Abstract
Context:
The discovery of hypercalcemic diseases due to loss-of-function mutations in 25-hydroxyvitamin D-24-hydroxylase has placed a new demand for sensitive and precise assays for 24,25-dihydroxyvitamin D [24,25-(OH)2D].
Objective:
We describe a novel liquid chromatography and tandem mass spectrometry-based method involving derivatization with DMEQ-TAD {4-[2-(6,7-dimethoxy-4-methyl-3,4-dihydroquinoxalinyl)ethyl]-1,2,4-triazoline-3,5-dione} to simultaneously assay multiple vitamin D metabolites including 25-hydroxyvitamin D (25-OH-D) and 24,25-(OH)2D using 100 μL of serum with a 5-minute run time.
Design:
The assay uses a newly synthesized internal standard d6-24,25-(OH)2D3 enabling the quantitation of 24,25-(OH)2D3 as well as the determination of the ratio of 25-OH-D3 to 24,25-(OH)2D3, a physiologically useful parameter.
Setting:
We report data on more than 1000 normal and disease samples involving vitamin D deficiency or hypercalcemia in addition to studies involving knockout mouse models.
Results:
The assay showed good correlation with samples from quality assurance schemes for 25-OH-D (25-OH-D2 and 25-OH-D3) determination (−2% to −5% bias) and exhibited low inter- and intraassay coefficients of variation (4%–7%) and lower limits of quantitation of 0.25–0.45 nmol/L. In clinical studies, we found a strong correlation between serum levels of 25-OH-D3 and 24,25-(OH)2D3 (r2 = 0.80) in subjects over a broad range of 25-OH-D3 values and a marked lack of production of 24,25-(OH)2D3 below 25 nmol/L of 25-OH-D. The ratio of 25-OH-D3 to 24,25-(OH)2D3, which remained less than 25 in vitamin D-sufficient subjects (serum 25-OH-D < 50 nmol/L) but was greatly elevated (80–100) in patients with idiopathic infantile hypercalcemia.
Conclusions:
The new method showed good utility in clinical settings involving vitamin D deficiency; supplementation with vitamin D and idiopathic infantile hypercalcemia, as well as in animal models with ablation of selected cytochrome P450-containing enzymes involved in vitamin D metabolism.
Serum 25-hydroxyvitamin D (25-OH-D) is widely accepted as a biomarker of vitamin D status (1). Vitamin D plays important roles in the body including the regulation of calcium/phosphate homeostasis and the cell cycle (2). Over the past 10 years, many epidemiological studies have demonstrated an inverse correlation between serum 25-OH-D levels and a broad range of important disease states (3, 4), which has in turn emphasized the importance of adequate vitamin D status (5). Currently, serum 25-OH-D is routinely measured by methods involving RIA, HPLC, or liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based technologies (6).
Recently some of us (7) showed that loss-of-function mutations of 25-hydroxyvitamin D-24-hydroxylase (CYP24A1) in a group of German, Turkish, and Russian children are associated with the hypercalcemic condition, idiopathic infantile hypercalcemia (IIH). With this newly acquired knowledge about the importance of the catabolic enzyme CYP24A1 (8), there has developed renewed interest in measuring its main circulating product, 24,25-dihydroxyvitamin D3 [24,25-(OH)2D3]. Competitive binding assays based on the strong binding of 24,25-(OH)2D3 to the plasma vitamin D binding globulin allowed for early estimates of the levels of this catabolite (9). However, methods required precise chromatography steps to resolve 24,25-(OH)2D3 from its more abundant precursor, 25-OH-D3 and from the other dihydroxyvitamin D metabolites including 25,26-dihydroxyvitamin D3 and 25-OH-D3-26,23-lactone, which are often increased in serum from hypervitaminotic D animals (10). In patients receiving vitamin D2, the analytical problem of accurately assaying 24-hydroxylated vitamin D2 and D3 becomes even more complex and time consuming.
The emergence of LC-MS/MS as a tool to quantify a wide range of clinically important bioactive hormones and metabolites has been a major advance in clinical chemistry. Tandem mass spectrometry provides the ideal universal detector for vitamin D metabolites separated by conventional liquid chromatography techniques (11). Its only drawback has been the identification of interfering substances such as 3-epi-25-OH-D3 in a 25-OH-D assay (12) and 4,25-(OH)2D3 in 1,25 dihydroxyvitamin D3 [1,25-(OH)2D3] assay (13), which make the chromatographic step particularly important.
In this paper, we describe a sample preparation and derivatization method that allows the simultaneous assay in serum in both humans and mice of all abundant metabolites of both vitamin D2 and D3 and the accurate measurement of 24,25-(OH)2D3 and 25-OH-D3 using a specific deuterated internal standard, whose synthesis is described. In addition, we demonstrate that simultaneous assay of multiple vitamin D metabolites including serum 24,25-(OH)2D3 and the 25-OH-D3 to 24,25-(OH)2D3 ratio provide evidence for the presence of loss-of-function mutations of CYP24A1; provide evidence for an assessment tool for establishing the degree of vitamin D deficiency; and provide information on the catabolism of vitamin D during vitamin D supplementation.
Materials and Methods
Human and animal studies
Serum samples were analyzed from the following: 1) 163 healthy, white, postmenopausal women who were part of a 1-year randomized prospective, placebo-controlled VIDOS (Vitamin D Supplementation in Older Subjects) clinical trial approved by the Creighton University Institutional Review Board and published previously (14); 2) 110 healthy older African American women who were enrolled in a randomized, double-blind vitamin D supplementation trial at Creighton Medical Center and Indiana University Medical Center published previously (15); and 3) 198 healthy young women (119 Caucasian and 79 African American) enrolled in a 1-year prospective, randomized, placebo controlled vitamin D supplementation trial published previously (16). Serum samples from patients with idiopathic infantile hypercalcemia were provided by Schlingmann et al (7) as previously described. Mouse sera were provided by Dr Hector F. DeLuca and Dr René St-Arnaud; and all mice were managed in compliance with protocols approved by the University of Wisconsin Research Animal Resources Center or McGill University Animal Care Committee as previously described (17, 18).
