This study shows that PHYTOCHROME INTERACTING FACTOR1 forms a complex with CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1)/SUPPRESSOR OF PHYA and enhances the substrate recruitment and autoubiquitylation and transubiquitylation activity of COP1. The data support a genetic and biochemical model in light signaling pathways and expand the regulatory roles of bHLH factors as potential cofactors of E3 ligases.
Abstract
CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) is a RING/WD40 repeat–containing ubiquitin E3 ligase that is conserved from plants to humans. COP1 forms complexes with SUPPRESSOR OF PHYTOCHROME A (SPA) proteins, and these complexes degrade positively acting transcription factors in the dark to repress photomorphogenesis. Phytochrome-interacting basic helix-loop-helix transcription factors (PIFs) also repress photomorphogenesis in the dark. In response to light, the phytochrome family of sensory photoreceptors simultaneously inactivates COP1-SPA complexes and induces the rapid degradation of PIFs to promote photomorphogenesis. However, the functional relationship between PIFs and COP1-SPA complexes is still unknown. Here, we present genetic evidence that the pif and cop1/spa Arabidopsis thaliana mutants synergistically promote photomorphogenesis in the dark. LONG HYPOCOTYL5 (HY5) is stabilized in the cop1 pif1, spa123 pif1, and pif double, triple, and quadruple mutants in the dark. Moreover, the hy5 mutant suppresses the constitutive photomorphogenic phenotypes of the pifq mutant in the dark. PIF1 forms complexes with COP1, HY5, and SPA1 and enhances the substrate recruitment and autoubiquitylation and transubiquitylation activities of COP1. These data uncover a novel function of PIFs as the potential cofactors of COP1 and provide a genetic and biochemical model of how PIFs and COP1-SPA complexes synergistically repress photomorphogenesis in the dark.
INTRODUCTION
Plants are sessile and photoautotrophic organisms and therefore have evolved a variety of mechanisms to optimize their growth and development to the ambient light environment. Several classes of photoreceptors enable plants to sense and respond to light. Among these, phytochromes sense red and far-red light, cryptochromes and phototropins sense UV-A/blue light, and UVR8 senses UV-B light. Light-induced activation of these photoreceptors promotes photomorphogenic development throughout the life cycle of plants, including seed germination, seedling deetiolation, the shade avoidance response, phototropism, and flowering (Schaefer and Nagy, 2006; Bae and Choi, 2008; Franklin and Quail, 2010; Chen and Chory, 2011).
Phytochromes are chromoproteins encoded by a small gene family (PHYA to PHYE in Arabidopsis thaliana) that exist as the red light–absorbing inactive Pr form in the dark (Mathews and Sharrock, 1997). Upon light absorption, a conformation shift to the far-red light–absorbing biologically active Pfr form triggers their nuclear translocation and photobody (speckle) formation (Nagatani, 2004; Chen and Chory, 2011). Within the nucleus, the activated Pfr physically interacts with multiple proteins, including a small group of basic helix-loop-helix (bHLH) transcription factors called Phytochrome Interacting Factors (PIFs; PIF1 and PIF3 to PIF8) (Quail, 2000; Huq and Quail, 2002, 2005; Huq et al., 2004; Duek and Fankhauser, 2005; M. Chen et al., 2010; Galvão et al., 2012; Paik et al., 2012). PIFs constitutively accumulate in the nucleus in the dark and inhibit photomorphogenesis (Castillon et al., 2007; Henriques et al., 2009; Leivar and Quail, 2011). Upon light exposure, the physical interaction between Pfr and PIFs triggers a cascade of events, including light-induced phosphorylation, ubiquitylation, and 26S proteasome–mediated degradation of PIFs (Bauer et al., 2004; Shen et al., 2005, 2008; Al-Sady et al., 2006; Bu et al., 2011). The removal of PIFs after light exposure results in large-scale changes in gene expression that promote photomorphogenic development (Leivar et al., 2009; Shin et al., 2009; Leivar and Quail, 2011; Zhang et al., 2013; Leivar and Monte, 2014). Consistently, the reduction in PIF level in the pifq (pif1 pif3 pif4 pif5) quadruple mutant or the overexpression of a truncated form of PIF1 results in photomorphogenic development in the dark (Leivar et al., 2008b; Shen et al., 2008; Shin et al., 2009).
In addition to PIFs, previous genetic studies have identified 11 CONSTITUTIVE PHOTOMORPHOGENIC/DEETIOLATED/FUSCA (COP/DET/FUS) genes that act as negative regulators of photomorphogenesis (Deng et al., 1992; Hoecker, 2005; Henriques et al., 2009; Lau and Deng, 2012). Among these COP/DET/FUS proteins, COP1 is a RING-type E3 ligase that is evolutionarily conserved from plants to vertebrates (Deng et al., 1992; Osterlund et al., 2000; Bianchi et al., 2003; Dornan et al., 2004; Yi and Deng, 2005; Lau and Deng, 2012). COP1 plays a central role in repressing photomorphogenesis in the dark by forming multiple complexes with SUPPRESSOR OF PHYTOCHROME A-105 (SPA1-4) family members in a tissue- and developmental stage–specific manner (Deng et al., 1992; Hoecker et al., 1999; Osterlund et al., 2000; Laubinger et al., 2004; Zhu et al., 2008; Lau and Deng, 2012). These complexes ubiquitylate and degrade positively acting transcription factors such as LONG HYPOCOTYL5 (HY5)/ LONG AFTER FAR-RED LIGHT1 (LAF1)/LONG HYPOCOTYL IN FAR-RED1 (HFR1) to repress photomorphogenesis in the dark (Hoecker et al., 1999; Saijo et al., 2003; Seo et al., 2003; Jang et al., 2005; Yang et al., 2005; Zhu et al., 2008). COP1-SPA complexes also associate with Cullin4, COP9 signalosome (CSN), and the CDD (for COP10, DDB1a, and DET1) complexes, and all three subcomplexes synergistically repress photomorphogenesis in the dark (H.D. Chen et al., 2010). Under prolonged light conditions, COP1 is depleted from the nucleus using a nuclear exclusion mechanism that allows these target proteins to accumulate and promote photomorphogenesis (Osterlund and Deng, 1998; Subramanian et al., 2004; Pacín et al., 2013).
The COP1-SPA complexes also regulate the photoreceptor levels in light. phyA, a type I light-labile phytochrome, is degraded in light in a COP1-dependent manner (Seo et al., 2004). COP1 also degrades type II light-stable phytochromes (phyB to phyE) under light. Strikingly, PIFs interact with both COP1 and phyB and enhance the polyubiquitylation of phyB by COP1 in vitro (Jang et al., 2010). These data are consistent with an increased abundance of phyB in pif mutants resulting in their hypersensitive phenotypes under continuous red light (Leivar et al., 2008a; Leivar and Quail, 2011). Therefore, COP1 desensitizes the signaling pathway by regulating the abundance of the photoreceptors.
The above studies show that PIFs and COP1-SPA complexes act as key regulators of photomorphogenesis in the dark. However, the functional relationship between PIFs and COP1-SPA complexes is still unknown. Previously, it was shown that PIF3 is unstable in cop1 and spa mutants (Bauer et al., 2004; Leivar et al., 2008b), suggesting that PIFs might act downstream of COP1-SPA complexes to repress photomorphogenesis. Here, we provide genetic and biochemical evidence that these factors function synergistically as well as additively to repress photomorphogenesis in the dark. Our results have uncovered a novel biochemical function of this group of bHLH transcription factors in optimizing the photomorphogenic development of plants.
