Protein stability of jasmonate (JA)-related MYC transcription factors is regulated by light quality through the corresponding photoreceptors and by CONSTITUTIVE PHOTOMORPHOGENIC1. Shade reduces MYC protein abundance and increases the levels of their JAZ repressors, therefore inhibiting JA-dependent defenses, which underlies the trade-off between shade avoidance and JA-mediated responses.
Abstract
Reduction of the red/far-red (R/FR) light ratio that occurs in dense canopies promotes plant growth to outcompete neighbors but has a repressive effect on jasmonate (JA)–dependent defenses. The molecular mechanism underlying this trade-off is not well understood. We found that the JA-related transcription factors MYC2, MYC3, and MYC4 are short-lived proteins degraded by the proteasome, and stabilized by JA and light, in Arabidopsis thaliana. Dark and CONSTITUTIVE PHOTOMORPHOGENIC1 destabilize MYC2, MYC3, and MYC4, whereas R and blue (B) lights stabilize them through the activation of the corresponding photoreceptors. Consistently, phytochrome B inactivation by monochromatic FR light or shade (FR-enriched light) destabilizes these three proteins and reduces their stabilization by JA. In contrast to MYCs, simulated shade conditions stabilize seven of their 10 JAZ repressors tested and reduce their degradation by JA. MYC2, MYC3, and MYC4 are required for JA-mediated defenses against the necrotrophic pathogen Botrytis cinerea and for the shade-triggered increased susceptibility, indicating that this negative effect of shade on defense is likely mediated by shade-triggered inactivation of MYC2, MYC3, and MYC4. The opposite regulation of protein stability of MYCs and JAZs by FR-enriched light help explain (on the molecular level) the long-standing observation that canopy shade represses JA-mediated defenses, facilitating reallocation of resources from defense to growth.
INTRODUCTION
The phytohormone jasmonoyl-l-isoleucine (JA-Ile) is an oxylipin that regulates many developmental and stress responses throughout the entire plant’s life cycle (Wasternack, 2007; Balbi and Devoto, 2008; Browse and Howe, 2008; Kazan and Manners, 2008; Bari and Jones, 2009; Browse, 2009; Reinbothe et al., 2009). Synthesis of JA-Ile in response to developmental cues allows plant adaptation to changing environments through a massive transcriptional reprogramming (Reymond et al., 2004; Devoto et al., 2005; Mandaokar et al., 2006; Pauwels et al., 2008).
Several transcription factors (TFs) responsible for activation of different jasmonate (JA)–mediated responses have been identified (Lorenzo et al., 2004; Cheng et al., 2011; Fernández-Calvo et al., 2011; Niu et al., 2011; Pauwels and Goossens, 2011; Qi et al., 2011; Song et al., 2011, 2013; Zhu et al., 2011; Nakata and Ohme-Takagi, 2013; Sasaki-Sekimoto et al., 2013; Fonseca et al., 2014). In basal conditions, activity of these TFs is prevented by JAZ repressors that recruit the general corepressors TOPLESS and TOPLESS-related proteins through interaction with the adaptor protein NINJA (Pauwels et al., 2010) or directly in the case of JAZ8 (Shyu et al., 2012). JAZ repressors are direct targets of the E3-ubiquitin ligase SCFCOI1 (Skp1-Cul1-F-box protein Coronatine-Insensitive1 [COI1]; Xie et al., 1998; Chini et al., 2007; Thines et al., 2007; Yan et al., 2007). Upon elicitation by stress or developmental cues, the biologically active epimer of JA-Ile, (+)-7-iso-JA-Ile, is synthesized by JASMONATE RESISTANT1 (Fonseca et al., 2009a, 2009b; Suza et al., 2010) and perceived by a coreceptor formed by COI1 and its JAZ targets (Xie et al., 1998; Chini et al., 2007; Thines et al., 2007; Katsir et al., 2008; Fonseca et al., 2009a, 2009b; Sheard et al., 2010). JA-Ile triggers binding of JAZ proteins to COI1 and their subsequent ubiquitination and degradation by the 26S proteasome (Chini et al., 2007; Maor et al., 2007; Thines et al., 2007; Yan et al., 2007; Saracco et al., 2009). Degradation of JAZ repressors liberates the TFs from the NINJA/TOPLESS corepressor complex and activates the transcriptional responses mediated by the hormone.
MYC2 was the first known target of JAZ repressors and is a key transcriptional regulator of JA-mediated gene expression that belongs to the bHLH family of transcription factors (Boter et al., 2004; Lorenzo et al., 2004; Chini et al., 2007). MYC2 regulates JA-dependent developmental processes and JA-triggered defenses against insect herbivory in a partially redundant manner with MYC3 and MYC4 (Fernández-Calvo et al., 2011; Schweizer et al., 2013). Phosphorylation- and proteasome-dependent turnover of MYC2 is coupled with its function (Zhai et al., 2013) and protein levels are regulated by JA and circadianly (Shin et al., 2012; Zhai et al., 2013). In addition to the JA pathway, MYC2 is involved in the regulation of other processes such as responses to abscisic acid, ethylene, or blue light (Abe et al., 2003; Yadav et al., 2005; Song et al., 2014). Therefore, MYC2 is currently seen as a node of convergence of several pathways, whose activity needs to be tightly regulated.
Integration of environmental cues through plant signaling pathways is essential for plant adaptation and survival in nature. Among environmental signals, light is probably the most influential factor modulating plant growth and development. Plants use light as a source of energy for photosynthesis and as a signal to coordinate adaptive responses to environmental changes. Thus, plants have developed extremely sensitive photoreceptors to detect variations in light conditions. Phytochromes are red (R) and far-red (FR) light photoreceptors that can be found in two photoreversible forms, Pr and Pfr. The inactive form, Pr, can absorb R light and converts into the active Pfr form, which reverts to the Pr form by absorption of FR quanta (Franklin and Quail, 2010). Phytochrome A (phyA) is photo-labile and degrades after R light absorption. In Arabidopsis thaliana, there are four additional phytochromes, phyB, C, D, and E. Upon activation by R light, phytochromes enter the nucleus and repress the activity of PIF TFs, which are growth-promoting proteins (Franklin and Quail, 2010; Leivar and Quail, 2011). In the dark, PIFs promote skotomorphogenesis (developmental growth in the dark), whereas positive regulators of photomorphogenesis, such as the TFs HY5, HYH, LAF1, and HFR1, are continuously degraded in the nucleus through the action of CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) (Deng et al., 1991; Ballesteros et al., 2001; Duek and Fankhauser, 2003; Huq, 2006; Lorrain et al., 2006). COP1 is a RING-finger E3 ubiquitin ligase that acts in the dark as a repressor of light signaling. Light perception by photoreceptors inhibits COP1 activity (at least in part due to nuclear exclusion), allowing accumulation of positive regulators of photomorphogenesis (von Arnim and Deng, 1994; Yi and Deng, 2005).
Shade from neighboring plants reduces plant access to the light resource. Hence, plants have developed mechanisms to detect and avoid shading. Chlorophylls absorb R light; thus, in dense canopies, light is enriched in FR (Smith, 2000; Franklin, 2008; Ballaré, 2009). The reduction in R/FR ratio is sensed by photoreceptors, mainly phyB, and alert plants of neighbor competitors, triggering a suite of developmental responses known as shade avoidance syndrome (SAS), which include elongation of stems and petioles, hyponasty (upward bending of leaves), and early flowering (Franklin, 2008; Ballaré, 2009; Martínez-García et al., 2010; Pierik and de Wit, 2013).
In spite of the importance of light in plant development, the molecular mechanisms by which light signaling orchestrates hormonal pathways are not fully understood. Extensive crosstalk between light and jasmonate signaling has been described (Moreno et al., 2009; Robson et al., 2010; Ballaré, 2011; Kazan and Manners, 2011; Suzuki et al., 2011). COI1 and other components of the JA pathway are required for some aspects of photomorphogenesis, SAS, and nodulation (Robson et al., 2010; Suzuki et al., 2011). Moreover, both phyA and phyB photoreceptors are required for full JA sensitivity (Moreno et al., 2009; Robson et al., 2010; Kazan and Manners, 2011; Suzuki et al., 2011; Xie et al., 2011; Cerrudo et al., 2012;). Interestingly, a mechanistic link between phyA and JA signaling has already been reported, based on the requirement of phyA for JA-triggered JAZ1 protein degradation (Robson et al., 2010). However, the molecular mechanism by which phyB and SAS regulate JA responses is unknown, although it has been shown to require JAZ10 expression and is independent of SA (Cerrudo et al., 2012).
Here, we analyzed the protein stability of MYC2, MYC3, and MYC4 in Arabidopsis under different light regimes and found that they are short-lived proteins degraded in the dark and stabilized by light and JA. phyB plays a major role in MYCs stability; consistently, phyB inactivation by FR-enriched light (shade; low R/FR ratios) reduces MYC proteins levels and JA-mediated plant defenses. We also found that, in contrast to MYCs, shade stabilizes JAZ repressors and reduces their degradation by JA. This opposite regulation by shade of MYC2, MYC3, and MYC4 TFs and their JAZ repressors explains at the molecular level the shade-triggered repression of JA sensitivity and JA-mediated defenses.
RESULTS
MYC2, MYC3, and MYC4 Are Short-Lived Proteins Degraded by the Proteasome
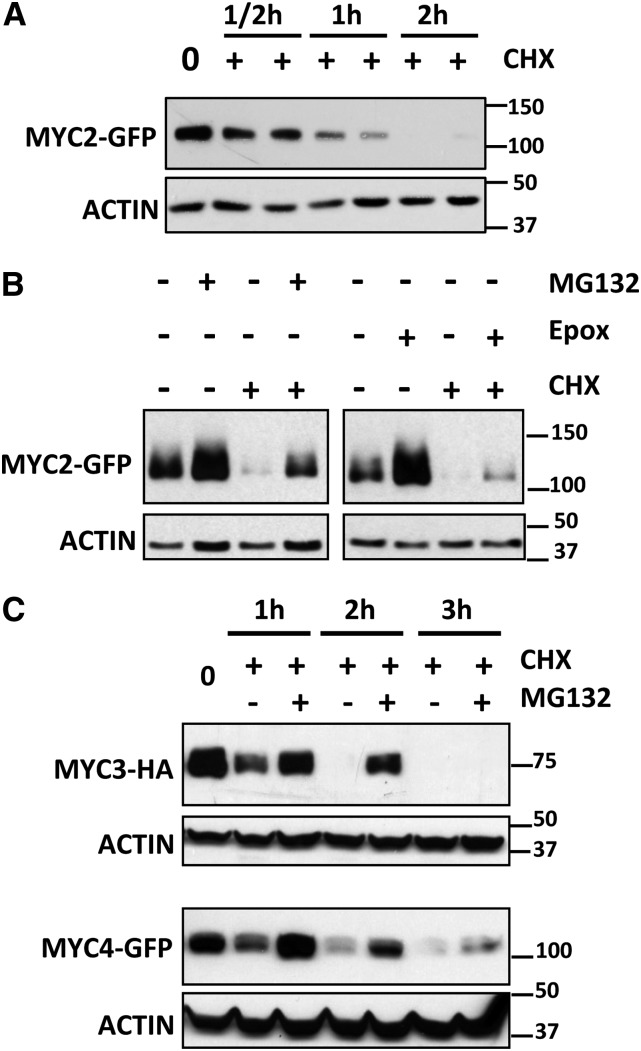
To further explore MYC2 function and regulation, we obtained transgenic Arabidopsis plants constitutively expressing a fully functional MYC2-green fluorescent protein (GFP) fusion protein (Chini et al., 2009). GFP fluorescence in the transgenic lines was low compared with the transgene expression levels mediated by the 35S promoter (Chini et al., 2009), which suggested that protein stability could be regulated. Therefore, we analyzed MYC2 protein stability after inhibition of translation by cycloheximide (CHX) treatment. It is worth noting that MYC2 transcript levels vary during the day (www.genevestigator.com/gv; Shin et al., 2012). Therefore, to avoid variation in protein levels due to transcriptional regulation, we analyzed the levels of MYC2-GFP protein (and MYC3-HA and MYC4-GFP) constitutively expressed from the strong 35S promoter in all our experiments. Levels of MYC2-GFP in the transgenic plants decrease quickly after inhibition of translation (CHX treatment) compared with GFP control, indicating that MYC2 is a short-lived protein (Figure 1A; Supplemental Figure 1A). MYC2-GFP could not be detected after 2 h of CHX treatment. However, pretreatment of seedlings with proteasome inhibitors such as MG132 or epoxomicine increased MYC2-GFP accumulation in basal conditions and delayed degradation upon CHX treatment, suggesting the involvement of the 26S proteasome in the regulation of MYC2 stability (Figure 1B). These results are in line with those recently published by Zhai et al. (2013), showing that MYC2 protein stability is regulated by phosphorylation-coupled proteolysis through the proteasome.
