Abstract
Context:
CD34+ fibrocytes, bone marrow-derived progenitor cells, infiltrate orbital connective tissue in thyroid-associated ophthalmopathy, a manifestation of Graves' disease. In the orbit, they become CD34+ fibroblasts and coexist with native CD34− fibroblasts. Fibrocytes have been shown to express TSH receptor and thyroglobulin.
Objective:
The objective of the study was to determine whether a broader repertoire of thyroid protein expression can be detected in fibrocytes and whether a common factor is responsible.
Design/Setting/Participants:
Fibrocytes and fibroblasts were collected and analyzed from healthy individuals and those with Graves' disease in an academic clinical practice.
Main Outcome Measures:
Real-time PCR, Western blot analysis, gene promoter analysis, cell transfections, and flow cytometric cell sorting were performed.
Results:
We detect two additional thyroid proteins expressed by fibrocytes, namely sodium-iodide symporter and thyroperoxidase. The autoimmune regulator (AIRE) protein appears necessary for this expression. AIRE expression in fibrocytes results from an active AIRE gene promoter and stable AIRE mRNA. Knocking down AIRE with a targeting small interfering RNA reduces the expression of these thyroid proteins in fibrocytes as well as the transcription factors paired box-8 and thyroid transcription factor-1. When compared with an unaffected first-degree relative, levels of these proteins are substantially reduced in fibrocytes from an individual with an inactivating AIRE mutation. Levels of AIRE and the thyroid proteins are lower in orbital fibroblasts from patients with thyroid-associated ophthalmopathy than in fibrocytes. However, when mixed fibroblast populations are sorted into pure CD34+ and CD34− subsets, the levels of these proteins are dramatically increased selectively in CD34+ fibroblasts.
Conclusions:
Fibrocytes express four proteins, the aggregate expression of which was previously thought to be restricted to thyroid epithelium. These proteins represent the necessary molecular biosynthetic machinery necessary for thyroid hormone production. Our findings implicate AIRE in the promiscuous expression of thyroid proteins in fibrocytes.
Fibrocytes are bone marrow-derived progenitor cells of monocyte lineage (1) that display a characteristic array of markers, including CD45, CD11b, CD34, CXCR4, and collagen1 (2). They traffic to sites of injury, infiltrate tissue, and orchestrate remodeling (3). They participate in normal wound healing and in scar formation (4). Antigens can be presented by fibrocytes to T cells as a consequence of high constitutive level major histocompatibility complex class II display (5). They express several costimulatory molecules and cytokines, synthesize vitronectin, and can differentiate into fat cells and myofibroblasts (6). The pathogenesis of rheumatoid arthritis (7) and interstitial lung fibrosis (8) has been linked to the activities of fibrocytes. Identifying the details surrounding involvement of fibrocytes in physiological and disease-related processes should provide important insights into their roles in immune function.
Another autoimmune process in which fibrocytes have been implicated is Graves' disease (GD), a condition in which the thyroid becomes overactive as a consequence of disease-specific activating antibodies targeting the TSH receptor (TSHR) (9). Thirty percent to 50% of patients with GD manifest expansion, inflammation, and remodeling of orbital connective tissue (10). This disfiguring process is known as thyroid-associated ophthalmopathy (TAO). Activated T cells infiltrate the orbit in TAO, but the immunological basis for their trafficking is uncertain (11). Moreover, details concerning the connections between TAO and the processes occurring within the thyroid in GD have yet to be identified. Recently we reported that circulating fibrocytes become more abundant in GD (12, 13). They apparently infiltrate orbital connective tissue (12) and can also be detected in the thyroid (13). In orbit, they can be recognized as CD34+ fibroblasts which comprise a subset of cells distinct from native CD34− fibroblasts. In addition to the characteristic profile of markers associated with their lineage, TSHR and thyroglobulin (Tg) have recently been detected in fibrocytes cultivated from peripheral blood mononuclear cells (PBMCs) (14). Orthodoxy had previously taught that these two proteins are expressed exclusively by thyroid epithelium. However, evidence that they might be distributed more widely, albeit at relatively modest levels, surfaced several years ago. Most notably, low levels of TSHR or its encoding mRNA were detected in orbital connective tissue, especially in tissues derived from individuals with TAO (15). Tg was also detected in the TAO orbit but was thought to originate in the thyroid (16). In addition, functional sodium/iodide symporter (NIS) and thyroperoxidase (TPO) have been detected in tissues outside the thyroid (17, 18). But the aggregate expression of these four proteins remains a signature widely viewed as occurring uniquely in thyroid epithelial cells.
The autoimmune regulator (AIRE) gene encodes an unorthodox transcription factor, the expression of which was thought to be limited to thymic medullary epithelial cells (mTECs) (19, 20). AIRE protein localizes to the nucleus in thymus where its actions on target gene transcription drive expression of thousands of proteins. Among them are those considered to be specific to specialized peripheral tissues (19–22). Thymic education results from the actions of AIRE, leading to deletion of autoreactive thymocytes. This in turn establishes central immune tolerance (23). In addition to its role in supporting target gene transcription, AIRE appears to regulate mTEC differentiation (24). The scope of cells in which AIRE has been detected includes several with relatively low-level expression, such as monocytes, dendritic cells, and lymph node stromal cells (25–27). The function of AIRE in extrathymic cells remains uncertain. A prominent example of AIRE expression in the periphery was described by Anderson and colleagues (25), who have identified extrathymic AIRE-expressing cells (eTACs) in lymphoid tissues. These cells can interact with and instigate the deletion of naïve antigen-specific T cells. It is clear that normal immune function is critically dependent on the wild-type AIRE protein. Mutated AIRE causes the central defect in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APS-1) (19, 20). Finding additional evidence for AIRE playing a role in organ-specific autoimmunity through activities outside the thymus could further our understanding of immune function.
We now provide evidence that fibrocytes, regardless of whether they derive from healthy donors or those with GD, express NIS and TPO in addition to TSHR and Tg. Furthermore, we demonstrate that these cells express AIRE and that knocking down this transcription factor results in substantial reduction in the level of these proteins. The aggregate expression of these four proteins has traditionally defined the unique biosynthetic repertoire of thyroid epithelium. These findings identify a heretofore unrecognized but potentially important role for AIRE in supporting the promiscuous expression of thyroid proteins in fibrocytes. The presence of ectopic thyroid antigens outside the gland may underlie abnormal immune responses occurring in GD and TAO and potentially in other forms of thyroid autoimmunity. Our detection of these proteins in fibrocytes from healthy individuals suggests that other factors, such as the particular T cell repertoire associated with disease susceptibility, might determine the development of autoimmune disease.