Materials
25-OH-D3 and 25-OH-D2 calibrators (6PLUS1) were purchased from Chromsystems. A six-level calibrator set for 24,25-(OH)2D3 was generated in-house using a pool of human serum containing 6.5 ng/mL 25-OH-D3 (courtesy of Dr Jackie Berry, University of Manchester, Manchester, United Kingdom). An artificial sample matrix was generated using 20% human serum in 0.1% BSA dissolved in PBS and supplemented with synthetic 24,25-(OH)2D3. Internal standards d3-25-OH-D3 and d3-25-OH-D2 were purchased from IsoSciences. The synthesis of d6-24,25-(OH)2D3 is described below. All LC-MS/MS solvents, additives, and extraction solvents were Optima liquid chromatography-mass spectrometry grade and purchased from Fisher, with the exception of methyl tertiary butyl ether, which was purchased from Sigma. The Cookson reagent, DMEQ-TAD {4-[2-(6,7-dimethoxy-4-methyl-3,4-dihydroquinoxalinyl)ethyl]-1,2,4-triazoline-3,5-dione} was purchased from Key Synthesis.
Synthesis of d6-24,25-(OH)2D3
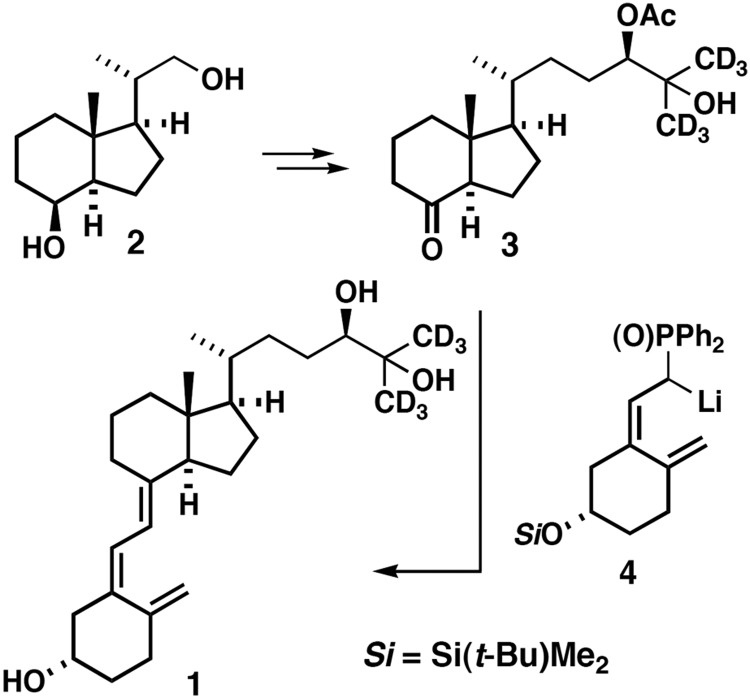
The synthesis of the deuterated internal standard, 24R,25-dihydroxyvitamin D3 (26,26,26,27,27,27-d6) (Figure 1) (1, seocalciferol-d6) starts with inhoffen-lythgoe diol (2), which can be prepared from vitamin D2 (19) and converted to the acetate 3 by known procedures (20). Ketone 3 was coupled with the phosphine anion 4 in tetrahydrofuran (THF) at −78°C to afford, after desilylation with tetrabutyl-ammonium fluoride in THF and subsequent saponification with sodium methoxide in THF, the desired deuterated vitamin D3 metabolite 1 (purity information provided in Supplemental Table 1).
Figure 1.
Synthesis of d6-24,25-(OH)2D3 used in the assay of serum 24,25-(OH)2D. See accompanying text for a more detailed description of the route of synthesis.
Serum preparation for ultraperformance liquid chromatography (UPLC)-tandem mass spectrometry
In microcentrifuge tubes, 100-μL aliquots of test serum or calibrator were diluted with 200 μL of water and supplemented with the following internal standards: 80 ng/mL d3-25-OH-D3, 65 ng/mL d3-25-OH-D2, and 6 ng/mL d6-24,25-(OH)2D3. A 100-μL volume of 0.1 M HCl was added, and protein precipitation was carried out by adding 0.2 M zinc sulfate and 450 μL of methanol, with vortexing after addition of each component. The mixture was centrifuged at 12 000 × g for 10 minutes, and the supernatant was transferred to borosilicate glass tubes. Organic extraction was carried out by adding 700 μL of hexane and 700 μL of methyl tertiary butyl ether, with vortexing after the addition of each component. The upper organic phase was transferred to LC-MS/MS sample vials and dried under a stream of prepurified N2 at 37°C. Samples were derivatized by redissolving the dry residue in 25 μL of 0.1 mg/mL DMEQ-TAD in ethyl acetate and incubating for 30 minutes at room temperature in the dark. A second aliquot of DMEQ-TAD was added and allowed to incubate for an additional 60 minutes (21, 22). A 40-μL volume of ethanol was added, and the derivatized extract was dried and redissolved in 60 μL of 60:40 (vol/vol) methanol/water running solvent.
