Abstract
Aim—To determine the liver cell populations that express the phylogenetically conserved cytosolic protein stathmin during liver regeneration.
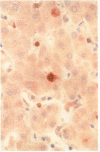
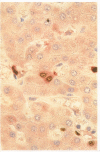
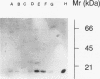
Methods—Double immunostaining for stathmin and the Ki67 antigen was performed on sections of formaldehyde fixed, paraffin wax embedded tissues from 31 liver specimens. These included a variety of disease conditions characterised by some degree of hepatocyte regeneration. Quantitative western blot analysis was performed on 22 of these specimens.
Results—Variable amounts of stathmin protein were detected by western blotting in all of the specimens examined. Stathmin was not detected in three cases of histologically normal liver. On immunostaining, stathmin was demonstrated in a proportion of hepatocytes as well as lymphoid inflammatory cells and other tissue elements. In all cases most of these stathmin positive cells showed nuclear positivity for the Ki67 antigen.
Conclusions—Stathmin is expressed by proliferating hepatocytes but not by resting hepatocytes. Thus, it is likely that the protein has a function important to cell proliferation as opposed to cell differentiation.
Keywords: Stathmin
Keywords: p19
Keywords: prosolin
Keywords: metablastin
Keywords: LAP18
Keywords: liver
Keywords: proliferation
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beretta L., Dobránsky T., Sobel A. Multiple phosphorylation of stathmin. Identification of four sites phosphorylated in intact cells and in vitro by cyclic AMP-dependent protein kinase and p34cdc2. J Biol Chem. 1993 Sep 25;268(27):20076–20084. [PubMed] [Google Scholar]
- Brattsand G., Roos G., Marklund U., Ueda H., Landberg G., Nånberg E., Sideras P., Gullberg M. Quantitative analysis of the expression and regulation of an activation-regulated phosphoprotein (oncoprotein 18) in normal and neoplastic cells. Leukemia. 1993 Apr;7(4):569–579. [PubMed] [Google Scholar]
- Braverman R., Bhattacharya B., Feuerstein N., Cooper H. L. Identification and characterization of the nonphosphorylated precursor of pp17, a phosphoprotein associated with phorbol ester induction of growth arrest and monocytic differentiation in HL-60 promyelocytic leukemia cells. J Biol Chem. 1986 Oct 25;261(30):14342–14348. [PubMed] [Google Scholar]
- Brown G., Bunce C. M., Rowlands D. C., Williams G. R. All-trans retinoic acid and 1 alpha,25-dihydroxyvitamin D3 co-operate to promote differentiation of the human promyeloid leukemia cell line HL60 to monocytes. Leukemia. 1994 May;8(5):806–815. [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Cooper H. L., McDuffie E., Braverman R. Human peripheral lymphocyte growth regulation and response to phorbol esters is linked to synthesis and phosphorylation of the cytosolic protein, prosolin. J Immunol. 1989 Aug 1;143(3):956–963. [PubMed] [Google Scholar]
- Gerdes J., Li L., Schlueter C., Duchrow M., Wohlenberg C., Gerlach C., Stahmer I., Kloth S., Brandt E., Flad H. D. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991 Apr;138(4):867–873. [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Gullberg M., Noreus K., Brattsand G., Friedrich B., Shingler V. Purification and characterization of a 19-kilodalton intracellular protein. An activation-regulated putative protein kinase C substrate of T lymphocytes. J Biol Chem. 1990 Oct 15;265(29):17499–17505. [PubMed] [Google Scholar]
- Hanash S. M., Strahler J. R., Kuick R., Chu E. H., Nichols D. Identification of a polypeptide associated with the malignant phenotype in acute leukemia. J Biol Chem. 1988 Sep 15;263(26):12813–12815. [PubMed] [Google Scholar]
- Harrison R. F., Reynolds G. M., Rowlands D. C. Immunohistochemical evidence for the expression of proliferating cell nuclear antigen (PCNA) by non-proliferating hepatocytes adjacent to metastatic tumours and in inflammatory conditions. J Pathol. 1993 Oct;171(2):115–122. doi: 10.1002/path.1711710208. [DOI] [PubMed] [Google Scholar]
