Abstract
Context:
In order to characterize diurnal changes in central leptin and its target neuropeptide, proopiomelanocortin (POMC), we measured leptin and POMC in cerebrospinal fluid (CSF) as related to changes in plasma leptin and soluble leptin receptor (sOB-R) levels. CSF and plasma levels of 20 amino acids (AA) were also measured because AA can affect brain POMC.
Design and Participants:
Stored CSF and plasma samples obtained from eight healthy subjects who served as controls for a previous study were evaluated. CSF was collected hourly over 33 h via indwelling subarachnoid catheter. Leptin, sOB-R, and POMC were measured by sensitive ELISA and AA by gas chromatography-mass spectrometry.
Results:
There was a diurnal rhythm for plasma leptin with a peak at 2200 h (144% of baseline) and there was a similar diurnal rhythm for CSF leptin with a peak (117%) 3–5 h after the plasma peak. Plasma sOB-R was lowest at 0300 h and correlated negatively with plasma and CSF leptin. A diurnal rhythm for POMC in CSF was also detected with a peak (125%) at 0100 h. A positive correlation existed between CSF POMC and leptin in individual subjects over time. CSF levels of many AA increased at night. There was a significant correlation between CSF POMC and 10 AA, including leucine, isoleucine, tryptophan, and tyrosine.
Conclusions:
Diurnal changes occur in leptin and POMC in human CSF that likely reflect changes in central leptin and melanocortin activity. Our results suggest that nocturnal elevations in leptin, AA, and POMC may help to suppress appetite and feeding at night.
Leptin and ingested nutrients regulate key brain neurons, including hypothalamic proopiomelanocortin (POMC) neurons that maintain energy balance (1, 2). Leptin enters the brain by a receptor-mediated saturable transport mechanism (3). The short form of the leptin receptor, found in the choroid plexus, has been implicated in transporting leptin into the brain (4). The soluble leptin receptor (sOB-R), another isoform of the leptin receptor that circulates in blood, can also affect leptin transport into the brain. There is evidence that sOB-R functions as a leptin-binding protein that can delay leptin clearance from the circulation. In animals, sOB-R can inhibit leptin transport into the brain (5) and affect energy balance (6). In humans, plasma sOB-R levels correlate negatively with BMI and increase with fasting (7, 8). A diurnal rhythm for plasma leptin is well established in humans with highest levels in the evening (9, 10). There is also a rhythm for sOB-R that is opposite to that of leptin that could affect leptin transport into brain (8). It is unknown to what extent central leptin levels change with respect to these circadian variations in plasma leptin and sOB-R levels. Cerebrospinal fluid (CSF) leptin has been used as a surrogate for brain leptin but previous studies of leptin in human CSF have been limited by assay sensitivity for the low levels of leptin present in CSF. We have therefore used a sensitive ELISA to measure leptin in human CSF as related to changes in plasma leptin and sOB-R levels.
POMC neurons play a critical role in responding to leptin as well as to other hormonal and nutrient signals, and disruption of these neurons causes obesity in humans and animals. Leptin stimulates POMC and the POMC-derived peptide, α-MSH, which inhibits food intake and stimulates energy expenditure (11). In rodents, circadian changes in hypothalamic POMC have been reported but it is unknown whether there are circadian changes in brain POMC in the human (12). To assess potential circadian changes in brain POMC in the human we measured POMC in CSF as a surrogate for brain POMC. We have chosen to assess POMC by measuring the intact POMC precursor using a specific ELISA. The POMC prohormone is the predominant POMC peptide in human CSF, with levels up to 50-fold higher than its processed peptide products (13). Previous studies in the rodent have shown that levels of the intact POMC prohormone in CSF can serve as a measure of hypothalamic POMC activity (14). We have also measured plasma and CSF levels of 20 amino acids (AAs) to characterize the relative differences in peripheral and central concentrations because there is evidence that AAs may influence brain signaling pathways, and branched-chain amino acids (BCAA) have been shown to affect brain POMC in the rodent (15, 16). The purpose of this study was to test the hypothesis that there are diurnal changes in CSF leptin and POMC in humans that reflect changes in brain leptin and POMC activity, and to examine relationships with plasma sOB-R and plasma and CSF AA levels. Accordingly, we have measured plasma leptin, sOB-R, and AA levels every 1–2 h and CSF leptin, POMC, and AA levels every hour over a 33-h period in eight healthy subjects.
Materials and Methods
Subjects
Stored CSF and plasma samples were obtained from eight subjects in good general health (5 males, 3 females), mean age 34.5 years (range, 23–49 years), who were recruited from the community and served as controls for an amyloid-β metabolism study conducted at Washington University School of Medicine, St Louis, MO (17). Individual BMI values and fasting serum insulin and leptin levels are depicted in Table 1. These studies were approved by the Washington University Human Studies Committee and the Clinical Research Unit (CRU) Advisory Committee. Informed written consent was obtained from all participants.
Table 1.