Liquid chromatography and tandem mass spectrometry
LC-MS/MS analysis was performed using an Acquity UPLC connected in-line with a Xevo TQ-S mass spectrometer in electrospray positive mode (Waters). Chromatographic separations were achieved using a BEH-Phenyl UPLC column (1.7 μm, 2.1 × 50 mm) (Waters) and methanol/water-based gradient solvent system. The serum extract run on LC-MS/MS is the equivalent of 17 μL of serum or approximately one sixth of the 100-μL sample extracted and the chromatography runtime is less than 5 minutes. Further details of the chromatography, optimized mass spectrometer settings, transitions for multiple reaction monitoring, and data management are provided in Supplemental Table 2, A and B.
Results
Optimization and performance of the simultaneous vitamin D metabolite assay
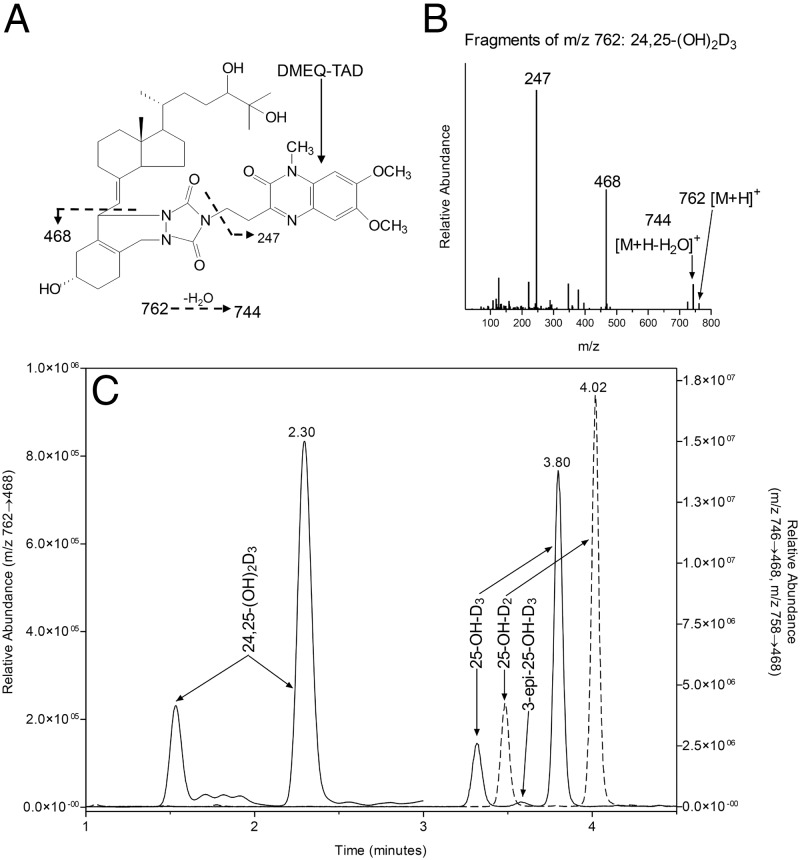
Derivatization of vitamin D metabolites with DMEQ-TAD offers the advantage of improving ionization efficiency relative to native metabolites and increasing molecular mass by 363 Da, to a region of the mass spectrum in which there is reduced background. The major characteristic ions for derivatized 25-OH-D3, 25-OH-D2, and 24,25-(OH)2D3 were their molecular ions [M+H]+ at a mass/charge (m/z) ratio of 746.6, 758.6 and 762.6 respectively, and when subjected to collision-induced dissociation under optimized conditions they yield an A-ring/DMEQ-TAD fragment (m/z 468) and a DMEQ-TAD fragment (m/z 247) as the major products. The fragmentation pattern for the 24,25-(OH)2D3 adduct is shown in Figure 2, A and B. The m/z 468 was selected as the fragment ion for multiple reaction monitoring (MRM) analysis because of greater specificity and lower background as compared with the fragment at m/z 247. DMEQ-TAD adducts of the target analytes consisted of 6R and 6S isomers, of which the more abundant 6S isomer was used for quantitation. In a representative serum sample, the 6S isomers of the DMEQ-TAD adducts of 25-OH-D3, 25-OH-D2, and 24,25-(OH)2D3 eluted at 3.80, 4.02, and 2.30 minutes, respectively, as shown in Figure 2B. The peak at 3.58 minutes comigrated with synthetic 3-epi-25-OH-D3, characterized by a single broad peak, suggesting coelution of the 6R and 6S isomers for this analyte. 3-epi-25-OH-D3 is a known isomer of 25-OH-D3. Because these two analytes were chromatographically resolved, the presence of 3-epi-25-OH-D3 did not confound the 25-OH-D3 measurement using the current assay.
Figure 2.
A, Structure and fragmentation of DMEQ-TAD adduct of 24,25-(OH)2D3 used for detection in the LC-MS/MS assay. B, Mass spectrum of the DMEQ-TAD adduct of 24,25-(OH)2D3 indicating that a m/z of 468 is a major fragment of the molecular ion m/z 762. C, Composite chromatogram from superimposition of MRM at 746->468 for 25-OH-D3 and 3-epi-25-OH-D3, at 758->468 for 25-OH-D2 and at 762->468 for 24,25-(OH)2D3 in a serum extract.
