Abstract
Aim—To examine the expression of CD40 and B7 (CD80) antigens and the CD40 ligand in Hodgkin's disease.
Methods—Antigen and ligand expression was studied in 17 cases of Hodgkin's disease using immunohistochemistry. The study included 11 cases of Hodgkin's disease in which latent Epstein-Barr virus (EBV) infection could be demonstrated within tumour cells by in situ hybridisation for the EBV encoded early RNAs (EBERs).
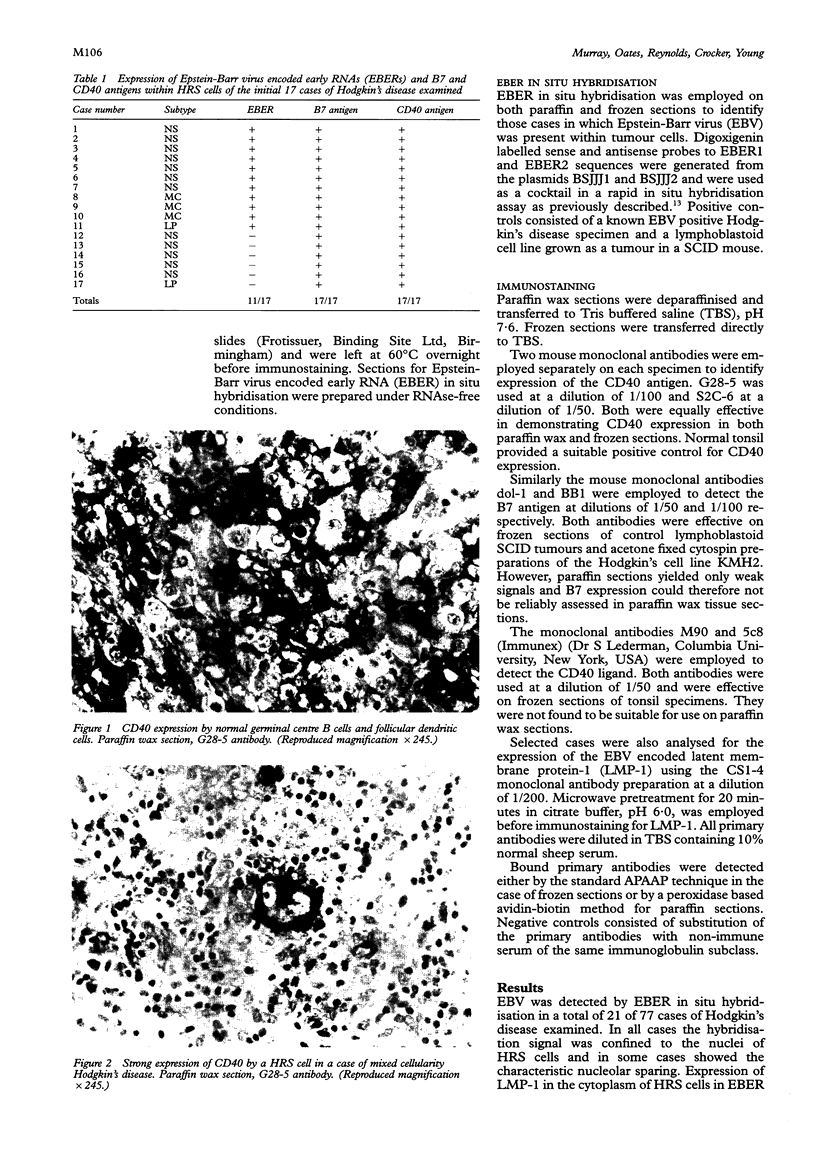
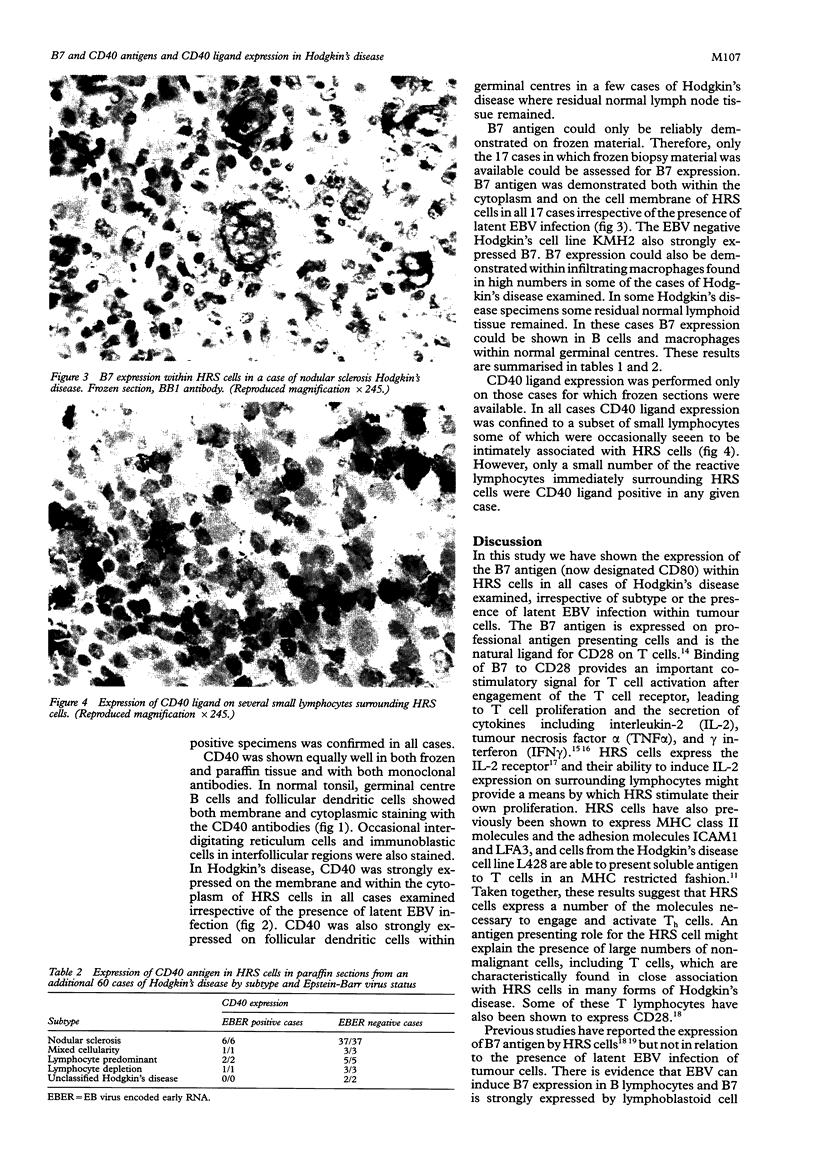
Results—In all cases, irrespective of EBV status, Reed-Sternberg cells and their variants (HRS cells) showed strong expression of both B7 and CD40 antigens. CD40 ligand expression was not shown in HRS cells but was confined to a subset of small lymphocytes some of which were seen to be in intimate contact with HRS cells.
Paraffin wax sections from a further 60 cases of Hodgkin's disease were examined for CD40 and EBER expression alone. The CD40 antigen was identified in HRS cells in all of these cases irrespective of EBER expression.
Conclusions—As CD40 and B7 expression are features of professional antigen presenting cells, these results provide further evidence that HRS cells may have antigen presenting properties and that this may contribute to the characteristic recruitment and activation of non-malignant lymphocytes which is a feature of Hodgkin's disease. The ability of HRS cells to activate Th cells may in turn contribute to their own survival through the induction of the gp39/CD40 pathway.
Keywords: Hodgkin's disease
Keywords: B7 (CD80) antigen
Keywords: CD40 antigen
Keywords: CD40 ligand
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel C. A., Pringle J. H., Naylor J., West K. P., Lauder I. Analysis of antigen receptor genes in Hodgkin's disease. J Clin Pathol. 1993 Apr;46(4):337–340. doi: 10.1136/jcp.46.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage R. J., Fanslow W. C., Strockbine L., Sato T. A., Clifford K. N., Macduff B. M., Anderson D. M., Gimpel S. D., Davis-Smith T., Maliszewski C. R. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992 May 7;357(6373):80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Barletta J. M., Kingma D. W., Ling Y., Charache P., Mann R. B., Ambinder R. F. Rapid in situ hybridization for the diagnosis of latent Epstein-Barr virus infection. Mol Cell Probes. 1993 Apr;7(2):105–109. doi: 10.1006/mcpr.1993.1014. [DOI] [PubMed] [Google Scholar]