RESULTS
PIFs and COP1/SPA Proteins Synergistically Repress Photomorphogenesis in the Dark
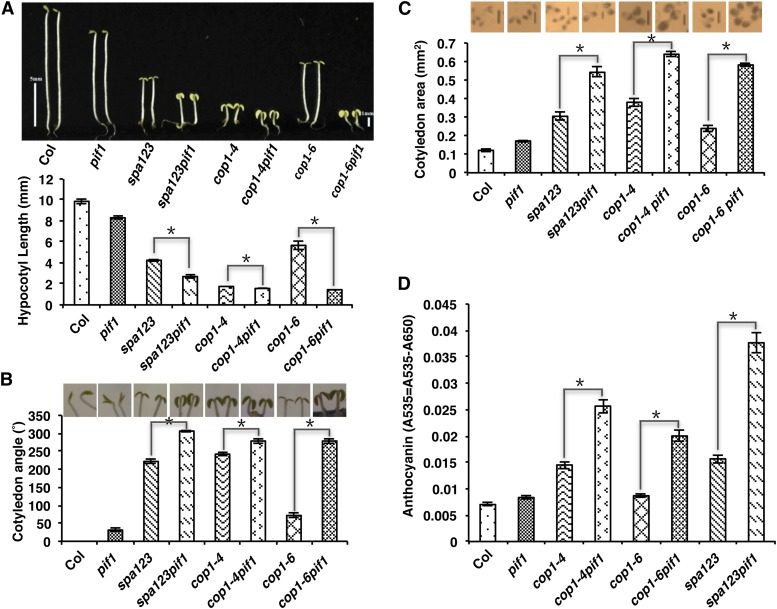
To examine the genetic relationship between PIFs and COP1/SPA proteins, we generated various combinations of pifs, cop1, and spa Arabidopsis mutants. Because both PIFs and COP1/SPA proteins repress photomorphogenesis in the dark, we examined deetiolation phenotypes including hypocotyl length, cotyledon angle, cotyledon area, anthocyanin level, and gene expression phenotypes for wild-type (Columbia-0 [Col-0]) and mutant seedlings grown in darkness. As observed previously, the wild type and pif1 displayed etiolated phenotypes, while cop1-4, cop1-6, and spa123 (spa1 spa2 spa3) displayed deetiolated phenotypes under these conditions (Figures 1A to 1D). Strikingly, the cop1-6 pif1 double and spa123 pif1 quadruple mutants displayed synergistic deetiolation phenotypes including shorter hypocotyls, higher cotyledon angles, more expanded cotyledon areas, and higher anthocyanin levels compared with cop1-6 and spa123, respectively (Figures 1A to 1D). cop1-4 pif1 also displayed enhanced deetiolation phenotypes compared with cop1-4 (Figures 1A to 1D); however, because cop1-4 is a strong allele lacking the WD40 region of COP1, the synergistic effect was not observed for all these phenotypes. Chlorophyll and carotenoid levels were also higher in the cop1-4 pif1, cop1-6 pif1, and spa123 pif1 mutant backgrounds compared with the cop1-4, cop1-6, and spa123 mutant backgrounds, respectively (Supplemental Figures 1A and 1B). Among all PIFs, only PIF1 was shown to repress seed germination by directly regulating genes in the gibberellin and abscisic acid pathways in the dark (Oh et al., 2004, 2009). Examination of the seed germination phenotypes for cop1-4, cop1-6, spa123, and their combinations with pif1 revealed that the germination rates of cop1-4 pif1, cop1-6 pif1, and spa123 pif1 did not differ from those of pif1 (Supplemental Figure 1C). Taken together, these data show that PIF1 and COP1/SPA synergistically repress photomorphogenesis in the dark.
Figure 1.
pif1 and cop1/spa123 Mutants Synergistically Promote Photomorphogenesis in the Dark.
(A) to (C) Top, visible phenotypes of the wild type (Col), pif1, cop1, and spa123 and various combinations of pif1, cop1, and spa123 mutants as indicated. Seeds of various genotypes were grown on MS medium without Suc for 5 d in the dark. Photographs show the hypocotyl lengths (A), cotyledon angles (B), and cotyledon areas (C) of the genotypes as indicated. Bottom, bar graphs show the mean hypocotyl lengths (A), cotyledon angles (B), and cotyledon areas (C) of the above genotypes (n > 30, three biological replicates). Error bars indicate sd. Asterisks indicate significant differences (P < 0.05). Bars = 1 mm.
(D) Mean anthocyanin levels of the wild type, pif1, cop1, and spa123 and various combinations of pif1, cop1, and spa123 mutants as indicated. Seedlings of various genotypes were grown on MS medium with Suc for 3 d in the dark (n = 50, three replicates). Error bars indicate sd. Asterisks indicate significant differences (P < 0.05).
[See online article for color version of this figure.]
To examine if other PIFs can repress photomorphogenesis synergistically with COP1, we generated a series of higher order mutants among cop1-4, cop1-6, and three additional pif (pif3, pif4, and pif5) mutants. Phenotypic analyses showed that a combination of cop1-6 and pif1, pif3, or pif4 displays equal synergistic enhancement of photomorphogenesis compared with cop1-6 in the dark (Supplemental Figures 2B to 4B). Moreover, the various mutant combinations of pifs with cop1-6 (cop1-6 pif13, cop1-6 pif14, cop1-6 pif15, cop1-6 pif134, cop1-6 pif135, cop1-6 pif145, and cop1-6 pifq) displayed increasing levels of photomorphogenesis compared with cop1-6 pif double mutants (cop1-6 pif1, cop1-6 pif3, and cop1-6 pif4) in the dark (Supplemental Figures 2B to 4B). The higher order mutant combinations with cop1-4 also displayed increased photomorphogenesis in the dark (Supplemental Figures 2A to 4A); however, because cop1-4 is a strong allele, the synergistic deetiolation phenotype of cop1-4 and pif combinations was not observed. Taken together, these data suggest that all four PIFs synergistically repress photomorphogenesis with COP1/SPA proteins individually. However, additional PIFs incrementally repress photomorphogenesis in the dark in an additive manner.
pif1 and cop1/spa123 Mutants Display Synergistic Promotion of Light-Regulated Gene Expression in the Dark
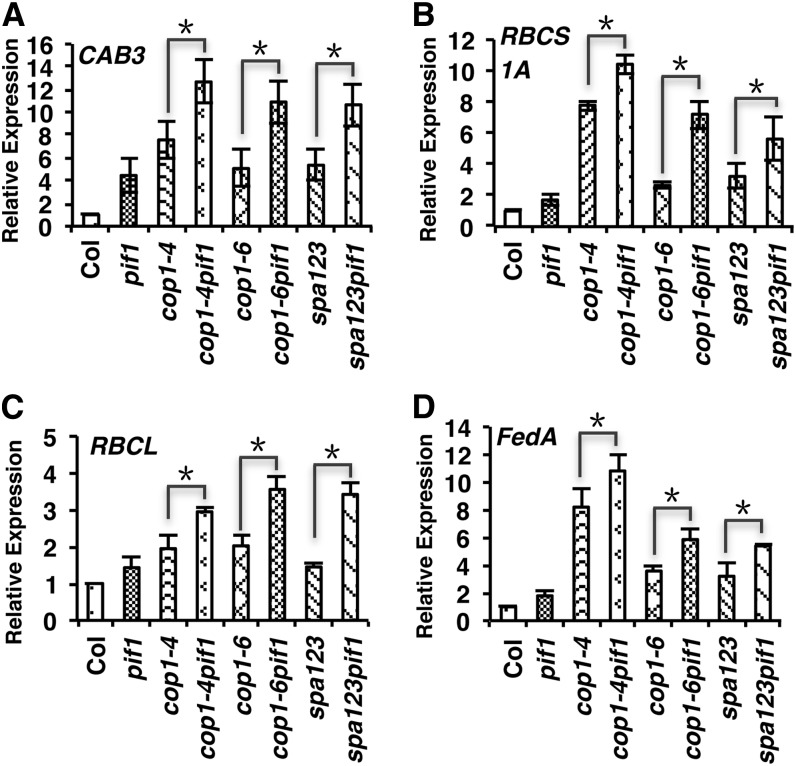
To determine if the above mutant combinations also display synergistic phenotypes at the molecular level, we examined the expression levels of a selected group of genes that are usually expressed in light-grown seedlings. As expected, cop1-4, cop1-6, and spa123 displayed higher expression of these genes compared with the wild type under these conditions (Figures 2A to 2D). Consistent with the above results, the expression of these genes was much higher in the cop1-4 pif1, cop1-6 pif1, and spa123 pif1 backgrounds compared with that in the cop1-4, cop1-6, and spa123 backgrounds, respectively (Figures 2A to 2D). These data suggest that PIFs and COP1/SPA proteins synergistically repress both the morphological and molecular phenotypes in darkness.
Figure 2.
pif1 and cop1/spa123 Mutants Display Synergistic Promotion of Light-Regulated Gene Expression in the Dark.
Bar graphs show the expression of CAB3 (A), RBCS1A (B), RBCL (C) and FedA (D) transcript levels in the wild type (Col), pif1, cop1, and spa123 and various combinations of pif1, cop1, and spa123 mutants as indicated. Total RNA was isolated from 4-d-old dark-grown seedlings for qRT-PCR assays (n = 3 independent biological repeats). PP2A was used as an internal control. The wild-type value was set as 1, and the relative gene expression levels were calculated. Error bars indicate sd. Asterisks indicate significant differences (P < 0.05).
