summarizes the definitions of the 3 clinical categories used for risk stratification: clinical illness, equivocal presentation, and well appearing. The details of how we arrived at these groups, which included the use of recursive partitioning, logistic regression, visual examination of predictor-outcome relationship grids, and input from practicing neonatologists, are provided in the Supplemental Information.
TABLE 1.
Hierarchical Classification of Clinical Signsa
| Clinical Presentationb | Description |
|---|---|
| Clinical illness | In the first 12 h of age, the infant had a 5-min Apgar <5; received nasal continuous positive airway pressure or mechanical ventilation; received continuous infusion of vasoactive drugs; had a clinical seizure; or had significant respiratory distress (nasal flaring, grunting, or retractions were present and the infant received supplemental oxygen within the first 6 h) |
| Equivocal presentation | In the first 12 h of age, the infant experienced at least 2 instances of 1 of the following, with “instance”c meaning that there were ≥2 measurements ≥2 h apart: |
| Heart rate ≥160 | |
| Respiratory rate ≥60 | |
| Temperature ≥100.4°F or <97.5°F | |
| Respiratory distress (grunting, flaring, or retracting) | |
| Well appearing | The infant did not fall into one of the above 2 groups in the first 12 h of age |
Clinical presentations shown in the table are mutually exclusive and the scheme is applied sequentially, with infants removed from the group before applying the next category.
To quantify the duration of clinical instability and avoid classifying infants based on transient abnormalities, we used the concept of “instance” for vital signs and respiratory distress (grunting, flaring, or retractions). An instance was the occurrence of a second abnormal value at least 2 hours after the first occurrence of an abnormal index value. Abnormal values after the index measurement counted as a new instance if they were followed by another abnormal measurement at least 2 hours later. Instances were sign-specific (ie, an abnormal temperature followed by an abnormal respiratory rate 2.1 hours later would not count as an instance for either temperature or respiratory rate).
See text, Supplemental Information, and reference 2 for a description of data collection protocol.
We calculated likelihood ratios (LRs) by using Bayes’ rule and the number needed to treat (NNT) in a given group of infants by dividing 1000 by the incidence rate per thousand live births. The Supplemental Information describes how we calculated 95% confidence intervals (CIs) around incidence rates, LRs, and NNTs.
Results
Table 2 summarizes the characteristics of the cases and controls. Maternal data are in our previous report.2 Table 2 shows that case infants tended to have lower gestational age, birth weight, and Apgar scores. The table also shows that significant clinical signs and major deteriorations tended to occur very early (before 6 hours of age).
TABLE 2.
Description of Study Cohorta
| Controls (n = 1063) | Patients (n = 350) | |
|---|---|---|
| Maternal ethnicity, % | ||
| Asian | 15.3 | 14.3 |
| Black | 8.2 | 10.0 |
| Hispanic | 20.9 | 18.9 |
| White | 49.0 | 47.1 |
| Other | 6.6 | 9.7 |
| Multiple gestation, % | 2.8 | 2.0 |
| Gestational age, wk, % | ||
| 34–36 | 6.5 | 14.0 |
| 37–40 | 79.7 | 67.1 |
| ≥41 | 13.8 | 18.9 |
| Male, % | 51.3 | 53.7 |
| Birth weight, g, mean ± SD | 3440 ± 521 | 3399 ± 593 |
| Birth weight <2500 g, % | 3.8 | 6.9 |
| Cesarean delivery, % | 19.5 | 39.4 |
| Apgar score <7 at 5 min, % | 0.7 | 10.9 |
| Died, % | 0.0 | 1.1 |
| Statusb at 6 h of age, % | ||
| Clinical illness | 1.8 | 24.0 |
| Equivocal presentation | 5.6 | 18.6 |
| Well appearing | 92.7 | 57.4 |
| Statusb at 12 h of age, % | ||
| Clinical illness | 2.0 | 27.1 |
| Equivocal presentation | 2.5 | 17.4 |
| Well appearing | 95.6 | 55.4 |
| Statusb at 24 h of age, % | ||
| Clinical illness | 2.2 | 29.4 |
| Equivocal presentation | 0.6 | 2.3 |
| Well appearing | 97.3 | 68.3 |
Additional detail on study cohort can be found in citation 2. Tabular data disaggregating derivation and validation cohorts are in the Supplemental Information.
