Abstract
Context:
In vitro and animal studies have reported conflicting results regarding an independent role for FSH in the regulation of bone turnover.
Objective:
Our objective was to test the hypothesis that suppressing serum FSH while holding serum gonadal steroid levels stable in the eugonadal range will affect biochemical markers of bone metabolism in healthy men.
Participants, Design, and Setting:
Eugonadal men aged 20 to 50 years participated in this randomized controlled trial at a tertiary care academic teaching hospital.
Interventions:
Participants received monthly GnRH analog injections to suppress FSH secretion plus daily topical testosterone gel in prespecified doses (intervention group). Controls received matching placebos (control group). Subjects in the intervention group were individually matched with subjects in the control group to ensure that the mean testosterone and estradiol levels (measured every 4 weeks during the 16-week study period) in the 2 groups were similar.
Main Outcome Measures:
Biochemical markers of bone resorption (serum N-terminal telopeptide and C-terminal telopeptide), bone formation (serum osteocalcin), and FSH were measured at baseline and after 16 weeks of treatment.
Results:
Serum FSH declined by 2% in the control group and by 60% in the intervention group (P < .0001 for the between-group difference). Despite the substantial suppression of serum FSH in the intervention group, serum N-terminal telopeptide, C-terminal telopeptide, and osteocalcin did not change in the intervention group, nor were any between-group differences observed.
Conclusion:
When gonadal steroid levels are held constant, short-to midterm suppression of FSH does not affect bone turnover in men. FSH does not appear to be a significant regulator of bone metabolism in eugonadal men.
Although hypogonadal bone loss has traditionally been attributed primarily to the decline in gonadal steroid levels, recent observations have led to the hypothesis that other hormonal factors may also play a role in hypogonadal bone loss in both men and women. For example, bone loss begins in the perimenopausal period before estrogen levels fall but after inhibin and progesterone levels decline and FSH rises (1–8). Although there are reports suggesting a significant role for each of these factors in the pathogenesis of perimenopausal bone loss, FSH has been studied most extensively (9–12). Several epidemiological studies have reported that FSH is positively associated with bone resorption and inversely associated with bone mineral density (BMD) (7, 13). Additionally, in vitro experiments and several, but not all, mouse models have suggested that FSH may directly mediate osteoclast function and bone resorption in both male and female animals (14–16). Conversely, a recent experimental study in humans reported that when FSH levels are suppressed in postmenopausal women via GnRH analog administration, bone turnover does not decrease as would be predicted from the animal data (17). Some investigators have suggested that the lack of effect of FSH suppression in these subjects may have been due, at least in part, to the very high baseline bone turnover rate in these postmenopausal women (18). To investigate the potential independent role of FSH on bone turnover in eugonadal men, we suppressed serum FSH levels while maintaining serum testosterone and estradiol levels in the normal adult male reference range. In doing so, we were able to assess the impact of selectively lowering serum FSH on biochemical markers of bone resorption and bone formation in eugonadal men and directly test the hypothesis that FSH is an independent regulator of skeletal metabolism in men.
Subjects and Methods
This study was performed as part of a larger 16-week study called Hypogonadism in Men (HIM) examining the dose-response relationships between testosterone and a variety of outcome measures in healthy young men (19). Subjects in this larger study were treated with monthly goserelin acetate (3.6 mg Zoladex; AstraZeneca Pharmaceuticals LP) and 1 of 5 doses of topical testosterone (AndroGel; AbbVie Inc) and compared with a control group that received placebos for both the GnRH agonist and for the testosterone gel.
Of the 37 subjects enrolled in the placebo group of the HIM study, 6 subjects did not complete the study or did not have usable N-terminal telopeptide (NTX) data, 1 subject was incidentally discovered to be hypogonadal, and 1 subject could not be matched with an intervention group subject using the criteria described below. These 29 placebo group subjects (control group) were individually matched with subjects who received both a monthly injection of goserelin acetate and topical testosterone at a dose of either 5 or 10 mg daily (intervention group). Each control group subject was matched with an intervention group subject whose mean serum testosterone levels were within 100 ng/dL and whose mean serum estradiol levels were within 5 pg/mL.
