Abstract
Context:
Vitamin D deficiency, defined by the total serum 25-hydroxyvitamin D [25(OH)D] level, is common and more prevalent among Blacks than whites. Vitamin D–binding protein (DBP) levels vary with race and may modulate “bioavailable” levels of 25(OH)D.
Objective:
To determine the effect of DBP levels on the functional response to vitamin D.
Setting and Design:
A randomized, placebo-controlled trial of vitamin D repletion for 2 mo, which took place at an outpatient research unit. Participants included 150 vitamin D–deficient (25(OH)D <20 ng/mL) adults. Participants were randomly assigned to receive either 50,000 IU of vitamin D3 or placebo weekly for 8 weeks. This is a post-hoc analysis using DBP, 25(OH)D, PTH, and calcium levels.
Results:
Blacks had lower total 25(OH)D (12 vs 15 ng/mL, P < .001) and DBP levels (119 vs 234 μg/mL, P < .001) than non-Blacks. DBP levels were similar before and after vitamin D3 or placebo treatment (r = 0.98, P < .001). Baseline total 25(OH)D levels were a significant determinant of baseline PTH levels (P < .001). The change in total 25(OH)D was associated with the change in PTH (P < 0.001) and calcium levels (P < .05). In contrast, DBP levels were not a determinant of baseline PTH (P = .57) nor significantly related to changes in either PTH (P = .53) or calcium levels (P = .88).
Conclusions:
DBP levels are stable in Blacks and non-Blacks, and do not change with correction of vitamin D deficiency. Even for individuals with total 25(OH)D levels < 20 ng/mL, Blacks have significantly lower DBP levels than non-Blacks. However, within this range of total 25(OH)D, DBP levels do not influence the effect of vitamin D repletion on PTH or calcium levels.
The serum level of total 25-hydroxyvitamin D (25(OH)D) is the standard clinical criterion for determining an individual's vitamin D status. Commonly used ranges include 30–100 ng/mL as sufficient, 20–30 ng/mL as insufficient, and < 20 ng/mL as deficient (1); however, the optimal level of 25(OH)D remains unclear (2). The application of a universally appropriate “normal” level is complicated by well-described population-level disparities in 25(OH)D levels, particularly between Blacks and whites (3, 4). Indeed, there are no guidelines specific to age, race, or sex for targeting 25(OH)D levels.
As a lipophilic hormone, less than 1% of 25(OH)D is free in plasma (5). Approximately 90% is bound to vitamin D–binding protein (DBP), and the remainder to albumin. Genetic variation in the DBP gene, Gc, is associated with 25(OH)D levels (6, 7). Further, single-nucleotide polymorphisms (SNPs) in Gc are known to modulate DBP levels and affinity for 25(OH)D (8). However, whether DBP levels have an effect on the physiologic effects of vitamin D repletion has not been studied.
One of the most sensitive biomarkers of vitamin D activity in vivo is the PTH level, which displays an inverse relationship with the total serum 25(OH)D level (9). This is a causal relationship where PTH levels decline in response to vitamin D repletion. We had previously conducted a randomized controlled trial (RCT) of vitamin D repletion in vitamin D deficient adults (10). To determine whether DBP levels influence a functional endpoint of vitamin D therapy—the response in PTH levels—we have measured DBP levels and performed secondary analyses of our RCT.
Materials and Methods
Study population
Methods regarding the conduct of the RCT were previously published (10). Briefly, 151 adults with a serum 25(OH)D level ≤20 ng/mL were recruited from a racially diverse population and enrolled between October 2009 and May 2011. Participants were randomly assigned to receive either vitamin D3 50,000 U or placebo (The BTR Group) weekly for 8 weeks. Participants were asked to self identify by race, and were classified as Black, white, or Asian. Asian participants (n = 8) had baseline DBP (250 μg/mL) and 25(OH)D levels (17.7 ng/mL) similar to white participants. Thus, for subsequent analyses, these participants were combined into one group as “non-Blacks.” One individual did not have baseline calcium or PTH values recorded and was omitted from the analyses. Participants were not allowed to consume more than 400 U of vitamin D daily and most participants did not take any supplemental vitamin D. Participants did not record food diaries, although the mean change in 25(OH)D over the entire study period in the placebo group was less than 1 ng/mL, suggesting that neither dietary vitamin D intake nor sun exposure were significant. Participants were primarily (>80%) recruited in the autumn and winter months from November to March. All research participants provided written informed consent for research procedures. The research protocol was approved by Rockefeller University's Institutional Review Board. This study was listed on ClinicalTrials.gov (Identifier NCT01008384).