PIFs Promote COP1/SPA-Mediated Degradation of HY5 Posttranslationally in the Dark
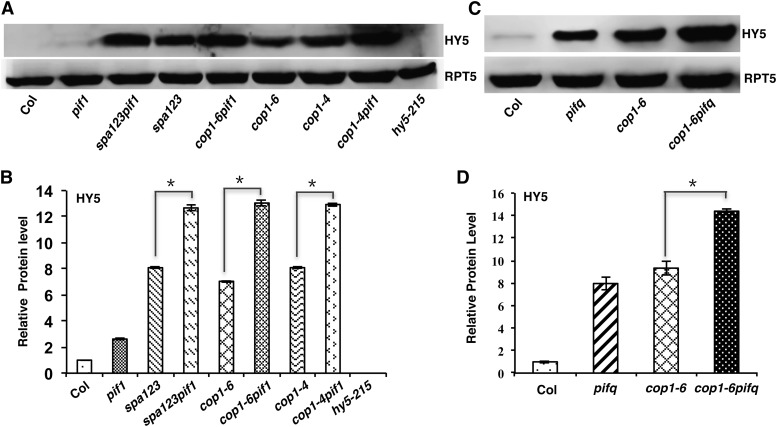
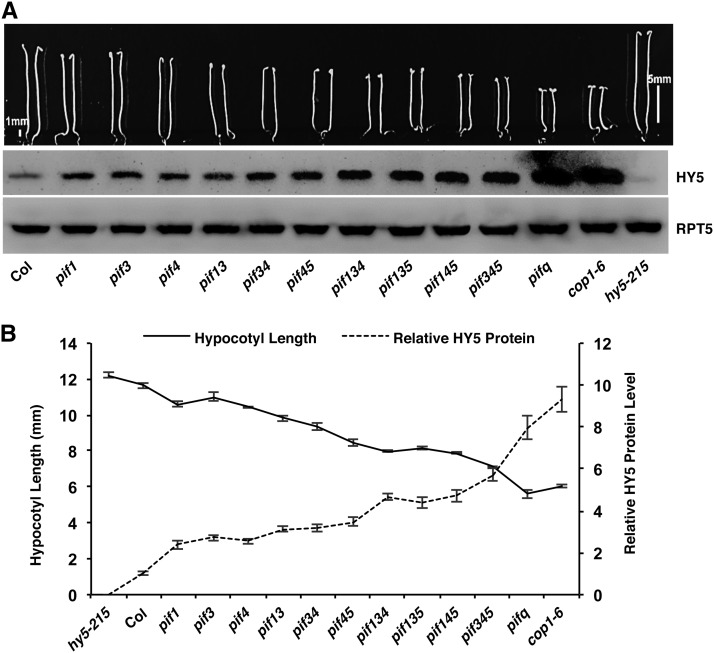
Previous studies have shown that COP1-SPA complexes interact with the positively acting transcription factors (e.g., HY5, HFR1, LAF1, and others) and induce their degradation through the ubiquitin/26S proteasome pathway in the dark (Osterlund et al., 2000; Saijo et al., 2003; Seo et al., 2003; Jang et al., 2005; Yang et al., 2005; Lau and Deng, 2012). HY5 is a bZIP transcription factor whose abundance directly correlates with the level of photomorphogenesis (Osterlund et al., 2000). To determine if the synergistic promotion of photomorphogenesis observed in the various cop1 pif and spa123 pif1 mutants is due to an increased abundance of HY5 in the dark, we examined HY5 protein and mRNA levels in cop1, spa123, pif1, and the corresponding higher order mutants. The results show that the HY5 protein, but not the HY5 mRNA, is synergistically stabilized in the cop1-4 pif1, cop1-6 pif1, and spa123 pif1 backgrounds compared with that in the cop1-4, cop1-6, and spa123 backgrounds, respectively (Figures 3A and 3B; Supplemental Figure 5). Because pifq displays constitutive photomorphogenesis similar to cop1, we examined the HY5 level in pifq, cop1-6, and cop1-6 pifq mutants. Strikingly, HY5 is much more abundant in the pifq background compared with the wild type. In addition, the HY5 level is much higher in the cop1-6 pifq background compared with that in the cop1-6 and pifq backgrounds (Figure 3C). However, the HY5 mRNA level is reduced in the pifq background compared with the wild type (Supplemental Figure 5) (Zhang et al., 2013), suggesting that PIFs regulate the HY5 level posttranslationally in an additive manner in darkness. Because HY5 is more abundant in the pifq background, we examined the HY5 level in the various pif single, double, triple, and quadruple mutant backgrounds. The results show that HY5 is progressively more abundant in the higher order pif mutants compared with the wild type and pif single mutants (Figure 4A, bottom). Strikingly, the hypocotyl lengths of these mutants correlated inversely with the HY5 level in various pif mutant combinations compared with the wild type (Figures 4A and 4B). These data also suggest that the constitutive photomorphogenic phenotype of pifq might be partly due to an increased abundance of HY5 in the dark.
Figure 3.
PIFs Promote COP1- and SPA-Mediated Degradation of HY5 in the Dark Posttranslationally.
(A) Immunoblot showing the HY5 level in various genotypes as indicated. Total protein was extracted from 5-d-old seedlings grown on MS medium without Suc in darkness. Total protein was separated on an 8% SDS-PAGE gel, blotted onto a PVDF membrane, and probed with anti-HY5 or anti-RPT5 antibody.
(B) Bar graph showing the HY5 protein levels in the indicated mutants. For protein quantitation, HY5 band intensities were quantified from three independent blots using ImageJ and normalized against RPT5 levels. The wild-type value (Col) was set as 1, and the relative protein levels were calculated. Error bars indicate sd. Asterisks indicate significant differences (P < 0.05).
(C) Immunoblot showing the HY5 level in the wild-type, pifq, cop1-6, and cop1-6 pifq backgrounds. The RPT5 blot shows a loading control. Seedlings were grown in the dark as described above.
(D) Bar graph showing the HY5 protein levels in the wild-type, pifq, cop1-6, and cop1-6 pifq backgrounds. Error bars indicate sd. Asterisks indicate significant differences (P < 0.05).
Figure 4.
pifs Redundantly Regulate the Level of HY5 in the Dark.
(A) Top, visible phenotypes of the wild type (Col), various pif single, double, triple, and quadruple mutants, and cop1-6 and hy5-215 as controls. Seeds of various genotypes were grown on MS medium without Suc for 5 d in the dark. Bottom, immunoblot showing the HY5 level in the above genotypes. The RPT5 blot shows a loading control. Total protein was extracted from 5-d-old dark-grown seedlings grown on MS medium without Suc.
(B) Line graph showing the inverse correlation between the hypocotyl lengths and the HY5 levels in the above genotypes. Band intensities were quantified from three independent blots using ImageJ and normalized against RPT5 levels. The wild-type value was set as 1, and the relative protein levels were calculated. Error bars indicate sd.
hy5-215 Partially Suppresses the Synergistic Promotion of Photomorphogenesis in the cop1-6 pif1 Background
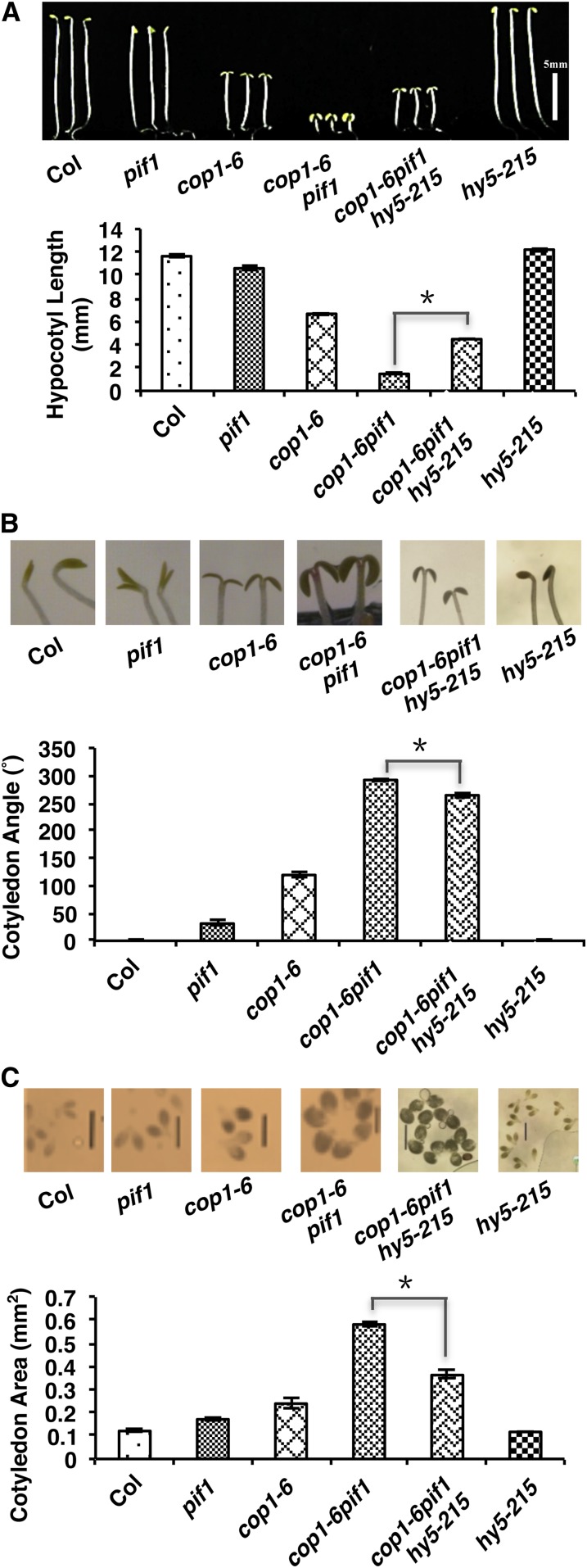
To provide genetic evidence that the increased abundance of HY5 in the dark contributes to the synergistic repression of photomorphogenesis by PIFs and COP1, we generated a cop1-6 pif1 hy5-215 triple mutant. Phenotypic analyses showed that all three deetiolation phenotypes (shorter hypocotyl and expanded cotyledon angle and area) are partially suppressed in the cop1-6 pif1 hy5-215 triple mutant compared with those in the cop1-6 pif1 double mutant (Figures 5A to 5C). These data suggest that the increased amount of HY5 in the dark indeed contributes to the synergistic promotion of photomorphogenesis in the cop1-6 pif1 double mutant in the dark.