Materials and Methods
Materials
Rabbit anti-Tg antibody (catalog number ab92467), rabbit anti-TPO monoclonal antibody (catalog number ab109383), and mouse anti-NIS monoclonal antibody (catalog number ab17795) were purchased from Abcam. Mouse anti-CD34 IgG 1 fluorescein isothiocyanate was from BD (catalog number 555822) and anti-TSHR phycoerythrin was supplied by Santa Cruz Biotechnology (catalog number sc-53542). Specific small interfering RNA (siRNA) targeting human AIRE (catalog number L-010993) and its scrambled control (catalog number D-001810–10-5) were from Dharmacon. The siRNA comprises a pool of the following four target sequences: 1) GAAGAAUGAGGACGAGUGU, 2) CAACAGUCCAGGAGGUGCA, 3) GUGCUGCGGUGUACUCACU, and 4) GUGCGGAGAUGGUACGGAC. Fetal bovine serum (FBS) was from Life Technologies. 5,6-Dichlorobenzimidazole came from Cayman Chemicals (catalog number 10010302). Plasmid pAP1200 containing a 1200-bp fragment of the human AIRE gene promoter was a kind gift from Professor Pärt Peterson (Tartu, Estonia).
Fibrocyte and fibroblast cultivation
Human tissue and venous blood were collected from study participants after obtaining their informed consent in a manner approved by the Institutional Review Board of the University of Michigan Health System. Twenty-five strains of fibrocytes were generated from blood generously provided by the American Red Cross, while 11 strains came from euthyroid subjects with GD who manifested TAO. They were isolated from PBMCs and cultivated using the methods first described by Bucala et al (1). Twenty-four-well plates were inoculated with 5 × 106 PBMCs in DMEM with 10% FBS. After 12–14 days in culture, adherent monolayers (<5% of the starting population) were rinsed and removed by scraping or were treated with accutase (Millipore catalog number 1000449). Culture purity was confirmed by fluorescence-activated cell sorter (FACS) analysis to be greater than 90% fibrocytes. Cell viability was greater than 90% by trypan blue exclusion.
Orbital fibroblasts (OF) were cultivated from surgical waste obtained from seven individuals either with TAO undergoing orbital decompression surgery (GD-OF) or from healthy individuals during cosmetic procedures (H-OF). Dermal fibroblasts (DF) derived from three healthy donors or were purchased from the American Type Tissue Collection. Fibroblasts were used between the second and 11th passages, an interval during which they maintained stable phenotypes (28).
Western blot and immunoprecipitation
Protein analysis was conducted using fibrocytes derived from Red Cross leukocyte filters. Each was from a different donor. For Western blots, monolayers were solubilized in lysis buffer (Invitrogen; catalog number FNN0011) containing 1 mM phenylmethanesulfonylfluoride and protease inhibiter cocktail (Sigma). Samples were sonicated on ice and centrifuged for 10 minutes at 10 000 × g and supernatants were collected. Homogenized human thyroid and FRTL-5 cells served as positive controls. Protein concentrations were determined using the Dc protein assay kit (Bio-Rad Laboratories). Samples were boiled in Laemmli buffer with 8 M urea, and 75 μg protein was layered on 4%–15% Tris-glycine gels, subjected to SDS-PAGE, and transferred to polyvinyl difluoride membrane (Millipore; catalog number IPFL00010). Primary rabbit anti-Tg antibody (1:10 000) and mouse anti-NIS antibody (1:100) were incubated overnight at 4°C. Washed membranes were incubated with horseradish peroxidase-conjugated antirabbit secondary antibody (Cell Signaling; catalog number 7047) and horseradish peroxidase-conjugated antimouse secondary antibody (DAKO; catalog number P0447). ECL plus reagent (Thermo scientific; catalog number 32106) was used for signal generation. Anti-β-actin (Santa Cruz Biotechnology) was used as a loading control. Antibodies were validated prior to their use.
Metabolic labeling was conducted to increase sensitivity for detecting TPO. Confluent cultures were incubated with [35S]methionine (PerkinElmer; 40 μCi/mL) in methionine- and cystein-free DMEM medium (Invitrogen) supplemented with 10% FBS for 48 hours. Cell pellets were collected by centrifugation and stored at −70°C. They were resuspended in 100 μL ice-cold RIPA buffer (Thermo Scientific) with protease inhibitor cocktail and incubated at 4°C for 10 minutes. Cell lysates were precleaned by adding rabbit IgG (1:20) (Sigma; catalog number18140). After 1 hour incubation at 4°C, 50 μL protein A agarose beads (Santa Cruz Biotechnology; catalog number SC-2001) were added for the final 30 minutes. Samples were centrifuged at 14 000 × g, and supernatants were then subjected to immunoprecipitation with anti-TPO antibody overnight at 4°C followed by the addition of bead slurry (50 μL) for the final 4 hours. Immunoprecipitates were rinsed and resuspended in Laemmli buffer (Bio-Rad Laboratories) and subjected to SDS-PAGE followed by autoradiography. Radioactivity was quantified by liquid scintillation spectrometry (Winspectral; PerkinElmer).
Flow cytometric detection of NIS and TSHR
Low levels of NIS protein coupled with relatively few cells recovered after transfection necessitated the alteration of our detection strategy to one using flow cytometry on an LSR II (Becton Dickinson). TSHR has been quantified reliably in our laboratory by flow cytometry (12, 14).