Laboratory analysis
Subjects were asked to fast for 8 hours prior to study visits. Fasting serum samples were obtained before and after treatment. 25(OH)D, PTH, calcium, and creatinine levels were measured by the Memorial Sloan Kettering Cancer Center clinical laboratory concurrent with study visits. 25(OH)D levels were determined using the Diasorin LIASON automated chemiluminescent immunoassay. In addition, serum was aliquoted and stored at −80 C for future analysis.
A frozen aliquot, which had not been previously thawed, from baseline and after treatment, was used for each participant to determine DBP levels by a commercial ELISA per the manufacturer's instructions (R&D Systems). All DBP measurements were made in duplicate, and averaged values were used for subsequent analyses. Pre- and post-treatment sera from each participant were assayed on the same ELISA plate. The mean coefficient of variation for duplicate measurements was 3.1%.
Calculation of bioavailable 25(OH)D
Bioavailable 25(OH)D levels were calculated using total 25(OH)D, DBP, and albumin levels (11). A genotype-nonspecific affinity constant between DBP and 25(OH)D was used for all participants (7 × 10−8 M−1) (8).
Statistical analysis
Comparisons between the vitamin D repletion and placebo groups were made using the unpaired Student t test. Comparisons for within group changes were made using the paired Student t test. Univariate correlations were assessed by calculating Pearson correlation coefficients. Multivariate analyses were performed using linear regression models. P values for the race: group and time: group interactions were determined by two-factor fixed-effects ANOVA. P values <.05 were considered significant. Regression analysis results were not affected whether Asians were analyzed together with whites as “non-Blacks.” Statistical analyses were conducted using Tibco S+ software (version 8.2).
Results
Baseline characteristics of Black and non-Black participants are shown in Table 1. Baseline characteristics of placebo and vitamin D groups have been previously reported (10). 25(OH)D, DBP, and albumin levels were significantly lower in Blacks than in non-Blacks. To test whether DBP levels were influenced by changes in 25(OH)D levels and the stability of DBP levels over time, we measured DBP levels before and after treatment with either vitamin D3 or placebo. Pre- and post-treatment DBP values were strongly correlated with each other (r = 0.98, P < .001; Figure 1 and Supplemental Table 1). The treatment interval was 8 weeks, which is considerably longer than the 2- to 3-day half-life of DBP (12). A high degree of stability was observed regardless of whether participants received placebo or vitamin D3. The lack of effect of treatment and high stability of DBP levels was observed for Blacks and non-Blacks alike. Therefore, while DBP levels may be a determinant of 25(OH)D levels, the converse relationship is untrue.
Table 1.
Changes in 25(OH)D, PTH, and Calcium in Response to Vitamin D3 Versus Placebo
| Parameter | Blacks (n = 71) | P Value | Non-Blacks (n = 79) | P Value | P Value for Race: Group Interaction |
|---|---|---|---|---|---|
| 25(OH)D (ng/mL) | 28 [24 to 33] | <.001 | 30 [26 to 34] | <.001 | .56 |
| PTH, pg/mL | −21 [−7 to −34] | .003 | −9 [0 to −18] | .05 | .15 |
| Calcium, mg/dL | 0.2 [0 to 0.3] | .05 | 0.1 [−0.1 to 0.2] | .20 | .45 |
Data is shown as mean value of change (95% confidence interval).
Figure 1.