Subject Characteristics
| Subject | Sex | Age (y) | BMI (kg/m2) | Fasting Insulin (μU/mL) | Plasma Leptin (ng/mL) |
|---|---|---|---|---|---|
| 1 | F | 41 | 30.9 | 24.1 | 45.4 |
| 2 | F | 27 | 25.0 | 8.7 | 14.8 |
| 3 | F | 27 | 23.3 | 6.1 | 10.5 |
| 4 | M | 39 | 31.0 | 10.4 | 7.8 |
| 5 | M | 46 | 28.9 | 5.4 | 3.2 |
| 6 | M | 49 | 32.6 | 11.3 | 9.0 |
| 7 | M | 24 | 23.8 | 7.2 | 5.7 |
| 8 | M | 23 | 27.3 | 7.1 | 4.9 |
| Mean | 34.5 | 27.8 | 10.0 | 12.7 |
BMI, body mass index; F, female; M, male.
Protocol
Study subjects were admitted to the CRU at 0700 h after fasting from 2000 h the previous evening. An iv catheter was placed in an antecubital vein to obtain blood samples. An intrathecal catheter was placed at the L3-L4 interspace using a Touhy needle for chronic CSF sampling. CSF samples (6 mL) were obtained hourly throughout the study for up to 36 h. Blood samples were obtained hourly for 16 h and then every 2 h. CSF and plasma samples were centrifuged and frozen immediately at −80 C. Aliquots of CSF and plasma (0.5 mL) were obtained over a 33-h period for the current study. The CSF samples had been previously thawed once and then quickly refrozen at −80 C. The CRU Research Kitchen provided meals (60% carbohydrate, 20% fat, 20% protein). However, dietary protein was low at breakfast and lunch on day 1 (5% of total energy) to achieve a low leucine diet during 13C6-leucine infusion as part of another protocol to study fractional production and clearance rates of amyloid-ß (17). Meals were provided at 0900, 1300, and 1800 h with snacks at 1100, 1500, and 2000 h. Subjects consumed an average of 90.1% (range, 65.2–106%) of their calculated energy requirements, determined by the Harris-Benedict equation, and were allowed to maintain their own natural light-dark and sleep cycles.
Assays
CSF leptin was measured by a highly sensitive ELISA (R&D Systems) with a sensitivity of 7 pg/mL (18). Plasma leptin was measured by RIA (Millipore Corporation). The Bland-Altman analysis suggested that the two leptin assays provided similar measures. The bias was −0.62; the 95% limits of agreement between assays ranged from −4.0–2.7 and no measures fell outside these limits. The correlation between plasma leptin measured by RIA and ELISA was r = 0.98, P < .0001. Plasma sOB-R was measured by ELISA (R&D Systems); sensitivity, 0.3 ng/mL. CSF POMC was measured by two-site ELISA with antibodies provided by Anne White; the capture monoclonal antibody was directed at ACTH (10–18); detection antibody was directed against γ-MSH (18, 19). There is 100% cross-reactivity with 22K pro-ACTH but none with ACTH, α-MSH, γ-MSH, or ß-endorphin. Affinity-purified human 31K POMC was used for standards. Assay sensitivity was 8 fmol/mL. We have previously shown by gel filtration that most the POMC immunoactivity detected in CSF elutes in the same position as 31K POMC standard (13, 18). AAs were measured at the University of Michigan Metabolomics Core Laboratory after purification and derivatization of 100-μL samples of plasma or CSF via gas chromatography-mass spectrometry (Agilent 6890) using a modified EZ:faast kit (Phenomenex); 13C-labeled AAs were used as internal standards (20). Serum insulin was measured by solid-phase enzyme-labeled chemiluminescent immunometric assay, Immulite1000 (Siemens Healthcare Diagnostics).
Statistical analysis
Data were analyzed over time by one-way repeated measures ANOVA and Dunnett's multiple comparison test. Correlations were determined by linear regression analysis with Pearson's correlation. Cosinor analysis was performed as previously described and was used to analyze the 33-h patterns of plasma and CSF leptin and CSF POMC (21). A cosine transformation was applied to the time variable using 24 h as the default circadian cycle and the GraphPad Prism version 6.0b for Mac (Graphpad Software) was used to estimate the parameters of the circadian patterns for leptin and POMC fluctuations. The mesor (midline of the oscillation), amplitude (distance between the peak and mesor), and acrophase (the time corresponding to the peak of the curve) were calculated for each subject and averaged for the group.
