Abstract
Despite an increasingly detailed understanding of endogenous and environmental growth-controlling signals and their signaling networks, little is known on how these networks are integrated with the cell expansion machinery. Members of the CrRLK1L family control cell wall properties and cell expansion in a variety of developmental and environmental contexts. Two recent reports provide exciting new insights into the mode of action of these RLKs. One study shows that one family member, FERONIA (FER), is required for the production of hydroxyl radicals in the female gametophyte, which causes pollen tube rupture and sperm cell release during fertilization. Another study shows that FER is a receptor for a signaling peptide (Rapid Alkalinization Factor 1 [RALF1]) that triggers cell wall alkalinization and growth arrest, possibly through the inhibition of plasma membrane H+-ATPase activity. RALF1 belongs to a large gene family, with a wide range of expression patterns. Other CrRLK1L family members therefore may also be receptors for RALF-like peptides. These findings have important implications for our understanding of the control of cell wall integrity during growth and raise new intriguing questions.
FERONIA CONTROLS CELL EXPANSION AND CELL-TO-CELL COMMUNICATION
The receptor-like kinase FERONIA (FER), named after an Etruscan goddess of fertility, was originally identified as a factor required for the communication between the male and female gametophytes during fertilization. Ovules lacking FER fail to induce pollen tube bursting and sperm cell release and attract supernumerary pollen tubes leading to a characteristic phenotype in which pollen tubes pile up inside the mutant female gametophyte (Huck et al., 2003; Rotman et al., 2003; Escobar-Restrepo et al., 2007). Later, it was shown that FER is required for correct polar targeting of NORTIA, a multi-spanning membrane protein of the mildew resistance locus O (MLO) family, which is also required for pollen tube bursting and fertilization (Kessler et al., 2010). Furthermore, FER has fertilization-independent functions, as fer mutants generally show reduced growth, defective root hairs, and increased resistance to powdery mildew (Guo et al., 2009; Duan et al., 2010; Kessler et al., 2010; Duan et al., 2014).
FER belongs to a 17-member family of proteins in Arabidopsis thaliana named CrRLK1L (named after the first member characterized in Catharanthus roseus cell cultures), several members of which have been implicated in the regulation of cell elongation (see Table 1 for a summary of phenotypes observed in CrRLK1L mutant alleles). CrRLK1L family members that have been studied are part of a signaling module that involves the recruitment of guanidine nucleotide exchange factors of the plant-type Rho GTPase, the activation of NADPH oxidases and, thus, the generation of extracellular reactive oxygen species (ROS), and an increase in intracellular Ca2+ levels (Duan et al., 2010, 2014; Denness et al., 2011; Boisson-Dernier et al., 2013). Depending on the cellular context, this can have various consequences for cell elongation and cellular integrity (see below). The function of CrRLK1L family proteins has been discussed by excellent recent reviews (Boisson-Dernier et al., 2011; Cheung and Wu, 2011; Lindner et al., 2012a). Here, we concentrate on new perspectives and questions that have been raised by several exciting recent studies.
Table 1. Overview of CrRLK1L Family Member Mutant Phenotypes.
| Allele | Phenotype | Mutation | Ecotype | Reference |
|---|---|---|---|---|
| FER | ||||
| fer | Pollen tube overgrowth, multiple pollen tubes, reduced stature, increased cell death, increased ROS burst and MAPK activation upon flg22 in leaves, reduced bacterial proliferation, resistant to powdery mildew, defective NTA localization | 4-bp insertion, frame shift | Ler | Huck et al. (2003); Escobar-Restrepo et al. (2007); Keinath et al. (2010) |
| Ds transposon | Kessler et al. (2010) | |||
| srn/sir | Pollen tube overgrowth, multiple pollen tubes, defective root hairs, larger seeds, bigger cotyledons, reduced ROS in female gametophyte, hypersensitive to ABA, autonomous endosperm development | 1-bp deletion, frame shift | C24 | Rotman et al. (2003) |
| γ-Ray irradiation | Escobar-Restrepo et al. (2007); Rotman et al. (2008); Duan et al. (2010, 2014); Yu et al. (2014) | |||
| fer-2 | Reduced hypocotyl length, brassinosteroid insensitivity, ethylene hypersensitivity | tDNA insertion | Col-0 | Deslauriers and Larsen (2010) |
| Activation tagging | ||||
| fer-3 | Reduced hypocotyl length | tDNA insertion in 5′ part | Col-0 | Deslauriers and Larsen (2010) |
| fer-4 | Reduced stature, defective root hairs, defective trichomes, reduced fertility, reduced auxin and RAC/ROP signaling, reduced ROS in roots/female gametophyte, larger seeds, bigger cotyledons, RALF insensitive, longer root length under blue light, increased capacity to acidify medium, hypersensitive to LiCl, pollen tube overgrowth, reduced ROS in female gametophyte, hypersensitive to ABA | tDNA insertion in 5′ part | Col-0 | Duan et al. (2010); Yu et al. (2012, 2014); Huang at al. (2013); Duan et al. (2014); Haruta et al. (2014) |