Serum testosterone and estradiol were measured at weeks 0, 4, 8, 12, and 16. The serum NTX, C-terminal telopeptide (CTX), osteocalcin, and FSH were measured at 0 and 16 weeks. The study was approved by the Partners Healthcare Institutional Review Board, and all subjects provided written informed consent.
Assays
Serum testosterone was measured with the Siemens Centaur XP solid-phase chemiluminescent immunoassay (interassay coefficient of variation [CV] <10%). Total testosterone was remeasured by liquid chromatography-tandem mass spectroscopy (LC/MS/MS) at all time points from 5 randomly selected men in each of the 5 groups in HIM. The correlation between the testosterone assays was 0.93, and the assays gave very similar results (TRIA = 0.98TLC/MS/MS + 21, where T is testosterone). Serum estradiol was measured using LC/MS/MS (assay sensitivity is 1.25 pg/mL, interassay CV <10%). Serum FSH was measured using the Beckman-Coulter Access chemiluminescent immunoassay (interassay CV <10%). Serum NTX was measured using an ELISA (Wampole Laboratories) with an interassay CV of <8%. Serum CTX was measured using Nordic Biosciences CrossLaps ELISA (interassay CV <8%). Serum osteocalcin was measured with an ELISA (ALPCO Diagnostics) with an interassay CV of <10%. 25-Hydroxyvitamin D (25(OH)D) was initially measured using an RIA (Diasorin) with an interassay CV of 9% to 13%. After January 2006, 25(OH)D levels were measured using a chemiluminescent assay (LIAISON; Diasorin) with an interassay CV of 12% to 16%.
Statistical analyses
The primary outcome variable was the change in serum NTX during the study period. Within-group changes from baseline to week 16 were assessed using paired t tests. Because each subject in the intervention group was individually matched with a subject in the placebo group, between-group differences in the change from baseline to week 16 for each endpoint were also assessed using paired t tests. For each subject, serum testosterone and estradiol levels from weeks 4, 8, 12, and 16 were averaged to determine the gonadal steroid levels while on the study protocol. P values <.05 (two-sided) were considered statistically significant. With 80% power at a 5% significance level, the detectable size of the between-group mean NTX percent change was at least 21.6%. Unless noted otherwise, data are reported as mean ± SD.
Results
Baseline characteristics of the study subjects are shown in Table 1. Subjects in the intervention group were significantly older than the control group. There were no other differences between groups in baseline parameters, including serum testosterone, estradiol, and FSH levels.
Table 1.
Baseline Characteristics of the Subjects in the Intervention and Control Groups
| Control (n = 29) | Intervention (n = 29) | P Value | |
|---|---|---|---|
| Age, y | 30 ± 6 | 35 ± 7 | .0005 |
| Height, cm | 178 ± 7 | 175 ± 8 | .17 |
| Weight, kg | 80 ± 18 | 84 ± 18 | .50 |
| Body mass index, kg/m2 | 25.4 ± 5.0 | 27.2 ± 4.8 | .17 |
| Calcium, mg/dL | 9.6 ± 0.3 | 9.5 ± 0.3 | .09 |
| PTH, pg/mL | 39.5 ± 10.5 | 44.4 ± 14.9 | .14 |
| 25(OH)D, ng/mL | 32 ± 21 | 28 ± 14 | .40 |
| TSH, μU/mL | 1.6 ± 0. 8 | 1.6 ± 0.6 | .97 |
| Creatinine, mg/dL | 1.1 ± 0.1 | 1.1 ± 0.1 | .30 |
| Testosterone, ng/dL | 583 ± 204 | 511 ± 148 | .11 |
| Estradiol, pg/mL | 30 ± 10 | 30 ± 8 | .80 |
| FSH, U/L | 4.8 ± 2.0 | 5.2 ± 4.5 | .69 |
| NTX, nM BCE | 12.6 ± 5.7 | 13.9 ± 5.5 | .30 |
| CTX, pg/mL | 0.75 ± 0.29 | 0.65 ± 0.37 | .21 |
| Osteocalcin, ng/mL | 15.5 ± 4.5 | 14.6 ± 4.9 | .47 |
P values refer to between-group differences at study entry.