Figure 5.
hy5-215 Partially Suppresses the Synergistic Promotion of Photomorphogenesis in the cop1-6 pif1 Background.
(A) Top, photographs of 5-d-old dark-grown seedlings of the wild type (Col), pif1, cop1-6, cop1-6 pif1, hy5-215, and cop1-6 pif1 hy5-215. Bottom, bar graph showing hypocotyl lengths of various genotypes as indicated.
(B) Top, photographs of cotyledon angles of 5-d-old dark-grown seedlings. Bottom, bar graph showing cotyledon angles of various genotypes as indicated.
(C) Top, photographs of cotyledon areas of 5-d-old dark-grown seedlings. Bottom, bar graph showing cotyledon areas of various genotypes as indicated. Bars = 1 mm.
Error bars indicate sd. Asterisks indicate significant differences (P < 0.05); n > 30, three biological replicates.
[See online article for color version of this figure.]
hy5-215 Partially Suppresses the Constitutive Photomorphogenic Phenotypes of pifq
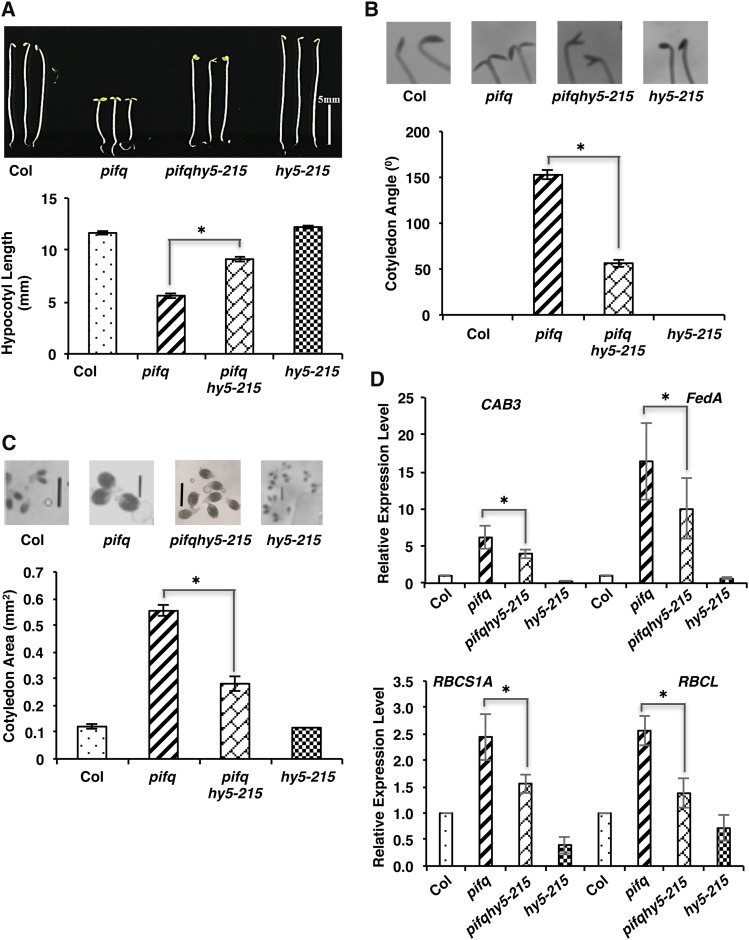
The suppression of cop1-6 pif1 phenotypes by hy5-215 shown above could still be due to the suppression of only the cop1-6 phenotypes by hy5-215, as shown previously (Ang et al., 1998). To provide direct genetic evidence that the increased abundance of HY5 in pifq in the dark contributes to the constitutive photomorphogenic phenotypes of pifq, we generated the pifq hy5-215 quintuple mutant. Phenotypic analyses showed that both the deetiolation and gene expression phenotypes are partially suppressed in the pifq hy5-215 quintuple mutant compared with those in the pifq background (Figures 6A to 6D). These data strongly suggest that the increased amount of HY5 in pifq in the dark contributes to the constitutive photomorphogenic phenotypes of pifq in darkness.
Figure 6.
hy5-215 Partially Suppresses the Constitutive Photomorphogenic Phenotypes of pifq.
(A) Top, photographs of 5-d-old dark-grown seedlings of wild type (Col), pifq, pifq hy5-215, and hy5-215. Bottom, bar graph showing hypocotyl lengths of various genotypes as indicated.
(B) Top, photographs of cotyledon angles of 5-d-old dark-grown seedlings. Bottom, bar graph showing cotyledon angles of various genotypes as indicated.
(C) Top, photographs of cotyledon areas of 5-d-old dark-grown seedlings. Bottom, bar graph showing cotyledon areas of various genotypes as indicated. Bars = 1 mm.
Error bars indicate sd. Asterisks indicate significant differences (P < 0.05); n > 30, three biological replicates.
(D) Bar graphs showing the expression of CAB3, FedA, RBCS1A, and RBCL transcript levels in the wild type, pifq, pifq hy5-215, and hy5-215 as indicated. Total RNA was isolated from 4-d-old dark-grown seedlings for qRT-PCR assays (n = 3 independent biological repeats). PP2A was used as an internal control. The wild-type value was set as 1, and the relative gene expression levels were calculated. Error bars indicate sd. Asterisks indicate significant differences (P < 0.05).
[See online article for color version of this figure.]
PIF1 Interacts with COP1, HY5, and SPA1
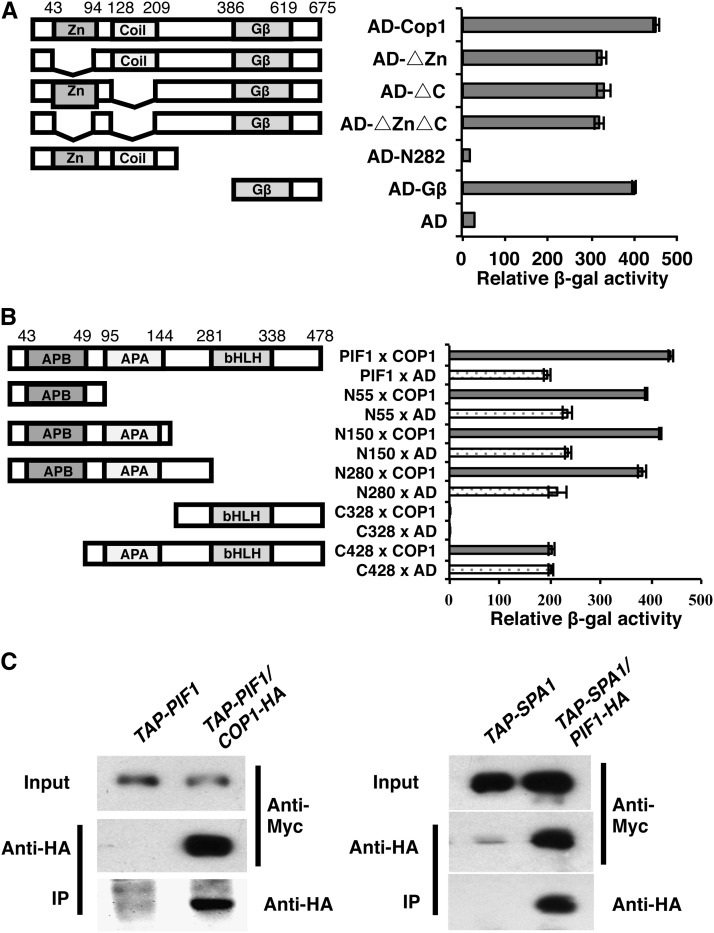
Because HY5 is more abundant in cop1 pif1 as well as in pifq, we examined if PIF1 could interact with COP1, SPA1, and HY5. Yeast two-hybrid assays show that PIF1 robustly interacts with COP1 (Figures 7A and 7B). Domain-mapping analyses show that the WD40 repeat domain of COP1 is both necessary and sufficient for the interaction with full-length PIF1 (Figure 7A). Conversely, the N-terminal 55 amino acids containing the active phytochrome binding (APB) domain of PIF1 is both necessary and sufficient for the interaction with full-length COP1 (Figure 7B; Supplemental Figure 6). Recently, the bHLH domain of PIF1 was shown to interact with the bZIP domain of HY5 in vitro and in vivo (Chen et al., 2013). We also examined the interaction between PIF1 and HY5 using yeast two-hybrid assays. The results show that PIF1 interacts with the bZIP domain of HY5, confirming the previous report (Supplemental Figure 7).