| fer-5 | Reduced stature, defective root hairs, reduced auxin and RAC/ROP signaling, reduced ROS in roots/female gametophyte, partially insensitive to RALF, hypersensitive to ABA | tDNA insertion in 3′ part | Col-0 | Duan et al. (2010) |
| Haruta et al. (2014) | ||||
| Yu et al. (2014) | ||||
| fer amiRNA | Reduced growth, rounded leaves, reduced cell elongation in hypocotyl and petioles | amiRNA under control of BRI1 promoter | Col-0 | Guo et al. (2009b) |
| THE1 | ||||
| the1-1 | Suppresses growth defect and ectopic lignification of prc1-1, reverts gene expression changes in prc1-1 | G110A EMS allele | prc1-1 (Col-0) | Hématy et al. (2007) |
| Denness et al. (2011) | ||||
| the1-2 | Suppresses growth defect of prc1-1 | G448A EMS allele | prc1-1 (Col-0) | Hématy et al. (2007) |
| the1-3 | Suppresses growth defect and ectopic lignification of several cell wall–damaging mutants, reverts gene expression changes in prc1-8 | tDNA | Ws | Hématy et al (2007) |
| the1-4 | Reduced stature and cell elongation when combined with herk, enhances bri1-5 when combined with herk1, suppresses bes1-1D when combined with herk1, altered gene expression when combined with herk1 | tDNA | Col-0 | Guo et al. (2009b) |
| ANX1/2 | ||||
| anx1,2 | Reduced fertility, pollen tube burst | tDNAs | Col-0 | Miyazaki et al. (2009) |
| anx1-2, 2-2 | Reduced fertility, pollen tube burst | tDNAs | Col-0 | Boisson-Dernier et al. (2009); Boisson-Dernier et al. (2013) |
| HERK1/2 | ||||
| herk1 | Reduced stature and cell elongation when combined with the1-4, enhances bri1-5 when combined with the1-4, suppresses bes1-1D when combined with the1-4, altered gene expression when combined with the1-4 | tDNA | Col-0 | Guo et al. (2009b) |
| herk2 | Reduced stature when combined with herk1 and the1-4 | Guo et al. (2009a) | ||
| ERULUS | ||||
| eru1 | Defective root hairs | tDNA | Col-0 | Haruta et al. (2014) |
| eru2 | Defective root hairs | tDNA | Col-0 | Haruta et al. (2014) |
References in bold denote first description of the respective mutant alleles. Col-0, Columbia-0; Ler, Landsberg erecta; Ws, Wassilewskija; amiRNA,artificial microRNA.
FER FUNCTIONS
FER Controls Hydroxyl Radical Production by the Female Gametophyte, Which Induces Rupture of the Pollen Tube
FER is expressed in most tissues except for the male gametophyte. In the female gametophyte, FER is highly expressed, in particular in the synergid cells that line the ovular aperture, consistent with its role in inducing pollen tube rupture. Recently, Duan et al. (2014) investigated the molecular mechanism of this process. They confirmed previous observations (Martin et al., 2013) that ROS transiently accumulate at the so-called filiform apparatus, a domain in synergid cells with abundant membrane protrusions at the entrance of the female gametophyte. ROS accumulation depends on plasma membrane–associated NADPH-oxidase activity, correlates in space and time with FER protein accumulation (as shown with a FER-GFP fusion), and is not observed in fer mutants. Using elegant assay systems (a pistil feeding assay and a semi–in vivo pollen tube growth system), the authors show that promoting superoxide production, adding H202 or promoting ·OH formation triggers rapid pollen tube bursting. Inversely, the pharmacological inhibition of NADPH oxidase activity (which produces superoxide) as well as the scavenging of H202 or, most significantly, ·OH in the female gametophyte prevents pollen tube bursting. These results indicate that superoxide or H202 primarily act via their conversion into hydroxyl radicals, which therefore are the most likely reactive oxygen species to induce pollen tube rupture in this context. Since ·OH radicals are very short-lived (the average diffusion distance is <10 nm; Roots and Okada, 1975), they would constitute an effective messenger to signal the close proximity of the pollen tube to its target. The identity of the female gametophyte-expressed NADPH-oxidase(s) responsible for FER-dependent ROS production remains to be determined. Finally, the authors show that Ca2+ influx into the pollen tube is induced by ROS application and is required for ROS-induced bursting. It is intriguing in this context that rboh-deficient mutant pollen tubes (see below; Boisson-Dernier et al., 2013; Lassig et al., 2014) and root hairs (Duan et al., 2010) with low internal levels of ROS also display loss of integrity. It is possible, however, that the tip-localized bursting of the pollen tube and the subsequent extrusion of the sperm cells involves a specialized process that is distinct from that underlying the premature loss of integrity of the pollen tube observed in rboh-deficient mutants.