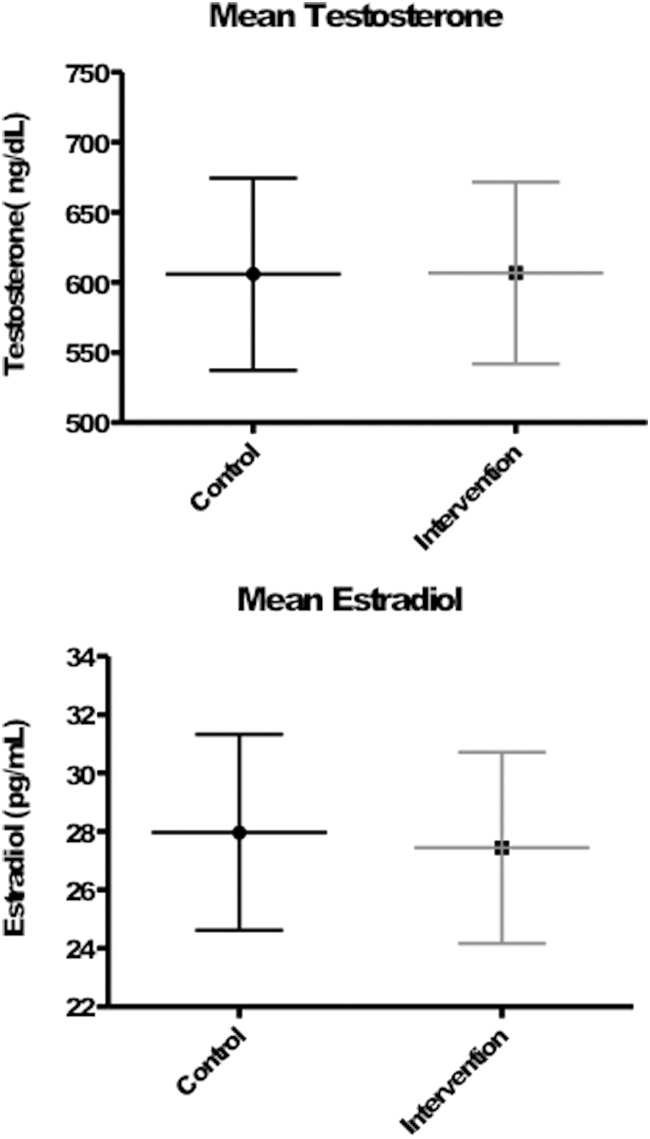
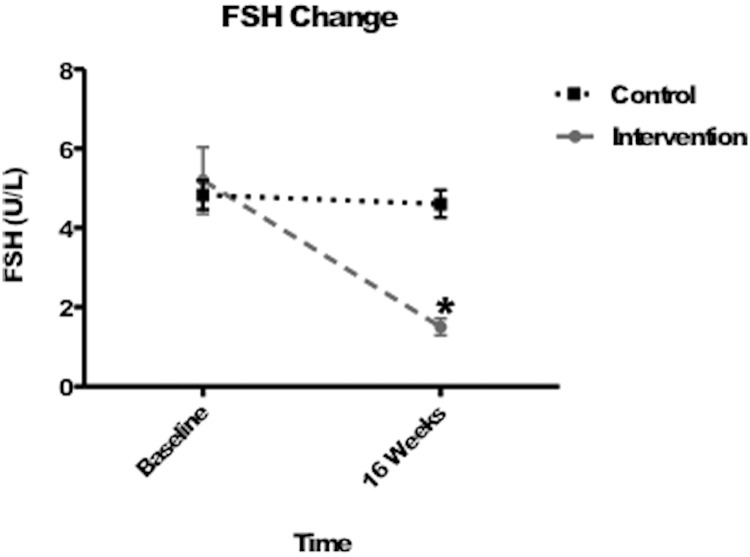
Figure 1 shows the mean gonadal steroid levels achieved during the study period and the change in FSH levels in each group, respectively. As a consequence of our matching procedure, mean serum testosterone and estradiol levels achieved during the 16-week treatment period did not differ between groups (P = .94 and P = .26, respectively) and were normal in all subjects. In the intervention group, mean FSH levels decreased from 5.2 ± 4.5 IU/L at baseline to 1.5 ± 1.2 IU/L at week 16 (mean percent change of −60.3% ± 32.7%, P < .0001 for within-group comparison), whereas mean FSH levels fell minimally from 4.8 ± 2.0 IU/L at baseline to 4.6 ± 1.9 IU/L at week 16 in the control group (−1.7% ± 17.0%, P = .046 for within-group comparison and P < .0001 for comparison of change between intervention group and control group; Figure 2).