Clinical status based on available data closest to the infant’s specified age in hours. See Table 1 for the specific description of the hierarchical classification of clinical signs.
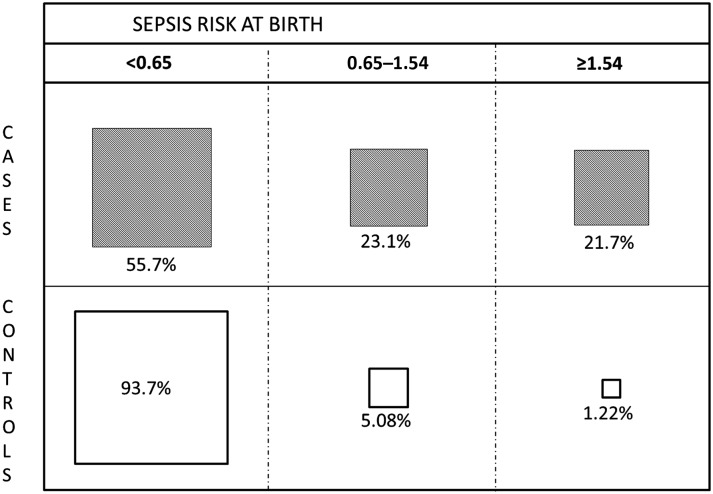
Our previously described model of risk stratification using only sepsis risk at birth that uses only objective maternal data are shown in Fig 1. The figure demonstrates how 2 cutoffs obtained from recursive partitioning initially divided newborns into 3 preliminary risk groups. The infant’s clinical condition also provides information on risk of EOS. The LRs for the 3 clinical categories shown on Table 1 were as follows: clinical illness, 14.5 (95% CI 10.2–21.2); equivocal presentation, 3.75 (2.83–5.00), and well appearing, 0.36 (0.31–0.41). When these LRs are combined with sepsis risk at birth, a PP of EOS can be calculated, permitting much better risk stratification than using either consideration alone (Table 3). This table expresses the results as rates per thousand live births and NNT. Given 350 cases of 608 014 births, the population rate is 0.58 per thousand live births (95% CI 0.52–0.64) and the population NNT is 1737 (1562–1923) (ie, this is the number of infants one would treat with systemic antibiotics per infant with EOS if the entire population were treated).
FIGURE 1.
Sepsis risk at birth ranges identified via recursive partitioning. The boxes are drawn to scale and show the percentage distribution of patients (shaded boxes) and controls (clear boxes), with the sepsis risk at birth per thousand live births from the maternal model (see citation 2) at the top. The figure shows the highly uneven distribution of EOS cases in the study population of infants born at ≥34 weeks’ gestation.
TABLE 3.