Figure 1.
MYC2, MYC3, and MYC4 Are Short-Lived Proteins Degraded by the Proteasome.
Immunoblot analysis of MYC2-GFP, MYC3-HA, and MYC4-GFP and actin protein levels in 35S:MYC2-GFP, 35S:MYC3-HA, and 35S:MYC4-GFP transgenic plants.
(A) Seven-day-old 35S:MYC2-GFP seedlings were treated with 50 μM CHX and harvested at the indicated times.
(B) Seven-day-old 35S:MYC2-GFP seedlings were incubated overnight with 50 μM MG132, 20 nM epoxomicin (Epox), or mock treated and treated the next morning with 50 μM CHX for 1 h and harvested.
(C) Seven-day-old 35S:MYC3-HA or 35S:MYC4-GFP seedlings were incubated overnight with 50 μM MG132 (or mock) and then treated with 50 μM CHX and harvested at the indicated times
Protein molecular mass (in kilodaltons for all figures) is shown on the right. These experiments were repeated three times with similar results.
Similar analyses of MYC3 and MYC4 proteins using 35S:MYC3-HA and 35S:MYC4-GFP transgenic plants confirmed that both proteins are also short lived and regulated through proteasomal degradation (Figure 1C).
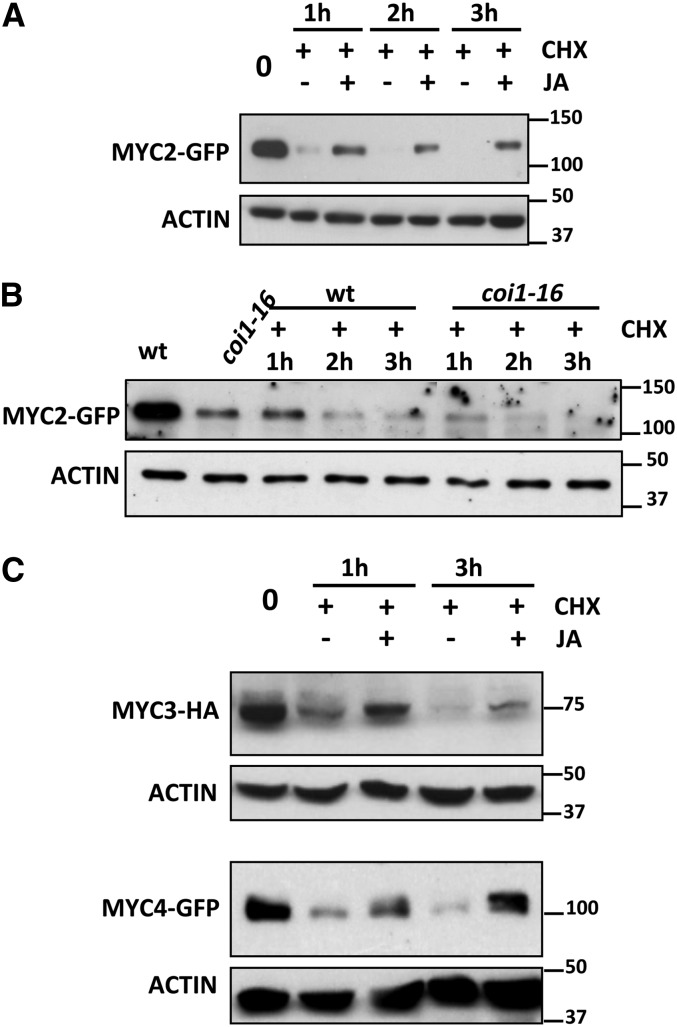
JA Increases MYC2, MYC3, and MYC4 Stability
Regulation of MYC2, MYC3, and MYC4 stability could be an interesting key mechanism of crosstalk between JA and other signaling pathways. To find signals regulating MYC2 stability, we tested the effects of different hormones, such as JA, salicylic acid (SA), gibberellin, abscisic acid, or the ethylene precursor ACC. None of these molecules, except JA, had any significant effect in the degradation rate of MYC2. Treatment with JA, however, prevented the quick decline of the protein and stabilized it (Figure 2A; Supplemental Figure 1B). Consistent with the JA-promoted stabilization, the level of MYC2-GFP protein was lower in JA-insensitive coi1-16 mutants than in the wild-type background, indicating that stability of MYC2-GFP requires hormone perception and a functional JA pathway (Figure 2B).
Figure 2.
JA Stabilizes MYC2, MYC3, and MYC4 Protein Levels.
(A) Immunoblot analysis of MYC2-GFP and actin protein levels in 35S:MYC2-GFP transgenic plants. Seven-day-old seedlings were treated with 50 μM CHX and 50 μM JA or mock (dimethylformamide) and harvested at the indicated times.
(B) Immunoblot analysis of MYC2-GFP and actin protein levels in 35S:MYC2-GFP transgenic plants in wild-type and coi1-16 backgrounds. Seven-day-old seedlings were treated with 50 μM CHX and harvested at the indicated times.
(C) Immunoblot analysis of MYC3-HA, MYC4-GFP, and actin proteins levels in 35S:MYC3-HA and 35S:MYC4-GFP transgenic plants. Seven-day-old seedlings were treated with 50 μM CHX and 50 μM JA or mock (dimethylformamide) and harvested at the indicated times.
Protein molecular mass is shown on the right. These experiments were repeated three times with similar results.
Similar to MYC2, proteasomal degradation of MYC3-HA or MYC4-GFP could be prevented or attenuated by JA treatment (Figure 2C).
Dark and FR Light Reduce MYC2 Protein Levels
In addition to hormone signaling, MYC2 is involved in the regulation of plant responses to blue (B) light (Yadav et al., 2005). Therefore, we analyzed the stability of MYC2 during day/night cycles and the effect of different light wavelengths on MYC2 stability. As shown in Supplemental Figure 2, protein levels varied during the day/night cycle, increasing during the day, with maximum levels around dusk and decreasing during the night, with minimums around dawn. These results suggested that MYC2 might be regulated either by light, the circadian clock, or both. The circadian regulation has been already confirmed by Shin et al. (2012), which showed that MYC2 transcript and protein levels are regulated circadianly by TIME FOR COFFEE.
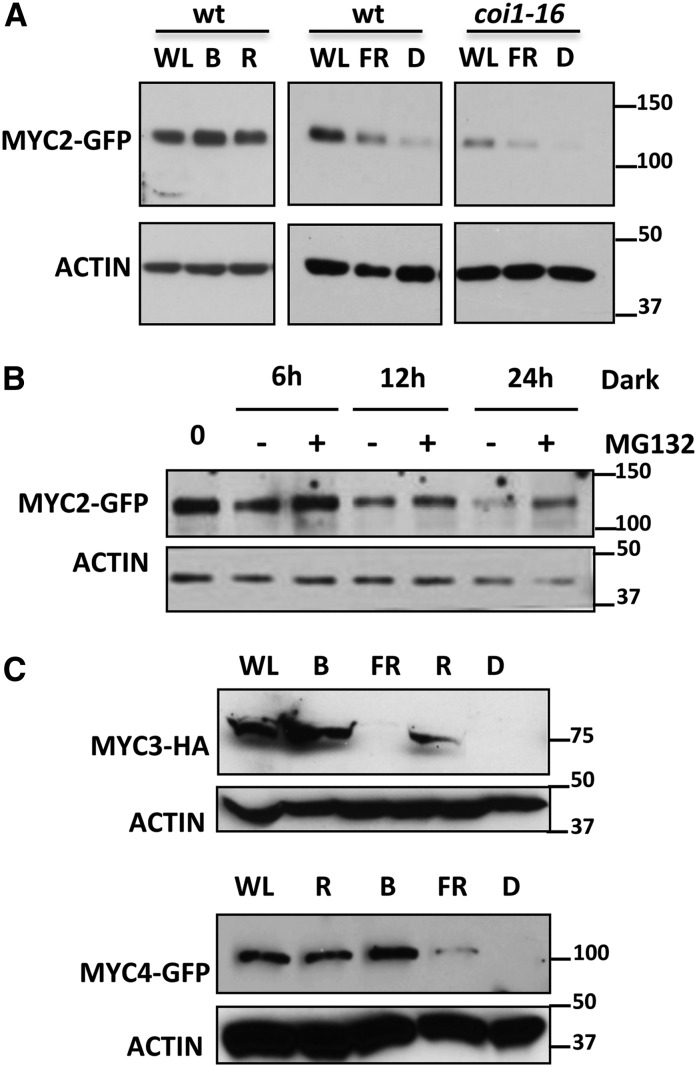
To test if MYC2 stability could be regulated by light quality, we transferred seedlings from white light (WL) to darkness or to different types of monochromatic light and analyzed protein levels after 24 h to avoid circadian variations. As shown in Figure 3A and Supplemental Figure 1C, dark and FR light had a strong effect, reducing the amount of MYC2, in spite of the constitutive (35S) expression of the transgene. In contrast, R and B light maintained MYC2 levels that were similar to those detected under WL. This reduction of MYC2 levels in the dark requires proteasomal activity (Figure 3B) and is independent of COI1, since a similar depletion of MYC2 could be detected in the coi1-16 mutant background (Figure 3A).
Figure 3.
MYC2, MYC3, and MYC4 Are Stabilized under B or R Light and Destabilized in Dark or FR Light.
(A) Immunoblot analysis of MYC2-GFP and actin protein levels in 35S:MYC2-GFP transgenic plants in wild-type and coi1-16 backgrounds. Seedlings were grown in white light/dark cycles (WL/D, 16/8 h) for 4 d and transferred 24 h to WL, B, R, or FR light or to dark (D). Protein molecular mass is shown on the right side.
(B) Immunoblot analysis of MYC2-GFP and actin protein levels in 35S:MYC2-GFP transgenic plants grown in light/dark cycles (WL/D, 16/8 h) for 4 d and transferred to dark for the indicated times in the presence or absence of 50 μM of the proteasome inhibitor MG132.
(C) Immunoblot analysis of MYC3-HA, MYC4-GFP, and actin protein levels in 35S:MYC3-HA or 35S:MYC4-GFP transgenic plants. Seedlings were grown in white light/dark cycles (WL/D, 16/8 h) for 4 d and transferred 24 h to WL, B, R, or FR light or to dark (D).