Figure 1.
Mean serum testosterone (top) and estradiol (bottom) levels with 95% confidence intervals during the study period. P values are nonsignificant for both between-group comparisons.
Figure 2.
Change in serum FSH levels in the intervention and placebo groups. *, Statistical significance of between-group differences in FSH from baseline to week 16, P < .0001.
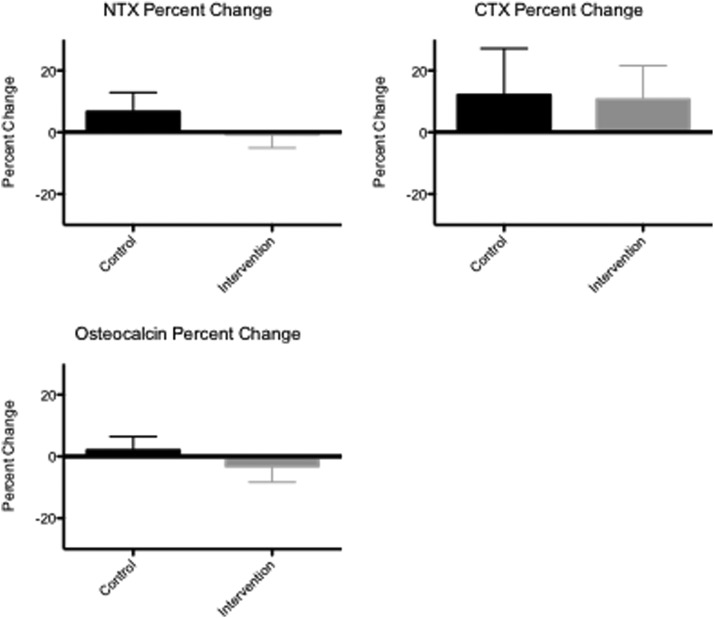
Figure 3 shows the changes in bone turnover markers during the study. Mean serum NTX, CTX, and osteocalcin levels did not change significantly within the control group (P = .99, .76, and .95, respectively) or the intervention group (P = .45, .44, and .63, respectively), and the magnitude of the changes in serum NTX, CTX, and osteocalcin did not differ significantly between groups (P = .43 for NTX, P = .77 for CTX, and P = .49 for osteocalcin).
Figure 3.
Change in serum NTX (top left), CTX (top right), and osteocalcin (bottom) levels from baseline to study completion in the intervention and placebo groups. The P value is nonsignificant for the difference in between-group change and within-group comparison in all groups.
Discussion
This study demonstrates that suppressing FSH for 16 weeks does not affect bone turnover in eugonadal men. Serum testosterone and estradiol levels were carefully matched between groups, eliminating the potential confounding influence of differences in gonadal steroid levels and making this model well suited to assess the independent effect of lowering FSH on bone turnover. Despite a 60% decrease in serum FSH in the intervention group, no change was observed in serum NTX, CTX, or osteocalcin levels. These results demonstrate that FSH is not a major regulator of bone turnover in men, at least in the eugonadal setting.