- Curran R. C., Jones E. L. Dendritic cells and B lymphocytes in Hodgkin's disease. Lancet. 1977 Aug 13;2(8033):349–349. doi: 10.1016/s0140-6736(77)91502-1. [DOI] [PubMed] [Google Scholar]
- Delabie J., Ceuppens J. L., Vandenberghe P., de Boer M., Coorevits L., De Wolf-Peeters C. The B7/BB1 antigen is expressed by Reed-Sternberg cells of Hodgkin's disease and contributes to the stimulating capacity of Hodgkin's disease-derived cell lines. Blood. 1993 Nov 1;82(9):2845–2852. [PubMed] [Google Scholar]
- Delsol G., Meggetto F., Brousset P., Cohen-Knafo E., al Saati T., Rochaix P., Gorguet B., Rubin B., Voigt J. J., Chittal S. Relation of follicular dendritic reticulum cells to Reed-Sternberg cells of Hodgkin's disease with emphasis on the expression of CD21 antigen. Am J Pathol. 1993 Jun;142(6):1729–1738. [PMC free article] [PubMed] [Google Scholar]
- Fisher R. I., Bostick-Bruton F., Sauder D. N., Scala G., Diehl V. Neoplastic cells obtained from Hodgkin's disease are potent stimulators of human primary mixed lymphocyte cultures. J Immunol. 1983 Jun;130(6):2666–2670. [PubMed] [Google Scholar]
- Fisher R. I., Cossman J., Diehl V., Volkman D. J. Antigen presentation by Hodgkin's disease cells. J Immunol. 1985 Nov;135(5):3568–3571. [PubMed] [Google Scholar]
- Gimmi C. D., Freeman G. J., Gribben J. G., Sugita K., Freedman A. S., Morimoto C., Nadler L. M. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6575–6579. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesser H., Feller A. C., Mak T. W., Lennert K. Clonal rearrangements of T-cell receptor and immunoglobulin genes and immunophenotypic antigen expression in different subclasses of Hodgkin's disease. Int J Cancer. 1987 Aug 15;40(2):157–160. doi: 10.1002/ijc.2910400205. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Hsu P. L. Lymphocyte functional antigens stabilize agglutination between Reed-Sternberg cells and T cells, but are not responsible for homotypic binding of Hodgkin's Reed-Sternberg cells. Am J Pathol. 1990 Sep;137(3):563–574. [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E., Stites D. P., Levy R., Warnke R. Exogenous immunoglobulin and the macrophage origin of Reed-Sternberg cells in Hodgkin's disease. N Engl J Med. 1978 Nov 30;299(22):1208–1214. doi: 10.1056/NEJM197811302992203. [DOI] [PubMed] [Google Scholar]