Protein molecular mass is shown on the right side. These experiments were repeated four times with similar results.
Similar to MYC2, dark and FR light strongly reduced MYC3-HA and MYC4-GFP protein levels, whereas R and B light maintained similar levels of both proteins to those in WL (Figure 3C).
Destabilization of MYC2 by Dark or FR Does Not Require phyA
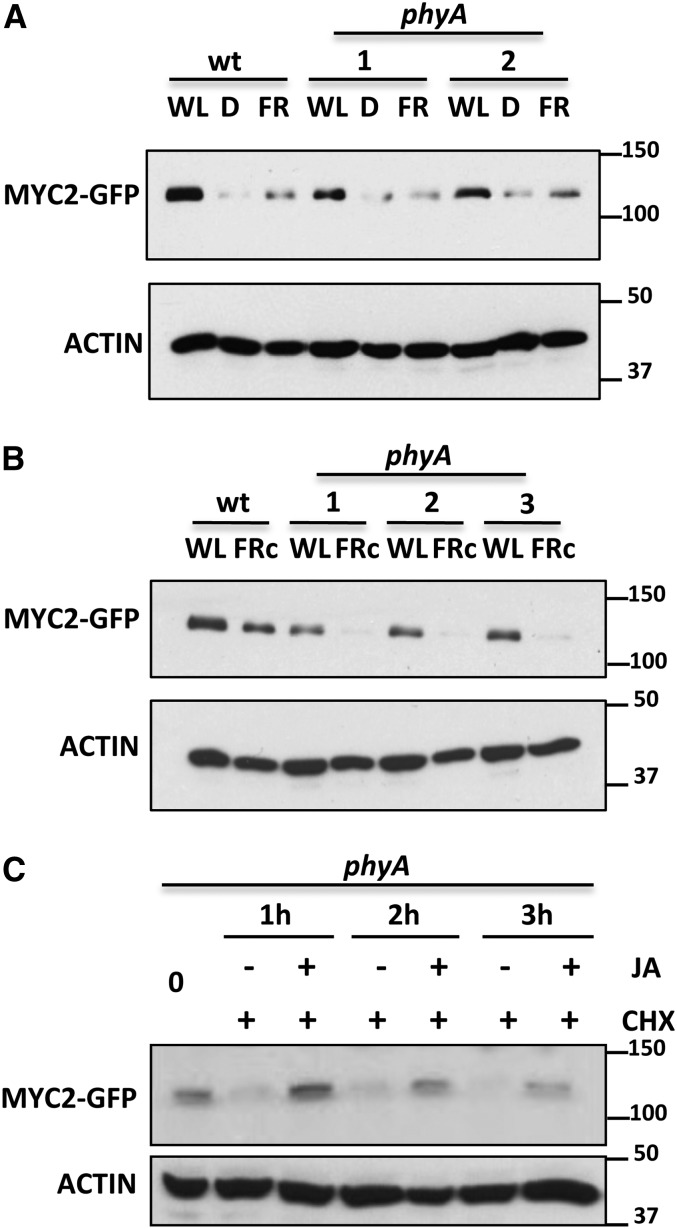
It has been previously reported that JA-triggered JAZ degradation requires phyA (Robson et al., 2010). To test if phyA is also involved in MYC2 destabilization under dark or FR conditions, we introgressed the MYC2-GFP construct into the phyA-211 background by direct crossing and selection of F2 double homozygotes. As shown in Figure 4A and Supplemental Figure 3, in deetiolated seedlings, the levels of MYC2 in the phyA background were similar to those in wild-type seedlings in all conditions tested (WL, dark, and FR and in time-course experiments during day/night cycles), suggesting that phyA is not required for the destabilization effect of dark or FR on MYC2 protein levels in deetiolated seedlings. In fact, MYC2 levels in WL-grown plants were slightly lower in phyA than in wild-type plants (Figures 4A and 4B), suggesting that phyA is required for full stability of MYC2. To test this, we analyzed the levels of MYC2 protein in seedlings germinated and grown in continuous FR (FRc). As shown in Figure 4B, the levels of MYC2 protein were much lower in phyA mutant seedlings grown in FRc than in wild-type plants, indicating a positive effect of phyA on MYC2 stability. Altogether, these results indicate that phyA does not mediate destabilization of MYC2, but rather is required for full MYC2 stability. In other words, since FR activates phyA, the effect of FR reducing MYC2 stability cannot be mediated by increased phyA activation. Therefore, this negative effect of FR on MYC2 levels is likely due to FR-mediated inactivation of other photoreceptors.
Figure 4.
phyA Is Required for MYC2 Stability under Monochromatic FRc.
(A) and (B) Immunoblot analysis of MYC2-GFP and actin protein levels in 35S:MYC2-GFP transgenic plants in wild-type and phyA backgrounds (two independent lines were tested). Seedlings were grown in white light/dark cycles (WL/D, 16/8 h) for 4 d and transferred 24 h to WL, FR, or dark (D) (A) or germinated and grown in FRc (B).
(C) Immunoblot analysis of MYC2-GFP and actin protein levels in 35S:MYC2-GFP transgenic plants in phyA background. Seedlings were treated with 50 μM CHX and with (or without) 50 μM JA for the indicated times
Protein molecular mass is shown on the right side. The experiments were repeated twice with similar results.
We also tested the effect of JA on MYC2 stability in the phyA background and found that, similar to wild-type seedlings, JA treatment reduces MYC2-GFP protein decline in this mutant background, indicating that phyA is also not required for JA-mediated stabilization of MYC2 (Figure 4C).
Light-Activated phyB Is Required for MYC2 Stability
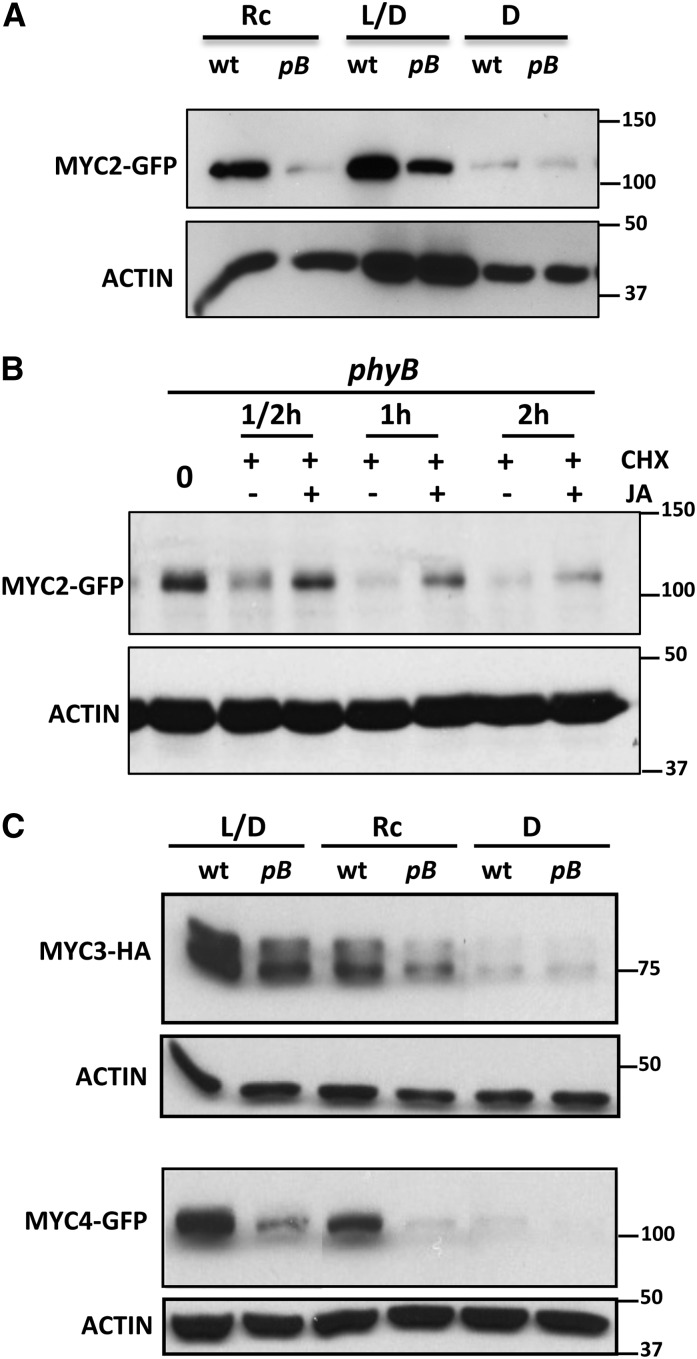
The positive effect of phyA on MYC2 stability suggested that the destabilization of MYC2 by FRc could be mediated by the inactivation of other photoreceptors by this wavelength. To test this hypothesis, we analyzed the effect of phyB mutations on MYC2 stability by introgressing the MYC2-GFP construct into the phyB-9 background. As shown in Figure 5A, the amount of MYC2-GFP in seedlings grown in light/dark cycles (L/D) was higher in the wild type than in phyB-9 background, indicating that phyB is required for MYC2 protein stability. This effect was even more evident in seedlings germinated and grown in continuous red light, a condition in which phyB was absolutely required for MYC2 protein accumulation (Figure 5A). Consistent with the requirement of phyB, the amount of MYC2-GFP was lower in etiolated seedlings (D; Figure 5A) or in the dark (Figure 3A), where phyB is inactive, than in L/D- or continuous red light–grown plants. Consistent with the hypothesis that destabilization of MYC2 by FRc could be mediated by inactivation of phyB (and likely other phytochromes), the effect of FRc on reducing MYC2 levels was lower (2.4-fold) in the phyB background compared with the wild type (13.7-fold; Supplemental Figure 4). Interestingly, however, some reduction of MYC2 levels by FRc was also observed in phyB mutants, suggesting that other phytochromes act redundantly with phyB in regulating MYC2 stability.
Figure 5.
phyB Is Required for MYC2 Stability.
(A) Immunoblot analysis of MYC2-GFP and actin protein levels in 35S:MYC2-GFP transgenic plants in wild-type and phyB backgrounds (pB). Seedlings were grown for 4 d in continuous red light (Rc), white light/dark cycles (L/D, 16/8 h), or in the dark (D) and harvested.
(B) JA stabilizes MYC2 in the phyB background. Immunoblot analysis of MYC2-GFP and actin protein levels in 35S:MYC2-GFP transgenic plants in phyB background. Seven-day-old seedlings were treated with 50 μM CHX and 50 μM JA or mock (dimethylformamide) and harvested at the indicated times.
(C) Immunoblot analysis of MYC3-HA, MYC4-GFP, and actin protein levels in 35S:MYC3-HA and 35S:MYC4-GFP transgenic plants in wild-type and phyB backgrounds (pB). Seedlings were grown for 4 d in continuous red light (Rc), white light/dark cycles (L/D, 16/8 h), or in the dark (D) and harvested.
Protein molecular mass is shown on the right side. These experiments were repeated three times with similar results.
To further analyze the effect of the phyB mutation on MYC2 stability, we checked MYC2 levels throughout a complete day/night cycle and compared them with levels in the wild-type background. As shown in Supplemental Figure 3, levels of MYC2 were lower in phyB-9 than in the wild-type background in all time points analyzed.
These results further indicate that phyB is required for MYC2 accumulation. Interestingly, JA treatment can also delay MYC2 degradation by the proteasome in the phyb background (Figure 5B), indicating that the effect of JA is independent of phyB.
Similar experiments using 35S:MYC3-HA and 35S:MYC4-GFP constructs introgressed into the phyB-9 background gave essentially similar results to those of MYC2 (Figure 5C) and demonstrated that phyB is also essential for MYC3 and MYC4 stability.