Updated Posterior Probability and NNTa
| Clinical Presentationb | Previous Probability (Sepsis Risk at Birth, Based on Maternal Risk Factorsb) Rate per 1000 Live Births | ||
|---|---|---|---|
| <0.65 | 0.65–1.54 | ≥1.54 | |
| Well appearing | |||
| PP | 0.11 (0.08–0.13) | 1.08 (0.70–1.65) | 6.74 (3.09–16.06) |
| NNT | 9370 (7418–12 073) | 923 (605–1428) | 148 (62–323) |
| Equivocal presentation | |||
| PP | 1.31 (0.93–1.84) | 11.07 (5.02–27.74) | |
| NNT | 763 (543–1076) | 90 (36–199) | |
| Clinical illness | |||
| PP | 4.66 (2.80–8.04) | 62.94 (12.94–583.72) | |
| NNT | 214 (124–357) | 16 (2–77) | |
In this table, the columns show 3 sepsis risk at birth ranges calculated based on maternal risk factors (see citation 2), which constitute the initial previous probability for a given neonate. These are then combined with the infant’s clinical presentation (rows) to generate an updated PP and the NNT. The updated PPs, with their associated 95% CIs in parentheses, are expressed as the rate of sepsis per 1000 live births. The NNT (total number of newborns one would need to treat to ensure that all cases of sepsis were treated within a given risk group) is estimated by dividing 1000 by the rate per thousand live births. For the entire study population, in which the incidence was 0.58/1000 (350 cases in a population of 608 014), the number NNT is 1737 (95% CI 1562–1.923). See text for details on how we estimated 95% CIs. Some cells were combined because of very small numbers. For example, only 2.9% of all infants (but 42% of all sepsis cases) showed clinical illness; within this group, infants with a sepsis risk at birth of ≥1.54/1000, who constituted 0.2% of all live births (but 8.3% of all sepsis cases), had a PP of 25.4/1000. Detailed breakdowns for all clinical presentations are provided in the Supplemental Information.
See text and Supplemental Information for a description of how sepsis risk at birth ranges were established. The hierarchical, mutually exclusive clinical categorizations are described in Table 1; a description of their development is in the Supplementarl Information.
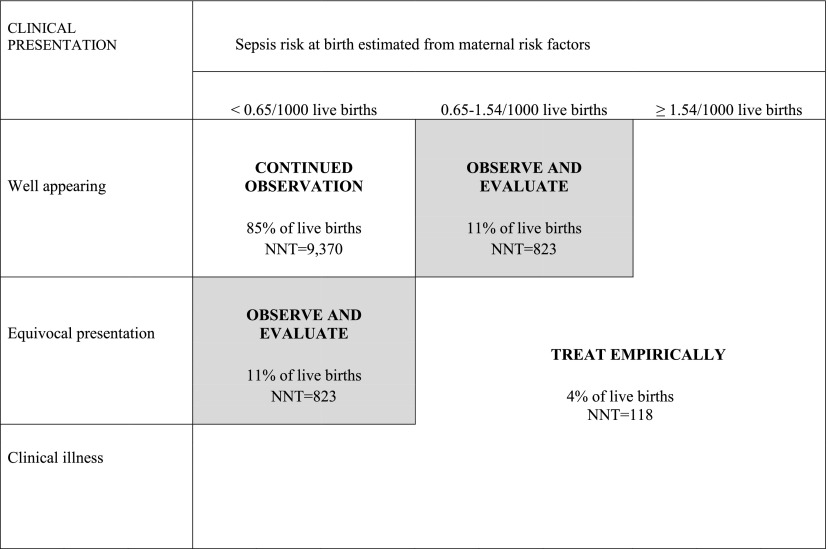
The explicit goal of our study was to define a simple strategy to guide newborn clinical care, which is shown in Figure 2. The high-risk group consists of infants with clinical illness, sepsis risk at birth ≥1.54, or the combination of an equivocal presentation and sepsis risk at birth ≥0.65/1000. This group has an NNT of 118 (95% CI 87–158). It seems reasonable to suggest that infants in this group, which represent 4% of all live births, should enter a clinical pathway that includes immediate treatment with systemic antibiotics pending negative culture results. The middle group (11% of live births) has an NNT of 823 (639–1063) and consists of infants with a sepsis risk at birth ≥0.65/1000 or an equivocal presentation. In these infants, a prudent course of action would be to formally evaluate them with a blood culture and to keep them in the hospital by using a more rigorous observation protocol (eg, more frequent vital signs and repeated examination). If these infants’ clinical status changed or if a culture came back positive, systemic antibiotics could then be started. Because the number of infants with positive cultures is low, the number of infants treated in this group would be very low and would have a negligible contribution to the total number of infants treated. The low-risk group, which would be placed into a continued observation pathway, consists of infants with low a priori risk (sepsis risk at birth <0.65/1000) who had <2 clinical abnormalities in the first 12 hours. This group constitutes 85% of all live births and has a sepsis incidence rate of 0.11/1000 (0.08–0.13). The NNT for this group is 9370 (7418–12 073).