RNA preparation and real-time PCR
Total cellular RNA was extracted with RNeasy (QIAGEN). RNA (200 ng) was reverse transcribed, and real-time PCR was performed with an Applied Biosystems instrument using a QuantiTect SybrGreen PCR kit (QIAGEN; catalog number 204143). The following primer sequences, synthesized by Life Technologies, were used: Tg, forward, 5′-GAGCCCTACCTCTTCTGGCA-3′, and reverse, 5′-GAGGTCCTCATTCCTCAGCC-3′; TSHR, forward, 5′-AGCCACTGCTGTGCTTTTAAG-3′, and reverse, 5′-CCAAAACCAATGATCTCATCC-3′; TPO, forward, 5′-AACAACAGAGACCACCCCAGAT-3′, and reverse, 5′-TGACTGAAGCCGTCCTCATAGAC-3′; AIRE, forward, 5′-CCCTACTGTGTGTGGGTCCT-3′, and reverse, 5′-ACGTCTCCTGAGCAGGATCT-3′; prostaglandin endoperoxide H synthase-2 (PGHS-2), forward, 5′-GAGCAG GCAGATGAAATG-3′, and reverse, 5′-TACCAG AAGGGCAGGATAC-3′; paired box protein-8 (PAX8), forward, 5′-CAGGCATGGTGGCAGGAAGT-3′, and reverse, 5′-ACAGATGGTCAAAGGCCGTG-3′. NIS mRNA was quantified with the Taqman technique using forward, 5′-CCCTCATCCTGAACCAAG-TG-3′, reverse, 5′-GATCCGGGAGTGGTTCTG-3′, and probe, FAM-CTGGACATCTGGGCGTCGCTCTAMARA. SYBR Green human 18S rRNA was from QIAGEN (catalog number PPH05666E), and TaqMan human 18S rRNA primers were from Life Technologies (catalog number Hs99999901-s1). Thyroid transcription factor 1 (TTF-1) SYBR Green primers were from QIAGEN (catalog number QT00010682). Commercially purchased human thymus RNA (CLONTECH) served as a positive control for AIRE transcript quantification. Sample values were generated against a standard curve and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) signals.
Cell transfections
Activity of the Aire gene promoter was assessed in fibrocytes, OFs, and DFs using a reporter plasmid in which the 1200-bp fragment, extending from −1200 nt to +1 nt and was fused to the luciferase coding region. Knockdown studies were conducted by transfecting fibrocytes with specific siRNA targeting AIRE. Transfections into fibrocytes, OF, and DF were conducted as described by Fernando et al (14) and Tsui et al (29), respectively. siRNA specifically targeting AIRE and its scrambled control were transfected into fibrocytes and fibroblasts as described previously (30).
mRNA stability assay
AIRE mRNA stability was assessed using a previously described method (31).
Statistics
Statistical significance was determined with the two-tailed Student's t test.
Results
Fibrocytes express multiple proteins thought to be restricted to thyroid tissue
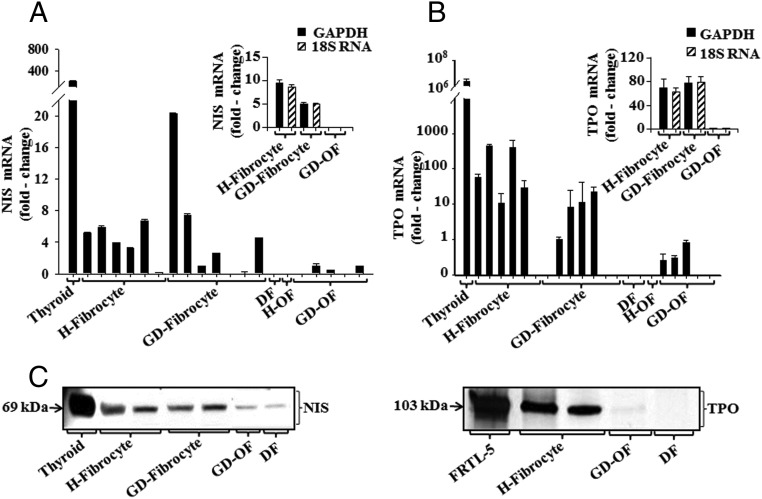
Recent reports from this laboratory group demonstrated the unanticipated expression of TSHR and Tg by fibrocytes (12, 14). Subsequent studies have revealed that both proteins are functionally active. Further examination of fibrocytes now discloses expression of a thyroid protein repertoire that also includes NIS and TPO (Figure 1). The respective mRNAs are easily detected, albeit at levels substantially lower than those found in thyroid tissue. NIS mRNA was detected in 11 of 13 fibrocyte culture strains, each from a different donor (Figure 1A). In contrast, the transcript was undetectable in DFs and either undetectable or expressed at extremely low levels in H-OF and GD-OF. TPO was also detected in a majority of fibrocyte strains but was found at much lower levels or not detected in OFs and DFs (Figure 1B). Expression of NIS and TPO mRNAs was equivalent, regardless of whether the results were normalized to GAPDH or 18S RNA (Figure 1, A and B, insets). The respective proteins can be readily detected by Western blot analysis in fibrocytes and migrate in electrophoretic patterns similar to those found in thyroid tissue and FRTL-5 cells in culture (Figure 1C). The dominant band for NIS appears at 69 kDa, whereas that for TPO resolved at 103 kDa. Despite the extremely low level of NIS mRNA, faint NIS protein can be identified in DFs and GD-OF, suggesting that these cells efficiently translate low abundance transcripts. This was found to be the case in our earlier studies examining Tg expression (14). TPO protein was essentially undetectable in any fibroblast strain. Thus, fibrocytes express several proteins that traditionally define the unique phenotype of thyroid epithelial cells and are essential for the generation of iodothyronines.
Figure 1.
Relative NIS (A) and TPO (B) mRNA levels in H-fibrocyte, GD-fibrocyte, DF, H-OF, and GD-OF. RNA was extracted from confluent culture as described in Figure 2 and subjected to real-time PCR. Values were normalized to respective GAPDH signals. Data are expressed as the mean ± SD of three independent determinations. Insets, Data are normalized to either GAPDH or 18S RNA. C, Detection of NIS and TPO proteins in the cell types indicated. For NIS detection, equivalent quantities of solubilized proteins were subjected to Western immunobloting and probed with an anti-NIS antibody. Membranes were reprobed with anti-β-actin antibody. For TPO, cells were labeled with [35S]methionine (20 μCi/mL) for 48 hours and subjected to immunoprecipitation using anti-TPO. Pulled-down proteins ran on 4%–15% SDS-PAGE, and gels were dried and analyzed using autoradiography.