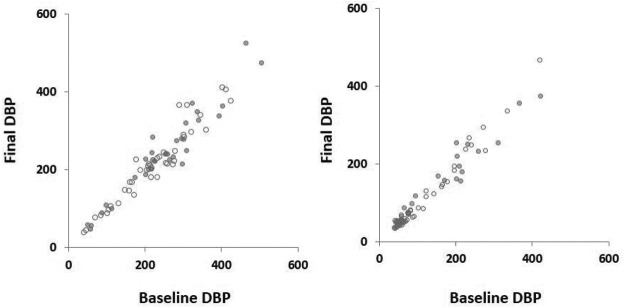
The correlation between baseline and final DBP levels (μg/mL) in Blacks (closed circles) and non-Blacks (open circles) receiving vitamin D3 (left) or placebo (right).
Consistent with prior studies and the known inhibitory effect of 25(OH)D on PTH production, both the treatment and placebo groups showed an inverse correlation between baseline PTH levels and baseline total 25(OH)D levels (r = −0.35, P < .001) (R2 for model = 0.28, P < .001; P for total 25(OH)D < .001). Because binding of 25(OH)D to DBP and albumin may modulate its physiologic activity, we tested whether DBP and albumin levels correlated with baseline PTH levels. Adding baseline DBP and albumin levels did not improve the model (R2 for model = 0.28, P < .001). Moreover, neither DBP (P = .57) nor albumin levels (P = .39) were significantly related to baseline PTH levels. Alternatively, using calculated “bioavailable” 25(OH)D in place of total 25(OH)D, DBP and albumin weakened the model (R2 for model = 0.23, P < .001). Although, “bioavailable” 25(OH) was correlated to baseline PTH (r = −0.25, P = .002), the relationship was not as strong in comparison to total 25(OH)D.
Treatment of vitamin D deficiency ameliorates hyperparathyroidism and can lower PTH levels, even within the normal range (14). Indeed, both Blacks and non-Blacks experienced a decline in PTH levels in response to vitamin D3 repletion (Table 2), and Blacks showed larger decline than non-Blacks, although this did not reach statistical significance (−21 vs −9 pg/mL, P = .15). This is consistent with Blacks starting at a lower total 25(OH)D level compared with non-Blacks (P < .001), while both groups achieved a similar change in total 25(OH)D levels (P = .56). Because of the strong association between race and DBP levels, we used linear regression to model the change in PTH levels. The change in total 25(OH)D, as well as sex, age, and baseline total 25(OH)D, PTH, calcium, creatinine, albumin, and DBP levels were used as potential predictors of the change in PTH levels (R2 for model = 0.44, P < .001; Table 3, Model3 change in serum PTH levels). Only the change in total 25(OH)D (P < .001) and baseline PTH levels (P < .001) were significant predictors of the change in PTH levels. Baseline DBP levels, however, were not predictive of changes in PTH (P = .53), nor were albumin levels (P = .57). When calculated “bioavailable” 25(OH)D is used instead, the model is not as strong as that using total 25(OH)D (Table 3, Model2 vs Model4 change in serum PTH levels). Though, there is a significant correlation between the change in “bioavailable” 25(OH)D and the change in PTH levels (P < .001).
Table 2.
Baseline Characteristics
| Characteristic | Blacks (n = 71) | Non-Blacks (n = 79) | P Value |
|---|---|---|---|
| Age, y | 46.0 ± 9.9 | 49.8 ± 13.5 | .06 |
| Women, % | 44 | 47 | .70 |
| Total 25(OH)D, ng/mL | 11.8 ± 5.2 | 15.4 ± 5.2 | <.001 |
| Calcium, mg/dL | 9.1 ± 0.3 | 9.0 ± 0.4 | .38 |
| PTH, pg/mL | 63 ± 35 | 58 ± 31 | .33 |
| DBP, μg/mL | 119 ± 93 | 234 ± 101 | <001 |
| Albumin, g/dL | 4.3 ± 0.3 | 4.4 ± 0.3 | .03 |
| Creatinine, mg/dL | 1.06 ± 0.24 | 1.02 ± 0.23 | .26 |
Data are shown as mean ± SD.
Table 3.
Linear Regression Models for the Changes in Serum PTH and Calcium Levels
| Model | R2 | P Value |
|---|---|---|
| Change in serum PTH levels | ||
| Model1a | 0.36 | <.001 |
| Model2b | 0.43 | <.001 |
| Model3c | 0.44 | <.001 |
| Model4d | 0.40 | <.001 |
| Change in serum calcium levels | ||
| Model1a | 0.28 | <.001 |
| Model2b | 0.31 | <.001 |
| Model3c | 0.35 | <.001 |
| Model4d | 0.29 | <.001 |
Age, sex, baseline PTH, calcium, and creatinine levels.