Figure 7.
PIF1 Interacts with COP1 and SPA1.
(A) The WD40 repeat domain of COP1 is necessary and sufficient to interact with PIF1 in yeast two-hybrid assays. The left panel shows the full-length and various deletion fragments of AD-COP1 fusion constructs. The zinc binding ring finger (Zn), the coiled-coil domain (Coil), and the WD-40 repeats (Gβ) of COP1 are as indicated. The right panel shows the corresponding β-galactosidase activities with LexA–full-length PIF1. Error bars represent sd (n = 3).
(B) The N-terminal 55 amino acids containing the APB domain of PIF1 is necessary and sufficient for the interaction with COP1 in yeast two-hybrid assays. The left panel shows the full-length and various deletion fragments of LexA-PIF1 fusion constructs. The APB, the active phyA binding (APA), and the bHLH domains of PIF1 are as indicated. The right panel shows the corresponding β-galactosidase activities with AD–full-length COP1. Error bars represent sd (n = 3).
(C) PIF1 interacts with COP1 and SPA1 in vivo. The left panel illustrates the interaction between PIF1 and COP1 in transgenic plants. Plants expressing TAP-PIF1 from the native PIF1 promoter and COP1-HA from the constitutively active 35S promoter or plants expressing only TAP-PIF1 were grown in the dark for 4 d before protein extraction. TAP-PIF1 was immunoprecipitated (IP) using anti-HA antibody and immunoblotted using anti-Myc or anti-HA antibody. The right panel illustrates the interaction between PIF1 and SPA1 in transgenic plants. Plants expressing PIF1-HA from the 35S promoter and TAP-SPA1 from the 35S promoter or plants expressing only TAP-SPA1 were grown in the dark for 4 d before protein extraction. TAP-SPA1 was immunoprecipitated using anti-HA antibody and immunoblotted using anti-Myc or anti-HA antibody.
To examine the physical interactions between PIF1 and COP1/SPA1 in vivo, we expressed fusion proteins in transgenic plants. In vivo coimmunoprecipitation (co-IP) assays show that COP1-HA (for hemagglutinin) robustly interacts with TAP-PIF1 (Figure 7C, left). In addition, PIF1-HA robustly coimmunoprecipitates TAP-SPA1, suggesting that PIF1-HA interacts with TAP-SPA1 in vivo (Figure 7C, right). Taken together, the yeast two-hybrid and in vivo co-IP assays provide strong evidence that PIF1 can associate with COP1, HY5, and SPA1.
PIF1 Enhances the Substrate Recruitment of COP1
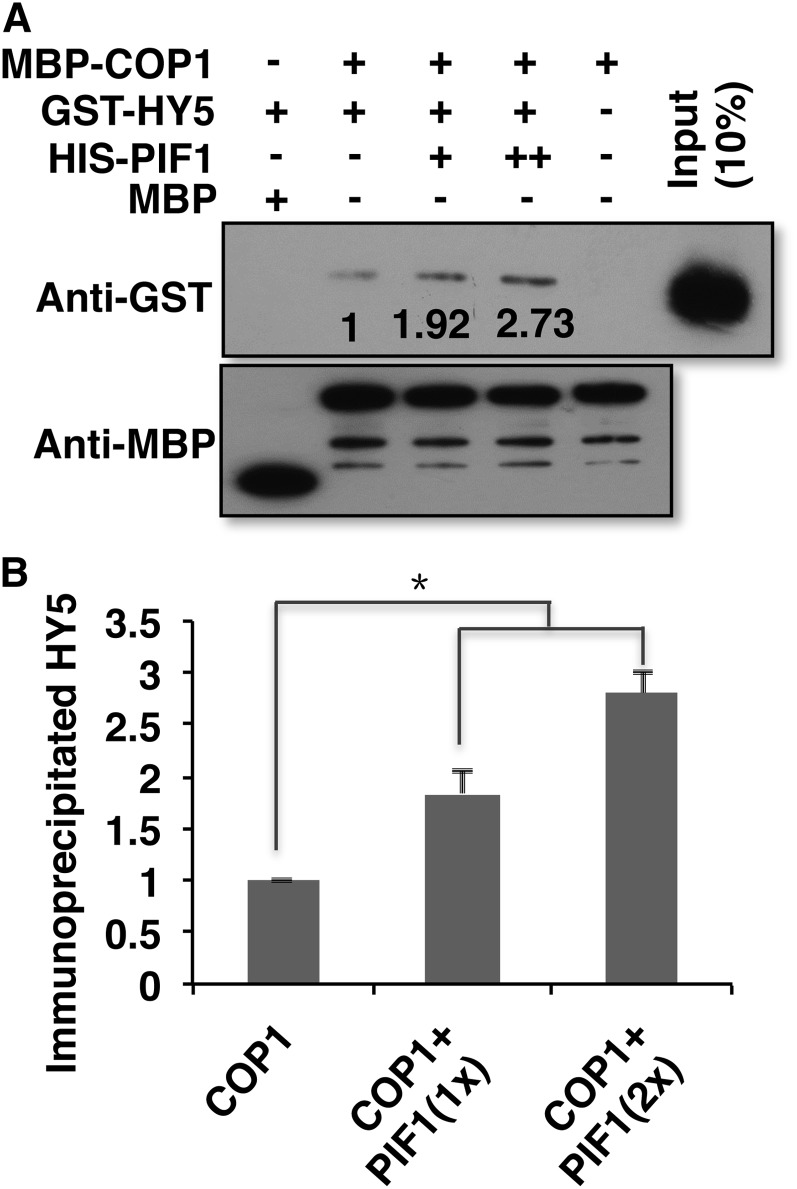
Because PIF1 interacts with both COP1 and HY5, and HY5 is more abundant in the cop1-6 pif1 background compared with the cop1-6 and pif1 backgrounds in the dark (Figure 3), we investigated the biochemical mechanisms by which PIF1 promotes the COP1-mediated degradation of HY5. To examine if PIF1 increases the substrate availability of COP1, we performed in vitro co-IP assays between COP1 and HY5 in the absence and presence of increasing concentrations of PIF1. The results show that PIF1 enhances the interaction between COP1 and HY5 more than 2-fold in vitro (Figures 8A and 8B). These data suggest that the enhanced interaction between COP1 and HY5 in the presence of PIFs might contribute to the enhanced degradation of HY5 by COP1 in the dark.
Figure 8.
PIF1 Promotes the Interaction between COP1 and HY5 in an in Vitro Pull-Down Assay.
Recombinant MBP-COP1, HIS-PIF1, and GST-HY5 fusion proteins were purified from Escherichia coli.
(A) GST-HY5 was precipitated by MBP-COP1 using maltose agarose beads in the absence and presence of increasing concentrations of HIS-PIF1. The pellet fraction was eluted and analyzed by immunoblotting using anti-GST and anti-MBP antibodies. Numbers on the blot indicate fold induction of the interaction between COP1 and HY5.
(B) Bar graph showing the enhancement of the interaction between COP1 and HY5 in the presence of PIF1. Error bars indicate se (n = 3). The asterisk indicates a significant difference (P < 0.05).