Over the calibration range of 25-OH-D3 (10–370 nmol/L), 25-OH-D2 (12–291 nmol/L), and 24,25-(OH)2D3 (1–28 nmol/L), the method was shown to be linear for each analyte giving representative r2 values of at least 0.997 (Supplemental Table 3). Lower limits of quantification as defined by signal to noise ratios of 10 or greater were estimated to be within the 0.1- to 0.2-ng/mL range and lower limits of detection (signal to noise ratio ≥ 3) were estimated to be as low as 0.04 ng/mL. Intraassay and interassay imprecision was determined by analyzing five replicates of a serum sample containing 55 nmol/L 25-OH-D3, 83 nmol/L 25-OH-D2, and 6 nmol/L of 24,25-(OH)2D3 on each of 14 assay days. The mean intraassay coefficients of variation for the target analytes ranged from 3% to 4% and interassay coefficients of variation ranged from 4% to 7%. For 25-OH-D3, and 25-OH-D2, accuracy was assessed by analysis of serum samples distributed by the Vitamin D External Quality Assessment Scheme (DEQAS) with each assay run. Mean discrepancies from the all-laboratory trimmed mean and the LC-MS/MS method mean were −2% and −5%, respectively, based on 77 DEQAS samples analyzed over 14 days of analysis. Performance of the total 25-OH-D LC-MS/MS assay based on 20 samples analyzed by the National Institute for Standards and Technology gold standard reference method between fall 2012 and summer 2013 was a mean bias of −4.18%.
Utility of simultaneous vitamin D metabolite assay in women supplemented with vitamin D
The simultaneous vitamin D metabolite assay method was used to analyze serum 25-OH-D and 24,25-dihydroxyvitamin D [24,25-(OH)2D] in a total of 694 samples from healthy women and in black and white postmenopausal osteopenic women before and after oral supplementation with between 0 and 4800 IU vitamin D3 for 1 year (13–15). In samples collected from white postmenopausal osteopenic women (13), serum 25-OH-D values determined by the simultaneous vitamin D metabolite assay agreed well with the values previously published using the Diasorin RIA method (n = 289; r2 = 0.817; slope = 0.902; y intercept = 17.3 nmol/L) (13). In the complete set of approximately 672 serum samples from three published studies (13–15), the relationship between serum 25-OH-D3 and serum 24,25-(OH)2D3 (Figure 3A) covered a range for serum 25-OH-D from 12.5 nmol/L to 200 nmol/L, reflecting the range of vitamin D supplementation and resulting in serum 24,25-(OH)2D levels in the range of 0.25–30 nmol/L. There was a strong correlation between serum 25-OH-D and 24,25-(OH)2D (n = 672, r2 = 0.80, slope = 0.13, x-intercept = 25.6 nmol/L), a relationship previously observed by others (23, 24). The intercept on the x-axis was 25 nmol/L demonstrating that serum 24,25-(OH)2D3 is zero in individuals with serum 25-OH-D levels less than 25 nmol/L, a value considered vitamin D deficient by current public health criteria (5). The relationship between the molar ratio of serum 25-OH-D to 24,25-(OH)2D to serum 25-OH-D is shown in Figure 3B and indicates that a ratio below 20 in a population corresponds to vitamin D sufficiency and a ratio above 20 suggests vitamin D insufficiency (25). Although less than 20% of these women exhibited measurable serum 25-OH-D2 at baseline, this metabolite was negligible (<5 nmol/L) after vitamin D3 supplementation for 1 year. 3-epi-25-OH-D3 was measurable in most patients and averaged 6% of the serum 25-OH-D3 value, in agreement with data from others (26, 27).
Figure 3.
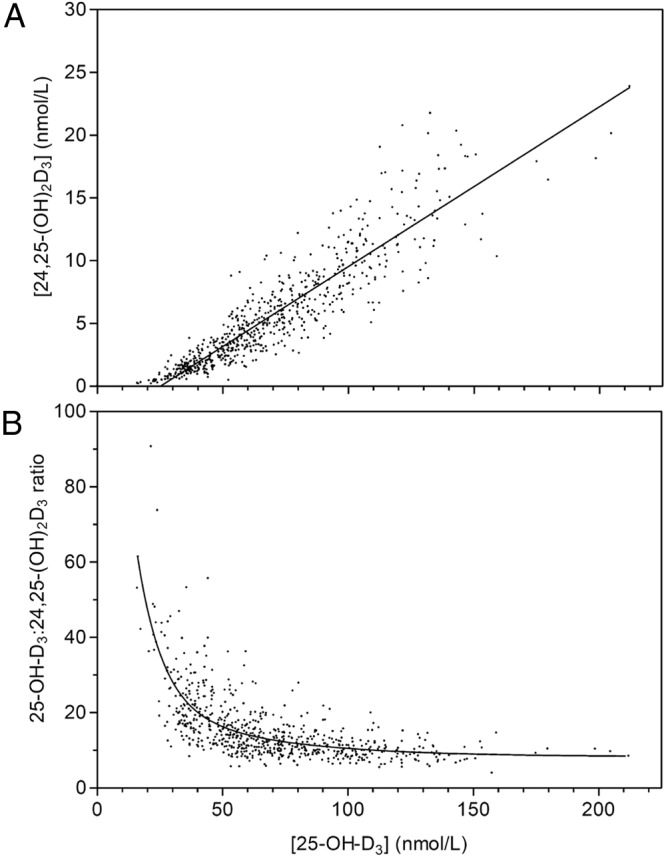
A, Plot of serum 24,25-(OH)2D3 vs serum 25-OH-D3 in 694 serum samples from three different clinical trials determined by LC-MS/MS. B, Lowess plot (Prism; GraphPad) of the ratio of serum 25-OH-D3 to 24,25-(OH)2D3 vs serum 25-OH-D3 in 694 serum samples from three different clinical trials.