The exact link between ·OH production and the observed Ca2+ influx and bursting during fertilization is not clear. Is the hydroxyl radical acting directly as a Ca2+ channel agonist, or is it acting indirectly through its loosening effect on cell walls? Previous work has shown that ·OH radicals are generated from H2O2 in the cell wall in the presence of trace elements of transition metals, such cupper or iron, through Fenton chemistry. These radicals can very locally cleave polysaccharides and promote wall relaxation (Fry, 1998; Fry et al., 2001; Schopfer, 2001). In this scenario, ·OH-mediated wall weakening might facilitate bursting once the pollen tube enters the presumably Ca2+-rich environment of the synergid cytoplasm, perhaps combined with the opening of stretch-activated Ca2+ channels in the pollen tube. It should be noted that ·OH-mediated cell wall loosening and peroxidase-mediated tightening (e.g., through tyrosine cross-linking of wall proteins or phenolic acids) would compete for available H2O2. ·OH radicals in principle should be produced in most cell walls, given the fact that most walls contain sufficient copper or iron and that very high amounts of scavenging solutes (e.g., cysteine or salicylate) are needed to remove ·OH completely. This raises the possibility that the balance between the growth-inhibiting or -promoting effect of ROS production depends on the peroxidase activity in the wall, perhaps as part of a complex with the NADPH-oxidase as observed during the formation of the Casparian strip in the endodermis (Lee et al., 2013). In this context, it will be interesting to investigate peroxidase activity in synergid cells during fertilization.
FER Is a RALF Receptor, Causing Cell Wall Alkalinization
Another recent study sheds a new and unexpected light on the role of FER (Haruta et al., 2014). The authors show that FER is a receptor for Rapid Alkalinization Factor 1 (RALF1), a 49–amino acid peptide that had been identified in tobacco (Nicotiana tabacum) leaf extracts by its ability to induce the rapid alkalinization of the culture medium of tobacco suspension-cultured cells (Pearce et al., 2001). RALF-like peptides are conserved among land plants, including mosses and lycophytes. In Arabidopsis, RALF1 is released from a 120–amino acid pre-propeptide by proteolytic processing (Matos et al., 2008) and overexpression or external application of the peptide results in growth inhibition (Bergonci et al., 2014; Haruta et al., 2014). In their quest for the mode of action of RALF1, Haruta et al. used an elegant quantitative phosphoproteomics approach to monitor early changes in protein phosphorylation in Arabidopsis seedlings treated with epitope-tagged RALF1. Within 5 min of RALF1 addition, a 2.5-fold or greater change in abundance in phosphopeptides was observed for five proteins, including FER, which was phosphorylated at three positions in the C terminus of the kinase domain.
The hypothesis that FER plays a role in RALF1 signaling was corroborated by the absence of RALF1-induced root growth inhibition in the loss-of-function mutant fer-4 and the reduced response in the hypomorphic mutant fer-5. Another RALF1-induced immediate response, the transient increase of cytosolic Ca2+, was also impaired in fer-4. The H+-ATPase AHA2 was also among the RALF1-induced phosphorylated proteins, however, with a phosphorylation site that is suggested to promote activity (Rudashevskaya et al., 2012), indicating that more work is needed to elucidate the mechanism of the proposed FER-mediated inhibition of ATPase activity. Moreover, it remains to be seen whether the activated FER kinase directly phosphorylates the H+-ATPase or whether another protein kinase is involved, for instance, the Ca2+-dependent protein kinase 9 (CPK9) that was also found to be phosphorylated upon RALF1 treatment. Finally, the authors show that FER is a bona fide RALF1 receptor by demonstrating the binding of RALF1 to FER produced in tobacco and to the FER ectodomain produced as a fusion protein in Escherichia coli.
Other CrRLK1L Family Members Also Play a Role in Growth Control
In addition to FER, several other CrRLK1L family members have been studied and shown to play a role in the control of cell expansion: ANXUR1 (ANX1), ANX2, THESEUS1 (THE1), HERKULES1 (HERK1), and HERK2.
ANX1 and 2 are pollen-specific RLKs that redundantly control polar growth of pollen tubes. Double mutant anx1/2 pollen tubes burst prematurely, whereas ANX1 overexpression causes pollen tube growth inhibition and the overaccumulation of secreted wall material (Boisson-Dernier et al., 2009, 2013; Miyazaki et al., 2009). Genetic analysis shows that ANX1-induced growth inhibition requires the activity of the partially redundant NADPH oxidases RbohH and RbohJ, which are responsible for ROS production close to the pollen tube tip (Boisson-Dernier et al., 2013). Both enzymes are located in the pollen tube tip and are activated by intracellular Ca2+ through direct binding (to a cytosolic EF hand motif) and Ca2+-dependent phosphorylation (Kaya et al., 2014). A recent report used kinematic analysis to study the link between Ca2+-induced NADPH oxidase activation, cell wall deposition, and growth control. Whereas wild-type pollen tubes in general elongate with a constant growth rate, rbohh/j mutants typically show oscillatory growth patterns, with rates exceeding that of the wild type, followed by growth arrest (Lassig et al., 2014). Interestingly, in rbohh/j, exocytosis of wall material, in particular pectate, appears to precede the growth burst, whereas intracellular Ca2+ levels peak together with, or slightly after, the growth peak (Lassig et al., 2014). These results are consistent with previous observations (brilliantly reviewed in Winship et al., 2011) showing that pectate deposition may have a cell wall–loosening and growth-promoting effect through the chelation of Ca2+ from load-bearing Ca2+-pectate cross-links (Proseus and Boyer, 2006, 2008, 2012; Rojas et al., 2011). In addition, the results indicate that the growth-dependent Ca2+ increase may be part of a feedback mechanism that dampens growth oscillations in wild-type pollen tubes through the activation of NADPH oxidases and ROS-dependent growth inhibition, thereby coordinating growth rate and exocytosis rate. ANX1/2 may impinge on this feedback loop through the promotion of NADPH oxidase activity either directly or indirectly for instance by promoting cytosolic Ca2+ increase.