Fibrocytes express AIRE
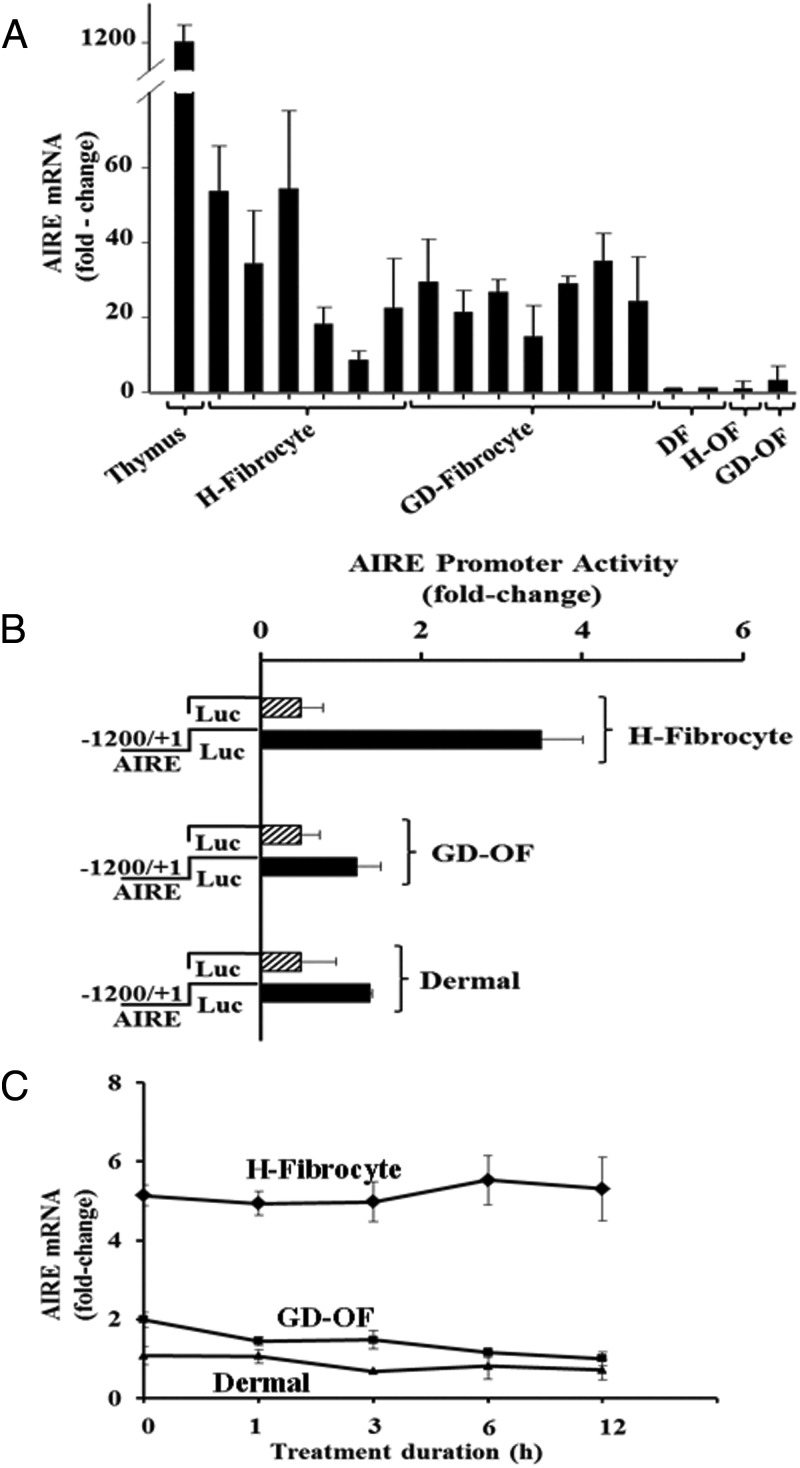
AIRE mRNA can also be detected in multiple fibrocyte strains. As Figure 2A demonstrates, the AIRE transcript is expressed in all 13 strains of fibrocytes, each from a different healthy donor (n = 6) or patient with GD (n = 7). AIRE transcript abundance in all of these strains is modest when compared with that in human thymus. Levels of AIRE mRNA in GD-OF, H-OF, and DFs are even lower. Expression of AIRE mRNA in fibrocytes can be attributed to an active AIRE gene promoter. A fragment of the human promoter exhibited substantially greater activity in fibrocytes than that observed in OFs and DFs when the construct was transiently transfected into these cell types (Figure 2B). It appears that AIRE mRNA is stable over 12 hours in all three cell types (Figure 2C).
Figure 2.
Human fibrocytes express AIRE mRNA, the consequence of an active AIRE gene promoter and a stable transcript. A, Relative AIRE mRNA abundance in thymus, H-fibrocyte, GD-fibrocyte, DF, H-OF, and GD-OF. Confluent cultures were lysed, and RNA was extracted, reverse transcribed, and subjected to real-time PCR. Values were normalized to their respective GAPDH signals. B, Fibrocytes, GD-OF, and DF were transfected with an empty vector or one fused to a fragment of the human AIRE gene promoter. After 24-hour incubations, cells were disrupted, and luciferase activity was quantified as described in Materials and Methods. Data are expressed as mean ± SD of triplicate determinations from one experiment, representative of three performed. C, AIRE mRNA stability was assessed as described in Materials and Methods. 5,6-Dichlorobenzimidazole was added at time 0, and replicate culture wells were harvested at the times indicated along the abscissa. AIRE mRNA was reverse transcribed and cDNA was subjected to RT-PCR.
