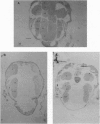
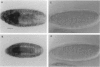
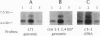Abstract
In Drosophila, stripe (sr) gene function is required for normal muscle development. Some mutations disrupt embryonic muscle development and are lethal. Other mutations cause total loss of only a single muscle in the adult. Molecular analysis shows that sr encodes a predicted protein containing a zinc finger motif. This motif is homologous to the DNA binding domains encoded by members of the early growth response (egr) gene family. In mammals, expression of egr genes is induced by intercellular signals, and there is evidence for their role in many developmental events. The identification of sr as an egr gene and its pattern of expression suggest that it functions in muscle development via intercellular communication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellen H. J., O'Kane C. J., Wilson C., Grossniklaus U., Pearson R. K., Gehring W. J. P-element-mediated enhancer detection: a versatile method to study development in Drosophila. Genes Dev. 1989 Sep;3(9):1288–1300. doi: 10.1101/gad.3.9.1288. [DOI] [PubMed] [Google Scholar]
- Celniker S. E., Lewis E. B. Molecular basis of transabdominal--a sexually dimorphic mutant of the bithorax complex of Drosophila. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1566–1570. doi: 10.1073/pnas.90.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker S. E., Lewis E. B. Transabdominal, a dominant mutant of the Bithorax Complex, produces a sexually dimorphic segmental transformation in Drosophila. Genes Dev. 1987 Apr;1(2):111–123. doi: 10.1101/gad.1.2.111. [DOI] [PubMed] [Google Scholar]
- Costello W. J., Wyman R. J. Development of an indirect flight muscle in a muscle-specific mutant of Drosophila melanogaster. Dev Biol. 1986 Nov;118(1):247–258. doi: 10.1016/0012-1606(86)90092-8. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Fernandes J., Bate M., Vijayraghavan K. Development of the indirect flight muscles of Drosophila. Development. 1991 Sep;113(1):67–77. doi: 10.1242/dev.113.1.67. [DOI] [PubMed] [Google Scholar]
- Gill G., Pascal E., Tseng Z. H., Tjian R. A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):192–196. doi: 10.1073/pnas.91.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber D. A., Buckler A. J., Glaser T., Call K. M., Pelletier J., Sohn R. L., Douglass E. C., Housman D. E. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms' tumor. Cell. 1990 Jun 29;61(7):1257–1269. doi: 10.1016/0092-8674(90)90690-g. [DOI] [PubMed] [Google Scholar]
- Hresko M. C., Williams B. D., Waterston R. H. Assembly of body wall muscle and muscle cell attachment structures in Caenorhabditis elegans. J Cell Biol. 1994 Feb;124(4):491–506. doi: 10.1083/jcb.124.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht J. D., Hanna-Rose W., Reddy J. C., English M. A., Ro M., Grossel M., Shaknovich R., Hansen U. Mapping and mutagenesis of the amino-terminal transcriptional repression domain of the Drosophila Krüppel protein. Mol Cell Biol. 1994 Jun;14(6):4057–4066. doi: 10.1128/mcb.14.6.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden S. L., Rauscher F. J., 3rd Positive and negative regulation of transcription and cell growth mediated by the EGR family of zinc-finger gene products. Ann N Y Acad Sci. 1993 Jun 11;684:75–84. doi: 10.1111/j.1749-6632.1993.tb32272.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Nguyen H. Q., Hoffman-Liebermann B., Liebermann D. A. The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993 Jan 29;72(2):197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- Rothman A., Wolner B., Button D., Taylor P. Immediate-early gene expression in response to hypertrophic and proliferative stimuli in pulmonary arterial smooth muscle cells. J Biol Chem. 1994 Mar 4;269(9):6399–6404. [PubMed] [Google Scholar]
- Schneider-Maunoury S., Topilko P., Seitandou T., Levi G., Cohen-Tannoudji M., Pournin S., Babinet C., Charnay P. Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell. 1993 Dec 17;75(6):1199–1214. doi: 10.1016/0092-8674(93)90329-o. [DOI] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P. Early transcriptional events in cell growth: the Egr family. J Am Soc Nephrol. 1990 Dec;1(6):859–866. doi: 10.1681/ASN.V16859. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Volk T., VijayRaghavan K. A central role for epidermal segment border cells in the induction of muscle patterning in the Drosophila embryo. Development. 1994 Jan;120(1):59–70. doi: 10.1242/dev.120.1.59. [DOI] [PubMed] [Google Scholar]
- de la Pompa J. L., Garcia J. R., Ferrús A. Genetic analysis of muscle development in Drosophila melanogaster. Dev Biol. 1989 Feb;131(2):439–454. doi: 10.1016/s0012-1606(89)80016-8. [DOI] [PubMed] [Google Scholar]