Based on previous in vitro and animal models, the potential role of FSH in the regulation of bone turnover has remained unclear. Some observations support the hypothesis that FSH can directly stimulate bone resorption. For example, FSH receptors have been identified in osteoclasts and mesenchymal stem cells but not on CD14+ cells, fibroblasts, or mature osteoblasts. In addition, FSH stimulates osteoclastogenesis in human mononuclear cell precursors in vitro in a dose-dependent fashion (14). Moreover, in mice with null mutations of the FSH receptor or the FSH β-subunit gene, areal and volumetric BMD are maintained at or above levels of wild-type mice, even though the mutant animals have features consistent with severe estrogen deficiency. Additionally, the same group reported that lumbar spine and proximal femur BMD are elevated in FSH-β haploinsufficient mice, which have apparently normal gonadal steroid levels despite a 50% reduction in FSH levels (14).
Although these reports appear to provide strong evidence for a direct effect of FSH on bone resorption, other findings from animal studies question this concept. First, serum testosterone levels are elevated in the female mice with null mutations in the FSH receptor or FSH-β genes, making it difficult to determine whether the absence of bone loss in the setting of estrogen deficiency is due to the lack of FSH signaling or the compensatory increase in testosterone (15). This group of investigators reported that BMD is actually modestly reduced in mice with null mutations of the FSH receptor gene and that ovariectomy induces further bone loss in these mice. In fact, ovariectomy in these FSH receptor-null mice resulted in bone mass that was indistinguishable from ovariectomized wild-type mice, suggesting that FSH is not required for estrogen-deficiency bone loss to occur (15).
Other mouse models investigating the effects of FSH signaling or FSH antagonist administration have also shown conflicting results, with one study demonstrating no effect of an FSH infusion, another showing increases in bone mass in mice with transgenic human FSH expression, and a recent study demonstrating protection from bone loss with FSH-β antibody administration, both by inhibition of bone resorption and stimulation of bone formation (20–22).
In humans, most of the evidence for a putative role for FSH in the pathogenesis of hypogonadal bone loss comes from observational studies. Several epidemiologic studies have found that associations between various reproductive hormones and changes in bone resorption markers and/or BMD during the menopause transition are stronger for FSH than for estradiol or testosterone (7, 13). However, the reported associations between reproductive hormones, including FSH, and bone loss are weak and cannot establish a causal relationship. It is possible that the previously reported association between FSH and perimenopausal bone loss simply reflects that FSH is a better integrator of declining ovarian function or represents an interaction with unmeasured confounders (23).
There are multiple reports in humans demonstrating that bone resorption increases and BMD decreases in men and in premenopausal women treated with GnRH agonists. GnRH agonists suppress FSH levels modestly while lowering gonadal steroids production to prepubertal levels (24–26). This model would seem to provide conclusive evidence indicating that a compensatory increase in FSH is not required for the development of hypogonadal bone loss in either gender. Other human interventional trials designed to more specifically evaluate an independent role for FSH in bone physiology have also not demonstrated an effect. In one study, a GnRH agonist administered to postmenopausal women resulted in an 86% reduction in FSH levels but no decrease in biochemical markers of bone turnover (17). In addition, infertile women undergoing in vitro fertilization who received a GnRH analog followed by recombinant FSH had no change in biochemical markers of bone turnover until estradiol levels increased (27). When these studies are considered in conjunction with the results of the present study, it appears that FSH neither plays a direct role in the pathogenesis of hypogonadal bone loss nor directly modulates bone homeostasis in eugonadal men.