FIGURE 2.
Quantitative Risk Stratification for EOS. Quantitative risk stratification schema for newborns >34 weeks’ gestation developed in this study. Stratification is based on clinical evolution in the first 12 hours of age (rows) and sepsis risk at birth estimated from maternal risk factors (columns). Infants who have a sepsis risk at birth of >1.54/1000 live births, or who have a sepsis risk at birth >0.65/1000 and an equivocal presentation fall into the “Treat Empirically” group, which has an NNT of 118 and accounts for 4% of all live births. Infants with an equivocal presentation (middle cell, far left column) or who are well appearing but whose sepsis risk at birth is 0.65 to 1.54/1000 (top cell, middle column) fall into the “Observe and Evaluate” group, which has an NNT of 823 and accounts for 11% of all live births. Last, the largest group, well-appearing infants with a sepsis risk at birth <0.65/1000, has an NNT of 9370 and accounts for 85% of all live births.
We conducted a second manual review of the records of the 55 newborns placed in the continued observation protocol. We found that 30 (54%) were completely asymptomatic throughout their hospital stay, 22 (41%) remained asymptomatic until after 24 hours, and 3 (5%) presented with sudden collapse or with a combination of major clinical signs not included in our classification scheme (eg, persistent cyanosis, poor perfusion, and hypoglycemia). Reasons for evaluation of the infants in the continued observation category included evaluation for the presence of isolated clinical signs (eg, a single measurement of fever, which does not count as an “instance” for our risk classification scheme) or risk factors (eg, low-grade maternal fever) present in the first few hours of life; evaluation because of maternal postpartum fever (6 infants); or the development of signs of illness beyond 12 hours of life, including 3 newborns who presented with sudden collapse. Further, of these 55 infants, 20 (36%) had GBS-positive cultures but their mothers had not been screened for carriage or tested negative. We recalculated these neonates’ sepsis risk at birth assuming a positive GBS screen (ie, simulating what would have happened had more effective universal screening been in place). In this simulation, the number of sepsis cases placed into the continued observation pathway fell from 55 to 45.
Discussion
We have developed a quantitative risk-stratification strategy of the risk of EOS in newborns of ≥34 weeks’ gestation that combines maternal risk factors with a newborn’s evolving clinical examination. It is clinically intuitive and, because maternal risk factors could be captured and analyzed electronically in real time, it can take maximum advantage of modern electronic medical records. Further, our strategy is not restricted to GBS sepsis. It could supplement existing care protocols by permitting clinicians to group infants into discrete, risk-based, subsets. In addition, from a methodological standpoint, our study is important in that it does not ignore a critical component of a newborn’s clinical examination: the passage of time. Previous studies have labeled infants as being symptomatic or asymptomatic but have not specified these terms with respect to duration of clinical signs.
Using our results clinically would require clinicians to be explicit about their value judgments and that they may need to involve parents in thinking through (1) the number of newborns they would be willing to treat to avoid missing 1 sepsis case, and (2) exactly how and how long low-risk infants should be observed. However, given frustration with existing approaches,15 this may not turn out to be a major problem, and the experience in KPNC suggests that clinicians will adapt. Currently in KPNC, while developers are embedding the maternal model into the Epic inpatient electronic medical record (www.epicsystems.com), clinicians access it via mobile phones17 or from the principal investigator’s Web page.16 To ensure that infants are placed in the right risk groups and that infants in the continued observation group are properly observed, KPNC clinician teams are developing specific protocols as well as electronic order sets that will mandate specific observation periods, time intervals between vital signs measurements, lighting conditions under which infants should be examined, and escalation protocols for transferring infants to a higher level of care.