Results
Plasma and CSF leptin levels and plasma sOB-R levels
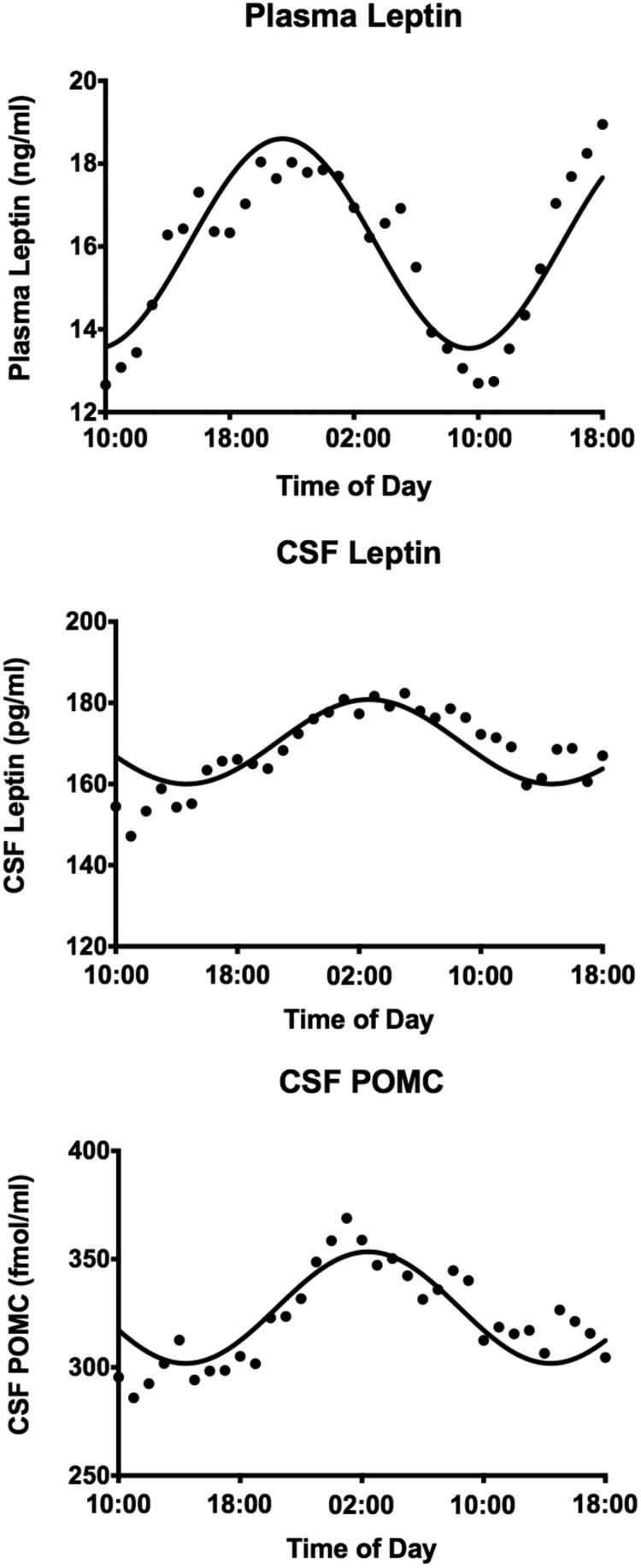
Mean plasma and CSF leptin levels as percent of their respective 1000-h baseline levels are shown in Figure 1. There was a diurnal rhythm for plasma leptin with a peak at 2200 h (144% of baseline); there was a similar rhythm for leptin in CSF with a peak 3–5 h (117% of baseline) after the plasma peak. The actual mean peak value for plasma leptin was 18.0 ± 7.2 ng/mL at 2200 h with a nadir of 12.7 ± 4.8 ng/mL at 1000 h. Peak CSF leptin was 182 ± 27 pg/mL at 0300 h with a nadir of 147 ± 18 pg/mL at 1100 h. CSF leptin levels were approximately 100-fold less than plasma levels. The nocturnal increase in plasma and CSF leptin was significant by ANOVA. Correlations between plasma and CSF leptin over 33 h are shown in Figure 1. The correlation between simultaneously collected plasma and CSF leptin was not significant; however, it became highly significant when CSF leptin levels were back-shifted by 2–5 h (r = 0.664, P < .001) (Figure 1). Cosinor analysis was used to assess the circadian pattern of plasma and CSF leptin (Figure 2). The data fit a cosine function with r2 = 0.808 for plasma leptin and 0.626 for CSF leptin.
Figure 1.
Mean plasma and CSF leptin levels (±SEM) over 33 h in eight subjects depicted as percent of their respective 1000-h baseline levels (left panel). Correlations between plasma and CSF leptin over the 33-h period are shown on the right. The correlation between simultaneously collected plasma and CSF leptin was not significant; however, it became highly significant when CSF leptin levels were back-shifted by 2–5 h to correct for the time delay for CSF flow to the lumbar intrathecal space (right panel).
Figure 2.
Cosinor fit of the plasma and CSF leptin and CSF POMC levels over time. Plasma leptin (upper panel): mesor, 16.1 ng/mL; amplitude, 2.5 ng/mL; acrophase, 2130 h; r2 = 0.808. CSF leptin (middle panel): mesor, 170 pg/mL; amplitude, 10.4 pg/mL; acrophase, 0248 h; r2 = 0.626. CSF POMC (lower panel): mesor, 328 fmol/mL; amplitude, 25.8 fmol/mL; acrophase, 0242 h; r2 = 0.704.
There was a positive correlation (r = 0.780, P = .02) of the mean plasma leptin level averaged over 33 h from each of the eight subjects with the respective mean CSF leptin level averaged over 33 h. However, there was a negative correlation between plasma leptin and the CSF/plasma leptin ratio (r = −0.874, P = .005), consistent with decreased percent transport of leptin into brain and CSF in individuals with higher plasma leptin levels. As expected, plasma leptin and fasting insulin were positively correlated (r = 0.917, P = .0014). Plasma insulin was negatively correlated with the CSF/plasma leptin ratio (r = −0.752, P = .03).