There are several potential limitations of this study. First, this is a study conducted in men with testosterone levels in the normal young-adult reference range, so results are applicable specifically to eugonadal men. In addition, the magnitude of the reduction in FSH in our study may have been insufficient to affect bone turnover. However, BMD is higher and bone resorption is lower in mice that are haploinsufficient for the β-subunit of FSH resulting in FSH levels that are reduced by only 50% (14). Thus, although in mice a 50% reduction in FSH is sufficient to suppress bone resorption in animals, in our study, bone resorption was not reduced despite a 60% reduction in FSH, suggesting a discrepancy between mouse and human physiology. It is conceivable that selection bias may have been introduced into our analysis in the matching of placebo group subjects with intervention group subjects. This appears unlikely, however, because when bone markers from all subjects treated with either 5 or 10 g of topical testosterone were analyzed without regard to matching, no changes in any biochemical marker of bone turnover were observed (19). Finally, we did not measure inhibin levels in this study. Inhibin B is the physiologically important inhibin in men, whereas inhibin A levels are undetectable (28, 29). Studies investigating the effects of the inhibins on bone turnover have shown varied results, with epidemiologic studies showing an association with both bone resorption and formation and in vivo studies primarily showing an effect on bone formation (9, 30, 31). Based on these data, we might expect a change in bone formation markers if decreasing inhibin B levels were influencing our results. We did not observe any change in osteocalcin levels; thus, the likelihood that inhibin B is serving as an unmeasured confounder in our study is low.
In conclusion, this study demonstrates that in healthy eugonadal men, bone turnover is unaffected by 16 weeks of FSH suppression. Thus, FSH does not appear to be an important regulator of skeletal metabolism in normal adult men. Further studies are needed to determine whether other peptide hormones, such as inhibins and activins, contribute to the pathogenesis of hypogonadal bone loss or the maintenance of normal adult bone homeostasis in men.
Acknowledgments
We thank Dr Robert Neer for his thoughtful comments during the preparation of this manuscript.
This work was supported by National Institutes of Health Grants R01 AG030545, K24 KD02759, and RR-1066. Additional support was provided by AstraZeneca and AbbVie Inc.
Disclosure Summary: A.V.U., H.L., and B.Z.L. have nothing to disclose. J.S.F. received research funding from AstraZeneca and Abbott Labs.
Footnotes
- BMD
- bone mineral density
- CTX
- C-terminal telopeptide
- CV
- coefficient of variation
- 25(OH)D
- 25-hydroxyvitamin D
- HIM
- Hypogonadism in Men
- LC/MS/MS
- liquid chromatography-tandem mass spectroscopy
- NTX
- N-terminal telopeptide.
References
- 1. Riggs BL, Wahner HW, Melton LJ, 3rd, Richelson LS, Judd HL, Offord KP. Rates of bone loss in the appendicular and axial skeletons of women. Evidence of substantial vertebral bone loss before menopause. J Clin Invest. 1986;77:1487–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riggs BL, Melton LJ, Robb RA, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Slemenda C, Hui SL, Longcope C, Johnston CC. Sex steroids and bone mass. A study of changes about the time of menopause. J Clin Invest. 1987;80:1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Slemenda C, Longcope C, Peacock M, Hui S, Johnston CC. Sex steroids, bone mass, and bone loss. A prospective study of pre-, peri-, and postmenopausal women. J Clin Invest. 1996;97:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sowers MR, Zheng H, Jannausch ML, et al. Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. J Clin Endocrinol Metab. 2010;95:2155–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sowers MR, Zheng H, McConnell D, Nan B, Harlow S, Randolph JF., Jr Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab. 2008;93:3958–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sowers MR, Jannausch M, McConnell D, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91:1261–1267 [DOI] [PubMed] [Google Scholar]