Cryptochromes Are Required for B Light Stabilization of MYC2
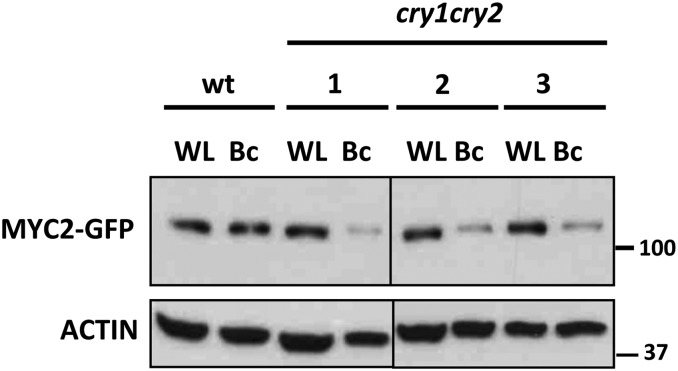
Since MYC2, MYC3, and MYC4 accumulate in monochromatic B light (Figure 3), we also tested the contribution of cryptochromes to the stability of MYC2 by introgressing the 35S:MYC2-GFP construct into the cryptochrome-deficient cry1 cry2 double mutant background and selecting F2 triple homozygotes. In WL, no differences in MYC2-GFP levels were observed between the wild-type and the cry1 cry2 backgrounds (Figure 6). However, under monochromatic B light, MYC2-GFP accumulation was compromised in the double cry mutant. These results suggest that B light stabilization of MYC2 (and likely MYC3 and MYC4) requires CRY1 and CRY2. However, in WL, the R component of light is sufficient to stabilize MYC2 even in the absence of these B light photoreceptors.
Figure 6.
Cryptochromes CRY1 and CRY2 Are Required for B Light–Mediated Stability of MYC2.
Immunoblot analysis of MYC2-GFP and actin protein levels in 35S:MYC2-GFP transgenic plants in wild-type and cry1 cry2 double mutant backgrounds (three independent transgenic lines are shown). Seedlings were grown for 4 d in white light/dark cycles (16/8 h; WL) or in monochromatic B light (Bc) and harvested. These experiments were repeated twice with similar results.
COP1 Is Required for MYC2 Degradation
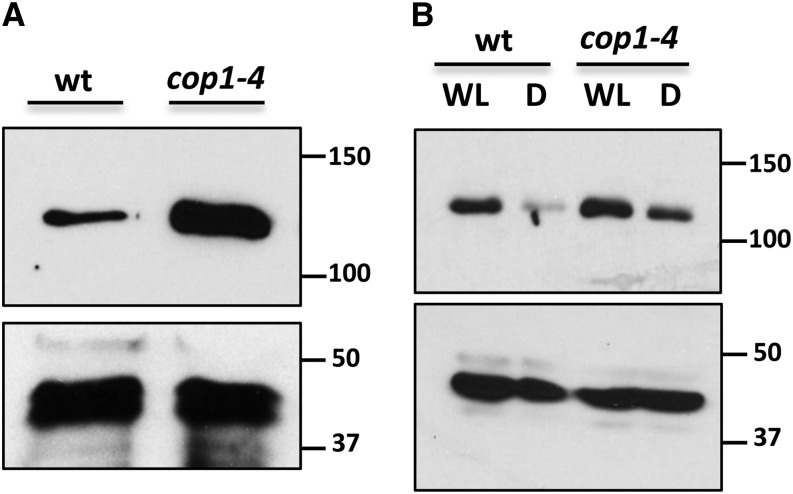
The negative effect of dark on MYC2 protein levels and its stabilization by R and B light resemble the behavior of positive regulators of photomorphogenesis, such as HY5 or HYH, which are direct targets of the E3 ubiquitin ligase COP1 (Holm et al., 2002). To check if the reduction of MYC2 levels in the dark could depend on COP1, we introgressed the MYC2-GFP construct into the cop1-4 background by crossing and selection of F2 double homozygotes. As shown in Figure 7A, in etiolated seedlings, the amount of MYC2-GFP in the cop1-4 background was much higher than in the wild-type background, indicating that COP1 is required for the degradation of MYC2 in the dark. Moreover, in deetiolated seedlings, the reduction of MYC2 levels after transferring the wild-type seedlings to dark is highly attenuated in the cop1-4 background (Figure 7B).
Figure 7.
COP1 Negatively Regulates MYC2 Accumulation.
Immunoblot analysis of MYC2-GFP and actin protein levels in 35S:MYC2-GFP transgenic plants in wild-type and cop1-4 backgrounds. Four-day-old etiolated seedlings (A) and seedlings grown in white light/dark cycles (16/8 h) for 6 d (WL) (B) were transferred 24 h to WL or to darkness (D). Protein molecular mass is shown on the right side. These experiments were repeated three times with similar results.
To further analyze the effect of the cop1 mutation on MYC2 stability, we checked MYC2 levels throughout a complete day/night cycle and compared them with levels in the wild-type background. As shown in Supplemental Figure 3, levels of MYC2 were lower in the wild type than in the cop1-4 background in all time points analyzed.
To test if MYC2 could be a direct target of COP1, we checked their interaction by yeast two-hybrid and pull-down experiments. None of these assays revealed a consistent and specific interaction, suggesting that the effect of COP1 on MYC2 stability is indirect.
JA-Dependent Defenses Repressed by Low R/FR Ratios Are Regulated by MYC2, MYC3, and MYC4
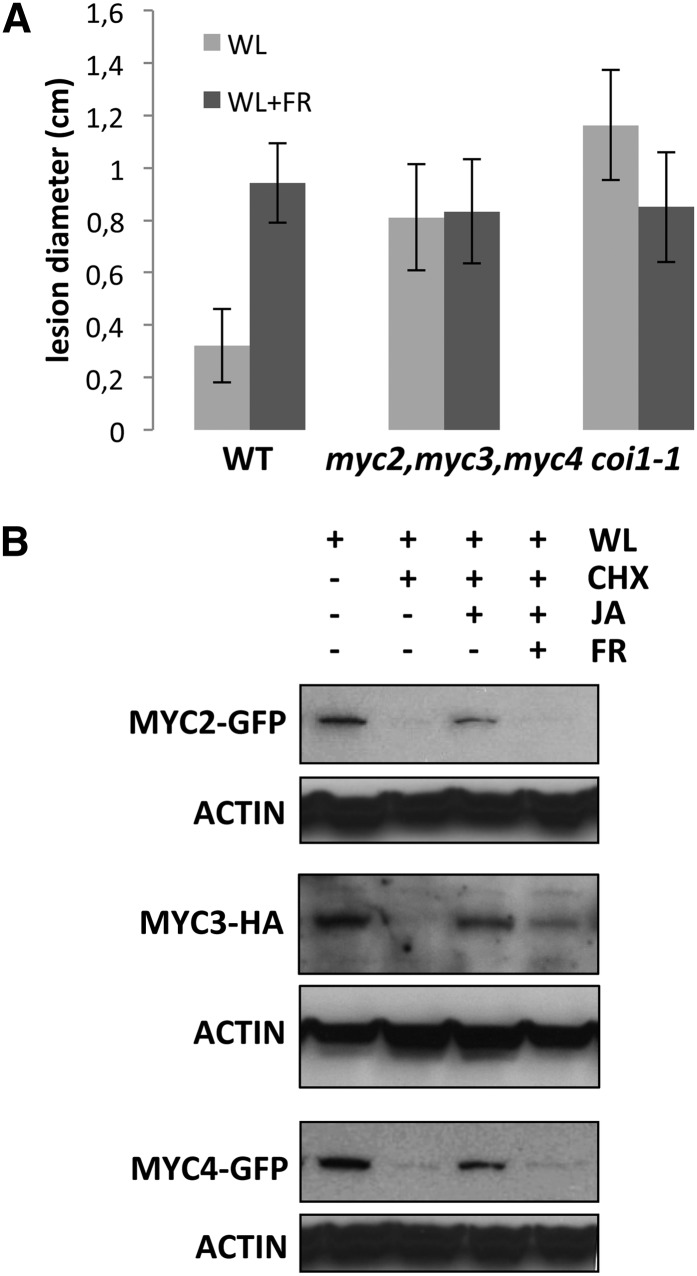
It has been shown that phyB inactivation by low R/FR ratios (shade or FR-enriched light) negatively affects JA-dependent defenses against insects and necrotrophic fungus (i.e., Botrytis cinerea; Moreno et al., 2009; Cerrudo et al., 2012; de Wit et al., 2013). We have previously reported that MYC2, MYC3, and MYC4 activate JA-dependent defenses against insect herbivory (Fernández-Calvo et al., 2011). To test if these three TFs are also responsible for the activation of JA-dependent (low R/FR-inhibited) defenses against B. cinerea, we analyzed disease progression in wild-type and triple myc2 myc3 myc4 and coi1 mutants in WL or simulated shade conditions. Consistent with previous reports (Cerrudo et al., 2012; de Wit et al., 2013), wild-type plants were significantly more susceptible in simulated shade than in WL conditions. Supplemented FR light was ineffective on coi1 mutants (Figure 8A), indicating that this effect is dependent on JA pathway–mediated defenses. Remarkably, simulated shade was also ineffective at increasing susceptibility in the triple myc2 myc3 myc4 mutant (Figure 8A). These results indicate that MYC2, MYC3, and MYC4 mediate JA-mediated defenses against B. cinerea and suggest that the shade-triggered susceptibility is likely achieved by FR-mediated inactivation of these TFs.
Figure 8.
MYC2, MYC3, and MYC4 Mediate JA Defenses against B. cinerea and Are Destabilized in Simulated Shade (FR-Enriched WL).
(A) Disease symptoms (lesion diameter) of 4-week-old wild-type plants, coi1-1 mutants, and myc2 myc3 myc4 triple mutants 3 d after inoculation of the leaves with 106 spores/mL of B. cinerea under WL or FR-enriched WL (WL+FR). Data values represent the mean of three independent experiments with similar results. Error bars represent sd.
(B) Immunoblot analysis of MYC2-GFP, MYC3-HA, MYC4-GFP, and actin protein levels in 35S:MYC2-GFP, 35S:MYC3-HA, and 35S:MYC4-GFP transgenic plants. Seedlings grown in white light/dark cycles (16/8 h) were exposed to WL or FR-enriched white light (FR) for 4 h and then treated with 50 μM CHX and/or 50 μM JA and harvested after 60 min. These experiments were repeated three times with similar results.
Low R/FR Ratios Reduce MYC Protein Levels and the Stabilizing Effect of JA
The reduction of MYC2, MYC3, and MYC4 protein levels in FRc and the requirement of these TFs for resistance against B. cinerea prompted us to test if the effect of low R/FR ratios on JA-dependent defenses could be mediated by regulation of MYC2, MYC3, and MYC4 proteins stability by shade. We analyzed MYC proteins levels in seedlings grown on L/D cycles and transferred to WL or WL supplemented with FR for 4 h. As shown in Supplemental Figure 5, the amount of MYC2 and MYC4 in FR-supplemented seedlings was lower than in the WL-grown plants in all time points tested. In the case of MYC3, protein levels in these conditions (FR-supplemented) recovered at 60 min, suggesting a slightly different regulation of MYC3 compared with MYC2 and MYC4. More importantly, stabilization of all three proteins by JA treatment was strongly impaired under FR-supplemented conditions (Figure 8B; Supplemental Figure 5). These results indicated that FR-enriched radiation quickly reduces MYC2 and MYC4 stability and partially prevents the stabilization effect of JA on all three proteins.