Knocking down AIRE in fibrocytes disrupts the expression of thyroid specific proteins
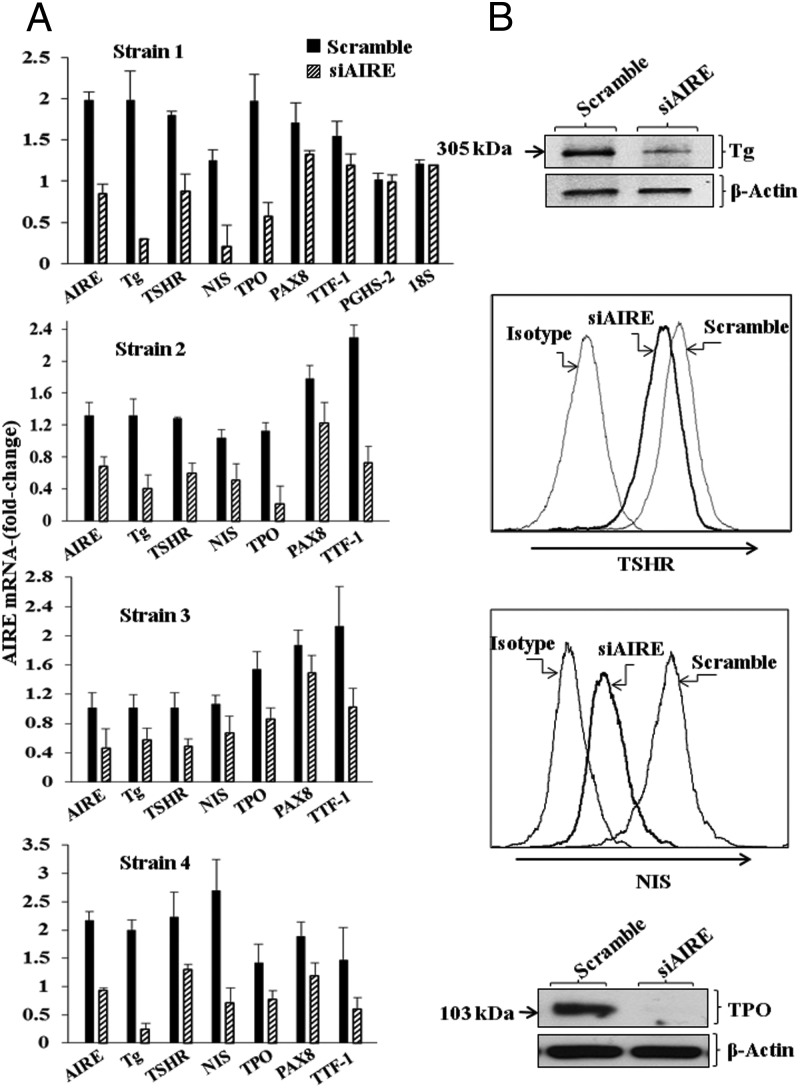
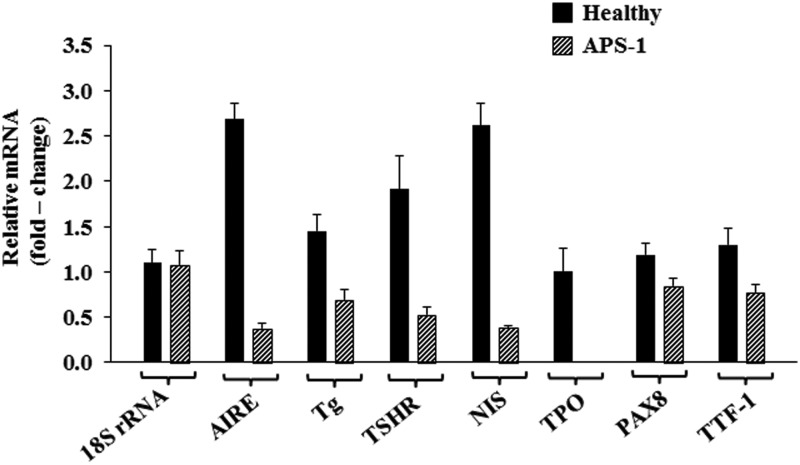
To directly determine whether AIRE played an important role in the promiscuous expression of TSHR, Tg, NIS, and TPO, fibrocytes were transfected with a specific siRNA targeting AIRE. Knocking down AIRE expression in a total of four fibrocyte strains, each from a different donor, substantially reduced transcripts encoding all four thyroid proteins (Figure 3A). The reductions in these steady-state mRNA levels were as follows: AIRE, 54% (P < .001); TSHR, 71% (P < .001 vs control); Tg, 50% (P < .001); NIS, 61% (P < .01); TPO, 60% (P < .01); PAX8, 28% (P < .01); and TTF-1, 50% (P < .01). In contrast, levels of PGHS-2, the inflammatory cyclooxygenase (31), and 18S RNA were unaffected by knocking down AIRE (Figure 3A). Levels of the respective proteins were similarly reduced, as the data in Figure 3B demonstrate. We next interrogated fibrocytes from an individual with APS-1 and those of her unaffected mother. Levels of AIRE expression were substantially lower in the proband, as expected (Figure 4). Those of TSHR mRNA and Tg mRNA were 73% (P < .01) and 52% (P < .01) below levels in the healthy control, whereas NIS mRNA and TPO mRNA were 85% (P < .01) and 100% (P < .001) lower, respectively (Figure 4). Thus, it would appear that the reduction of AIRE levels, either by knocking them down or as a consequence of a naturally occurring AIRE mutation, can be linked to substantially attenuated expression of all four thyroid proteins.
Figure 3.
Interfering with AIRE expression knocked-down levels of AIRE, Tg, TSHR, NIS, TPO, PAX8, and TTF-1 but not PGHS-2 or 18S RNA. A, Fibrocytes from four different donors were transfected, either with scrambled (control) siRNA or siRNA directed against AIRE, as described in Materials and Methods. Cultures were then incubated for 48 hours, and RNA was harvested and then subjected to real-time PCR. Data are expressed as mean ± SD of three independent determinations. B, Fibrocyte cultures were transfected with either scrambled siRNA or siRNA targeting AIRE for 96 hours as described in Materials and Methods. Tg protein was quantified by labeling with [35S]methionine (40 μCi/mL) and then immunoprecipitated, as in the legend to Figure 1. TSHR and NIS were quantified by flow cytometry (14). Levels of TPO were assessed by Western blotting.
Figure 4.
Fibrocytes derived from an individual with APS-1 express lower levels of AIRE, TSHR, Tg, NIS, TPO, PAX8, and TTF-1 than do those from an unaffected first-degree relative. Cells were prepared as described in Materials and Methods from PBMCs derived from an individual with APS-1 and her unaffected mother. The proband is heterozygous for two mutations, a C>T substitution at bp 769 and a 13-base deletion from bp 967 to bp 979. Cultures were harvested after 14 days and were interrogated by real-time PCR using the specific primer sets described. Data are expressed as mean ± SD (n = 6 independent determinations).