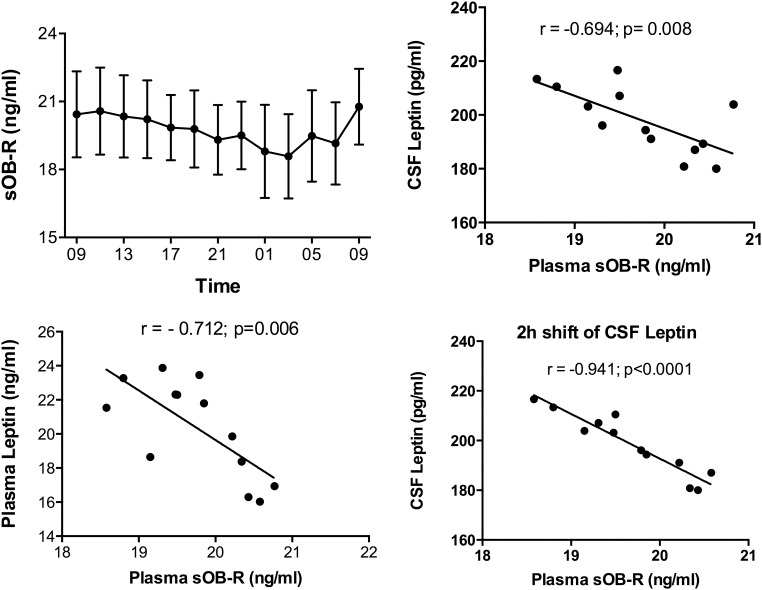
Mean plasma sOB-R levels changed significantly over time (P = .003) with a nadir at 0300 h when plasma and CSF leptin levels were elevated (Figure 3). There was a negative correlation between plasma leptin and sOB-R (r = −0.712; P = .006). A negative correlation between plasma sOB-R and CSF leptin was also noted (r= −0.694; P = .008) and became stronger when CSF leptin levels were shifted by 2h (r = −0.941; P < .0001) (Figure 3).
Figure 3.
Mean plasma sOB-R levels showing a significant change over time (P = .003) with a nadir at 0300 h (left upper panel). There was a significant negative correlation between plasma leptin and sOB-R levels (r = −0.712, P = .006) (left lower panel). A negative correlation between plasma sOB-R and CSF leptin was also noted that became stronger when CSF leptin levels were shifted by 2 h (right panels).
CSF POMC levels
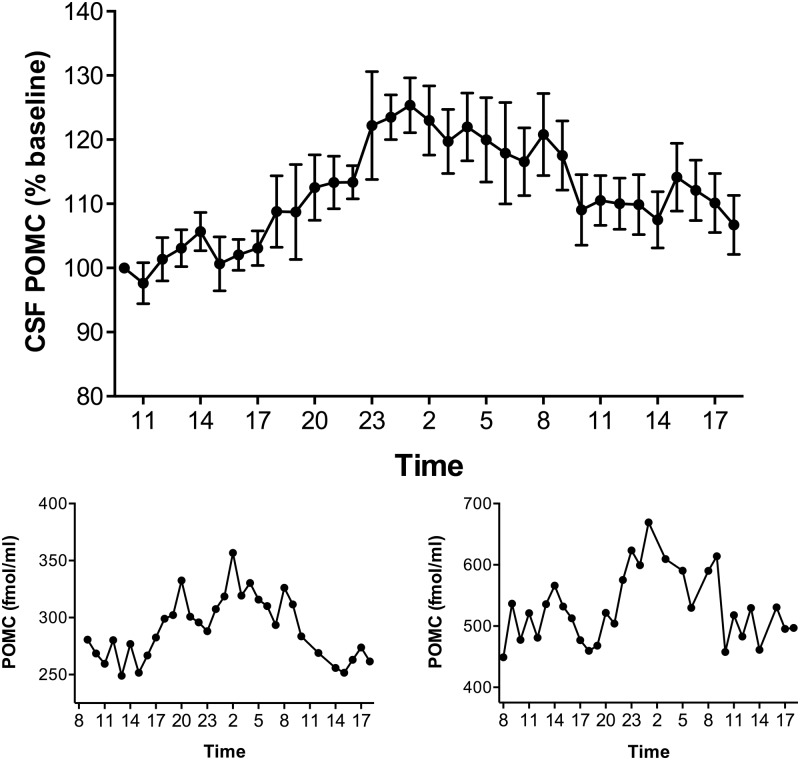
Mean CSF POMC as percent of the respective 1000-h baseline level is shown in Figure 4. There was a diurnal rhythm for CSF POMC with a peak between 2300 h and 0200 h (125% of baseline). The actual mean peak level of POMC of 369 ± 59 fmol/mL occurred at 0100 h with a nadir of 286 ± 45 fmol/mL at 1100 h. Individual CSF POMC profiles from two subjects are also depicted in Figure 4. The nocturnal elevation of CSF POMC was significant by ANOVA (P < .001). Cosinor analysis was used to assess the circadian pattern of CSF POMC (Figure 2). The data fitted a cosine function with r2 = 0.704. There was a strong positive correlation between CSF POMC and leptin when changes were evaluated over the 33-h time course (r = 0.755; P < .001). In contrast, when a single mean CSF POMC value from each of the eight subjects was correlated with their respective mean CSF leptin level, there was a nonsignificant negative correlation (r = −0.429; P = .28). Similar nonsignificant negative correlations were noted when CSF POMC was correlated with BMI and fasting insulin levels.