Simulated Shade Increases the Abundance of JAZ Repressors
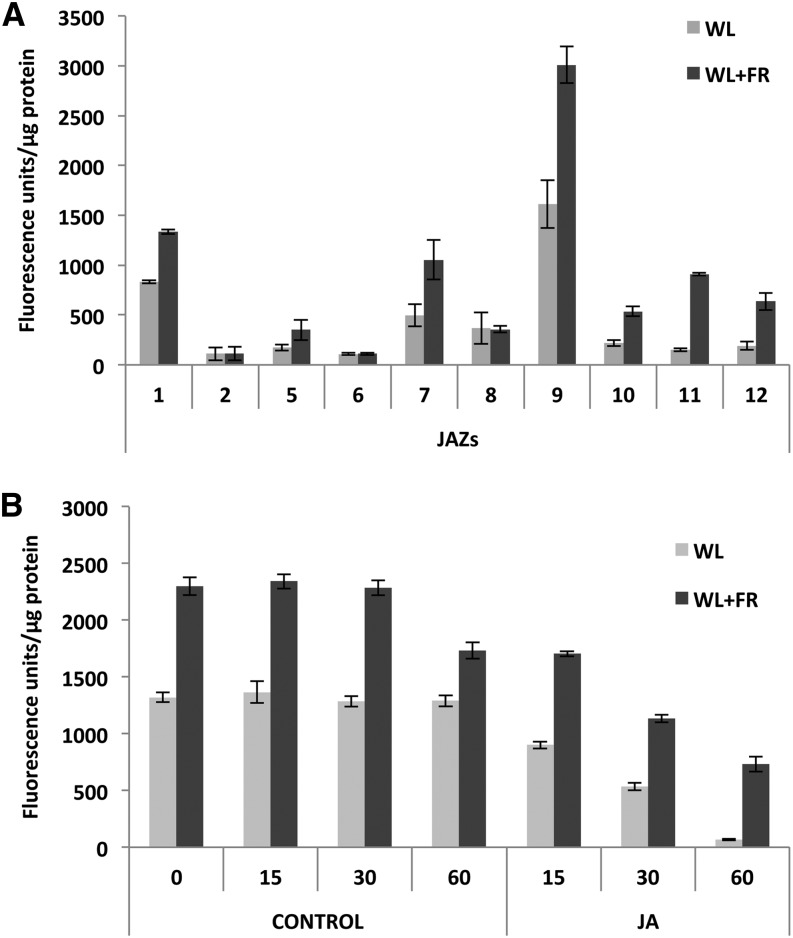
Previous work has suggested a relationship between light quality and JAZ1 protein stability, since wounding or JA-triggered degradation of JAZ1 requires a functional phyA photoreceptor (Robson et al., 2010). Therefore, we next analyzed the effect of simulated shade (FR-enriched WL) on JAZ protein levels. To avoid transcription-dependent variations, we generated independent transgenic plants constitutively expressing 10 of the 12 JAZ genes under the 35S cauliflower mosaic virus promoter. As shown in Figure 9A, the protein levels of 7 of the 10 JAZ tested were significantly increased under simulated shade conditions (WL+FR) compared with WL. Only JAZ2, JAZ6, and JAZ8 levels were unchanged, similar to the 35S:β-glucuronidase (GUS) control (Supplemental Figure 6). Moreover, time-course degradation experiments using the 35S:JAZ1-GUS line showed that although JA promotes degradation of JAZ1 under FR-enriched light, the levels of the protein are much higher in all time points tested, compared with those in WL. Therefore, these results suggest that simulated shade conditions (FR-enriched WL; low R/FR ratios) stabilize JAZ proteins and reduce JA-triggered degradation, thus contributing to reduce JA sensitivity. We cannot exclude the possibility that shade increases JAZ translation.
Figure 9.
JAZ Proteins Are Stabilized in Simulated Shade (FR-Enriched WL).
(A) Quantification of GUS activity of JAZ-GUS fusion proteins in 35S:JAZ-GUS transgenic plants. Seedlings grown in white light/dark cycles (16/8 h) were exposed to WL or FR-enriched white light (WL+FR) for 4 h. Error bars represent sd.
(B) Time-course quantification of GUS activity after JA-triggered degradation of JAZ1-GUS fusion proteins in 35S:JAZ1-GUS transgenic plants. Error bars represent sd.
These experiments were repeated four times with similar results.
DISCUSSION
Integration of external cues with basal developmental programs depends on phytohormone networks and is essential for plants survival in nature. Light is probably the most influential external factor for plant growth and development. However, the molecular mechanisms by which plants orchestrate the integration between light signals and phytohormone networks are not well understood.
The reduction in R/FR ratios that occurs in dense canopies due to the absorption of R light by chlorophylls and reflection of FR light alerts the plants to the presence of neighboring competitors. In fully deetiolated plants, this reduction in R/FR ratio inactivates phyB (Smith, 1995; Ballaré, 1999; Franklin, 2008), which reduces plant defenses and promotes growth, thus balancing the use of resources toward growth to outcompete neighbors and reach the light resource (Moreno et al., 2009; Ballaré, 2011). Inactivation of phyB by low R/FR ratios promotes a reduction in the plant sensitivity to JA and JA-dependent defense gene expression, lowering plant resistance to insect herbivory and necrotrophic pathogens (Moreno et al., 2009; Cerrudo et al., 2012; de Wit et al., 2013). To date, three partially redundant TFs, MYC2, MYC3, and MYC4, targets of JAZs repressors, have been shown to be involved in the activation of JA-mediated defense responses to herbivory (Fernández-Calvo et al., 2011). In this work, we found that these three MYC TFs are also required for JA-mediated defenses against the necrotrophic pathogen B. cinerea and discovered a mechanism of regulation of these TFs and their JAZ repressors by light quality. Reduction of MYC2, MYC3, and MYC4 protein stability under conditions that inactivate phyB and enhancement of JAZs protein levels may help explain, at the molecular level, the negative effect of low R/FR ratios on the reduction of plant sensitivity to JA and the increased susceptibility to insects and necrotrophic pathogens.
To avoid bias by transcriptional regulation, we analyzed protein levels in constitutive, 35S promoter–driven expression transgenic genotypes in all experiments. The results show that MYC2, MYC3, and MYC4 are short-lived proteins degraded by the proteasome in response to light quality. MYCs can be stabilized by R or B light, whereas darkness or FR radiation promotes their degradation. Consistently, R light–absorbing phytochromes phyA and phyB as well as B light–absorbing cryptochromes CRY1 and CRY2 are required for MYC protein stability. The effect of phyB, however, is much stronger than that of phyA, which correlates with the fact that phyA is a photolabile receptor whose accumulation in deetiolated seedling is very low (Sharrock and Clack, 2002). Therefore, the negative effect of FR (and darkness) on MYCs protein accumulation in deetiolated seedlings can be explained by the negative effect of this radiation (and darkness) on phyB activity.
However, FR light also has a phyA-dependent positive effect on MYC2 stability. This is particularly clear when plants are germinated and grown in FRc, which activates phyA (Figure 4B). In these conditions, MYC2 protein levels in wild-type seedlings are much higher than in phyA mutants, indicating that FR light has a dual effect on MYC2 (and presumably on MYC3 and MYC4) levels, one stabilizing the protein through the action of phyA and another one destabilizing it through the inactivation of phyB. Therefore, FR light finely tunes MYC protein levels depending on the relative amounts of photoreceptors. In fully deetiolated seedlings, where phyA levels are very low, the main effect of FR will be destabilization of MYC2, MYC3, and MYC4 through inactivation of the major photoreceptor phyB. However, this negative effect of FR on MYC2, MYC3, and MYC4 levels could be reduced in conditions in which phyA accumulates (etiolated or partially deetiolated plants). In such conditions, the positive effect of phyA on MYC2 stability seems to be coordinated with destabilization of its repressors, since it has been shown that phyA is required for JAZ1 degradation (Robson et al., 2010). Thus, phyA appears to play an important role in the activation of JA responses, as it is required for stabilization of MYC TFs and destabilization of their repressors.
Stabilization of MYC2, MYC3, and MYC4 by different light qualities and destabilization in the dark is reminiscent of the behavior of COP1 targets such as HY5 or HYH (Holm et al., 2002). Consistently, MYC2 levels are constitutively high in etiolated cop1-4 mutants, suggesting that MYC2 may be a target of COP1. Protein interaction assays in vitro (yeast two-hybrid and pull-down assays), however, failed to show a reproducible direct interaction between COP1 and MYC2, MYC3, or MYC4. These results suggest that the regulation may be indirect or that interaction between COP1 and MYCs may require additional factors. This is the case of the interaction between COP1 and GI, which requires EARLY FLOWERING3 (Yu et al., 2008). Moreover, members of the SPA protein family (SPA1-SPA4), which are DWD proteins like COP1, can physically interact with COP1 and, together, they constitute functional E3-ubiquitin ligase complexes (Zhu et al., 2008; Chen et al., 2010). MYC2 has been functionally related to SPA1; both proteins act redundantly or synergistically in the regulation of photomorphogenic responses, which opens the possibility that SPA proteins might be required for interaction between COP1 and MYC2 in vivo (Gangappa et al., 2010). Therefore, elucidation of the mechanism of MYCs regulation by COP1 may be complex and will require further research.
The requirement of R light–absorbing photoreceptors phyA and phyB and B light photoreceptors Cry1 and Cry2 for MYC protein stability is consistent with the hypothesis that MYCs may be targets of COP1. Light (R among other wavelengths) perceived by photoreceptors promotes the exclusion of COP1 from the nucleus (von Arnim and Deng, 1994). Therefore, the negative effect of phytochromes on COP1 is consistent with stabilization of MYCs by R light and destabilization by FR. Similarly, stabilization of MYC2 by B light could be explained by the negative effect of cryptochromes (Cry1 and Cry2) on COP1 activity (Wang et al., 2001).
Thus, in the dark, COP1 would actively trigger MYC2, MYC3, and MYC4 degradation. Exposure to R or B light would activate phyB or the cryptochromes, respectively, and inactivate COP1, thus allowing MYCs accumulation. FR light, however, has a dual effect on MYC stability that depends on its effect on phyA or phyB. In etiolated seedlings, exposure to FR light would activate phyA and therefore inactivate COP1, thus explaining the positive effect of FRc on MYC2 stability in situations where phyA accumulates. In contrast, inactivation of phyB by FR light (or by low R/FR ratios) would activate COP1 by shifting the nuclear-cytoplasmic equilibrium of COP1 toward the nucleus (Pacín et al., 2013), therefore increasing MYC2, MYC3, and MYC4 degradation. Therefore, the ultimate effect of FR on MYCs stability will depend on the balance of activation/ deactivation of phyA and phyB.
In this context, in FR-enriched light conditions (shade; low R/FR ratios), the main effect of FR light on fully deetiolated plants, in which the levels of the photo-labile phyA is very low, is the inactivation of phyB, and, therefore, the destabilization of MYC2, MYC3, and MYC4 (Figure 10). Moreover, in these shade conditions, reduction of MYC levels is paralleled by the stabilization of their JAZ repressors, thus contributing to reduce JA sensitivity and JA-mediated defenses (Figure 10).