Model1 + baseline 25(OH)D and Δ 25(OH)D levels.
Model2 + baseline DBP and albumin levels.
Model1 + baseline “bioavailable” 25(OH)D and Δ”bioavailable”25(OH)D levels.
A causal relationship also exists between vitamin D repletion and an increase in serum calcium levels via an increase in gut calcium absorption (15, 16). The change in total 25(OH)D, as well as sex, age, baseline 25(OH)D, PTH, calcium, creatinine, DBP, and albumin levels were used as potential predictors of the change in serum calcium (R2 = 0.35, P for the model < .001; Table 3, Model3 change in serum calcium levels). Only the change in total 25(OH)D (P < .05), and baseline calcium (P < .001), albumin (P = .004), and PTH levels (P = .02) were significant predictors of the change in serum calcium levels. However, DBP levels (P = .88) were not significantly related. Using calculated “bioavailable” 25(OH)D (Table 3, Model4 change in serum calcium levels) showed no relationship between the change in calcium and the change in “bioavailable” 25(OH)D (P = .39). Also, the resultant model was inferior to that using total 25(OH)D (Table 3, Model2 change in serum calcium levels).
Discussion
We have conducted a secondary analysis of an RCT comparing the effect of short-term vitamin D3 supplementation to placebo in vitamin D–deficient subjects. By measuring serum DBP levels, we have identified several relationships. First, we have shown that non-Blacks with total 25(OH)D levels well below 20 ng/mL still have 2-fold higher DBP levels compared with vitamin D–deficient Blacks. Powe et al (11) found that DBP levels in Blacks were 50% of those found in whites, which is nearly identical to the 51% ratio in our study. Further, at least over an 8-week interval, DBP levels are stable in Blacks and non-Blacks and are not influenced even by large changes in 25(OH)D levels. Regression analysis shows that unlike total 25(OH)D levels, DBP levels are not related to baseline PTH levels. In addition, changes in PTH and calcium levels in response to vitamin D3 repletion are not related to baseline DBP levels. In contrast, the change in total 25(OH)D was significantly associated with these classic metrics of mineral metabolism. Although “bioavailable” 25(OH)D was correlated with PTH levels, and the change in “bioavailable” 25(OH)D levels was correlated with the change in PTH levels, the relationships were weaker than those of similar comparisons with total 25(OH)D. Therefore, although there is a wide range of DBP levels and a strong association between race and DBP levels, our study provides no evidence that DBP levels modulate the physiologic effect of vitamin D repletion in vitamin D–deficient subjects.
Recently, an analysis of more than 2000 community-dwelling white and Black adults demonstrated a strong association between race, Gc genotype, and DBP levels (11). This study investigated 2 Gc SNPs (rs7041 and rs4588) that accounted for nearly 80% of the variation in DBP levels. The lower level and higher-affinity isoforms of DBP are more common in Blacks. The authors concluded that although Blacks have lower total 25(OH)D levels compared with whites, accounting for differences in DBP levels and affinity for 25(OH)D, they speculated that the effective physiologic 25(OH)D levels were comparable. The proxy for effective 25(OH)D used was “bioavailable” 25(OH)D, defined as free and albumin bound, but not DBP bound, 25(OH)D. Indeed for some cell types, such as monocytes, the presence of DBP attenuates the response to 25(OH)D (17). Further, studies from DBP-null mice demonstrate that a vitamin D–replete diet can protect against signs of vitamin D deficiency, including elevations in PTH (18). This suggests that DBP is not a necessary requirement for vitamin D bioactivity, and in some systems may even reduce activity. However, under normal physiologic conditions in humans, the acquisition of 25(OH)D by some cells requires it to be bound to DBP (19–21). For example, renal proximal tubular cells acquire 25(OH)D by receptor-mediated endocytosis of the DBP-25(OH)D complex through the megalin/cubulin receptor (22). 25(OH)D acquired in this manner is the substrate for 1-α-hydroxylation of 25(OH)D, which results in production of 1,25-dihydroxyvitamin D [1,25(OH)2D]. This is the most active endogenous vitamin D metabolite, and the kidney is the dominant source of 1,25(OH)2D in circulation. Thus, although DBP is not required for 25(OH)D uptake by all cell types, 25(OH)D bound to DBP cannot be regarded as physiologically unavailable (23). In fact, the mechanism of renal handling of vitamin D shows that 25(OH)D bound to DBP may be the more important determinant of “bioavailable” 25(OH)D. This interpretation—that total 25(OH)D levels are the best metric of vitamin D status—is supported by our regression analyses. Total 25(OH)D, of which 85–90% is DBP bound, was consistently associated with canonical metrics of vitamin D activity, whereas DBP levels were not.