THE1 was identified through a genetic screen for suppressors of reduced growth in the cellulose-synthase deficient mutant cesa6prc1-1 (Hématy et al., 2007). Strikingly, the1 mutants do not restore cellulose content itself but diminish the secondary effects of reduced cellulose synthesis such as ROS production, growth inhibition, ectopic lignin accumulation, expression of stress-related genes, and the synthesis of defense compounds. In the absence of cell wall damage, the1 mutants do not show an apparent phenotype; hence, THE1 is thought to sense the integrity of the cell wall and trigger ROS-mediated growth inhibition (Hématy et al., 2007; Denness et al., 2011). Another family member, HERK1, is thought to be partially redundant with THE1 in the absence of cell wall damage, as shown by the elongation defects in leaves and petioles of double mutants (Guo et al., 2009). The phenotype of these double mutants is very similar to that of fer, suggesting a role of the three RLKs in a common pathway. Finally, based on the gene expression patterns, other family members may have potential roles in responses to abiotic or biotic stresses (Lindner et al., 2012a).
PERSPECTIVES
Open Questions on CrRLK1L Signaling
The identification of RALF1 as a FER ligand raises the question how the signal is transduced. Exposure to RALF1 leads to a rapid FER-dependent intracellular Ca2+ increase (Haruta et al., 2014). However, it is at present unclear whether this is mediated by RAC/ROP signaling or whether it depends on RALF1-induced, NADPH oxidase–dependent ROS production.
While extracellular signals are often linked to cell reprogramming involving transcription factor targets, it is at present unclear whether there is a direct, dedicated pathway from FER or other CrRLK1Ls to transcription factors. The transcriptome of plants treated with RALF1 has been analyzed (Haruta et al., 2014), but it would be interesting to know which proportion of the gene expression changes compared with the wild type are dependent on FER, AHA2, and NADPH oxidases, for example.
A number of additional proteins have been identified, which might be involved in CrRLK1L signaling pathways. For example, NORTIA (NTA), a member of the MLO family, was discovered in a screen for fer-like pollen tube overgrowth phenotypes (Kessler et al., 2010). Remarkably, NTA becomes polarly localized in synergid cells upon pollen tube arrival, and this relocalization strictly depends on the presence of FER, suggesting that the two proteins act together in pollen tube reception, possibly in the same complex. Other mutants also cause a pollen tube phenotype similar to that of fer, such as mutants for the glycosyl-phosphatidylinositol-anchored protein LORELEI (LRE) and the UDP-glycosyltransferase TURAN (Capron et al., 2008; Tsukamoto et al., 2010; Lindner et al., 2012b). Like FER, LRE was shown to bind the small GTPase ROP2 and to be required for the production of female gametophytic ROS, suggesting that FER and LRE act in the same pathway presumably through a transient ROP2 signaling complex (Duan et al., 2014). Furthermore, a similar phenotype was discovered in the mutant abstinence by mutual consent (amc), in which a protein with homology to yeast PEROXIN13 (PEX13) is affected (Boisson-Dernier et al., 2008). PEX13 is involved in peroxisomal protein import, which might suggest a link to the generation or scavenging of ROS. However, as the name suggest, amc differs from the above-mentioned mutants in that the mutant allele has to be present in both male and female gametophyte (Boisson-Dernier et al., 2008) to elicit an otherwise identical phenotype.
CrRLK1L Proteins with Multiple or Complex Ligands?
Based on the cellular phenotypes of anx1,2 and fer mutants and the nature of the screen that led to the identification of THE1, it was originally suggested that CrRLK1L proteins might be sensing the cell wall state by interacting with extracellular carbohydrates. This is also suggested by the presence of GFP-tagged THE1 in so-called Hechtian strands that connect the protoplast to the cell wall of plasmolyzed cells. The discovery of malectin-like domains in CrRLK1Ls further supported this assumption (Boisson-Dernier et al., 2011). Indeed, malectin is a carbohydrate binding protein originally discovered in Xenopus laevis, whose homologs in mammals are involved in endoplasmic reticulum quality control through interaction with N-linked glycans (Schallus et al., 2008; Galli et al., 2011). The discovery that RALF1 binds to FER thus challenges the common view that a given receptor is matched with precisely one ligand. It is not known with which part of the 400–amino acid FER ectodomain RALF1 interacts, and it is certainly possible that binding occurs outside of the two ∼165–amino acid malectin-like domains. Furthermore, it is conceivable that the malectin domains function differently in plants and animals since the observed sequence conservation is only limited (Boisson-Dernier et al., 2011). What seems clear, however, is that glycosylation does not play a role in RALF1 function since (1) the mature peptide lacks predicted N-linked glycosylation sites, and (2) RALF1 peptide produced in E. coli, and thus in the absence of glycosylation, was biologically active and able to bind to FER (Haruta et al., 2014). Taken together, this opens the interesting possibility that CrRLK1Ls might have more than one ligand, as frequently observed with mammalian receptor proteins (Noy, 2007; Pi and Quarles, 2012). Alternatively, the malectin domain could function in tethering the proteins to the extracellular matrix, which could be tested for instance with fluorescence recovery after photobleaching experiments (Martinière et al., 2012).