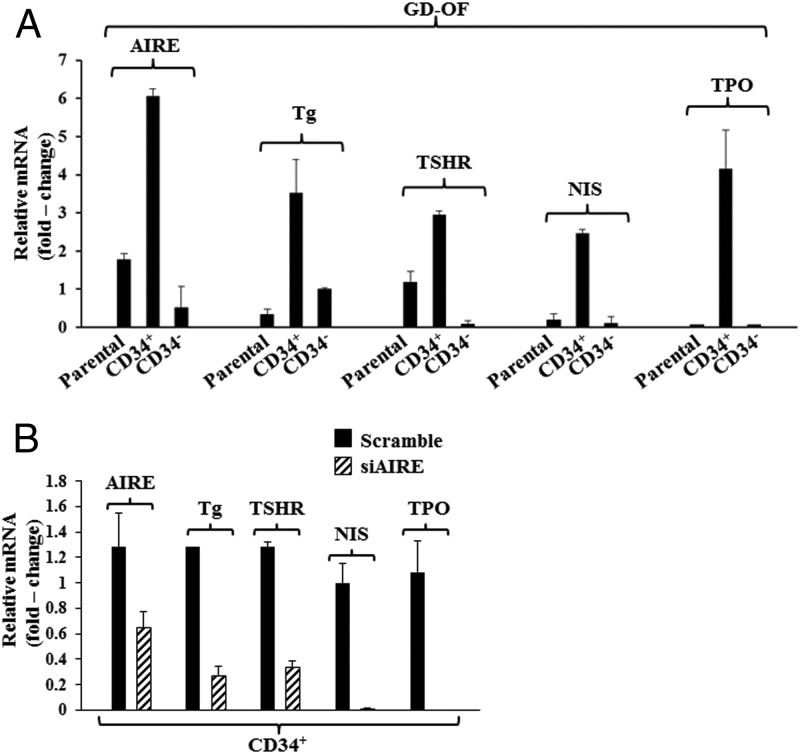
Separating GD-OF into pure CD34+ fibroblasts results in substantial increases in AIRE
Parental strains of GD-OF comprise a mixture of CD34+ and CD34− cells (12). This admixture of fibroblasts expresses substantially lower levels of TSHR and Tg than do fibrocytes. Expression of these proteins localizes to the CD34+ subset (14). Moreover, CD34− fibroblasts appear to negatively influence this expression. To determine whether these differences also might be attributed to CD34− GD-OF lowering AIRE expression, mixed parental GD-OF were sorted into pure CD34+ and CD34− subsets. As the results in Figure 5A clearly demonstrate, pure CD34+ GD-OF express substantially higher levels of AIRE as well as Tg, TSHR, NIS, and TPO than do either parental GD-OF or pure subsets of CD34− GD-OF. To determine whether an increase in AIRE per se drives the increased thyroid protein expression in pure CD34+ fibroblasts, this subset was transfected with siRNA targeting AIRE immediately after the cell sort. Knocking down AIRE substantially attenuated the increase in mRNAs encoding all four thyroid proteins observed in the sorted CD34+ cells transfected with scrambled siRNA (Figure 5B). Thus, the enhanced expression of thyroid-specific proteins after GD-OF sorting into pure CD34+ fibroblasts can be directly attributed to enhanced AIRE expression.
Figure 5.
Sorting GD-OF into pure CD34+ subsets results in dramatic elevations in AIRE, Tg, TSHR, NIS, and TPO mRNA levels, the consequence of increased levels of AIRE. A, Parental strain of GD-OF (containing a mixture of CD34+ and CD34− fibroblasts) was sorted into pure CD34+ and CD34− subsets by FACS on a FACSAria III instrument (BD). These were cultured for 48 hours, RNA extracted, reverse transcribed, and subjected to real time-PCR for the amplicons indicated. B, Transfecting pure CD34+ GD-OF with siRNA targeting human AIRE attenuates the up-regulation of AIRE, Tg, TSHR, NIS, and TPO. Cells were sorted as in panel A and cultured with either scrambled siRNA or siRNA targeting AIRE. RNA was extracted and subjected to RT-PCR. Data are expressed as mean ± SD of three independent determinations.
Discussion
The findings we report here provide completely new insights into potential functions of AIRE in extrathymic tissues and may hold broad implications for tissue-specific autoimmunity. In particular, we identify the previously unrecognized ability of fibrocytes to express AIRE. Furthermore, the results implicate that protein in the promiscuous expression of TSHR, Tg, NIS, and TPO. Thus, it is possible that AIRE serves as a master switch in controlling the expression of thyroid proteins in fibrocytes. Since the initial identification of AIRE in thymic medullary epithelial cells, it has been detected in other cell types and tissues, often at relatively low levels (25–27, 32). These include differentiated dendritic cells, eTACs, circulating monocytes, spleen, and lymph nodes. Assigning function to extrathymic AIRE has proven challenging.
Levels of the AIRE transcript in fibrocytes are modest compared with those found in the human thymus. On the other hand, they are considerably higher than those found in fibroblasts. The mechanisms underlying AIRE gene expression in any cell type are incompletely understood. The gene promoter has been characterized, the minimal promoter mapped, and a functional TATA box identified. Furthermore, cis-acting binding sites for activator protein-1, specificity protein-1, nuclear factor Y, and Ets have been identified (33). Evidence that CpG methylation plays a role in regulating AIRE gene expression has emerged (34, 35) The current studies reveal that AIRE expression in fibrocytes can be attributed to an active gene promoter (Figure 2B). The molecular basis for the constitutively active AIRE gene promoter in fibrocytes will require further study.
Similar uncertainty exists with regard to the mechanisms through which AIRE activates target genes. Pitkanen et al (36) demonstrated that although AIRE protein activates gene transcription in transient expression assays, it lacks classical DNA binding motifs. These findings are consistent with the concepts proposed by Giraud et al (37), who found that AIRE might not be acting through the recognition of specific promoter sites but instead binds RNA polymerase II indiscriminately and releases stalled enzyme at the transcription start sites of target genes. In this paradigm AIRE does not participate in polymerase II recruitment but facilitates the association of positive transcription elongation factor to a preassembled preinitiation complex (36, 37). AIRE may bind chromatin by recognizing the amino terminus of unmethylated histone H3 through plant homeodomain 1, an essential but insufficient condition for target gene activation (38–40). Whether AIRE activates target genes in fibrocytes in a manner similar to that already identified in mTECs remains to be determined. However, the current studies suggest that reduction of AIRE levels, either by treating fibrocytes with siRNA (Figure 3A) or resulting from a naturally occurring mutation (Figure 4), attenuates PAX8 and TTF-1 expression. These factors act synergistically by physically and functionally interacting to promote thyroid specific gene transcription in thyroid (41). Further studies will thus be necessary to determine whether these transcription factors play a role in fibrocyte gene expression.