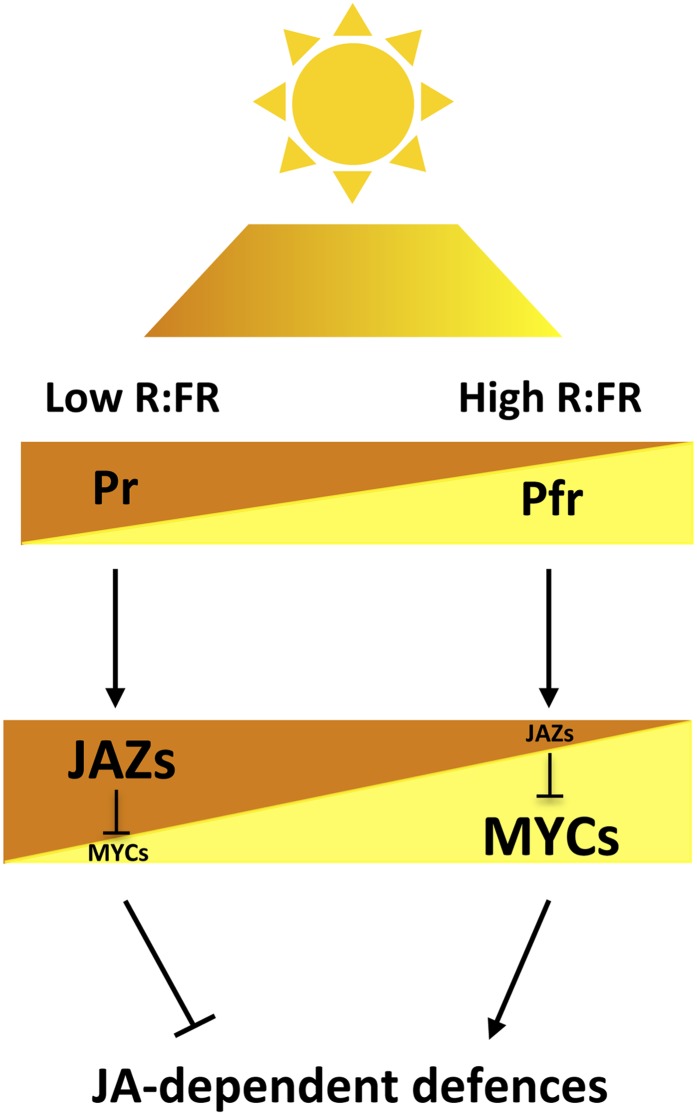
Figure 10.
Schematic Model of Regulation of JA-Mediated Defenses by Light Quality, through the Modulation of MYC and JAZ Stability.
R/FR ratios determine the balance of activation/deactivation (Pfr/Pr) of phytochromes, which differentially regulate the stability of MYC TFs and their JAZ repressors and, therefore, the defense output of the plant. Ambient light (high R/FR ratios; represented in yellow/light gray) shifts the Pr/Pfr equilibrium to the active Pfr form, which enhance JA-dependent defenses by mediating MYCs stabilization and allowing JA-mediated degradation of their JAZ repressors. Conversely, in shade conditions (low R/FR ratios; represented in brown/dark gray), the phytochrome equilibrium is shifted toward the inactive Pr form, thus reducing JA-dependent defenses by destabilizing MYCs and stabilizing JAZs. Thus, the balance of active phytochromes, which depends on the R/FR ratio, regulates the relative amount of MYCs and JAZ proteins and therefore defines the JA-dependent defense output of the plant. Brown (dark) and yellow (light) triangles represent the relative amount of the corresponding proteins (Pr and Pfr forms of phytochromes, as well as JAZ and MYC proteins) in each condition (shade, brown or dark gray; light, yellow or light gray)
[See online article for color version of this figure.]
Consistent with these results, the repressive effect of low R/FR on JA-dependent defenses against B. cinerea has been recently shown to be independent of SA (Cerrudo et al., 2012), suggesting that it is a direct effect of phytochromes on components of the JA signaling pathway, and not the consequence of a negative crosstalk between JA and other hormonal pathways (at least salicylic acid). Moreover, this effect requires JAZ10 expression (Cerrudo et al., 2012). This is fully consistent with our results, since we have previously demonstrated that JAZ10 is a transcriptional target of MYC2, MYC3, and MYC4 (Chini et al., 2007).
Daily MYC2 accumulation pattern suggest that JA-mediated defenses should be stronger at the middle of the day, when MYC2 levels are higher. This is exactly the case in the only two examples reported so far and regarding JA-dependent insect resistance against cabbage looper (Trichoplusia ni) and JA-dependent susceptibility to Pseudomonas syringae (Goodspeed et al., 2012; Shin et al., 2012). In both cases, the JA-dependent response is maximal at mid-day, coincident with maximal MYC2 levels (Shin et al., 2012; this work). Interestingly, insect feeding behavior of cabbage looper has been shown to be circadianly rhythmic, and Arabidopsis plants synchronize JA-mediated defense with insect circadian behavior (Goodspeed et al., 2012). Therefore, light- and circadian-regulated in-phase accumulation of MYC2 explains molecularly this maximal activation of JA-dependent defenses and provides a circadian clock–mediated selective advantage to plants through anticipation of an enhanced defense against herbivory (Goodspeed et al., 2012).
Reduction of defenses by low R/FR ratios represents a trade-off of balancing resources toward growth. However, plants cannot become completely defenseless because this would be counteradaptative; therefore, this balance has to be finely tuned. We have found that MYC2, MYC3, and MYC4 can also be stabilized by JA treatment. JA biosynthesis is induced when plants are challenged by environmental stresses, such as pathogens or insects attack (Wasternack, 2007). Therefore, stabilization of MYCs by JA provides a mechanism of fine-tuning that allows the plant to partially restore defenses when needed. In spite of this, however, stabilization of MYC2, MYC3, and MYC4 by JA under low R/FR ratios or in phyB mutant background does not reach the same levels as in WL conditions, explaining why defenses in canopy shade conditions are not as effective as in WL. In line with this, JAZ repressors are stabilized under low R/FR ratios and the protein levels are higher even after JA treatment, which adds to the destabilization of MYCs to reduce JA sensitivity and the activation of JA-dependent defenses. Thus, in summary, when plants detect a threat to their ability to reach the light, they dedicate their resources to growth. This modification of the balance between defense and growth represents a trade-off, and there is a reduction of defenses that involves the degradation of key components of the JA defense pathway, MYC2, MYC3, and MYC4, and the stabilization of their JAZ repressors (Figure 10). If the plant is attacked by pathogens or insects, the synthesis of JA allows partial restoration of defenses (through the partial stabilization of MYCs and reduced degradation of JAZs), so that plant can still dedicate resources to growth and reach light without being completely defenseless.
An alternative mechanism for balancing defense and growth has been already proposed based on the interaction between JAZ and DELLA proteins (Hou et al., 2010; Yang et al., 2012). DELLAs are repressors of the growth-promoting PIF transcription factors (de Lucas et al., 2008; Feng et al., 2008). Thus, gibberellin-triggered degradation of DELLAs (Silverstone et al., 2001; Davière et al., 2008) prioritizes growth over defense by liberating PIFs and JAZs. JAZ, in turn, will enhance repression of MYCs and therefore diminish JA-mediated defenses. In contrast, situations that increase DELLA levels, such as gibberellin depletion, would reduce free JAZ protein levels (through interaction with DELLAs), thus liberating MYCs and favoring defenses (Hou et al., 2010). Similarly, JA-promoted depletion of JAZ would release DELLAs from the JAZ-DELLA complexes and MYCs from the JAZ-MYC complexes, thus repressing PIFs and prioritizing defense over growth (Yang et al., 2012). Our results are fully compatible with this balancing model and add a regulatory layer to integrate light regulation. Phytochrome activation by light promotes destabilization of PIFs (Lau and Deng, 2010; Chen and Chory, 2011; Leivar and Quail, 2011) and stabilization of MYC2, MYC3, and MYC4 (this work), thus prioritizing defense over growth. In these conditions (light), reduction of PIF protein levels by light would release DELLA proteins that will compete with MYCs for interaction with JAZ, therefore reducing the amount of JAZ repressors available and further potentiating MYC-dependent defenses. In contrast, in the dark, PIFs are stabilized, whereas MYCs are destabilized, thus prioritizing growth over defense. In this situation (dark), reduction of MYC levels would release JAZ repressors that could now interact with DELLA proteins, further potentiating PIF activity.
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana Columbia-0 ecotype is the genetic background of the wild type, mutants, and transgenic lines used throughout the work. Seeds were surface-sterilized with a vapor phase of chlorine gas for 3 h in a bell jar. After sterilization, seeds were vernalized at 4°C for 3 d in the dark. Seedlings were grown in 0.5× Murashige and Skoog (MS) medium with 1% sucrose and 0.6% agar at 21°C under a 16-h-light/8-h-dark cycle in a growth chamber.
The 35S:MYC2-GFP transgene previously reported (Chini et al., 2009) was introgressed by direct crossing into coi1-16, cop1-4, phyB-9, and phyA-211 mutant backgrounds. Similarly, 35S:MYC3-HA and 35S:MYC4-GFP transgenes (Fernández-Calvo et al., 2011) were also introgressed into phyB-9 mutant background. F2 segregating progenies were grown in 40 μM hygromycin plates to select for the different constructs. Mutant phenotypes were selected by growing the seedlings on 50 μM JA plates for 8 d (for coi1-16) or for 4 d in the dark (cop1-4), or monochromatic R (32 μmoles m−2 s−1; phyB-9) or FR light (46 μmoles m−2 s−1; phyA-211), using a plant growth chamber from Percival Scientific. Homozygosity of the constructs was determined in the F3 lines, and F3 seedlings were used for the experiments.
To generate 35S:JAZ-GUS transgenic Arabidopsis lines, the full-length JAZ coding sequences were amplified with Expand High Fidelity polymerase (Roche) using Gateway-compatible primers (see Supplemental Table 1 online) and cloned into pDONR207 using the Gateway system (Invitrogen) as previously described (Chini et al., 2009). The JAZ coding sequences were transferred into destination vector pMDC140 (Curtis and Grossniklaus, 2003), sequence verified, and transformed into Agrobacterium tumefaciens strain GV3101. Columbia-0 plants were transformed by floral dipping (Clough and Bent, 1998), and several transgenic lines were selected for hygromycin resistance. Only homozygous lines carrying one insertion were used for further analysis.
To test daily variation of MYC2 protein levels, 35S:MYC2-GFP transgenic seedlings were grown under 16-h-light/8-h-dark cycles for 6 d. Samples were harvested 3, 6, 9, 12, 15, 18, 21, and 24 h, respectively, after dawn (growth chamber illumination). The first six samples were harvested during the day, and the last three samples were harvested in the dark.
Chemical Treatments
Arabidopsis seedlings were germinated and grown on filter paper in MS plates. Seven-day-old seedlings were transferred on the filter paper to new empty plates and immediately treated with liquid MS media containing 50 μM CHX, 50 μM MG132, 20 nM epoxomicin, or 50 μM JA and harvested at indicated times. CHX was dissolved in 100% ethanol, MG132, and epoxomicin in dimethyl sulfoxide and JA in dimethylformamide. The chemicals were provided by Sigma-Aldrich.
Light Treatments
Seedlings were grown at 21°C and exposed to continuous monochromatic B (470 nm; 19 μmoles m−2 s−1), R (670 nm; 32 μmoles m−2 s−1), or FR light (730 nm; 46 μmoles m−2 s−1) in a growth chamber equipped with light emitting diodes from Percival Scientific (model E-30-LED).
WL- or FR-enriched treatments were performed as described by González-Grandío et al. (2013). Briefly, seedlings were grown in a growth chamber under WL/dark cycles (16/8 h) and exposed for 4 h to white light (100 μmoles m−2 s−1 PAR; 11.71 R/FR ratio) or FR-enriched WL (FR light emitting diodes; 100 μmoles m−2 s−1 PAR; 0.20 R/FR ratio).