Reconciliation of Cellular and Organismal Phenotypes
Possibly related to our incomplete understanding of CrRLK1L downstream signaling, it is challenging to reconcile the presumed role of these proteins at the cellular level with the phenotypes observed in mutant plants. For example, in pollen tubes and in roots, FER seems to have an inhibiting effect on cell elongation, whereas the phenotype of numerous fer mutants seems to suggest a positive effect on growth (Guo et al., 2009; Deslauriers and Larsen, 2010; Duan et al., 2010; Kessler et al., 2010) (see also Table 1). However, this discrepancy is also observed with well-characterized growth promoting agents, e.g., auxin or brassinosteroids, that have growth-inhibiting effects depending on their concentration and the tissue in question. Therefore, the capacity to promote growth is certainly context dependent. In addition, one of the immediate results of CrRLK1L activation is the generation of ROS, which can be rapidly interconverted and can also have opposing effects depending on the precise species produced. As an alternative explanation for the apparent incongruity of the different fer phenotypes in light of the new evidence for FER being a negative regulator of growth (Haruta et al., 2014), reduced stature of fer mutants might be a secondary effect on development, perhaps through an imbalance in ROS production, increased cell death (Keinath et al., 2010), or hyperpolarization of the plasma membrane (Haruta et al., 2014). Thus, despite the recent advances, more insight into CrRLK1L signaling is needed to understand the biological functions of these receptors.
Regulation of RALFs
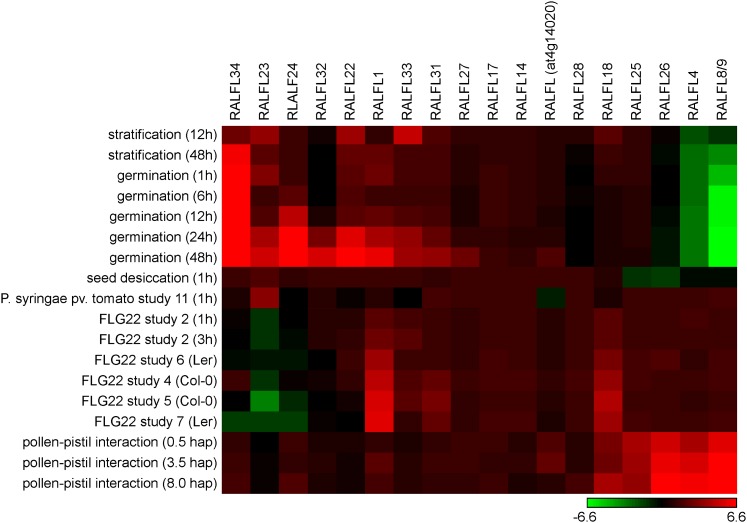
In light of the findings by Haruta et al. (2014), attempts to understand the physiological role of FER, and possibly other CrRLK1Ls, must take into account the observation that RALF family members are subject to complex regulation. Unfortunately, only 18 of at least 34 family members are represented on the commonly used ATH1 gene chip. But among those few, interesting patterns of expression can be found in public databases. Several Arabidopsis RALF peptide genes (RALFL4, RALFL12, RALFL17, RALFL25, and RALFL26) are strongly and specifically expressed in pollen where they potentially play a role in growth control (Covey et al., 2010). These peptides are candidate ligands for ANX1 and 2. Interestingly, expression of several members of the RALF family is also increased upon pollen–pistil interaction, which would be in line with a role in pollen tube integrity control or reception (Boavida et al., 2011). With respect to the latter process, it is interesting to note that according to a recent study, at least two RALFs are specifically expressed in synergid cells (RALFL14 and RALFL18; Wuest et al., 2010) where FER exerts its pollen tube reception control.
Environmental, hormonal, and defense-related cues also affect RALF transcript abundance. Germination and abscisic acid (ABA) treatment induce a number of RALF members, whereas the expression of others is reduced by the same conditions. Some RALFs, including RALF1, are upregulated upon treatment with the bacterial elicitor flg22 (Figure 1). Interestingly, FER mutants appear to be indistinguishable from the wild type with respect to flg22-induced growth inhibition (Haruta et al., 2014), but have a strongly increased response to the elicitor in terms of ROS production and mitogen-activated protein kinase (MAPK) activation (Keinath et al., 2010), also suggesting a link between RALF1 and elicitor responses.
Figure 1.
Cluster Analysis of RALF-Like Peptide Expression under Influence of Selected Stimuli.
A heat map was generated using matrix2png (http://www.chibi.ubc.ca/matrix2png/bin/matrix2png.cgi) and data extracted from public databases with the Genevestigator tool (Hruz et al., 2008). Color code indicates fold change in expression compared with control.
Another interesting aspect of RALF regulation concerns the maturation of the pro-peptide into the active form. RALFs have conserved basic amino acid motifs, which suggest potential processing by members of the subtilase protease family. Indeed, RALF23 is processed by Site-1 protease (S1P) at the motif RRIL (Srivastava et al., 2009). Intriguingly, the same motif is present in the FER ligand RALF1 and was shown to be required for RALF1 processing (Matos et al., 2008). S1P is localized in the Golgi apparatus and possibly also in other endomembrane compartments, such as the trans-Golgi network, but seems to be absent from the plasma membrane (Liu et al., 2007). This raises the question whether RALF processing occurs “per default” or whether the activity of S1P (or that of other subtilases putatively involved in RALF processing) is somehow controlled, adding another layer of regulation to this growth controlling module.