The current observations raise the question of whether promiscuously expressed thyroid proteins might play some role in the pathogenesis of GD and TAO. Antibodies directed at TSHR, Tg, and TPO are frequently detected in individuals manifesting thyroid autoimmunity. Expression of these proteins within the orbit in conjunction with AIRE might provoke antigen-specific T cell activation and immune reactivity through antigen presentation. Thus, the high constitutive levels of major histocompatibility complex class II displayed on fibrocytes and their ability to present antigen (5) might help explain the localized autoimmunity associated with TAO. It is possible that the consequences of central and peripheral AIRE expression might prove very different. Although AIRE plays a critical role in promoting immune tolerance in thymus, its chronic promotion of autoantigens by fibrocytes could instead result in immune reactivity. Importantly, circulating fibrocytes from healthy donors also express these proteins. This underscores the likelihood that T cell repertoires associated with susceptibility to GD will prove essential for fibrocyte-provoked immune reactivity leading to disease.
The current observations identifying a heretofore unrecognized function for AIRE in fibrocytes suggest a number of potentially interesting directions for future inquiry. Several reports have appeared, suggesting a wider distribution of thyroid proteins beyond the gland. TSHR has been detected in several fatty connective tissue depots (15). More recently, transcripts for TSHR, Tg, NIS, and TPO have been found in skin biopsies (42) and tissues from the right ventricle of the heart (18). Extrathyroidal generation of thyroid hormone was first suggested by the seminal work of Taurog and Evans (43). They demonstrated that thyroidectomized rats in which all thyroid tissue had been removed responded to iodide administration with thyromimetic activity and the production of T4. Moreover, parenteral delivery of 131I to these rats resulted in detectable plasma 131I-labeled T4. Although potential incompleteness of the thyroidectomy could have confounded the interpretation of their findings, their work in aggregate brought attention to the potential for thyroid hormone production outside the gland. Because fibrocytes appear to express the necessary molecular machinery, it is possible that they could produce thyroid hormones or related molecules under certain conditions. In addition to its impact on proteins important to thyroid function, additional consequences of AIRE expression must be considered. For instance, it is possible that AIRE drives other tissue-specific proteins in fibrocytes in addition to those identified here. In particular, it may drive those associated with autoimmunity other than GD (44). The large repertoire of gene targets identified previously in mTECs and eTACs makes this possibility worthy of further investigation.
Acknowledgments
We are grateful to Professors Mitsuru Matsumoto (Tokushima, Japan), Pärt Peterson (Tartu, Estonia), Mark Anderson (San Francisco, California), and Massimo Pietropaolo (Ann Arbor, Michigan) for helpful discussions and the provision of valuable materials.
This work was supported by National Institutes of Health Grants EY008976, EY011708, and DK063121; Core Center for Vision Grant EY007003 from the National Eye Institute; an unrestricted grant from Research to Prevent Blindness; and the Bell Charitable Foundation.
Disclosure Summary: All authors have nothing to declare.
Footnotes
- AIRE
- autoimmune regulator
- APS-1
- autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy
- DF
- dermal fibroblast
- eTac
- extrathymic AIRE-expressing cells
- FACS
- fluorescence-activated cell sorter
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GD
- Graves' disease
- GD-OF
- GD-derived orbital fibroblasts
- H-OF
- healthy donor-derived orbital fibroblasts
- mTec
- thymic medullary epithelial cells
- NIS
- sodium/iodide symporter
- OF
- orbital fibroblast
- PAX8
- paired box protein 8
- PBMC
- peripheral blood mononuclear cells
- PGHS-2
- prostaglandin endoperoxide H synthase-2
- siRNA
- small interfering RNA
- TAO
- thyroid-associated ophthalmopathy
- Tg
- thyroglobulin
- TPO
- thyroperoxidase
- TSHR
- thyrotropin receptor
- TTF-1
- thyroid transcription factor-1.
References
- 1. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81 [PMC free article] [PubMed] [Google Scholar]
- 2. Pilling D, Fan T, Huang D, Kaul B, Gomer R.H. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475–e7475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang J, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Improvement in postburn hypertrophic scar after treatment with IFN-α2b is associated with decreased fibrocytes. J Interferon Cytokine Res. 2007;27:921–930 [DOI] [PubMed] [Google Scholar]
- 5. Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94:6307–6312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-β and peroxisome proliferator-activated receptor γ. J Biol Chem. 2007;282:22910–22920 [DOI] [PubMed] [Google Scholar]
- 7. Galligan CL, Siminovitch KA, Keystone EC, Bykerk V, Perez OD, Fish EN. Fibrocyte activation in rheumatoid arthritis. Rheumatology (Oxford). 2010;49:640–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108 [DOI] [PubMed] [Google Scholar]
- 9. Zakarija M, Jin S, McKenzie JM. Evidence supporting the identity in Graves' disease of thyroid-stimulating antibody and thyroid growth-promoting immunoglobulin G as assayed in FRTL5 cells. J Clin Invest. 1988;81:879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kazim M, Goldberg RA, Smith TJ. Insights into the pathogenesis of thyroid-associated orbitopathy: evolving rationale for therapy. Arch Ophthalmol. 2002;120:380–386 [DOI] [PubMed] [Google Scholar]
- 11. Grubeck-Loebenstein B, Trieb K, Sztankay A, Holter W, Anderl H, Wick G. Retrobulbar T cells from patients with Graves' ophthalmopathy are CD8+ and specifically recognize autologous fibroblasts. J Clin Invest. 1994;93:2738–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gillespie EF, Papageorgiou KI, Fernando R, et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated opthalmopathy leads to chemokine production. J Clin Endocrinol Metab. 2012;97:E740–E746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fernando R, Atkins S, Raychaudhuri N, et al. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci USA. 2012;109:7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feliciello A, Porcellini A, Ciullo I, Bonavolontà G, Avvedimento EV, Fenzi G. Expression of thyrotropin-receptor mRNA in healthy and Graves' disease retro-orbital tissue. Lancet. 1993;342:337–338 [DOI] [PubMed] [Google Scholar]