Utility of simultaneous vitamin D metabolite assay in patients with IIH due to loss-of-function CYP24A1 mutations
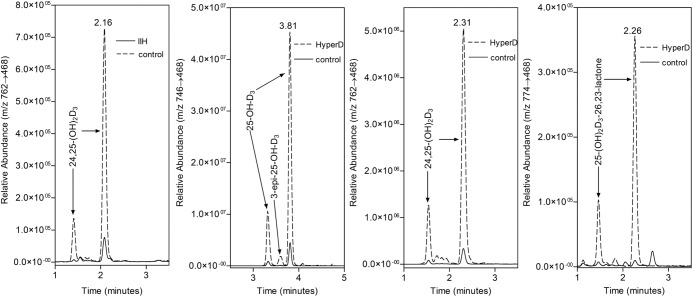
The simultaneous assay was used to measure vitamin D metabolite levels in serum samples from two patients previously diagnosed with IIH due to CYP24A1 mutations (16) (Table 1). Both patients with loss-of-function mutations of CYP24A1 had virtually undetectable levels of serum 24,25-(OH)2D3. Whether these measurable traces of 24,25-(OH)2D3 in IIH patients(Figure 4A) are small amounts of the metabolite made by other cytochrome P450s or represent other interfering dihydroxyvitamin D metabolites with the same retention time and mass spectral characteristics is unknown at this time (28, 29). Over the years, there have been reports that cytochrome P450s, other than CYP24A1, can generate 24,25-(OH)2D3, but those reports are mainly from in vitro studies (8, 28) and it is difficult to judge whether this can occur in vivo. On the other hand, if the trace metabolite is not 24,25-(OH)2D3 but an interfering metabolite, it is not 1,25-(OH)2D3, which runs with a different retention time on liquid chromatography [DMEQ adducts of 1,25-(OH)2D3 = 2.43 and 2.81 min; DMEQ adducts of 24,25-(OH)2D3 = 2.30 and 1.6 min]. Considering that the normal production of 24,25-(OH)2D3 is down-regulated completely when serum 25-OH-D3 levels fall into the vitamin D deficient range, we propose that the ratio of 25-OH-D3 to 24,25-(OH)2D3 is a more accurate parameter to use to express the absence of 24,25-(OH)2D3 in patients with IIH, especially because some of these patients have low vitamin D status (16). In both cases, the ratio of 25-OH-D3 to 24,25-(OH)2D3 was in the 90–120 range, whereas normal values for this ratio rarely rise above 20.
Table 1.
LC-MS/MS Analysis of Vitamin D Metabolites in Patients With Hypercalcemia
| Patient | 25-OH-D3, nmol/L | 24,25-(OH)2D3, nmol/L | 25-OH-D3 to 24,25-(OH)2D3 ratio |
|---|---|---|---|
| IIH1 | 94.8 | 0.9 | 98.7 |
| IIH2 | 81.2 | 0.7 | 112.8 |
| Hypervitaminosis D | 420.0 | 34.0 | 12.35 |
| Control | 53.5 | 5.0 | 10.6 |
Figure 4.
A. MRM of 24,25-(OH)2D3 in a serum extract from a patient with IIH due to loss-of-function mutation in CYP24A1 as compared with an unaffected sibling. B–D, MRMs of 25-OH-D3, 24,25-(OH)2D3 and 25-OH-D3-26,23-lactone in a serum extract from a hypervitaminotic D patient as compared with a normal vitamin D control. Note that all three metabolites are elevated in the hypervitaminotic D patient. MRM for 25-OH-D3-26,23-lactone uses m/z 774->468.
Utility of simultaneous vitamin D metabolite assay in patients with hypervitaminosis D
The simultaneous vitamin D metabolite assay was used to assess a patient with hypercalcemia due to an excessive intake of vitamin D3 (30). The 78-year-old patient presented with severe hypercalcemia (total calcium = 3.95 mM) and serum 25-OH-D of 788 nmol/L (normal range 82.5–250 nmol/L) and 1,25-(OH)2D of 18.3 pM (normal range 45–160 pM), by hospital liquid chromatography-mass spectrometry and RIA methods, respectively, suggesting hypervitaminosis D. Analysis of a subsequent serum sample using the simultaneous LC-MS/MS assay gave a 25-OH-D3 value of 420 nmol/L; a 24,25-(OH)2D3 value of 34 nmol/L (Figure 4, B and C) and a molar ratio of 25-OH-D3 to 24,25-(OH)2D3 of 12.35, suggesting no defect in CYP24A1 activity. Consistent with previous animal studies of hypervitaminosis D (31), the hypervitaminotic D patient also showed detectable amounts of the 25-OH-D3-26,23-lactone, another metabolic product of CYP24A1 (Figure 4D). The ability to detect multiple metabolites simultaneously is a major advantage of the use of DMEQ-TAD (21, 22).
Utility of simultaneous vitamin D metabolite assay in rodent serum samples
Rodent studies are often limited by the volumes of serum/plasma available for analysis and often necessitate sample pooling to allow for detection of vitamin D metabolites. The simultaneous vitamin D metabolite assay was used to analyze 100-μL samples from individual mice in a metabolic study involving wild-type, CYP2R1 knockout, CYP27A1 knockout, and CYP2R1/CYP27A1 double-knockout mice (17) (Table 2). The simultaneous vitamin D metabolite assay gave serum 25-OH-D3 results in line with the other methods used, but, in addition, the simultaneous vitamin D metabolite assay provided serum 24,25-(OH)2D3 and 25-OH-D3-26,23-lactone levels. In a further study, analysis of serum from CYP24A1 knockout mice and their heterozygous littermates showed widely different levels of 25-OH-D3, 24,25-(OH)2D3, and 25-OH-D3-26,23-lactone in the two genotypes (Table 2). Homozygous CYP24A1 knockout mice exhibited 6-fold higher levels of 25-OH-D3 than littermates and virtually undetectable levels of serum 24,25-(OH)2D3, similar to the values observed in human IIH patients with genetic blocks in CYP24A1. The small residual 24,25-(OH)2D3 peak evokes the same questions raised for IIH of whether this is an interfering substance or evidence of a non-CYP24A1 vitamin D 24-hydroxylase. These rodent studies confirmed earlier findings (10) that the normal mouse exhibits a different ratio of 25-OH-D3 to 24,25-(OH)2D3 compared with the human and a significantly increased production of 25-OH-D3-26,23-lactone.