As pointed out above, CrRLK1L/RALF function seems to be associated with cell wall integrity maintenance. In this respect, it is interesting to note that at least RALF4 is highly coregulated with pectin modifying enzymes (our unpublished data). Around 20% of the primary cell wall is homogalacturonan, the most abundant pectin species. Homogalacturonan is synthesized and secreted in an almost entirely methyl-esterified state, but can be selectively demethylesterified with a high spatio-temporal resolution by enzymes called pectin methyl esterases (PMEs), a process that is associated with growth transitions (Peaucelle et al., 2008; Pelletier et al., 2010). As PMEs have an alkaline pH optimum, and the removal of methyl groups leads to cell wall acidification and thus PME inhibition, it is possible that RALF receptor activation participates in pH homeostasis of the cell wall during growth.
Intriguingly, S1P not only processes RALFs as noted above, but also mediates the proteolytic processing of one type of PMEs, which is a prerequisite for their secretion to the apoplast (Wolf et al., 2009), again suggesting complex networks involving CrRLK1L/RALF signaling for the regulation of cell wall maintenance.
CONCLUSION
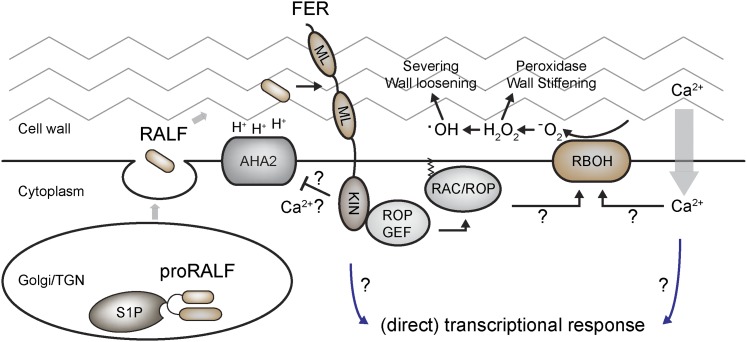
The CrRLK1L receptor family is involved in the regulation of cell expansion and cell-to-cell communication. At least some of the family members promote ROS production, which may be translated into wall loosening or wall stiffening depending on the ROS produced and the composition of the wall. We now know that FER is a receptor for a signaling peptide, RALF1, that induces Ca2+ influx and alkalinization of the apoplast through the inhibition of the H+-ATPase (summarized in Figure 2). This discovery opens up the possibility that other CrRLK1L family members may also be regulated by RALF-like peptides. The identification of the ligands for these receptors, for instance, using the approaches discussed in the accompanying Perspective (Butenko et al., 2014), should provide new insights into how various endogenous and environmental signals are translated into growth-controlling changes in cell wall properties.
Figure 2.
Schematic Representation of FER/RALF1 Signaling Components.
Speculative steps are indicated by question marks. See text for explanations.
AUTHOR CONTRIBUTIONS
S.W. and H.H. conceived and wrote the article.
Acknowledgments
We thank Qiaohong Duan and Alice Cheung for providing the image for the icon and for sharing unpublished information. We also thank Pierre Hilson for the critical reading of the article.
References
- Bergonci T., Ribeiro B., Ceciliato P.H., Guerrero-Abad J.C., Silva-Filho M.C., Moura D.S. (2014). Arabidopsis thaliana RALF1 opposes brassinosteroid effects on root cell elongation and lateral root formation. J. Exp. Bot. 65: 2219–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boavida L.C., Borges F., Becker J.D., Feijó J.A. (2011). Whole genome analysis of gene expression reveals coordinated activation of signaling and metabolic pathways during pollen-pistil interactions in Arabidopsis. Plant Physiol. 155: 2066–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A., Frietsch S., Kim T.H., Dizon M.B., Schroeder J.I. (2008). The peroxin loss-of-function mutation abstinence by mutual consent disrupts male-female gametophyte recognition. Curr. Biol. 18: 63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A., Kessler S.A., Grossniklaus U. (2011). The walls have ears: the role of plant CrRLK1Ls in sensing and transducing extracellular signals. J. Exp. Bot. 62: 1581–1591 [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A., Lituiev D.S., Nestorova A., Franck C.M., Thirugnanarajah S., Grossniklaus U. (2013). ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol. 11: e1001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A., Roy S., Kritsas K., Grobei M.A., Jaciubek M., Schroeder J.I., Grossniklaus U. (2009). Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development 136: 3279–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko M.A., Wildhagen M., Albert M., Jehle A., Kalbacher H., Aalen R.B., Felix G. (2014). Tools and strategies to match peptide-ligand receptor pairs. Plant Cell 26: 10.1105/tpc.113.120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron A., Gourgues M., Neiva L.S., Faure J.E., Berger F., Pagnussat G., Krishnan A., Alvarez-Mejia C., Vielle-Calzada J.P., Lee Y.R., Liu B., Sundaresan V. (2008). Maternal control of male-gamete delivery in Arabidopsis involves a putative GPI-anchored protein encoded by the LORELEI gene. Plant Cell 20: 3038–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A.Y., Wu H.M. (2011). THESEUS 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Curr. Opin. Plant Biol. 14: 632–641 [DOI] [PubMed] [Google Scholar]