- Lee S. P., Thomas W. A., Murray R. J., Khanim F., Kaur S., Young L. S., Rowe M., Kurilla M., Rickinson A. B. HLA A2.1-restricted cytotoxic T cells recognizing a range of Epstein-Barr virus isolates through a defined epitope in latent membrane protein LMP2. J Virol. 1993 Dec;67(12):7428–7435. doi: 10.1128/jvi.67.12.7428-7435.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Clark E. A., Ledbetter J. A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Freedman A. S., Aster J. C., Gribben J. G., Lee N. C., Rhynhart K. K., Banchereau J., Nadler L. M. In vivo expression of the B7 costimulatory molecule by subsets of antigen-presenting cells and the malignant cells of Hodgkin's disease. Blood. 1994 Feb 1;83(3):793–798. [PubMed] [Google Scholar]
- Pallesen G., Hamilton-Dutoit S. J., Rowe M., Young L. S. Expression of Epstein-Barr virus latent gene products in tumour cells of Hodgkin's disease. Lancet. 1991 Feb 9;337(8737):320–322. doi: 10.1016/0140-6736(91)90943-j. [DOI] [PubMed] [Google Scholar]
- Ranheim E. A., Kipps T. J. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993 Apr 1;177(4):925–935. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M. K., Armitage R. J., Strockbine L., Clifford K. N., Macduff B. M., Sato T. A., Maliszewski C. R., Fanslow W. C. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992 Dec 1;176(6):1543–1550. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Söderström K. O., Rinne R., Hopsu-Havu V. K., Järvinen M., Rinne A. Hodgkin's disease: a malignancy of follicular dendritic cells? Lancet. 1994 Feb 12;343(8894):422–423. doi: 10.1016/s0140-6736(94)91260-2. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Lindsten T., Ledbetter J. A., Kunkel S. L., Young H. A., Emerson S. G., Leiden J. M., June C. H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Nelson D. L., Fleisher T. A., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. II. Expression of Tac antigen on activated cytotoxic killer T cells, suppressor cells, and on one of two types of helper T cells. J Immunol. 1981 Apr;126(4):1398–1403. [PubMed] [Google Scholar]
- Wang F., Gregory C., Sample C., Rowe M., Liebowitz D., Murray R., Rickinson A., Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990 May;64(5):2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokochi T., Holly R. D., Clark E. A. B lymphoblast antigen (BB-1) expressed on Epstein-Barr virus-activated B cell blasts, B lymphoblastoid cell lines, and Burkitt's lymphomas. J Immunol. 1982 Feb;128(2):823–827. [PubMed] [Google Scholar]