Table 2.
LC-MS/MS Analysis of Serum Vitamin D Metabolites in Several CYP Knockout Mice
| Cyp2r1/Cyp27a1/CYP24a1 | 25-OH-D3, nmol/La | 24,25-(OH)2D3, nmol/La | 25-OH-D3-26, 23-Lactone, nmol/La | 25-OH-D3 to 24, 25-(OH)2D3 ratioa |
|---|---|---|---|---|
| ++/++/++ | 42.2 ± 3.4 | 28.9 ± 2.2 | 13.5 ± 1.4 | 1.5 ± 0.1 |
| +−/++/++ | 34.7 ± 3.3 | 25.5 ± 2.6 | 9.2 ± 2.1 | 1.4 ± 0.0 |
| −−/++/++ | 16.8 ± 2.5 | 13.5 ± 1.6 | 4.0 ± 0.9 | 1.2 ± 0.1 |
| ++/−−/++ | 176.3 ± 28.3 | 107.7 ± 6.4 | 42.1 ± 6.9 | 1.6 ± 0.2 |
| −−/−−/++ | 28.1 ± 3.8 | 25.8 ± 5.1 | 5.9 ± 0.9 | 1.1 ± 0.1 |
| ++/++/+− | 37.5 ± 3.6 | 19.2 ± 2.2 | 9.5 ± 0.8 | 2.0 ± 0.1 |
| ++/++/−− | 256.6 ± 23.1 | 2.9 ± 0.3 | 0.3 ± 0.0 | 87.6 ± 9.2 |
Mean ± SD, n = 3–8 animals.
Discussion
We describe here a novel, highly-sensitive LC-MS/MS-based method to simultaneously assay six different vitamin D metabolites including 25-OH-D3, 25-OH-D2, 3-epi-25-OH-D3, 24,25-(OH)2D3, 24,25-(OH)2D2, and 25-OH-D3-26,23-lactone in triplicate in 100-μL serum samples of human or animal origin. The new method has been tested on approximately 1000 clinical samples, and the method for 25-OH-D shows good correlation with the National Institute for Standards and Technology and DEQAS samples for 25-OH-D (25-OH-D2 and 25-OH-D3) determination, which is the only parameter with good external standardization available. Data for the accurate determination of serum 24,25-(OH)2D3 were made possible by the novel synthesis of d6-24,25-(OH)2D3 by our organic chemistry collaborators, and this can also now be used to estimate serum 24,25-(OH)2D2 and 25-OH-D3-26,23-lactone concentrations as well as the ratio of 25-OH-D3 to 24,25-(OH)2D3, which is a pathophysiologically useful ratio. The clinical utility of the new method is illustrated in several situations such as the following: 1) its usefulness as a novel approach for predicting vitamin D deficiency by an elevated 25-OH-D3 to 24,25-(OH)2D3 ratio, which is in our opinion is at least equivalent to the PTH to 25-OH-D3 plots commonly used; 2) its usefulness in aiding in the diagnosis of IIH due to loss-of-function CYP24A1 mutations; and 3) its usefulness in distinguishing CYP24A1 defects from hypervitaminosis D during vitamin D intoxication. Furthermore, we also show the value of the new method to study the vitamin D metabolite profile in the small blood volumes available in small animal models such as the knockout mouse.
The initial application of the new method was in the simultaneous determination of 25-OH-D3 and 24,25-(OH)2D3 in normal individuals over a wide range of vitamin D3 intakes (13–15). We chose to study a population of osteopenic women receiving vitamin D3 supplementation to gauge the importance of 24-hydroxylation in the catabolism of 25-OH-D3 and to relate the serum 25-OH-D levels, previously determined using a RIA (13–15). Results obtained using the RIA method correlated well with the LC-MS/MS technology. This was true both before and after supplementation with oral vitamin D3. The data, which include values for normal unsupplemented younger women, suggest that the new method works well over a wide serum 25-OH-D range from 12.5 nmol/L to 200 nmol/L. The ratio of 25-OH-D3 to 24,25-(OH)2D3 proved to be a valuable parameter in predicting vitamin D deficiency and showed consistent performance over a wide range of 25-OH-D values.
The new LC-MS/MS vitamin D metabolite assay was primarily developed for use in the diagnosis of IIH (16), in which the simultaneous analysis of 25-OH-D and 24,25-(OH)2D3 on small infant serum samples is critical in pinpointing defective CYP24A1 activity without the complications of vitamin D deficiency. The method works well in this application, giving absolute serum 24,25-(OH)2D values, as well as the ratio of 25-OH-D3 to 24,25-(OH)2D3, which together indicate the likelihood of IIH due to loss-of-function CYP24A1 mutation, which can be diagnosed only by expensive genetic testing. We conclude that 25-OH-D3 to 24,25-(OH)2D3 ratios greater than 80 are indicative of IIH due to inactivating mutations in CYP24A1. Furthermore, use of this method and determination of the 25-OH-D3 to 24,25-(OH)2D3 ratio allowed us to rule out CYP24A1 mutations in a patient with hypercalcemia resulting from vitamin D intoxication rather than an inability to catabolize 1,25-(OH)2D3 (7, 30). Taken together, the simultaneous assay of 25-OH-D3 and 24,25-(OH)2D3 is a valuable screening tool for patients presenting with hypercalcemia.