- Jones N. A., Lord J. M., Brown G. Changes in the phosphorylation status of a 19 kD cytosolic protein are linked to the growth arrest of HL-60 cells. Leuk Res. 1992;16(4):353–361. doi: 10.1016/0145-2126(92)90137-v. [DOI] [PubMed] [Google Scholar]
- Koppel J., Loyer P., Maucuer A., Rehák P., Manceau V., Guguen-Guillouzo C., Sobel A. Induction of stathmin expression during liver regeneration. FEBS Lett. 1993 Sep 27;331(1-2):65–70. doi: 10.1016/0014-5793(93)80298-9. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Wong A. K., Brown G. Changes in phosphoproteins during commitment of HL60 cells to monocyte differentiation: evidence for multiple protein kinase involvement. Exp Hematol. 1988 Aug;16(7):620–626. [PubMed] [Google Scholar]
- Marklund U., Brattsand G., Shingler V., Gullberg M. Serine 25 of oncoprotein 18 is a major cytosolic target for the mitogen-activated protein kinase. J Biol Chem. 1993 Jul 15;268(20):15039–15047. [PubMed] [Google Scholar]
- Melhem R. F., Strahler J. R., Hailat N., Zhu X. X., Hanash S. M. Involvement of OP18 in cell proliferation. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1649–1655. doi: 10.1016/0006-291x(91)91764-4. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G. K. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990 Feb 1;4(2):176–187. [PubMed] [Google Scholar]
- Mock B. A., Krall M. M., Padlan C., Dosik J. K., Schubart U. K. The gene for Lap18, leukemia-associated phosphoprotein p18 (metablastin), maps to distal mouse chromosome 4. Mamm Genome. 1993;4(8):461–462. doi: 10.1007/BF00296823. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Himi T., Peterson C., Mori N. Induction of stathmin mRNA during liver regeneration. FEBS Lett. 1993 Dec 20;336(1):8–12. doi: 10.1016/0014-5793(93)81598-t. [DOI] [PubMed] [Google Scholar]
- Peyron J. F., Aussel C., Ferrua B., Häring H., Fehlmann M. Phosphorylation of two cytosolic proteins. An early event of T-cell activation. Biochem J. 1989 Mar 1;258(2):505–510. doi: 10.1042/bj2580505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands D. C., Williams A., Jones N. A., Guest S. S., Reynolds G. M., Barber P. C., Brown G. Stathmin expression is a feature of proliferating cells of most, if not all, cell lineages. Lab Invest. 1995 Jan;72(1):100–113. [PubMed] [Google Scholar]
- Schubart U. K., Banerjee M. D., Eng J. Homology between the cDNAs encoding phosphoprotein p19 and SCG10 reveals a novel mammalian gene family preferentially expressed in developing brain. DNA. 1989 Jul-Aug;8(6):389–398. doi: 10.1089/dna.1.1989.8.389. [DOI] [PubMed] [Google Scholar]
- Schubart U. K. Expression of phosphoprotein p19 in brain, testis, and neuroendocrine tumor cells. Developmental regulation in rat brain. J Biol Chem. 1988 Aug 25;263(24):12156–12160. [PubMed] [Google Scholar]
- Schubart U. K., Xu J., Fan W., Cheng G., Goldstein H., Alpini G., Shafritz D. A., Amat J. A., Farooq M., Norton W. T. Widespread differentiation stage-specific expression of the gene encoding phosphoprotein p19 (metablastin) in mammalian cells. Differentiation. 1992 Sep;51(1):21–32. doi: 10.1111/j.1432-0436.1992.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Sobel A., Boutterin M. C., Beretta L., Chneiweiss H., Doye V., Peyro-Saint-Paul H. Intracellular substrates for extracellular signaling. Characterization of a ubiquitous, neuron-enriched phosphoprotein (stathmin). J Biol Chem. 1989 Mar 5;264(7):3765–3772. [PubMed] [Google Scholar]
- Strahler J. R., Lamb B. J., Ungar D. R., Fox D. A., Hanash S. M. Cell cycle progression is associated with distinct patterns of phosphorylation of Op18. Biochem Biophys Res Commun. 1992 May 29;185(1):197–203. doi: 10.1016/s0006-291x(05)80975-1. [DOI] [PubMed] [Google Scholar]
- Zhu X. X., Kozarsky K., Strahler J. R., Eckerskorn C., Lottspeich F., Melhem R., Lowe J., Fox D. A., Hanash S. M., Atweh G. F. Molecular cloning of a novel human leukemia-associated gene. Evidence of conservation in animal species. J Biol Chem. 1989 Aug 25;264(24):14556–14560. [PubMed] [Google Scholar]