- 8. Prior JC. Progesterone as a bone-trophic hormone. Endocr Rev. 1990;11:386–398 [DOI] [PubMed] [Google Scholar]
- 9. Perrien DS, Achenbach SJ, Bledsoe SE, et al. Bone turnover across the menopause transition: correlations with inhibins and follicle-stimulating hormone. J Clin Endocrinol Metab. 2006;91:1848–1854 [DOI] [PubMed] [Google Scholar]
- 10. Iqbal J, Blair HC, Zallone A, Sun L, Zaidi M. Further evidence that FSH causes bone loss independently of low estrogen. Endocrine. 2012;41:171–175 [DOI] [PubMed] [Google Scholar]
- 11. Agrawal M, Zhu G, Sun L, Zaidi M, Iqbal J. The role of FSH and TSH in bone loss and its clinical relevance. Curr Osteoporos Rep. 2010;8:205–211 [DOI] [PubMed] [Google Scholar]
- 12. Iqbal J, Sun L, Zaidi M. Commentary-FSH and bone 2010: evolving evidence. Eur J Endocrinol. 2010;163:173–176 [DOI] [PubMed] [Google Scholar]
- 13. Ebeling PR, Atley LM, Guthrie JR, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996;81:3366–3371 [DOI] [PubMed] [Google Scholar]
- 14. Sun L, Peng Y, Sharrow AC, et al. FSH directly regulates bone mass. Cell. 2006;125:247–260 [DOI] [PubMed] [Google Scholar]
- 15. Gao J, Tiwari-Pandey R, Samadfam R, et al. Altered ovarian function affects skeletal homeostasis independent of the action of follicle-stimulating hormone. Endocrinology. 2007;148:2613–2621 [DOI] [PubMed] [Google Scholar]
- 16. Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci U S A. 2006;103:14925–14930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drake MT, McCready LK, Hoey KA, Atkinson EJ, Khosla S. Effects of suppression of follicle-stimulating hormone secretion on bone resorption markers in postmenopausal women. J Clin Endocrinol Metab. 2010;95:5063–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu LL, Tourkova I, Yuen T, et al. Blocking FSH action attenuates osteoclastogenesis. Biochem Biophys Res Commun. 2012;422:54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ritter V, Thuering B, Saint Mezard P, et al. Follicle-stimulating hormone does not impact male bone mass in vivo or human male osteoclasts in vitro. Calcif Tissue Int. 2008;82:383–391 [DOI] [PubMed] [Google Scholar]
- 21. Allan CM, Kalak R, Dunstan CR, et al. Follicle-stimulating hormone increases bone mass in female mice. Proc Natl Acad Sci U S A. 2010;107:22629–22634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu LL, Blair H, Cao J, et al. Blocking antibody to the β-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci U S A. 2012;109:14574–14579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gourlay ML, Specker BL, Li C, et al. Follicle-stimulating hormone is independently associated with lean mass but not BMD in younger postmenopausal women. Bone. 2012;50:311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–955 [DOI] [PubMed] [Google Scholar]
- 25. Grossmann M, Zajac JD. Management of side effects of androgen deprivation therapy. Endocrinol Metab Clin North Am. 2011;40:655–671, x [DOI] [PubMed] [Google Scholar]
- 26. Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. ; Austrian Breast and Colorectal Cancer Study Group. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9:840–849 [DOI] [PubMed] [Google Scholar]
- 27. Omodei U, Mazziotti G, Donarini G, et al. Effects of recombinant follicle-stimulating hormone on bone turnover markers in infertile women undergoing in vitro fertilization procedure. J Clin Endocrinol Metab. 2013;98:330–336 [DOI] [PubMed] [Google Scholar]
- 28. Anawalt BD, Bebb RA, Matsumoto AM, et al. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab. 1996;81:3341–3345 [DOI] [PubMed] [Google Scholar]
- 29. Illingworth PJ, Groome NP, Byrd W, et al. Inhibin-B: a likely candidate for the physiologically important form of inhibin in men. J Clin Endocrinol Metab. 1996;81:1321–1325 [DOI] [PubMed] [Google Scholar]
- 30. Gaddy-Kurten D, Coker JK, Abe E, Jilka RL, Manolagas SC. Inhibin suppresses and activin stimulates osteoblastogenesis and osteoclastogenesis in murine bone marrow cultures. Endocrinology. 2002;143:74–83 [DOI] [PubMed] [Google Scholar]
- 31. Perrien DS, Akel NS, Edwards PK, et al. Inhibin A is an endocrine stimulator of bone mass and strength. Endocrinology. 2007;148:1654–1665 [DOI] [PubMed] [Google Scholar]