Figure 4.
Mean CSF POMC levels (±SEM) over 33 h in eight subjects depicted as percentage of their respective 1000-h baseline levels (upper panel). CSF POMC concentrations (fmol/mL) in two individual subjects are depicted below (lower panel).
Plasma and CSF amino acid levels
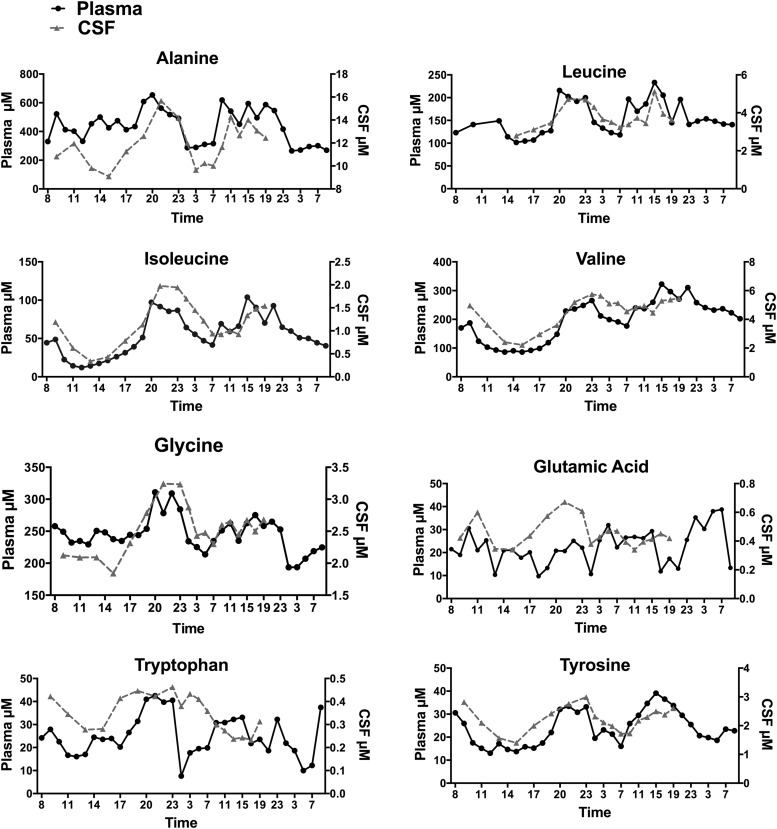
Twenty AAs were measured in plasma and CSF and correlated with CSF POMC and leptin levels. Selected plasma and CSF AA levels in an individual subject are shown in Figure 5. Mean plasma and CSF values of all 20 AAs in the eight subjects over 33 h are graphed in Supplemental Figure 1 and Supplemental Table 1. Mean CSF levels of several AAs increased at night, including leucine and isoleucine, which increased by 1.6- and 3.3-fold, respectively in the eight subjects; increases up to 2.5- and 6-fold were seen in individual subjects. Mean plasma and CSF AA levels in eight subjects along with peak and nadir levels and correlations between plasma and CSF AAs are shown in Supplemental Table 1. There were significant correlations between plasma and CSF levels of 13 AAs, including BCAAs. Mean CSF POMC and leptin levels in the eight subjects correlated with mean levels of several AAs in CSF (Supplemental Table 2). Significant correlations of CSF POMC were noted with CSF asparagine, isoleucine, leucine, lysine, methionine, ornithine, phenylalanine, sarcosine, tryptophan, and tyrosine. CSF leptin was significantly correlated with asparagine, glycine, isoleucine, leucine, lysine, methionine, ornithine phenylalanine, threonine, tryptophan, and tyrosine.
Figure 5.
Plasma and CSF AA levels. Changes in plasma and CSF levels of eight amino acids in an individual subject.
Discussion
This study demonstrates for the first time a circadian rhythm for leptin and its target neuropeptide, POMC, in human CSF and confirms the previously reported circadian rhythm for plasma leptin (9). The CSF leptin rhythm was considerably dampened compared with the plasma rhythm such that 24-h changes varied by only 20% in CSF compared with 44% in plasma. Although there was a similar diurnal rhythm for leptin in CSF and plasma, the CSF leptin peak occurred 3–5 h after the plasma peak. Furthermore, there was no correlation between plasma and CSF leptin levels over 33 h when simultaneously collected samples were compared; however, the correlation became highly significant when CSF leptin levels were back-shifted by 2–5 h to take into account the time needed for leptin transport into CSF and CSF flow to the lumbar intrathecal space. This time delay is consistent with prior protein labeling studies of ß-amyloid during 36-h collections in human CSF (21). A previous study by Wong et al (10) examined the 24-h pattern of leptin in plasma and CSF of human subjects and although they did detect a diurnal plasma rhythm, they did not detect a diurnal rhythm in CSF, nor did they find a significant correlation between plasma and CSF leptin (10). This result may have been due to the relatively poor sensitivity of the assay that was used to detect CSF leptin concentrations, which are 100-fold lower than plasma concentrations. In contrast, we used a highly sensitive leptin ELISA that easily detected the low levels of leptin present in CSF. Our CSF leptin data are consistent with diurnal regulation of brain leptin targets.