- Covey P.A., Subbaiah C.C., Parsons R.L., Pearce G., Lay F.T., Anderson M.A., Ryan C.A., Bedinger P.A. (2010). A pollen-specific RALF from tomato that regulates pollen tube elongation. Plant Physiol. 153: 703–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denness L., McKenna J.F., Segonzac C., Wormit A., Madhou P., Bennett M., Mansfield J., Zipfel C., Hamann T. (2011). Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 156: 1364–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers S.D., Larsen P.B. (2010). FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol. Plant 3: 626–640 [DOI] [PubMed] [Google Scholar]
- Duan Q., Kita D., Johnson E.A., Aggarwal M., Gates L., Wu H.M., Cheung A.Y. (2014). Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun 5: 3129. [DOI] [PubMed] [Google Scholar]
- Duan Q., Kita D., Li C., Cheung A.Y., Wu H.M. (2010). FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. USA 107: 17821–17826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Restrepo J.M., Huck N., Kessler S., Gagliardini V., Gheyselinck J., Yang W.C., Grossniklaus U. (2007). The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317: 656–660 [DOI] [PubMed] [Google Scholar]
- Fry S.C. (1998). Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem. J. 332: 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry S.C., Dumville J.C., Miller J.G. (2001). Fingerprinting of polysaccharides attacked by hydroxyl radicals in vitro and in the cell walls of ripening pear fruit. Biochem. J. 357: 729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli C., Bernasconi R., Soldà T., Calanca V., Molinari M. (2011). Malectin participates in a backup glycoprotein quality control pathway in the mammalian ER. PLoS ONE 6: e16304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Li L., Ye H., Yu X., Algreen A., Yin Y. (2009). Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 106: 7648–7653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M., Sabat G., Stecker K., Minkoff B.B., Sussman M.R. (2014). A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343: 408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy K., Sado P.E., Van Tuinen A., Rochange S., Desnos T., Balzergue S., Pelletier S., Renou J.P., Höfte H. (2007). A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 17: 922–931 [DOI] [PubMed] [Google Scholar]
- Hruz T., Laule O., Szabo G., Wessendorp F., Bleuler S., Oertle L., Widmayer P., Gruissem W. (2008). Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv. Bioinformatics 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G.Q., Li E., Ge F.R., Li S., Wang Q., Zhang C.Q., Zhang Y. (2013). Arabidopsis RopGEF4 and RopGEF10 are important for FERONIA-mediated developmental but not environmental regulation of root hair growth. New Phytol. 200: 1089–1101 [DOI] [PubMed] [Google Scholar]
- Huck N., Moore J.M., Federer M., Grossniklaus U. (2003). The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development 130: 2149–2159 [DOI] [PubMed] [Google Scholar]
- Kaya H., et al. (2014). Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26: 1069–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath N.F., Kierszniowska S., Lorek J., Bourdais G., Kessler S.A., Shimosato-Asano H., Grossniklaus U., Schulze W.X., Robatzek S., Panstruga R. (2010). PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J. Biol. Chem. 285: 39140–39149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S.A., Shimosato-Asano H., Keinath N.F., Wuest S.E., Ingram G., Panstruga R., Grossniklaus U. (2010). Conserved molecular components for pollen tube reception and fungal invasion. Science 330: 968–971 [DOI] [PubMed] [Google Scholar]
- Lassig R., Gutermuth T., Bey T.D., Konrad K.R., Romeis T. (2014). Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J. 78: 94–106 [DOI] [PubMed] [Google Scholar]
- Lee Y., Rubio M.C., Alassimone J., Geldner N. (2013). A mechanism for localized lignin deposition in the endodermis. Cell 153: 402–412 [DOI] [PubMed] [Google Scholar]
- Lindner H., Müller L.M., Boisson-Dernier A., Grossniklaus U. (2012a). CrRLK1L receptor-like kinases: not just another brick in the wall. Curr. Opin. Plant Biol. 15: 659–669 [DOI] [PubMed] [Google Scholar]
- Lindner H., Raissig M.T., Sailer C., Shimosato-Asano H., Bruggmann R., Grossniklaus U. (2012b). SNP-Ratio Mapping (SRM): identifying lethal alleles and mutations in complex genetic backgrounds by next-generation sequencing. Genetics 191: 1381–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.X., Srivastava R., Che P., Howell S.H. (2007). Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J. 51: 897–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M.V., Fiol D.F., Sundaresan V., Zabaleta E.J., Pagnussat G.C. (2013). oiwa, a female gametophytic mutant impaired in a mitochondrial manganese-superoxide dismutase, reveals crucial roles for reactive oxygen species during embryo sac development and fertilization in Arabidopsis. Plant Cell 25: 1573–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinière A., et al. (2012). Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proc. Natl. Acad. Sci. USA 109: 12805–12810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos J.L., Fiori C.S., Silva-Filho M.C., Moura D.S. (2008). A conserved dibasic site is essential for correct processing of the peptide hormone AtRALF1 in Arabidopsis thaliana. FEBS Lett. 582: 3343–3347 [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Murata T., Sakurai-Ozato N., Kubo M., Demura T., Fukuda H., Hasebe M. (2009). ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr. Biol. 19: 1327–1331 [DOI] [PubMed] [Google Scholar]