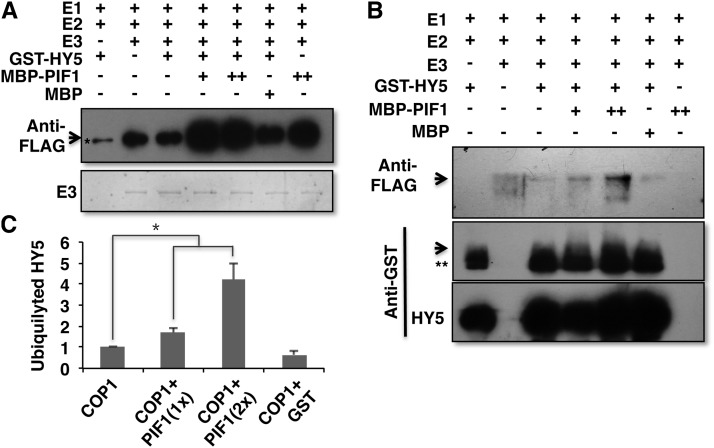
PIF1 Enhances the Autoubiquitylation and Transubiquitylation Activities of COP1
Previously, COP1 displayed ubiquitin ligase activity in vitro (Saijo et al., 2003; Seo et al., 2003). COP1 showed both autoubiquitylation and transubiquitylation to HY5, HFR1, LAF1, and others in vitro (Saijo et al., 2003; Seo et al., 2003; Yang et al., 2005; Lau and Deng, 2012). To examine the autoubiquitylation of COP1, we performed in vitro ubiquitylation assays in the absence and presence of increasing concentrations of PIF1. The results show that PIF1 enhances the autoubiquitylation activity of COP1 in a concentration-dependent manner (Figure 9A). HY5 and a control protein (maltose binding protein [MBP]) did not display any enhancement, as observed previously (Saijo et al., 2003), suggesting that PIF1 specifically enhances the autoubiquitylation activity of COP1.
Figure 9.
PIF1 Enhances the Autoubiquitylation and Transubiquitylation Activity of COP1.
Recombinant MBP-COP1, MBP-PIF1, and GST-HY5 fusion proteins were purified from E. coli.
(A) PIF1 promotes autoubiquitylation of COP1 in vitro. An in vitro ubiquitylation assay was performed using MBP-COP1 as E3, FLAG-ubiquitin, GST-HY5, and increasing concentrations of MBP-PIF1. MBP was used as a control. The arrow indicates autoubiquitylated MBP-COP1 detected by immunoblot using anti-FLAG antibody. The amount of MBP-COP1 (E3) in each lane is shown below on a Coomassie blue–stained gel. The asterisk indicates a nonspecific band.
(B) PIF1 promotes ubiquitylation of HY5 by COP1 in vitro. An in vitro ubiquitylation assay was performed as described above. Top, the arrow indicates ubiquitylated GST-HY5 detected by anti-FLAG antibody. Middle, the arrow indicates ubiquitylated GST-HY5 detected by immunoblot using anti-GST antibody. The double asterisk indicates a nonspecific band. Bottom, the amount of GST-HY5 in each lane is shown as detected by anti-GST antibody.
(C) Quantitation of the ubiquitylated HY5 level in the absence or presence of increasing concentrations of PIF1 or GST as a control protein. Error bars indicate se (n = 3). The asterisk indicates a significant difference (P < 0.05).
To investigate the transubiquitylation activity of COP1 to HY5, we performed in vitro transubiquitylation assays as described previously (Saijo et al., 2003; Seo et al., 2003). Immunoblotting with anti-FLAG antibody detecting ubiquitylated proteins showed that PIF1 enhances the transubiquitylation of COP1 to HY5 in a concentration-dependent manner (Figure 9B, top panel). Immunoblotting with anti-GST antibody also displayed the ubiquitylated GST-HY5 (Figure 9B, middle panel), where only the monoubiquitylation of GST-HY5 was observed under these conditions. This is consistent with the previous in vitro results showing only the monoubiquitylation of GST-HY5 by COP1 (Saijo et al., 2003). Quantitation of the transubiquitylated bands in the absence and presence of PIF1 shows that PIF1 enhances the transubiquitylation of HY5 by COP1 ∼2- to 4-fold in a concentration-dependent manner (Figure 9C). Overall, PIF1 promotes the COP1-mediated degradation of HY5 by increasing the affinity of COP1 to HY5, the autoubiquitylation of COP1, and the transubiquitylation activity of COP1 to HY5.
DISCUSSION
The genetic and biochemical data presented here provide strong evidence that PIFs and COP1/SPA proteins, two unrelated groups of negative regulators of photomorphogenesis, function independently as well as synergistically to repress photomorphogenesis in the dark (Supplemental Figure 8). Genetic analyses show that cop1-6 in combination with any of the three pif single mutants (pif1, pif3, and pif4) displayed synergistic photomorphogenic phenotypes, including the visible morphological (hypocotyl lengths, cotyledon angles, and cotyledon areas) and molecular (gene expression) phenotypes in the dark compared with cop1-6, pif1, pif3, and pif4 mutants (Figures 1 and 2; Supplemental Figures 2 to 4). Similarly, spa123 pif1 displayed strong synergistic morphological and molecular phenotypes compared with spa123 and pif1 (Figures 1 and 2; Supplemental Figures 2 to 4). HY5, a positively acting transcription factor, is synergistically stabilized posttranslationally in the cop1-6 pif1, cop1-4 pif1, and spa123 pif1 mutants compared with their genetic parents (Figure 3). PIF1 forms complexes with COP1, SPA1, and HY5 in yeast and in vivo (Figure 7; Supplemental Figures 6 and 7). In addition, hy5-215 suppresses the synergistic promotion of photomorphogenesis in the cop1-6 pif1 double mutants (Figure 5). Although the above data provide strong evidence that PIFs and COP1/SPA proteins function synergistically in repressing photomorphogenesis in the dark, the results with the cop1-4 allele were not as conclusive. The cop1-4 allele is predicted to encode a truncated protein lacking the WD40 region (PIF and HY5 interaction domain), raising the possibility that the observed synergistic phenotypes might be PIF independent. However, the truncated COP1-4 protein still has the interaction domain for SPA proteins and the E2 enzyme that binds to the RING domain of COP1 (McNellis et al., 1994). Because PIF1 interacts with SPA1 (Figure 7), one possibility is that this partial protein is still able to recruit HY5 through SPA and PIFs to promote the degradation of HY5. The HY5 level in cop1-4 pif1 versus cop1-4 as shown in Figure 3A supports this conclusion. Taken together, the comprehensive data presented here strongly suggest that PIFs and COP1/SPA proteins synergistically repress photomorphogenesis in the dark.
PIFs have been shown to regulate a large number of genes directly and indirectly in the dark (Leivar et al., 2009; Shin et al., 2009; Zhang et al., 2013). The majority of these genes promote photomorphogenic development and are repressed in the dark. Light-induced degradation of PIFs results in derepression of these genes and thereby promotes photomorphogenesis (Leivar and Quail, 2011). Therefore, the cop-like phenotype of pifq was thought to be mainly due to the release of PIFs’ transcriptional repression activity (Leivar et al., 2009; Shin et al., 2009; Leivar and Quail, 2011; Leivar and Monte, 2014). Strikingly, the genetic and biochemical data presented here show that the PIF-mediated repression of photomorphogenesis is also due to a posttranslational destabilization of HY5 by PIFs in the dark (Figures 3 and 4). HY5 is progressively more stabilized in the double, triple, and quadruple pif mutants compared with the wild type and the pif single mutants in the dark, paralleling the increasing photomorphogenic phenotypes of the double, triple, and quadruple pif mutants (Figure 4; Supplemental Figures 2B to 4B) (Leivar et al., 2008b; Chen et al., 2013). Moreover, hy5 suppresses both the morphological and molecular phenotypes of pifq in the dark (Figure 6). These data are consistent with a previous observation that the HY5 level directly correlates with the degree of photomorphogenesis (Figures 3 and 4) (Osterlund et al., 2000). Therefore, the cop-like phenotype of pifq is largely due to an increased abundance of HY5 in the dark. An additional level of complexity is that both PIFs and HY5 bind to the G-box (CACGTG) DNA sequence element in their target genes (Martínez-García et al., 2000; Jiao et al., 2007; Leivar et al., 2009; Shin et al., 2009; Zhang et al., 2011, 2013; Hornitschek et al., 2012; Chen et al., 2013), potentially regulating an overlapping set of target genes. Further studies are necessary to distinguish whether the large number of PIF target genes is directly regulated by PIFs and/or indirectly by destabilization of HY5, which can bind to the same target genes.