Application of our risk stratification could have a major effect on hospital use. Currently, ∼6% of newborns born at ≥34 weeks’ gestation are treated with systemic antibiotics in the neonatal period in KPNC and ∼10% in Brigham and Women’s Hospital. Using our strategy, this number would fall to 4%. It is likely that similar impacts would be seen in other settings. Extrapolating to the US birth cohort of ∼4 million births, this would mean 80 000 to 240 000 fewer infants treated each year. An additional benefit of our study is that its improved specification of sepsis risk can also be used for more rigorous delineation of neonatal subpopulations. This also could lead to more sophisticated studies using genomic and proteomic markers, which is important given that it is unlikely that perfect risk stratification can be achieved based on using clinical data only.
Certain important limitations of our study must be emphasized. The study cohort spanned periods during which both risk-based and screening-based approaches to GBS prophylaxis were used. Although the study by Bromberger et al20 showed no difference in the clinical presentation of newborns with GBS sepsis whose mothers did or did not receive intrapartum antibiotics, we cannot exclude the possibility that the clinical presentation of newborns with sepsis may be changing over time. It is also important to note that the relative contribution of GBS status to our predictive model is very small (2.3%); most of the predictive power of the maternal model comes from gestational age (16.7%), highest antepartum temperature (58.4%), and length of time since membranes ruptured (12.6%).2 Nonetheless, our approach would benefit from revalidation using data from centers adhering to the CDC’s recommended practices of 2010. Although it is based on a large cohort, our study may not be representative of all newborns. Thus, it should be validated prospectively, particularly in settings with different bacterial ecology, and, ideally, using a cluster randomized trial design in which our approach would be compared with the CDC recommended approach. Our team is in the process of developing a new cohort from KPNC, Kaiser Permanente Southern California, and the Brigham and Women’s Hospital in Boston. However, because our models are parsimonious, future validation or modification of our approach could be much simpler and could take advantage of existing collaborative structures in neonatology as well as the availability of comprehensive inpatient electronic medical records that were not available when we performed this study.
Our study also does not address how infants should be evaluated or treated. It does not specifically address the role of the complete blood count, a common component of current evaluation recommendations, although we have discussed this elsewhere.21 It is also true that collapsing maternal risk factors into 3 sepsis risk at birth categories does lose some information value, but the gain in simplicity is substantial.
Conclusions
Using sepsis risk at birth based on a maternal risk factors multivariate model and combining it with a newborn’s evolving clinical examination, we have defined a risk stratification strategy for EOS.
Supplementary Material
Acknowledgments
The authors thank Eric Eichenwald, MD, for his contributions to the initial design of this study and for many subsequent helpful discussions; Amy Zolit, Cat Magallon, and Dennis Andaya for chart review and data abstraction; Manuel Chinchilla, Issa Alaweel, and Marla Gardner for database construction and management; and Paul Hughes, Vineeta Vaidya, and Gregory Tomilonus for providing hospital demographic information. We also thank Dr Allen Fischer, KPNC Regional Director for Neonatology, for facilitating access to neonatologist panels, reviewing the manuscript, and multiple helpful discussions; Dr Tracy Lieu, Director of the Division of Research, for reviewing the manuscript; and Ms Rachel Lesser for formatting the manuscript.