- 16. Tao T-W, Cheng P-J, Pham H, Leu S-L, Kriss JP. Monoclonal antithyroglobulin antibodies derived from immunizations of mice with human eye muscle and thyroid membranes. J Clin Endocrinol Metab. 1986;63:577–582 [DOI] [PubMed] [Google Scholar]
- 17. Dohán O, Carrasco N. Advances in Na(+)/I(−) symporter (NIS) research in the thyroid and beyond. Mol Cell Endocrinol. 2003;213:59–70 [DOI] [PubMed] [Google Scholar]
- 18. Meischl C, Buermans H, Hazes T, et al. H9c2 cardiomyoblasts produce thyroid hormone. Am J Physiol Cell Physiol. 2008;294:C1227–C1233 [DOI] [PubMed] [Google Scholar]
- 19. Nagamine K, Peterson P, Scott HS, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398 [DOI] [PubMed] [Google Scholar]
- 20. The Finnish-German APECED Consortium. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403 [DOI] [PubMed] [Google Scholar]
- 21. Rinderle C, Christensen HM, Schweiger S, Lehrach H, Yaspo ML. AIRE encodes a nuclear protein co-localizing with cytoskeletal filaments: altered sub-cellular distribution of mutants lacking the PHD zinc fingers. Hum Mol Genet. 1999;8:277–290 [DOI] [PubMed] [Google Scholar]
- 22. Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self-shadow within the thymus by the AIRE protein. Science. 2002;298:1395–1401 [DOI] [PubMed] [Google Scholar]
- 23. Heino M, Peterson P, Kudoh J, et al. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem Biophys Res Commun. 1999;257:821–825 [DOI] [PubMed] [Google Scholar]
- 24. Yano M, Kuroda N, Han H, et al. AIRE controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J Exp Med. 2008;205:2827–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gardner JM, Devoss JJ, Friedman RS, et al. Deletional tolerance mediated by extrathymic AIRE-expressing cells. Science. 2008;321:843–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sillanpää N, Magureanu CG, Murumägi A, et al. Autoimmune regulator induced changes in the gene expression profile of human monocyte-dendritic cell-lineage. Mol Immunol. 2004;41:1185–1198 [DOI] [PubMed] [Google Scholar]
- 27. Kogawa K, Nagafuchi S, Katsuta H, et al. Expression of AIRE gene in peripheral monocyte/dendritic cell lineage. Immunol Lett. 2002;80:195–198 [DOI] [PubMed] [Google Scholar]
- 28. Smith TJ, Sempowski GD, Wang HS, Del Vecchio PJ, Lippe SD, Phipps RP. Evidence for cellular heterogeneity in primary cultures of human orbital fibroblasts. J Clin Endocrinol Metab. 1995;80:2620–2625 [DOI] [PubMed] [Google Scholar]
- 29. Tsui S, Fernando R, Chen B, Smith TJ. Divergent Sp1 protein levels may underlie differential expression of UDP glucose dehydrogenase by fibroblasts: role in susceptibility to orbital Graves' disease. J Biol Chem. 2011;286:24487–24499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raychaudhuri N, Fernando R, Smith TJ. Thyrotropin regulates IL-6 expression in CD34+ fibrocytes: clear delineation of its cAMP-independent actions. PLoS One. 2013;8:e75100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han R, Tsui S, Smith TJ. Up-regulation of prostaglandin E2 synthesis by interleukin-1β in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and gluthathione-dependent prostaglandin E2 synthase expression. J Biol Chem. 2002;277:16355–16364 [DOI] [PubMed] [Google Scholar]
- 32. Lee JW, Epardaud M, Sun J, Yonekura AR, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190 [DOI] [PubMed] [Google Scholar]
- 33. Murumägi A, Silvennoinen O, Peterson P. Ets transcription factors regulate AIRE gene promoter. Biochem Biophys Res Commun. 2006;348:768–774 [DOI] [PubMed] [Google Scholar]
- 34. Murumägi A, Vahamurto P, Peterson P. Characterization of regulatory elements and methylation pattern of the autoimmune regulator (AIRE) promoter. J Biol Chem. 2003;278:19784–19790 [DOI] [PubMed] [Google Scholar]
- 35. Kont V, Murumägi A, Tykocinski LO, et al. DNA methylation signatures of the AIRE promoter in thymic epithelial cells, thymomas and normal tissues. Mol Immunol. 2011;49:518–526 [DOI] [PubMed] [Google Scholar]
- 36. Pitkanen J, Vahamurto P, Krohn K, Peterson P. Subcellular localization of the autoimmune regulator protein. Characterization of nuclear targeting and transcriptional activation domain. J Biol Chem 2001;276:19597–19602 [DOI] [PubMed] [Google Scholar]
- 37. Giraud M, Yoshida H, Abramson J, et al. AIRE unleashes stalled RNA polymerase to induce ectopic gene expression in thymic epithelial cells. Proc Natl Acad Sci USA. 2012;109:535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oven I, Brdickova N, Kohoutek J, Vaupotic T, Narat M, Peterlin BM. AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol. 2007;27:8815–8823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zumer K, Low AK, Jiang H, Saksela K, Peterlin BM. Unmodified histone H3K4 and DNA-dependent protein kinase recruit autoimmune regulator to target genes. Mol Cell Biol. 2012;32:1354–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koh AS, Kuo AJ, Park SY, et al. AIRE employs a histone-binding module to mediate immunological tolerance, linking chromatin regulation with organ-specific autoimmunity. Proc Natl Acad Sci USA. 2008;105:15878–15883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palma T, Nitsch R, Mascia A, Nitsch L, Di Lauro R, Zannini M. The paired domain-containing factor Pax-8 and the heterodomain-containing factor TTF-1 directly interact and synergistically activate transcription. J Biol Chem. 2003;278:3395–3402 [DOI] [PubMed] [Google Scholar]
- 42. Cianfarani F, Baldini E, Cavalli A, et al. TSH receptor and thyroid-specific gene expression in human skin. J Invest Dermatol. 2010;130:93–101 [DOI] [PubMed] [Google Scholar]
- 43. Taurog A, Evans E. Extrathyroidal throxine formation in completely thyroidectomized rats. Endocrinology. 1967;80:915–925 [DOI] [PubMed] [Google Scholar]
- 44. Fernando R, Vonberg A, Atkins SJ, Pietropaolo S, Pietropaolo M, Smith TJ. Human fibrocytes express multiple antigens associated with autoimmune endocrine diseases. J Clin Endocrinol Metab. 2014;99:E796–E803 [DOI] [PMC free article] [PubMed] [Google Scholar]