We show here the utility of the new method for the analysis of vitamin D metabolites including 24,25-(OH)2D3 in rodent sera (17, 18). The example involving mouse sera shows that LC-MS/MS, unlike certain commercially available RIAs (32), is not sensitive to the presence of blood proteins from certain species. Also unlike commercially available RIAs, LC-MS/MS can resolve or remove the complications caused by the presence of high levels of 24,25-(OH)2D3 and 25-OH-D3-26,23-lactone found in serum from wild-type mice (9). The rodent studies using CYP24A1 reinforce the current dogma that this cytochrome P450 is responsible for the production of many side-chain hydroxylated metabolites including 24,25-(OH)2D3 and 25-OH-D3-26,23-lactone (7). The examples here show that in addition to the advantage the LC-MS/MS method measures multiple vitamin D metabolites, it is sensitive enough to allow convenient analyses on individual animals as opposed to pooled samples. Animal-to-animal variability in mice with a similar genotype and diet is small and allows for more rigorous statistical analysis.
LC-MS/MS methodology continues to improve in terms of sensitivity, and the addition of a derivatization step with DMEQ-TAD improves this by at least 10-fold over conventional LC-MS/MS using the native vitamin D metabolites. LC-MS/MS methods, including the current simultaneous assay, have proven to be superior to immunoassays in their detection and accurate measurement of 25-OH-D2 that is present at high concentrations in vitamin D2-treated patients (33, 34). Part of the utility of the LC-MS/MS method for 24,25-(OH)2D3 is that it uses much smaller aliquots of serum than competitive-binding assays that preceded it. In addition, because there are a variety of Cookson reagents/dienophiles now becoming available (35, 36), we can expect this technology to become even more sensitive in the years to come and these improvements will eventually allow for the routine inclusion of 1,25-(OH)2D3 in the metabolites that can be measured using a 100-μL serum aliquot.
Acknowledgments
G.J. acknowledges the support of the Waters Corp for the provision of the LC-MS/MS used in these studies.
J.C.G. was supported by Grant RO1-AG28168 from the National Institutes on Aging and Grant W81XWH-07–1-201 from the US Department of Defense. M.P. was supported by Grant UL1RR025761. A.M. thanks Xunta de Galicia (Project CN2012/074, Grant INCITE08PXIB209130PR), and the Spanish Ministry of Education and Innovation (Project Grant SAF2010–15291) for financial support. R.S. thanks Xunta de Galicia for an Angeles Alvariño grant.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- CYP24A1
- 25-hydroxyvitamin D-24-hydroxylase
- DEQAS
- Vitamin D External Quality Assessment Scheme
- IIH
- idiopathic infantile hypercalcemia
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- MRM
- multiple reaction monitoring
- m/z
- mass/charge
- 25-OH-D
- 25-hydroxyvitamin D
- 1,25-(OH)2D3
- 1,25 dihydroxyvitamin D3
- 24,25-(OH)2D
- 24,25-dihydroxyvitamin D
- 24,25-(OH)2D3
- 24,25-dihydroxyvitamin D3
- THF
- tetrahydrofuran
- UPLC
- ultraperformance liquid chromatography.
References
- 1. Jones G. Metabolism and biomarkers of vitamin D. Scand J Clin Lab Invest Suppl. 2012;243:7–13 [DOI] [PubMed] [Google Scholar]
- 2. Jones G, Strugnell S, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231 [DOI] [PubMed] [Google Scholar]
- 3. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 4. Jones G. Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels, extra-renal 1α-hydroxylase in the classical and non-classical actions of 1α,25-dihydroxyvitamin D3. Semin Dial. 2007;20:316–324 [DOI] [PubMed] [Google Scholar]
- 5. IOM (Institute of Medicine). Dietary Reference Intakes for Ca and Vitamin D. Washington, DC: The National Academies of Science; 2011 [Google Scholar]
- 6. Carter GD. 25-Hydroxyvitamin D: a difficult analyte. Clin Chem. 2012;58:486–488 [DOI] [PubMed] [Google Scholar]
- 7. Schlingmann KP, Kaufmann M, Weber S, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. 2011;365:410–421 [DOI] [PubMed] [Google Scholar]
- 8. Jones G, Kaufmann M, Prosser DE. 25-Hydroxyvitamin D3-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523:9–18 [DOI] [PubMed] [Google Scholar]
- 9. Haddad JG, Jr, Min C, Walgate J, Hahn T. Competition by 24,25-dihydroxycholecalciferol in the competitive protein binding radioassay of 25-hydroxycalciferol. J Clin Endocrinol Metab. 1976;43:712–715 [DOI] [PubMed] [Google Scholar]
- 10. Cunningham J, Coldwell RD, Jones G, Tenenhouse HS, Trafford DJ, Makin HL. Plasma 24,25-dihydroxyvitamin D3 concentrations in X-linked hypophosphatemic mice: studies using mass fragmentographic and radioreceptor assays. J Bone Miner Res. 1990;5:173–177 [DOI] [PubMed] [Google Scholar]
- 11. Makin HLJ, Jones G, Kaufmann M, Calverley M. Analysis of vitamins D, their metabolites, analogues. In: Makin HLJ, Gower DB, eds. Steroid Analysis. 2nd ed London: Springer (Chapman, Hall); 2010:967–1094 [Google Scholar]
- 12. Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91:3055–3061 [DOI] [PubMed] [Google Scholar]