Several studies have measured leptin in single human CSF samples and levels were shown to correlate strongly with plasma leptin (22–24). In our study, mean CSF and plasma leptin from each of the eight subjects was positively correlated; however, the CSF-to-plasma-leptin ratio was negatively correlated with plasma leptin. This confirms previous reports showing that the CSF-to-plasma-leptin ratio correlates negatively with plasma leptin and BMI (22, 23). This is consistent with a saturable transport process resulting in a relative decrease in the transport of leptin across the blood-brain barrier (BBB) in obesity. These data are consistent with studies in mice demonstrating that leptin enters the brain by a receptor-mediated saturable transport mechanism (3) and that there is diurnal variation in the influx of 125I-labeled leptin from the blood to the brain (25).
sOB-R may modulate leptin transport into the brain (5). In this study we report a negative correlation between plasma sOB-R and plasma leptin, consistent with previous reports, (7, 8) but in addition show a negative correlation between sOB-R and CSF leptin. sOB-R levels were lowest between 0100 and 0300 h when CSF leptin peaks, consistent with the hypothesis that sOB-R inhibits leptin transport into the brain. In rodents, sOB-R can inhibit the permeation of leptin across the BBB by reducing binding and endocytosis of leptin (5). However, the physiological role of sOB-R is not yet completely understood and some results are contradictory with respect to leptin transport into the brain and energy balance. In humans, plasma sOB-R correlates negatively with BMI and is inversely related to diabetes risk (26). Furthermore, transgenic overexpression of sOB-R in mice results in reduced body weight (6). Yet, sOB-R increases with fasting in humans (8). In addition, during pregnancy there is an increase in plasma leptin, but not in CSF leptin (18). A potential explanation for this discrepancy is the parallel increase in plasma sOB-R levels during pregnancy that may inhibit leptin transport into the brain and help to maintain positive energy balance. The results of the current study suggest that the fall in sOB-R levels during the night may serve to enhance leptin transport into the brain.
POMC is an important target for leptin and the POMC-derived MSH peptides play a critical role in regulating energy balance (27). In this study, POMC was measured by an ELISA that detects the intact POMC prohormone, which is the predominant form of POMC in human CSF (13). As previously reported, we detected relatively high levels of POMC in CSF (13, 18). Prior studies have shown that POMC is much higher in CSF than in plasma and that CSF and plasma levels of POMC peptides are regulated differently (13, 28). Furthermore, POMC peptides persist in human CSF after hypophysectomy, consistent with brain origin (28). A brain origin for CSF POMC is further supported by studies in rodents showing parallel changes in CSF and hypothalamic POMC levels (14). In the current study, there was a clear diurnal rhythm for POMC in CSF that is characterized by a nocturnal peak between 2300 and 0200 h. This is consistent with rodent studies that report diurnal changes in POMC expression in the hypothalamus with a nadir at 1800 h before the animals start feeding at night and provides additional support that CSF POMC reflects central melanocortin activity (12). The nocturnal peak of CSF POMC is thus likely to reflect brain POMC activity and may help to suppress appetite and feeding at night. There was a strong positive correlation between CSF POMC and leptin when changes were evaluated over the 33-h time course. This is consistent with the known stimulatory effects of leptin on POMC. However, because the relationship between leptin and POMC in different subjects may vary with adiposity, we also correlated mean CSF POMC from each of the eight subjects with their respective mean CSF leptin level and found a nonsignificant negative correlation. This trend is similar to a study that we performed in the monkey showing significant negative correlations between CSF POMC and leptin and between CSF POMC and BMI (29). In that study, obese animals with the highest BMI had lower POMC levels, possibly consistent with varying degrees of leptin resistance. However, in the current study, a positive correlation between CSF leptin and POMC is noted in individual subjects over time regardless of adiposity and the absolute CSF POMC or leptin concentration.
Nutrients including glucose, fatty acids, and AAs can also interact with melanocortin neurons (16, 30). We therefore measured AA levels in plasma and CSF and correlated them with CSF leptin and POMC. Although our hypotheses related primarily to the BCAA, tyrosine, and tryptophan, we report data on all 20 proteogenic AAs that were measured by the Phenomenex kit. There were significant positive correlations between CSF POMC and 10 of the 20 AAs measured over 33 h. The observed Pearson's correlation was reported for each AA without any correction for multiple comparisons given that AAs share similar structure, transport and metabolic fate, and are not truly independent measurements. An important focus was on the BCAA, and highly significant correlations were noted between CSF POMC and CSF leucine and isoleucine levels. In rodents, leucine has been shown to stimulate POMC and activate a hypothalamic-brainstem circuit that regulates food intake (16). Leucine can regulate food intake centrally via stimulation of the mTOR pathway (15) and chronic administration can attenuate diet-induced obesity (31). In the rat, plasma and brain levels of BCAA increase as dietary protein increases (32). In our study, CSF levels of BCAA increased in parallel with plasma levels: mean increases in CSF leucine and isoleucine were 1.6- and 3.3-fold respectively. However, in some subjects, CSF levels changed by as much as 6-fold with levels being highest at night. In a study in the monkey, plasma and CSF BCAA levels varied markedly with time of day and dietary protein content (33). The authors found that plasma and CSF leucine concentrations varied up to 4-fold. We speculate that the magnitude of the changes in CSF BCAA levels noted in both the human and monkey studies could potentially influence the mTOR pathway and neuropeptide activity in hypothalamic neurons. Another focus was on CSF tryptophan and tyrosine in view of rodent studies showing that dietary protein can affect brain concentrations of tryptophan and tyrosine, and their associated serotonin and catecholamine neurotransmitter products (34, 35). Both serotonergic and dopaminergic pathways have been implicated in the regulation of hypothalamic POMC (36, 37). It is therefore of interest that in our subjects CSF POMC correlated strongly with CSF tryptophan and tyrosine levels. Tyrosine has also been shown to increase leptin transport into brain (38). Plasma BCAAs are transported into brain by a transporter located on capillary endothelial cells at the BBB. This transporter is competitive and is shared by several large neutral AAs including tryptophan and tyrosine (39). Thus, increases in BCAA could negatively affect the transport of tryptophan and tyrosine. In our study, CSF levels of all of these AAs tended to increase at night and we did not detect negative correlation between CSF BCAA and tryptophan or tyrosine levels. Thus as with the BCAA, the changes in CSF tryptophan and tyrosine may be large enough to influence metabolic, and signaling pathways in the brain and may affect the nocturnal changes in POMC. Although our focus was on CSF AA, it is clear that other dietary nutrients could also affect nocturnal changes in CSF POMC. Furthermore, other neuroendocrine systems that are diurnally regulated may be involved in this process, and even sleep itself may affect brain diurnal rhythms (40).