The biochemical mechanisms by which PIFs destabilize HY5 appear to operate at least at three levels. First, PIF1 increases the affinity of COP1 for HY5, thereby increasing the substrate availability of COP1 for ubiquitylation (Figures 8A and 8B). Second, PIF1 promotes the autoubiquitylation of COP1 (Figure 9A). Finally, PIF1 also promotes the transubiquitylation of HY5 by COP1 (Figures 9B and 9C). These data suggest that PIFs function as integral cofactors for COP1 in regulating substrate degradation through the 26S proteasome pathway (Figure 10). A variety of mechanisms have been shown to regulate E3 ligase activity in eukaryotic cells. These include the enhancement of substrate recruitment and the stimulation of the autoubiquitylation and transubiquitylation activity of an E3 ligase. For example, Yin Yang enhances the affinity between p53 and its E3 ligase Human double minute2, thereby increasing the degradation of p53 (Sui et al., 2004). BRCA1-associated RING domain protein1, a ring finger protein, interacts with Breast cancer 1 (BRCA1), another ring finger E3 ligase, and this interaction enhances the autoubiquitylation of BRCA1 (Xia et al., 2003). Casitas B-lineage lymphoma c (Cbl-c), a ring ubiquitin E3 ligase, interacts with the Linl-1/Isl-1/Mec-3 domain–containing protein Hydrogen peroxide-inducible clone5 (Hic-5). Interaction between these proteins enhances the autoubiquitylation and transubiquitylation of Cbl-c (Ryan et al., 2012). In Arabidopsis, PIFs have been shown to promote the ubiquitylation of type II photoreceptors by COP1 in vitro and thereby destabilize phyB and other type II phytochromes under prolonged light conditions in a redundant manner (Leivar et al., 2008a; Jang et al., 2010). In addition, SPA proteins also promote the E3 ligase activity of COP1 (Saijo et al., 2003; Seo et al., 2003). However, these studies only showed PIF- and SPA-mediated enhancement of substrate ubiquitylation by COP1, possibly by increasing substrate recruitment. Our data suggest that PIFs utilize all three mechanisms to regulate the E3 ligase activity of COP1. The demonstration that PIF1 enhances substrate recruitment and stimulates the autoubiquitylation and transubiquitylation activity of COP1 suggests that PIF1 has multiple layers of regulation that potentially increase the diversity of COP1 substrates as well as the strength of their regulation by COP1 to fine-tune photomorphogenesis in plants. Because COP1 is conserved in plants and vertebrates (Yi and Deng, 2005), similar mechanisms also might function to fine-tune COP1-regulated processes in vertebrates. In addition, COP1 has been shown to target a plethora of substrates involved in various biological processes in plants (Lau and Deng, 2012). If PIFs interact with any of those substrates, PIFs might modulate the COP1 activity toward those substrates to optimize plant growth and development in response to light. Further studies are necessary to determine if PIFs have a much broader role in regulating plant growth and development than described previously.
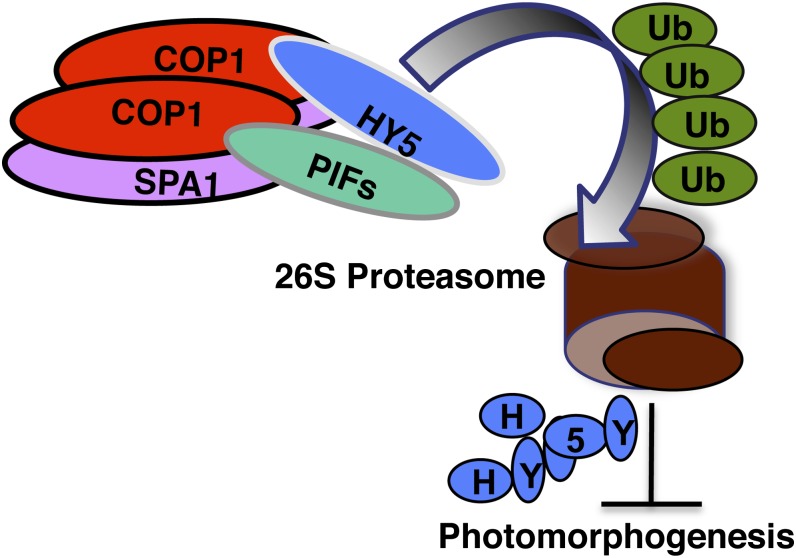
Figure 10.
Model of How PIF1 Promotes Substrate Recruitment and Autoubiquitylation and Transubiquitylation of HY5 to Repress Photomorphogenesis in the Dark.
The N-terminal 55 amino acids containing the APB domain of PIF1 interacts with the WD40 repeat domain of COP1, and the bHLH domain of PIF1 interacts with the bZIP domain of HY5 (Fig. 7; Supplemental Figure 7) (Chen et al., 2013). PIF1 also interacts with full-length SPA1 in vivo (Fig. 7). The N-terminal domain of HY5 interacts with the WD40 repeat domain of both COP1 and SPA1. In addition, both SPA1 and COP1 interact through their coiled-coil domains. The resulting complex promotes ubiquitylation and subsequent degradation of HY5 through the 26S proteasome–mediated pathway.
[See online article for color version of this figure.]
METHODS
Plant Materials, Growth Conditions, and Measurements
Seeds of wild-type (Col-0) Arabidopsis thaliana and various mutants (pif1, pif3, pif4, pif13, pif34, pif45, pif134, pif135, pif145, pif345, pifq, cop1-4, cop1-6, spa123, and hy5-215) in the Col-0 background were used (McNellis et al., 1994; Oyama et al., 1997; Laubinger et al., 2004; Leivar et al., 2008b). For the generation of different cop1, spa123, pif, and hy5-215 mutant combinations, cop1 and spa123 were first crossed with pif1, pif3, and pif4 to generate cop1-4 pif1, cop1-6 pif1, cop1-6 pif3, cop1-6 pif4, and spa123 pif1. The cop1-4 pif1 and cop1-6 pif1 double mutants were crossed with pifq and hy5-215 to obtain the F1 generation. Through genotyping and phenotypic characterization of the F2 population, we identified different mutant combinations (cop1-4 pif13, cop1-4 pif15, cop1-4 pif135, cop1-4 pifq, cop1-6 pif13, cop1-6 pif14, cop1-6 pif15, cop1-6 pif134, cop1-6 pif135, cop1-6 pif145, cop1-6 pifq, and cop1-6 pif1 hy5-215). To generate pifq hy5, pifq was crossed to hy5-215. pifq hy5 was selected by genotyping a large F2 population and further confirmed by genotyping and phenotypic analyses.
Plants were grown in Metro-Mix 200 soil (Sun Gro Horticulture) under 24 h of light at 24 ± 0.5°C. Seeds were surface-sterilized and plated on Murashige and Skoog (MS) growth medium containing 0.9% agar without Suc as described (Shen et al., 2005). After 3 to 4 d of moist chilling at 4°C in the dark to stratify, seeds were exposed to 3 h of white light at room temperature before being placed in the dark.
To measure hypocotyl lengths, cotyledon areas, and cotyledon angles, digital photographs of 5-d-old dark-grown seedlings were taken, and at least 30 seedlings were measured using the publicly available software ImageJ (http://rsb.info.nih.gov/ij/). The experiments were repeated at least three times.
To measure anthocyanin content, 50 seeds per genotype were plated in triplicate on growth medium supplemented with 2% Suc and induced to germinate as described above. Subsequently, plates were kept in darkness for 3 d. Anthocyanins were extracted under dim green safelight, and anthocyanin content was determined spectroscopically as described (Schmidt and Mohr, 1981).
To measure chlorophyll and carotenoid contents, the same amounts of seeds were surface-sterilized and plated on MS growth medium without Suc on filter paper. Plates were kept in darkness for 2.5 d before being exposed to white light for 5 h. To extract chlorophyll and carotenoid, 50 to 100 mg of fresh tissue was homogenized in liquid nitrogen, and then 400 μL of methanol was added to the powdered tissue and resuspended well. The samples were wrapped in aluminum foil to protect them from light and vortexed for 10 min. Then, 400 μL of 50 mM Tris-HCl, pH 7.5, and 1 M NaCl were added, and the samples were vortexed again for 10 min. Finally, 400 μL of chloroform was added before vortexing again for 5 min. The samples were left on ice for an additional 5 min before centrifuging at 16,000g for 5 min. The organic bottom phase was collected and dried in a SpeedVac at room temperature. The samples were resuspended in 50 to 150 μL of ethyl acetate, and then 750 μL of acetone was added before measuring absorbance at λ = 470, 644.8, and 661.8 nm to calculate the chlorophyll and carotenoid contents as described (Toledo-Ortiz et al., 2010).
The seed germination assay was performed as described (Oh et al., 2004). Briefly, triplicates of 60 seeds for each genotype were surface-sterilized and plated on MS medium within 1 h. The plates were then treated with far-red light (34 μmol·m−2·s−1) for 5 min before being placed in the dark at 21°C. Germination was scored after 6 d of growth.
RNA Extraction and Quantitative RT-PCR
The quantitative RT-PCR (qRT-PCR) analysis was performed as described with minor variations (Toledo-Ortiz et al., 2010). Total RNA was isolated from 4-d-old dark-grown seedlings using the Spectrum Plant Total RNA Kit (Sigma-Aldrich). One microgram of total RNA was treated with DNase I to eliminate genomic DNA and then reverse transcribed using SuperScript III (Life Technologies) as per the manufacturer’s protocol. Real-time PCR was performed using the Power SYBR Green RT-PCR Reagents Kit (Applied Biosystems) in a 7900HT Fast Real-Time PCR machine (Applied Biosystems). PP2A was used as a control to normalize the expression data. The resulting cycle threshold values were used to calculate the levels of expression of different genes relative to PP2A, as suggested by the manufacturer (Applied Biosystems). Primer sequences used for qRT-PCR are listed in Supplemental Table 1.