- Noy N. (2007). Ligand specificity of nuclear hormone receptors: sifting through promiscuity. Biochemistry 46: 13461–13467 [DOI] [PubMed] [Google Scholar]
- Pearce G., Moura D.S., Stratmann J., Ryan C.A., Jr (2001). RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc. Natl. Acad. Sci. USA 98: 12843–12847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucelle A., Louvet R., Johansen J.N., Höfte H., Laufs P., Pelloux J., Mouille G. (2008). Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr. Biol. 18: 1943–1948 [DOI] [PubMed] [Google Scholar]
- Pelletier S., et al. (2010). A role for pectin de-methylesterification in a developmentally regulated growth acceleration in dark-grown Arabidopsis hypocotyls. New Phytol. 188: 726–739 [DOI] [PubMed] [Google Scholar]
- Pi M., Quarles L.D. (2012). Multiligand specificity and wide tissue expression of GPRC6A reveals new endocrine networks. Endocrinology 153: 2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proseus T.E., Boyer J.S. (2006). Calcium pectate chemistry controls growth rate of Chara corallina. J. Exp. Bot. 57: 3989–4002 [DOI] [PubMed] [Google Scholar]
- Proseus T.E., Boyer J.S. (2008). Calcium pectate chemistry causes growth to be stored in Chara corallina: a test of the pectate cycle. Plant Cell Environ. 31: 1147–1155 [DOI] [PubMed] [Google Scholar]
- Proseus T.E., Boyer J.S. (2012). Pectate chemistry links cell expansion to wall deposition in Chara corallina. Plant Signal. Behav. 7: 1490–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E.R., Hotton S., Dumais J. (2011). Chemically mediated mechanical expansion of the pollen tube cell wall. Biophys. J. 101: 1844–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roots R., Okada S. (1975). Estimation of life times and diffusion distances of radicals involved in x-ray-induced DNA strand breaks of killing of mammalian cells. Radiat. Res. 64: 306–320 [PubMed] [Google Scholar]
- Rotman N., Gourgues M., Guitton A.E., Faure J.E., Berger F. (2008). A dialogue between the SIRENE pathway in synergids and the fertilization independent seed pathway in the central cell controls male gamete release during double fertilization in Arabidopsis. Mol. Plant 1: 659–666 [DOI] [PubMed] [Google Scholar]
- Rotman N., Rozier F., Boavida L., Dumas C., Berger F., Faure J.E. (2003). Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr. Biol. 13: 432–436 [DOI] [PubMed] [Google Scholar]
- Rudashevskaya E.L., Ye J., Jensen O.N., Fuglsang A.T., Palmgren M.G. (2012). Phosphosite mapping of P-type plasma membrane H+-ATPase in homologous and heterologous environments. J. Biol. Chem. 287: 4904–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallus T., Jaeckh C., Fehér K., Palma A.S., Liu Y., Simpson J.C., Mackeen M., Stier G., Gibson T.J., Feizi T., Pieler T., Muhle-Goll C. (2008). Malectin: a novel carbohydrate-binding protein of the endoplasmic reticulum and a candidate player in the early steps of protein N-glycosylation. Mol. Biol. Cell 19: 3404–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P. (2001). Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J. 28: 679–688 [DOI] [PubMed] [Google Scholar]
- Srivastava R., Liu J.X., Guo H., Yin Y., Howell S.H. (2009). Regulation and processing of a plant peptide hormone, AtRALF23, in Arabidopsis. Plant J. 59: 930–939 [DOI] [PubMed] [Google Scholar]
- Tsukamoto T., Qin Y., Huang Y., Dunatunga D., Palanivelu R. (2010). A role for LORELEI, a putative glycosylphosphatidylinositol-anchored protein, in Arabidopsis thaliana double fertilization and early seed development. Plant J. 62: 571–588 [DOI] [PubMed] [Google Scholar]
- Winship L.J., Obermeyer G., Geitmann A., Hepler P.K. (2011). Pollen tubes and the physical world. Trends Plant Sci. 16: 353–355 [DOI] [PubMed] [Google Scholar]
- Wolf S., Rausch T., Greiner S. (2009). The N-terminal pro region mediates retention of unprocessed type-I PME in the Golgi apparatus. Plant J. 58: 361–375 [DOI] [PubMed] [Google Scholar]
- Wuest S.E., Vijverberg K., Schmidt A., Weiss M., Gheyselinck J., Lohr M., Wellmer F., Rahnenführer J., von Mering C., Grossniklaus U. (2010). Arabidopsis female gametophyte gene expression map reveals similarities between plant and animal gametes. Curr. Biol. 20: 506–512 [DOI] [PubMed] [Google Scholar]
- Yu F., Li J., Huang Y., Liu L., Li D., Chen L., Luan S. (2014). FERONIA receptor kinase controls seed size in Arabidopsis thaliana. Mol. Plant 7: 920–922 [DOI] [PubMed] [Google Scholar]
- Yu F., et al. (2012). FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc. Natl. Acad. Sci. USA 109: 14693–14698 [DOI] [PMC free article] [PubMed] [Google Scholar]