In summary, significant diurnal changes in CSF leptin and POMC levels occur in normal subjects that likely reflect changes in brain leptin and melanocortin activity. In both cases a nocturnal peak was observed shortly after midnight. These changes were accompanied by changes in CSF levels of several AAs that could potentially affect brain signaling pathways involved with the regulation of energy balance including the melanocortin pathway. These results suggest that nocturnal elevations in brain leptin, AA, and POMC levels may help to suppress appetite and decrease feeding at night. It remains to be determined whether eating disorders, including night eating disorders, are characterized by disruption of these normal rhythms.
Acknowledgments
We thank Ms Rose Connors for her help with sample organization and shipment, Ms Shveta Dighe for technical assistance, and the nurses and dieticians in the CRU for help in performing the studies and the study participants. The authors are particularly grateful to Dr Abby Bloch for her help in fostering the collaboration among the Atkins Foundation Consortium.
This work was supported by NIH Grant RO1-DK093920 (S.L.W.), the Robert C. and Veronica Atkins Foundation Consortium (S.L.W, C.F.B., and S.K.), K23-AG0309460 (R.J.B.), R01-NS065667 (R.J.B.), DK 37948 (S.K.), DK 56341 (Washington University Nutrition Obesity Research Center), DK 26687 (New York Nutrition Obesity Research Center), DK089503 (Michigan Nutrition Obesity Research Center), U24-DK097153 (Michigan Regional Comprehensive Metabolomics Resource Core), A. Alfred Taubman Institute (C.F.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- amino acid
- BBB
- blood-brain barrier
- BCAA
- branched-chain amino acid
- CRU
- Clinical Research Unit
- CSF
- cerebrospinal fluid
- sOB-R
- soluble leptin receptor
- POMC
- proopiomelanocortin.
References
- 1. Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121:2087–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee M, Wardlaw SL. The central melanocortin system and the regulation of energy balance. Front Biosci. 2007;12:3994–4010 [DOI] [PubMed] [Google Scholar]
- 3. Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311 [DOI] [PubMed] [Google Scholar]
- 4. Hileman SM, Pierroz DD, Masuzaki H, et al. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775–783 [DOI] [PubMed] [Google Scholar]
- 5. Tu H, Kastin AJ, Hsuchou H, Pan W. Soluble receptor inhibits leptin transport. J Cell Physiol. 2008;214:301–305 [DOI] [PubMed] [Google Scholar]
- 6. Lou PH, Yang G, Huang L, et al. Reduced body weight and increased energy expenditure in transgenic mice over-expressing soluble leptin receptor. PloS one. 2010;5:e11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Dielen FM, van 't Veer C, Buurman WA, Greve JW. Leptin and soluble leptin receptor levels in obese and weight-losing individuals. J Clin Endocrinol Metab. 2002;87:1708–1716 [DOI] [PubMed] [Google Scholar]
- 8. Chan JL, Bluher S, Yiannakouris N, Suchard MA, Kratzsch J, Mantzoros CS. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes. 2002;51:2105–2112 [DOI] [PubMed] [Google Scholar]
- 9. Sinha MK, Ohannesian JP, Heiman ML, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong ML, Licinio J, Yildiz BO, et al. Simultaneous and continuous 24-hour plasma and cerebrospinal fluid leptin measurements: dissociation of concentrations in central and peripheral compartments. J Clin Endocrinol Metab. 2004;89:258–265 [DOI] [PubMed] [Google Scholar]
- 11. Korner J, Chua SC, Jr, Williams JA, Leibel RL, Wardlaw SL. Regulation of hypothalamic proopiomelanocortin by leptin in lean and obese rats. Neuroendocrinology. 1999;70:377–383 [DOI] [PubMed] [Google Scholar]
- 12. Steiner RA, Kabigting E, Lent K, Clifton DK. Diurnal rhythm in proopiomelanocortin mRNA in the arcuate nucleus of the male rat. J Neuroendocrinol. 1994;6:603–608 [DOI] [PubMed] [Google Scholar]
- 13. Tsigos C, Crosby SR, Gibson S, Young RJ, White A. Proopiomelanocortin is the predominant adrenocorticotropin-related peptide in human cerebrospinal fluid. J Clin Endocrinol Metab. 1993;76:620–624 [DOI] [PubMed] [Google Scholar]
- 14. Pritchard LE, Oliver RL, McLoughlin JD, et al. Proopiomelanocortin-derived peptides in rat cerebrospinal fluid and hypothalamic extracts: evidence that secretion is regulated with respect to energy balance. Endocrinology. 2003;144:760–766 [DOI] [PubMed] [Google Scholar]