Protein Gel Blotting Analysis
Eight to ten seedlings were harvested per sample, frozen in liquid nitrogen, and homogenized in 200 μL of 2× Laemmli SDS-PAGE protein loading buffer. The extracts were boiled at 95°C for 5 min and kept in ice. After centrifugation (13,000 rpm at room temperature) the supernatant was collected. A 20-μL volume of each sample was run into SDS-PAGE gel, transferred to nitrocellulose membrane (Bio-Rad), and incubated with anti-GFP (Milteny Biotec; dilution 1:1000) or anti-HA-horseradish peroxidase (Roche; dilution 1:1000) and monoclonal anti-ACTIN (produced in mouse, Sigma-Aldrich; dilution 1:2000) antibodies. Blots were developed using ECL (Pierce).
Botrytis cinerea Infection Analyses
For in planta analyses, seeds were sown on soil, vernalized for 5 d at 4°C, and grown in a chamber at 22°C and 70% relative humidity under a 16-h-light/8-h-dark photoperiod at 250 μE m−2 s−1 fluorescent illumination. Plants were treated and examined 4 weeks after seed germination. The fungal pathogen B. cinerea was kindly provided by E. Monte (CIALE). Plugs containing micelium were grown in PDA (OXOID) V8 8% (Campbell’s Soup Company). Spores were collected, resuspended in 15% glycerol, and stored at −80°C. A 10-μL drop of fungal spores dissolved in potato dextrose broth medium (Difco), containing 106 spores/mL of B. cinerea, was applied to three leaves per plant. Inoculated plants were covered with polypropylene to maintain 100% humidity. Fungal progression and infection symptoms were monitored at 3 d after fungal application. Symptoms were quantified by measuring lesion diameter.
Quantification of GUS Activity
The samples were immediately frozen after collection and the GUS activity was measured by fluorometry according to (Gallagher, 1992).
Yeast Two-Hybrid Assays
Yeast two-hybrid assays were performed with the Matchmaker LexA system (Clontech). MYC2, MYC3, MYC4, and HY5 coding sequences subcloned into pDONR207 were cloned into pB42-AD using Gateway (Invitrogen) LR reactions. COP1 coding sequence harboring K422E substitution was subcloned into pDONR207 and cloned into pGILDA vector to generate the bait fusion protein.
To assess protein interactions, the corresponding plasmids were cotransformed into Saccharomyces cerevisiae strain EGY48 (p8opLacZ) following standard heat shock protocols. Transformants were selected on SD-glucose medium supplemented with -Ura/-His/-Trp dropout solution. To test protein interactions transformed yeast strains were plated on SD-galactose/raffinose inducing medium containing -Ura/-His/-Trp dropout supplement and 80 μg/mL X-Gal. Plates were incubated at 28°C for 2 to 4 d.
Pull-Down Assays
MBP-MYC2, MBP-MYC3, and MBP-MYC4 fusion proteins were generated as described by Fernández-Calvo et al. (2011). Additionally, we generated MBP-HY5, MBP-MYC2ΔC, and MBP-MYC2ΔN fusion truncated proteins of MYC2 corresponding to N-terminal and C-terminal MYC2 sequences previously described (Chini et al., 2007). HY5 full-length, MYC2ΔC, and MYC2ΔN coding sequences were cloned into pDEST-TH1. Ten-day-old wild-type seedlings and a line expressing 35S:COP1-YFP (Oravecz et al., 2006) were used to perform pull-down experiments as described (Fonseca and Solano, 2013).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL libraries under the following accession numbers: MYC2 (Atg32640), MYC3 (At5g46760), MYC4 (At4g17880), phyA (At1g09570), phyB (At2g18790), CRY1 (At4g08920), CRY2 (At1g04400), COP1 (At2g32950), JAZ1 (At19180), JAZ2 (At1g74950), JAZ5 (At1g17380), JAZ6 (At1g72450), JAZ7 (At2g34600), JAZ8 (At1g30135), JAZ9 (At1g70700), JAZ10 (At5g13220), JAZ11 (At3g43440), and JAZ12 (At5g20900).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Immunoblot Analysis of GFP and MYC2-HA in 35S:GFP and 35S:MYC2-HA Transgenic Plants.
Supplemental Figure 2. Daily Variation of MYC2 Protein Levels.
Supplemental Figure 3. Effects of phyA, phyB, and cop1 Mutations in Daily Variation of MYC2 Protein Level.
Supplemental Figure 4. Immunoblot Analysis of MYC2-GFP and Actin Protein Levels in 35S:MYC2-GFP Transgenic Plants in Wild-Type and phyB Backgrounds.
Supplemental Figure 5. Immunoblot Analysis of MYC2-GFP, MYC3-HA, MYC4-GFP, and Actin Protein Levels in 35S:MYC2-GFP, 35S:MYC3-HA, and 35S:MYC4-GFP Transgenic Plants.
Supplemental Figure 6. Quantification of GUS Activity in Control Plants (35S:GUS Transgenics).
Supplemental Table 1. Primers Used to Amplify JAZ Sequences in the 35S:JAZ-GUS Constructs.
Supplementary Material
Acknowledgments
We thank Vicente Rubio (CNB-CSIC) for providing seeds of phyA-211, phyB-9, cry1 cry2, and cop1-4 and Eduardo González-Grandío and Pilar Cubas (CNB-CSIC) for helping with supplemented FR treatments. The fungal pathogen B. cinerea was kindly provided by E. Monte (CIALE). We also thank Christian Fankhauser (UNIL-Switzerland), Carlos Ballaré (IFEVA-Argentina), and Vicente Rubio for critical reading of the article and helpful suggestions. Research in R.S.’s lab was supported by grants from the Ministry of Science and Innovation to R.S. (BIO2010-21739, CSD2007-00057-B, and EUI2008-03666). A.C. was supported by a Ramon y Cajal Fellowship.
AUTHOR CONTRIBUTIONS
J.-M.C. and R.S. designed the research. J.-M.C., G.F.-B., A.C., P.F.-C., and M.D.-D. performed the research. J.-M.C., G.F.-B., A.C., P.F.-C., M.D.-D., and R.S analyzed the data.. J.-M.C., G.F.-B., A.C., P.F.-C., and M.D.-D. read and edited the article. R.S. wrote the article.
Glossary
- JA-Ile
jasmonoyl-l-isoleucine
- TF
transcription factor
- JA
jasmonate
- R
red
- FR
far-red
- SAS
shade avoidance syndrome
- CHX
cycloheximide
- B
blue
- WL
white light
- FRc
continuous FR
- L/D
light/dark
- MS
Murashige and Skoog
Footnotes
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Abe H., Urao T.I., Ito T., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. (2003). Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15: 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi V., Devoto A. (2008). Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol. 177: 301–318 [DOI] [PubMed] [Google Scholar]
- Ballaré C.L. (1999). Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci. 4: 201. [DOI] [PubMed] [Google Scholar]
- Ballaré C.L. (2009). Illuminated behaviour: phytochrome as a key regulator of light foraging and plant anti-herbivore defence. Plant Cell Environ. 32: 713–725 [DOI] [PubMed] [Google Scholar]
- Ballaré C.L. (2011). Jasmonate-induced defenses: a tale of intelligence, collaborators and rascals. Trends Plant Sci. 16: 249–257 [DOI] [PubMed] [Google Scholar]
- Ballesteros M.L., Bolle C., Lois L.M., Moore J.M., Vielle-Calzada J.P., Grossniklaus U., Chua N.H. (2001). LAF1, a MYB transcription activator for phytochrome A signaling. Genes Dev. 15: 2613–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R., Jones J.D. (2009). Role of plant hormones in plant defence responses. Plant Mol. Biol. 69: 473–488 [DOI] [PubMed] [Google Scholar]
- Boter M., Ruíz-Rivero O., Abdeen A., Prat S. (2004). Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev. 18: 1577–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J. (2009). Jasmonate passes muster: a receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 60: 183–205 [DOI] [PubMed] [Google Scholar]
- Browse J., Howe G.A. (2008). New weapons and a rapid response against insect attack. Plant Physiol. 146: 832–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrudo I., Keller M.M., Cargnel M.D., Demkura P.V., de Wit M., Patitucci M.S., Pierik R., Pieterse C.M., Ballaré C.L. (2012). Low red/far-red ratios reduce Arabidopsis resistance to Botrytis cinerea and jasmonate responses via a COI1-JAZ10-dependent, salicylic acid-independent mechanism. Plant Physiol. 158: 2042–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Huang X., Gusmaroli G., Terzaghi W., Lau O.S., Yanagawa Y., Zhang Y., Li J., Lee J.H., Zhu D., Deng X.W. (2010). Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA complexes to regulate photomorphogenesis and flowering time. Plant Cell 22: 108–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Chory J. (2011). Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 21: 664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Sun L., Qi T., Zhang B., Peng W., Liu Y., Xie D. (2011). The bHLH transcription factor MYC3 interacts with the Jasmonate ZIM-domain proteins to mediate jasmonate response in Arabidopsis. Mol. Plant 4: 279–288 [DOI] [PubMed] [Google Scholar]
- Chini A., Fonseca S., Chico J.M., Fernández-Calvo P., Solano R. (2009). The ZIM domain mediates homo- and heteromeric interactions between Arabidopsis JAZ proteins. Plant J. 59: 77–87 [DOI] [PubMed] [Google Scholar]
- Chini A., Fonseca S., Fernández G., Adie B., Chico J.M., Lorenzo O., García-Casado G., López-Vidriero I., Lozano F.M., Ponce M.R., Micol J.L., Solano R. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Curtis M.D., Grossniklaus U. (2003). A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davière J.M., de Lucas M., Prat S. (2008). Transcriptional factor interaction: a central step in DELLA function. Curr. Opin. Genet. Dev. 18: 295–303 [DOI] [PubMed] [Google Scholar]
- de Lucas M., Davière J.M., Rodríguez-Falcón M., Pontin M., Iglesias-Pedraz J.M., Lorrain S., Fankhauser C., Blázquez M.A., Titarenko E., Prat S. (2008). A molecular framework for light and gibberellin control of cell elongation. Nature 451: 480–484 [DOI] [PubMed] [Google Scholar]
- de Wit M., Spoel S.H., Sanchez-Perez G.F., Gommers C.M., Pieterse C.M., Voesenek L.A., Pierik R. (2013). Perception of low red:far-red ratio compromises both salicylic acid- and jasmonic acid-dependent pathogen defences in Arabidopsis. Plant J. 75: 90–103 [DOI] [PubMed] [Google Scholar]
- Deng X.W., Caspar T., Quail P.H. (1991). cop1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 5: 1172–1182 [DOI] [PubMed] [Google Scholar]
- Devoto A., Ellis C., Magusin A., Chang H.S., Chilcott C., Zhu T., Turner J.G. (2005). Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol. Biol. 58: 497–513 [DOI] [PubMed] [Google Scholar]
- Duek P.D., Fankhauser C. (2003). HFR1, a putative bHLH transcription factor, mediates both phytochrome A and cryptochrome signalling. Plant J. 34: 827–836 [DOI] [PubMed] [Google Scholar]
- Feng S., et al. (2008). Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451: 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Calvo P., et al. (2011). The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23: 701–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S., Solano R. (2013). Pull-down analysis of interactions among jasmonic acid core signaling proteins. Methods Mol. Biol. 1011: 159–171 [DOI] [PubMed] [Google Scholar]
- Fonseca S., Chini A., Hamberg M., Adie B., Porzel A., Kramell R., Miersch O., Wasternack C., Solano R. (2009a). (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 5: 344–350 [DOI] [PubMed] [Google Scholar]
- Fonseca S., Chico J.M., Solano R. (2009b). The jasmonate pathway: the ligand, the receptor and the core signalling module. Curr. Opin. Plant Biol. 12: 539–547 [DOI] [PubMed] [Google Scholar]
- Fonseca S., Fernández-Calvo P., Fernández G.M., Díez-Díaz M., Gimenez-Ibanez S., López-Vidriero I., Godoy M., Fernández-Barbero G., Van Leene J., De Jaeger G., Franco-Zorrilla J.M., Solano R. (2014). bHLH003, bHLH013 and bHLH017 are new targets of JAZ repressors negatively regulating JA responses. PLoS ONE 9: e86182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K.A. (2008). Shade avoidance. New Phytol. 179: 930–944 [DOI] [PubMed] [Google Scholar]