One limitation of our study is that we were unable to genotype participants for Gc polymorphisms, and thus could not precisely calculate what has been referred to as “bioavailable” 25(OH)D levels. However, we were able to assess the influence of DBP on the physiological response to vitamin D repletion in terms of changes in PTH and calcium levels, and we did calculate “bioavailable” 25(OH)D using the generic affinity constant for DBP and 25(OH)D (8, 11). The effect of Gc genotype on PTH levels in response to vitamin D has been studied before, although not in exclusively vitamin D–deficient subjects (24, 25). In one study, the major homozygote genotype for rs2282679 (a Gc SNP) that was associated with the largest increase in 25(OH)D levels in response to vitamin D also showed the largest decline in PTH levels. This is consistent with the known inverse relationship between PTH and total 25(OH)D. In the same population, the same relationships for an increase in 25(OH)D and decrease in PTH were seen for rs10741657, a SNP in CYP2R1, the 25-hydroxylase gene. Thus, the relationship between SNPs and the change in PTH may be more dependent on the change in total 25(OH)D and not DBP levels per se. Although unlikely, it is still possible that DBP levels can modify the effect of vitamin D repletion on outcome measures aside from PTH and calcium levels, such as fracture risk (26). Some studies have identified a correlation between bioavailable 25(OH)D and biomarkers of disease, such as bone mineral density (27). There is also data from animal models supporting a role for DBP in multiple sclerosis, a disease that also shows correlations with total 25(OH)D levels (28, 29). Indeed, DBP is also an acute-phase reactant and plays in important role in modulating inflammation by scavenging free actin and binding endotoxin, independent of its role in vitamin D metabolism (30). However, larger, longer-term prospective studies of the predictive value of DBP with vitamin D repletion are required to assess the relationship to health outcomes in humans.
Vitamin D metabolism plays a critical role in bone health and vitamin D deficiency has been associated with numerous diseases. However, simply raising 25(OH)D levels has been a surprisingly impotent therapeutic strategy (31–33). Therefore, it is essential to understand what the best biomarker of vitamin D status is. This is important not only to effectively screen for vitamin D deficiency, but also to assess the efficacy of supplementation. The discrepancy in total serum 25(OH)D levels between Blacks and whites can be partially explained by genetic variation in Gc. Accordingly, defining vitamin D status with the same total 25(OH)D threshold for Blacks and whites may not be prudent. However, “bioavailable” 25(OH)D is not necessarily a better reflection of vitamin D status. This calculated metric incorrectly incorporates the assumption that 25(OH)D bound to DBP is unavailable (34). As a validated bioassay of vitamin D activity, the change in PTH reflects end-organ responsiveness to vitamin D. In our study, Blacks had a mean 25(OH)D level of 12 ng/mL with a strong physiologic response to vitamin D supplementation (ie declines in serum PTH and an increase in serum calcium) independent of DBP levels. Using these functional endpoints, we have shown that for vitamin D deficient subjects undergoing repletion, total 25(OH)D is a true biomarker of vitamin D activity and that DBP levels do not seem to have an independent biologic effect. Thus, individuals with low total 25(OH)D can be functionally vitamin D deficient even with low DBP levels. For the clinical purpose of treating vitamin D deficiency, DBP levels may not be important.