Glossary
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- EOS
early-onset sepsis
- GBS
group B Streptococcus
- KPNC
Kaiser Permanente Northern California
- LRs
likelihood ratios
- NNT
number needed to treat
- PP
posterior probability
Footnotes
Dr Escobar was the principal investigator for the National Institute of General Medical Sciences grant, conceptualized and designed the study, obtained funding for the study, directed all phases of the study, and wrote the manuscript with input and approval from all other coauthors; Drs Puopolo, Newman, Zupancic, Lieberman, and Draper were coinvestigators, helped conceptualize the study and its data collection instruments and analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted; Mr Wi and Mr Turk are programmers who conducted analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted; Dr Kuzniewicz helped conceptualize study analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted; and Ms Walsh oversaw data quality audits for the study and assisted in oversight of the programmers’ work, reviewed and revised the manuscript, and approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institute of General Medical Sciences grant R01-GM-80180–3 (to Dr Escobar).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Weston EJ, Pondo T, Lewis MM, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J. 2011;30(11):937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puopolo KM, Draper D, Wi S, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics. 2011;128(5). Available at: www.pediatrics.org/cgi/content/full/128/5/e1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuchat A, Whitney C, Zangwill K, Centers for Disease Control and Prevention . Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996;45(RR-7):1–24 [PubMed] [Google Scholar]
- 4.Schuchat A, Zywicki SS, Dinsmoor MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics. 2000;105(1 pt 1):21–26 [DOI] [PubMed] [Google Scholar]
- 5.Bizzarro MJ, Raskind C, Baltimore RS, Gallagher PG. Seventy-five years of neonatal sepsis at Yale: 1928–2003. Pediatrics. 2005;116(3):595–602 [DOI] [PubMed] [Google Scholar]
- 6.Puopolo KM, Eichenwald EC. No change in the incidence of ampicillin-resistant, neonatal, early-onset sepsis over 18 years. Pediatrics. 2010;125(5). Available at: www.pediatrics.org/cgi/content/full/125/5/e1031 [DOI] [PubMed] [Google Scholar]
- 7.Cohen-Wolkowiez M, Moran C, Benjamin DK, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28(12):1052–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers of Disease Control and Prevention 2012. Active Bacterial Surveillance Report, Emerging Infections Program Network, Group B Streptococcus, 2010. http://www.cdc.gov/abcs/reports-findings/survreports/gbs10-orig.html. Accessed November 8, 2013
- 9.Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR-10):1–36 [PubMed]
- 10.Polin RA, Committee on Fetus and Newborn . Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129(5):1006–1015 [DOI] [PubMed] [Google Scholar]
- 11.Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1–22 [PubMed] [Google Scholar]
- 12.Johnson CE, Whitwell JK, Pethe K, Saxena K, Super DM. Term newborns who are at risk for sepsis: are lumbar punctures necessary? Pediatrics. 1997;99(4). Available at: www.pediatrics.org/cgi/content/full/99/4/E10 [DOI] [PubMed] [Google Scholar]
- 13.Escobar GJ, Li DK, Armstrong MA, et al. Neonatal sepsis workups in infants >/=2000 grams at birth: a population-based study. Pediatrics. 2000;106(2 pt 1):256–263 [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay S, Eichenwald EC, Puopolo KM. Neonatal early-onset sepsis evaluations among well-appearing infants: projected impact of changes in CDC GBS guidelines. J Perinatol. 2012;33(3):198–205 [DOI] [PMC free article] [PubMed]
- 15.Taylor JA, Opel DJ. Choriophobia: a 1-act play. Pediatrics. 2012;130(2):342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser Permanente, Division of Research. Probability of neonatal early-onset infection based on maternal risk factors. Available at: http://www.dor.kaiser.org/external/DORExternal/research/InfectionProbabilityCalculator.aspx, accessed March 30, 2013
- 17.newbornsepsiscalculator.org. Accessed November 8, 2013
- 18.Escobar GJ, Fischer A, Kremers R, Usatin MS, Macedo AM, Gardner MN. Rapid retrieval of neonatal outcomes data: the Kaiser Permanente Neonatal Minimum Data Set. Qual Manag Health Care. 1997;5(4):19–33 [PubMed] [Google Scholar]
- 19.Escobar GJ. The neonatal “sepsis work-up”: personal reflections on the development of an evidence-based approach toward newborn infections in a managed care organization. Pediatrics. 1999;103(1 suppl E):360–373 [PubMed] [Google Scholar]
- 20.Bromberger P, Lawrence JM, Braun D, Saunders B, Contreras R, Petitti DB. The influence of intrapartum antibiotics on the clinical spectrum of early-onset group B streptococcal infection in term infants. Pediatrics. 2000;106(2 pt 1):244–250 [DOI] [PubMed] [Google Scholar]
- 21.Newman TB, Puopolo KM, Wi S, Draper D, Escobar GJ. Interpreting complete blood counts soon after birth in newborns at risk for sepsis. Pediatrics. 2010;126(5):903–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.