- Franklin K.A., Quail P.H. (2010). Phytochrome functions in Arabidopsis development. J. Exp. Bot. 61: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher, S.R. (1992). Quantitation of GUS activity by fluorometry. In GUS Protocols: Using the GUS Gene as a Reporter of Gene Expression, S.R. Gallagher, ed (New York: Academic Press), pp. 47–59. [Google Scholar]
- Gangappa S.N., Prasad V.B., Chattopadhyay S. (2010). Functional interconnection of MYC2 and SPA1 in the photomorphogenic seedling development of Arabidopsis. Plant Physiol. 154: 1210–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Grandío E., Poza-Carrión C., Sorzano C.O., Cubas P. (2013). BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell 25: 834–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodspeed D., Chehab E.W., Min-Venditti A., Braam J., Covington M.F. (2012). Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc. Natl. Acad. Sci. USA 109: 4674–4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M., Ma L.G., Qu L.J., Deng X.W. (2002). Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 16: 1247–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Lee L.Y., Xia K., Yan Y., Yu H. (2010). DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev. Cell 19: 884–894 [DOI] [PubMed] [Google Scholar]
- Huq E. (2006). Degradation of negative regulators: a common theme in hormone and light signaling networks? Trends Plant Sci. 11: 4–7 [DOI] [PubMed] [Google Scholar]
- Katsir L., Schilmiller A.L., Staswick P.E., He S.Y., Howe G.A. (2008). COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. USA 105: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K., Manners J.M. (2008). Jasmonate signaling: toward an integrated view. Plant Physiol. 146: 1459–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K., Manners J.M. (2011). The interplay between light and jasmonate signalling during defence and development. J. Exp. Bot. 62: 4087–4100 [DOI] [PubMed] [Google Scholar]
- Lau O.S., Deng X.W. (2010). Plant hormone signaling lightens up: integrators of light and hormones. Curr. Opin. Plant Biol. 13: 571–577 [DOI] [PubMed] [Google Scholar]
- Leivar P., Quail P.H. (2011). PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O., Chico J.M., Sánchez-Serrano J.J., Solano R. (2004). JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16: 1938–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S., Genoud T., Fankhauser C. (2006). Let there be light in the nucleus! Curr. Opin. Plant Biol. 9: 509–514 [DOI] [PubMed] [Google Scholar]
- Mandaokar A., Thines B., Shin B., Lange B.M., Choi G., Koo Y.J., Yoo Y.J., Choi Y.D., Choi G., Browse J. (2006). Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. 46: 984–1008 [DOI] [PubMed] [Google Scholar]
- Maor R., Jones A., Nühse T.S., Studholme D.J., Peck S.C., Shirasu K. (2007). Multidimensional protein identification technology (MudPIT) analysis of ubiquitinated proteins in plants. Mol. Cell. Proteomics 6: 601–610 [DOI] [PubMed] [Google Scholar]
- Martínez-García J.F., Galstyan, A., Salla-Martret, M., Cifuentes-Esquivel, N., Gallemí, M., and Bou-Torrent, J. (2010). Regulatory components of shade avoidance syndrome. Adv. Bot. Res. 53: 65–116 [Google Scholar]
- Moreno J.E., Tao Y., Chory J., Ballaré C.L. (2009). Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc. Natl. Acad. Sci. USA 106: 4935–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata M., Ohme-Takagi M. (2013). Two bHLH-type transcription factors, JA-ASSOCIATED MYC2-LIKE2 and JAM3, are transcriptional repressors and affect male fertility. Plant Signal. Behav. 8: e26473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y., Figueroa P., Browse J. (2011). Characterization of JAZ-interacting bHLH transcription factors that regulate jasmonate responses in Arabidopsis. J. Exp. Bot. 62: 2143–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oravecz A., Baumann A., Máté Z., Brzezinska A., Molinier J., Oakeley E.J., Adám E., Schäfer E., Nagy F., Ulm R. (2006). CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell 18: 1975–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacín M., Legris M., Casal J.J. (2013). COP1 re-accumulates in the nucleus under shade. Plant J. 75: 631–641 [DOI] [PubMed] [Google Scholar]
- Pauwels L., Goossens A. (2011). The JAZ proteins: a crucial interface in the jasmonate signaling cascade. Plant Cell 23: 3089–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L., et al. (2010). NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464: 788–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L., Morreel K., De Witte E., Lammertyn F., Van Montagu M., Boerjan W., Inzé D., Goossens A. (2008). Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc. Natl. Acad. Sci. USA 105: 1380–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R., de Wit M. (December 9, 2013). Shade avoidance: phytochrome signalling and other aboveground neighbour detection cues. J. Exp. Bot. http//dx..org/. [DOI] [PubMed] [Google Scholar]
- Qi T., Song S., Ren Q., Wu D., Huang H., Chen Y., Fan M., Peng W., Ren C., Xie D. (2011). The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 23: 1795–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe C., Springer A., Samol I., Reinbothe S. (2009). Plant oxylipins: role of jasmonic acid during programmed cell death, defence and leaf senescence. FEBS J. 276: 4666–4681 [DOI] [PubMed] [Google Scholar]
- Reymond P., Bodenhausen N., Van Poecke R.M., Krishnamurthy V., Dicke M., Farmer E.E. (2004). A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell 16: 3132–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson F., Okamoto H., Patrick E., Harris S.R., Wasternack C., Brearley C., Turner J.G. (2010). Jasmonate and phytochrome A signaling in Arabidopsis wound and shade responses are integrated through JAZ1 stability. Plant Cell 22: 1143–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saracco S.A., Hansson M., Scalf M., Walker J.M., Smith L.M., Vierstra R.D. (2009). Tandem affinity purification and mass spectrometric analysis of ubiquitylated proteins in Arabidopsis. Plant J. 59: 344–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki-Sekimoto Y., Jikumaru Y., Obayashi T., Saito H., Masuda S., Kamiya Y., Ohta H., Shirasu K. (2013). Basic helix-loop-helix transcription factors JASMONATE-ASSOCIATED MYC2-LIKE1 (JAM1), JAM2, and JAM3 are negative regulators of jasmonate responses in Arabidopsis. Plant Physiol. 163: 291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F., Fernández-Calvo P., Zander M., Diez-Diaz M., Fonseca S., Glauser G., Lewsey M.G., Ecker J.R., Solano R., Reymond P. (2013). Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25: 3117–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock R.A., Clack T. (2002). Patterns of expression and normalized levels of the five Arabidopsis phytochromes. Plant Physiol. 130: 442–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard L.B., et al. (2010). Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Heidrich K., Sanchez-Villarreal A., Parker J.E., Davis S.J. (2012). TIME FOR COFFEE represses accumulation of the MYC2 transcription factor to provide time-of-day regulation of jasmonate signaling in Arabidopsis. Plant Cell 24: 2470–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu C., Figueroa P., Depew C.L., Cooke T.F., Sheard L.B., Moreno J.E., Katsir L., Zheng N., Browse J., Howe G.A. (2012). JAZ8 lacks a canonical degron and has an EAR motif that mediates transcriptional repression of jasmonate responses in Arabidopsis. Plant Cell 24: 536–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A.L., Jung H.S., Dill A., Kawaide H., Kamiya Y., Sun T.P. (2001). Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13: 1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. (1995). Physiological and ecological function within the phytochrome family. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46: 289–315 [Google Scholar]
- Smith H. (2000). Phytochromes and light signal perception by plants—an emerging synthesis. Nature 407: 585–591 [DOI] [PubMed] [Google Scholar]
- Song S., Huang H., Gao H., Wang J., Wu D., Liu X., Yang S., Zhai Q., Li C., Qi T., Xie D. (2014). Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 26: 263–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Qi T., Fan M., Zhang X., Gao H., Huang H., Wu D., Guo H., Xie D. (2013). The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet. 9: e1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Qi T., Huang H., Ren Q., Wu D., Chang C., Peng W., Liu Y., Peng J., Xie D. (2011). The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23: 1000–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suza W.P., Rowe M.L., Hamberg M., Staswick P.E. (2010). A tomato enzyme synthesizes (+)-7-iso-jasmonoyl-L-isoleucine in wounded leaves. Planta 231: 717–728 [DOI] [PubMed] [Google Scholar]
- Suzuki A., et al. (2011). Lotus japonicus nodulation is photomorphogenetically controlled by sensing the red/far red (R/FR) ratio through jasmonic acid (JA) signaling. Proc. Natl. Acad. Sci. USA 108: 16837–16842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G., Nomura K., He S.Y., Howe G.A., Browse J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- von Arnim A.G., Deng X.W. (1994). Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell 79: 1035–1045 [DOI] [PubMed] [Google Scholar]
- Wang H., Ma L.G., Li J.M., Zhao H.Y., Deng X.W. (2001). Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294: 154–158 [DOI] [PubMed] [Google Scholar]
- Wasternack C. (2007). Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. (Lond.) 100: 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D.X., Feys B.F., James S., Nieto-Rostro M., Turner J.G. (1998). COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094 [DOI] [PubMed] [Google Scholar]
- Xie X.Z., Xue Y.J., Zhou J.J., Zhang B., Chang H., Takano M. (2011). Phytochromes regulate SA and JA signaling pathways in rice and are required for developmentally controlled resistance to Magnaporthe grisea. Mol. Plant 4: 688–696 [DOI] [PubMed] [Google Scholar]
- Yadav V., Mallappa C., Gangappa S.N., Bhatia S., Chattopadhyay S. (2005). A basic helix-loop-helix transcription factor in Arabidopsis, MYC2, acts as a repressor of blue light-mediated photomorphogenic growth. Plant Cell 17: 1953–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Stolz S., Chételat A., Reymond P., Pagni M., Dubugnon L., Farmer E.E. (2007). A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19: 2470–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.L., et al. (2012). Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 109: E1192–E1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C., Deng X.W. (2005). COP1 - from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 15: 618–625 [DOI] [PubMed] [Google Scholar]
- Yu J.W., et al. (2008). COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol. Cell 32: 617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Q., Yan L., Tan D., Chen R., Sun J., Gao L., Dong M.Q., Wang Y., Li C. (2013). Phosphorylation-coupled proteolysis of the transcription factor MYC2 is important for jasmonate-signaled plant immunity. PLoS Genet. 9: e1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Maier A., Lee J.H., Laubinger S., Saijo Y., Wang H., Qu L.J., Hoecker U., Deng X.W. (2008). Biochemical characterization of Arabidopsis complexes containing CONSTITUTIVELY PHOTOMORPHOGENIC1 and SUPPRESSOR OF PHYA proteins in light control of plant development. Plant Cell 20: 2307–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., et al. (2011). Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 12539–12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.