Acknowledgments
This work was supported in part by Grant No. UL1 TR000043 from the National Center for Advancing Translational Sciences, National Institutes of Health. This work was also supported by the Center for Basic and Translational Research on Disorders of the Digestive System through the generosity of the Leona M. and Harry B. Helmsley Charitable Trust.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 25(OH)D
- 25-hydroxyvitamin D
- DBP
- vitamin D–binding protein
- RCT
- randomized controlled trial
- SNP
- single-nucleotide polymorphism.
References
- 1. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 2. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 4. Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22:1745–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–959 [DOI] [PubMed] [Google Scholar]
- 6. Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19:2739–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum Genet. 1993;92:183–188 [DOI] [PubMed] [Google Scholar]
- 9. Valcour A, Blocki F, Hawkins DM, Rao SD. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metab. 2012;97:3989–3995 [DOI] [PubMed] [Google Scholar]
- 10. Ponda MP, Dowd K, Finkielstein D, Holt PR, Breslow JL. The short-term effects of vitamin D repletion on cholesterol: a randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2012;32:2510–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta. 2006;372:33–42 [DOI] [PubMed] [Google Scholar]
- 13. Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254 [DOI] [PubMed] [Google Scholar]
- 14. Mawer EB, Davies M. Vitamin D nutrition and bone disease in adults. Rev Endocr Metab Disord. 2001;2:153–164 [DOI] [PubMed] [Google Scholar]
- 15. Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–146 [DOI] [PubMed] [Google Scholar]
- 16. Christakos S. Recent advances in our understanding of 1,25-dihydroxyvitamin D(3) regulation of intestinal calcium absorption. Arch Biochem Biophys. 2012;523:73–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chun RF, Lauridsen AL, Suon L, et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95:3368–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Safadi FF, Thornton P, Magiera H, et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103:239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515 [DOI] [PubMed] [Google Scholar]
- 20. Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr. 2006;136:2754–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abboud M, Puglisi DA, Davies BN, et al. Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology. 2013;154:3022–3030 [DOI] [PubMed] [Google Scholar]
- 22. Negri AL. Proximal tubule endocytic apparatus as the specific renal uptake mechanism for vitamin D-binding protein/25-(OH)D3 complex. Nephrology (Carlton). 2006;11:510–515 [DOI] [PubMed] [Google Scholar]
- 23. Lambert PW, Stern PH, Avioli RC, et al. Evidence for extrarenal production of 1 alpha, 25-dihydroxyvitamin D in man. J Clin Invest. 1982;69:722–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Didriksen A, Grimnes G, Hutchinson MS, et al. The serum 25-hydroxyvitamin D response to vitamin D supplementation is related to genetic factors, BMI, and baseline levels. Eur J Endocrinol. 2013;169:559–567 [DOI] [PubMed] [Google Scholar]
- 25. Nimitphong H, Saetung S, Chanprasertyotin S, Chailurkit LO, Ongphiphadhanakul B. Changes in circulating 25-hydroxyvitamin D according to vitamin D binding protein genotypes after vitamin D(3) or D(2)supplementation. Nutr J. 2013;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malik S, Fu L, Juras DJ, et al. Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit Rev Clin Lab Sci. 2013;50:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Powe CE, Ricciardi C, Berg AH, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26:1609–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang M, Qin Z, Zhu Y, et al. Vitamin D-binding protein in cerebrospinal fluid is associated with multiple sclerosis progression. Mol Neurobiol. 2013;47:946–956 [DOI] [PubMed] [Google Scholar]
- 29. Ascherio A, Munger KL, White R, et al. Vitamin D as an Early Predictor of Multiple Sclerosis Activity and Progression. JAMA Neurol. 2014;71:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chun RF. New perspectives on the vitamin D binding protein. Cell Biochem Funct. 2012;30:445–456 [DOI] [PubMed] [Google Scholar]
- 31. Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696 [DOI] [PubMed] [Google Scholar]
- 32. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683 [DOI] [PubMed] [Google Scholar]
- 33. Ponda MP, Huang X, Odeh MA, Breslow JL, Kaufman HW. Vitamin D may not improve lipid levels: a serial clinical laboratory data study. Circulation. 2012;126:270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: The free hormone hypothesis revisited [published online October 4, 2013]. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]