- 13. Wang Z, Senn T, Kalhorn T, et al. Simultaneous measurement of plasma vitamin D(3) metabolites, including 4β,25-dihydroxyvitamin D(3), using liquid chromatography-tandem mass spectrometry. Anal Biochem. 2011;418:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gallagher JC, Sai A, Templin T, 2nd, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012;156:425–427 [DOI] [PubMed] [Google Scholar]
- 15. Gallagher JC, Peacock M, Yalamanchili V, Smith LM. Effects of vitamin D supplementation in older African American women. J Clin Endocrinol Metab. 2013;98:1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gallagher JC, Jindal PS, Smith LM. Vitamin D supplementation in young White and African American women. J Bone Miner Res. 2014:29:173–181 [DOI] [PubMed] [Google Scholar]
- 17. Zhu J, Ochalek JT, Kaufmann M, Jones G, DeLuca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci USA. 2013;110:15650–15655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masuda S, Byford V, Arabian A, et al. Altered pharmacokinetics of 1α,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (CYP24A1) null mouse. Endocrinology. 2005;146:825–834 [DOI] [PubMed] [Google Scholar]
- 19. Sardina FJ, Mouriño A, Castedo L. Studies on the synthesis of side-chain metabolites of vitamin D. 2. Stereospecific synthesis of 25-hydroxyvitamin D2. J Org Chem. 1986;51:1264–1268 [Google Scholar]
- 20. Nicoletti D, Gregorio C, Mouriño A, Maestro M. A short practical approach to 24R,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2010;121:43–45 [DOI] [PubMed] [Google Scholar]
- 21. Higashi T, Awada D, Shimada K. Simultaneous determination of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 in human plasma by liquid chromatography-mass spectrometry employing derivatization with a Cookson-type reagent. Biol Pharm Bull. 2001;24:738–743 [DOI] [PubMed] [Google Scholar]
- 22. Aronov PA, Hall LM, Dettmer K, Stephensen CB, Hammock BD. Metabolic profiling of major vitamin D metabolites using Diels-Alder derivatization and ultra-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2008;391:1917–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wagner D, Hanwell HE, Schnabl K, et al. The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation. J Steroid Biochem Mol Biol. 2011;126(3–5):72–77 [DOI] [PubMed] [Google Scholar]
- 24. Kobold U. Approaches to measurement of vitamin D concentrations—mass spectrometry. Scand J Clin Lab Invest Suppl. 2012;243:54–59 [DOI] [PubMed] [Google Scholar]
- 25. Kaufmann M, Gallagher JC, Peacock M, Jones G. Ratio of serum 25-OH-D3: 24,25-(OH)2D3 is a novel and sensitive measure of predicting vitamin D deficiency. Paper presented at: Annual Meeting of the American Society for Bone and Mineral Research; October 4–7, 2013; Baltimore, MD [Google Scholar]
- 26. Strathmann FG, Sadilkova K, Laha TJ, et al. 3-epi-25 hydroxyvitamin D concentrations are not correlated with age in a cohort of infants and adults. Clin Chim Acta. 2012;413:203–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lensmeyer G, Poquette M, Wiebe D, Binkley N. The C-3 epimer of 25-hydroxyvitamin D(3) is present in adult serum. J Clin Endocrinol Metab. 2012;97:163–168 [DOI] [PubMed] [Google Scholar]
- 28. Nesterova G, Malicdan MC, Yasuda K, et al. 1,25-(OH)2D-24 Hydroxylase (CYP24A1) deficiency as a cause of nephrolithiasis. Clin J Am Soc Nephrol. 2013;8:649–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meusburger E, Mundlein A, Zitt E, Obermayer-Pietsch, Zotlot D, Lhotta K. Medullary nephrocalcinosis in an adult patient with idiopathic infantile hypercalcemia and a novel CYP24A1 mutation. Clin Kidney J. 2013;6(2):211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586S [DOI] [PubMed] [Google Scholar]
- 31. Shephard RM, Deluca HF. Plasma concentrations of vitamin D3 and its metabolites in the rat as influenced by vitamin D3 or 25-hydroxyvitamin D3 intakes. Arch Biochem Biophys. 1980;202:43–53 [DOI] [PubMed] [Google Scholar]
- 32. Horst RL. Exogenous versus endogenous recovery of 25-hydroxyvitamins D2 and D3 in human samples using high-performance liquid chromatography and the DiaSorin LIAISON Total-D Assay. J Steroid Biochem Mol Biol. 2010;121(1–2):180–182 [DOI] [PubMed] [Google Scholar]
- 33. Janssen MJ, Wielders JP, Bekker CC, et al. Multicenter comparison study of current methods to measure 25-hydroxyvitamin D in serum. Steroids. 2012;77:1366–1372 [DOI] [PubMed] [Google Scholar]
- 34. de Koning L, Al-Turkmani MR, Berg AH, Shkreta A, Law T, Kellogg MD. Variation in clinical vitamin D status by DiaSorin Liaison and LC-MS/MS in the presence of elevated 25-OH vitamin D2. Clin Chim Acta. 2013;415:54–58 [DOI] [PubMed] [Google Scholar]
- 35. Netzel BC, Cradic KW, Bro ET, et al. Increasing liquid chromatography-tandem mass spectrometry throughput by mass tagging: a sample-multiplexed high-throughput assay for 25-hydroxyvitamin D2 and D3. Clin Chem. 2011;57:431–440 [DOI] [PubMed] [Google Scholar]
- 36. Hedman CJ, Wiebe DA, Dey S, Plath J, Kemnitz JW, Ziegler TE. Development of a sensitive LC/MS/MS method for vitamin D metabolites: 1,25 dihydroxyvitamin D2&3 measurement using a novel derivatizing agent. J Chromatography B. 2014;953–954:62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]