- 15. Cota D, Proulx K, Smith KA, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930 [DOI] [PubMed] [Google Scholar]
- 16. Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci. 2009;29:8302–8311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Page-Wilson G, Reitman-Ivashkov E, Meece K, et al. Cerebrospinal fluid levels of leptin, proopiomelanocortin, and agouti-related protein in human pregnancy: evidence for leptin resistance. J Clin Endocrinol Metab. 2013;98:264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stovold R, Meredith SL, Bryant JL, et al. Neuroendocrine and epithelial phenotypes in small-cell lung cancer: implications for metastasis and survival in patients. Br J Cancer. 2013;108:1704–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kugler F, Graneis S, Schreiter PP, Stintzing FC, Carle R. Determination of free amino compounds in betalainic fruits and vegetables by gas chromatography with flame ionization and mass spectrometric detection. J Agric Food Chem. 2006;54:4311–4318 [DOI] [PubMed] [Google Scholar]
- 21. Huang Y, Potter R, Sigurdson W, et al. Effects of age and amyloid deposition on Aβ dynamics in the human central nervous system. Arch Neurol. 2012;69:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589–593 [DOI] [PubMed] [Google Scholar]
- 23. Caro JF, Kolaczynski JW, Nyce MR, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161 [DOI] [PubMed] [Google Scholar]
- 24. Hagan MM, Havel PJ, Seeley RJ, et al. Cerebrospinal fluid and plasma leptin measurements: covariability with dopamine and cortisol in fasting humans. J Clin Endocrinol Metab. 1999;84:3579–3585 [DOI] [PubMed] [Google Scholar]
- 25. Pan W, Kastin AJ. Diurnal variation of leptin entry from blood to brain involving partial saturation of the transport system. Life Sci. 2001;68:2705–2714 [DOI] [PubMed] [Google Scholar]
- 26. Sun Q, van Dam RM, Meigs JB, Franco OH, Mantzoros CS, Hu FB. Leptin and soluble leptin receptor levels in plasma and risk of type 2 diabetes in U.S. women: a prospective study. Diabetes. 2010;59:611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wardlaw SL. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur J Pharmacol. 2011;660:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schlachter LB, Wardlaw SL, Tindall GT, Frantz AG. Persistence of beta-endorphin in human cerebrospinal fluid after hypophysectomy. J Clin Endocrinol. 1983;57:221–224 [DOI] [PubMed] [Google Scholar]
- 29. Xiao E, Kim AJ, Dutia R, Conwell I, Ferin M, Wardlaw SL. Effects of estradiol on cerebrospinal fluid levels of agouti-related protein in ovariectomized rhesus monkeys. Endocrinology. 2010;151:1002–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci. 2005;8:579–584 [DOI] [PubMed] [Google Scholar]
- 31. Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–1654 [DOI] [PubMed] [Google Scholar]
- 32. Peters JC, Harper AE. Adaptation of rats to diets containing different levels of protein: effects on food intake, plasma and brain amino acid concentrations and brain neurotransmitter metabolism. J Nutr. 1985;115:382–398 [DOI] [PubMed] [Google Scholar]
- 33. Grimes MA, Cameron JL, Fernstrom JD. Cerebrospinal fluid concentrations of large neutral and basic amino acids in Macaca mulatta: diurnal variations and responses to chronic changes in dietary protein intake. Metabolism. 2009;58:129–140 [DOI] [PubMed] [Google Scholar]
- 34. Choi S, Disilvio B, Fernstrom MH, Fernstrom JD. Meal ingestion, amino acids and brain neurotransmitters: effects of dietary protein source on serotonin and catecholamine synthesis rates. Physiol Behav. 2009;98:156–162 [DOI] [PubMed] [Google Scholar]
- 35. Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr. 2007;137:1539S–1547S; discussion 1548S [DOI] [PubMed] [Google Scholar]
- 36. Xu Y, Jones JE, Kohno D, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matera C, Wardlaw SL. Dopamine and sex steroid regulation of POMC gene expression in the hypothalamus. Neuroendocrinology. 1993;58:493–500 [DOI] [PubMed] [Google Scholar]
- 38. Banks WA. Enhanced leptin transport across the blood-brain barrier by alpha 1-adrenergic agents. Brain Res. 2001;899:209–217 [DOI] [PubMed] [Google Scholar]
- 39. Fernstrom JD. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids. 2013;45:419–430 [DOI] [PubMed] [Google Scholar]
- 40. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]