Construction of Vectors and Generation of Transgenic Plants
The full-length PIF1 open reading frame (ORF) was amplified by PCR using the primers listed in Supplemental Table 1 and ligated into pENTR using the pENTR/D-TOPO Cloning Kit (Life Technologies; catalog No. K2400-20). The pENTR-PIF1 vector was recombined with pGWB14 (Nakagawa et al., 2007) to fuse a 3× HA tag at the C terminus of PIF1. The PIF1-HA-pGWB14 plasmid was transformed into the TAP-SPA1 transgenic line. Transgenic seeds were selected on hygromycin to obtain homozygous lines with single inserts. The COP1 ORF was PCR amplified and cloned into the pENTR vector as described above, and the resulting pENTR-COP1 vector was recombined with the pGWB14 vector to fuse the 3× HA tag at the C terminus of COP1. The COP1-HA-pGWB14 plasmid was transformed into TAP-PIF1 transgenic plants. Transgenic seeds were selected on hygromycin to obtain homozygous lines with single inserts.
Protein Extraction and Immunoblot Analyses
Seeds of various genotypes were grown in the dark for 5 d. For total protein extraction, 0.2 g of tissue was collected, ground in 800 μL of extraction buffer (1 M MOPS, pH 7.6, 0.5 M EDTA, pH 8, 50% glycerol, 10% SDS, 40 mM β-mercaptoethanol, 1× protease inhibitor cocktail [Sigma-Aldrich; catalog No. 59], 2 mM phenylmethylsulfonyl fluoride, 25 mM β-glycerophosphate, 10 mM NaF, and 2 mM sodium orthovanadate), and cleared by centrifugation at 14,000 rpm for 15 min at 4°C. After being boiled for 3 min, samples were centrifuged for 10 min and the total protein supernatants were separated on 8% SDS-PAGE gels, blotted onto polyvinylidene difluoride (PVDF) membranes, and probed with anti-HY5 (Hardtke et al., 2000) and anti-RPT5 (Enzo Life Sciences) antibodies. For the secondary antibody, anti-rabbit IgG-HRP conjugate (1:50,000) (Kirkegaard and Perry Laboratories) was used. Membranes were developed using the SuperSignal West Pico Chemiluminescent Substrate kit (Pierce Biotechnology) and visualized on x-ray film. The intensity of the HY5 and RPT5 bands from three independent blots was quantified using ImageJ software, and the HY5 values were divided by the RPT5 values to generate a ratio for each sample. The dark control for the wild-type sample was set to 1 from these ratios, and the relative values of the other samples were calculated based on the wild-type values. These relative values are shown as bar graphs in each figure under the blots.
Yeast Two-Hybrid Analyses
The full-length ORF and various truncated forms of PIF1 were amplified by PCR using the primers listed in Supplemental Table 1. These fragments were cloned into pEG202 and pJG4.5 (Ausubel et al., 1994) using the restriction sites included in the primers to generate LexA-PIF1, LexA-PIF1-N55, N150, N280, C328, C428, and AD-PIF1. All the clones were verified by restriction enzyme digestion and sequencing. Different HY5 and COP1 vectors used were as described previously (Ang et al., 1998; Saijo et al., 2003). These vectors were transformed into yeast strain EGY48-0 (Ausubel et al., 1994) and selected on minimal synthetic medium without Ura, His, and Trp for 3 d at 30°C. Colonies were cultured overnight in liquid synthetic medium without Ura, His, and Trp supplemented with 2% (w/v) Glc. Aliquots of overnight cultures were then transferred to medium supplemented with 2% (w/v) Gal and 1% (w/v) raffinose to induce the expression of the prey proteins. A β-galactosidase activity assay was performed as described (Saijo et al., 2003).
In Vivo/in Vitro co-IP Assays
For in vivo co-IP assays, seedlings were pretreated with MG132 as described (Shen et al., 2008). Total proteins were extracted from 0.4 g of dark-grown seedlings with 0.8 mL of native extraction buffer (50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1 mM EDTA, pH 8.0, 0.1% Tween 20, 1× protease inhibitor cocktail [Sigma-Aldrich; catalog No. P9599], 1 mM phenylmethylsulfonyl fluoride, 20 μM MG132, 25 mM β-glycerophosphate, 10 mM NaF, and 2 mM sodium orthovanadate) and cleared by centrifugation at 16,000g for 15 min at 4°C. Anti-HA (Sigma-Aldrich; catalog No. H6908) antibody was incubated with Dynabeads (20 μL/μg antibody; Life Technologies) for 30 min at 4°C, and the beads were washed twice with the extraction buffer to remove the unbound antibody. The bound antibody beads were added to 500 μg of total protein extracts in 0.8 mL and rotated for another 3 h at 4°C in the dark. The beads were collected using a magnet, washed three times with wash buffer, dissolved in 1× SDS loading buffer, and heated at 65°C for 5 min. The immunoprecipitated proteins were separated on a 6.5% SDS-PAGE gel, blotted onto PVDF membranes, and probed with either anti-MYC (Sigma-Aldrich; catalog No. M4439) or anti-HA (Covance; catalog No. 16B12) antibody. Membranes were developed and visualized on x-ray film as described above.
For in vitro co-IP assays, the full-length COP1 ORF was PCR amplified and cloned into pENTR using the pENTR/D-TOPO Cloning Kit (Life Technologies; catalog No. K2400-20). The pENTR-COP1 vector was recombined with the pVP13 destination vector (Jeon et al., 2005) to produce pVP-13-COP1. MBP-COP1 (Seo et al., 2003), GST fusion protein with Arabidopsis HY5 (Hardtke et al., 2000), and HIS-PIF1 (Bu et al., 2011) were prepared as described previously. All protein combinations were incubated with 20 μL of amylose resin in the binding buffer (50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 0.6% Tween 20, and 1 mM DTT) for 3 h. The beads were collected and washed six times with 5 min of rotation each time in binding buffer. The bound HY5 was detected by anti-GST-HRP conjugate (RPN1236; GE Healthcare Bio-Sciences). Membranes were developed and visualized as described above. The intensity of the GST-HY5 band from three independent blots was quantified using ImageJ software. The sample without PIF1 was set to 1 from these ratios, and the relative values of the other samples were calculated based on this value. These relative values are shown as bar graphs.
In Vitro Ubiquitylation Assays
The His-PIF1 (Bu et al., 2011), GST-HY5 (Hardtke et al., 2000), MBP-COP1 (Seo et al., 2003), and E2 At-UBC8 (Lee et al., 2009) were prepared as described previously. All in vitro ubiquitylation assay procedures were performed as described (Saijo et al., 2003). Briefly, 2 μg of FLAG-ubiquitin (U120; Boston Biochem), ∼25 ng of E1 (UBE1, E-305; Boston Biochem), ∼100 ng of E2 (At-UBC8), ∼600 ng of MBP-COP1, ∼400 ng of GST-HY5, and 100 to 200 ng of MBP-PIF1 were used in the reaction. The FLAG-ubiquitin–conjugated HY5 and COP1 were detected by immunoblot with anti-FLAG antibody (F1804; Sigma-Aldrich). Anti-GST-HRP conjugate was used for HY5 detection. The intensity of the GST-HY5 bands detected by anti-FLAG antibody from three independent blots was quantified using ImageJ software. The sample without PIF1 was set to 1, and the relative values of the other samples were calculated based on this value. These relative values are shown as bar graphs.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL data libraries under the following accession numbers: CAB3 (At1g29910), COP1 (At2g32950), CUL4 (At5g46210), FedA (At1g60950), HY5 (At5g11260), PIF1/PIL5 (At2g20180), PIF3 (At1g09530), PIF4 (At2g43010), PIF5/PIL6 (At3g59060), RBCL (Atcg00490), RBCS1A (At1g67090), SPA1 (At2g46340), SPA2 (At4g11110), SPA3 (At3g15354), UBC8 (At5g41700), and PP2A (At1g13320).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. pif1 Enhances the Accumulation of Chlorophyll and Carotenoid in the cop1 and spa123 Backgrounds in the Dark.
Supplemental Figure 2. pifs Enhance the Photomorphogenic Development Synergistically with cop1 and spa123 in the Dark.
Supplemental Figure 3. pifs Increase the Cotyledon Angle of Dark-Grown Seedlings Synergistically with cop1 and spa123.
Supplemental Figure 4. pifs Increase the Cotyledon Area of Dark-Grown Seedlings Synergistically with cop1 and spa123.
Supplemental Figure 5. HY5 mRNA Levels in Various Mutants Compared with the Wild Type.
Supplemental Figure 6. The APB Domain of PIF1 Is Necessary for Interaction with the Full-Length COP1 in Yeast.
Supplemental Figure 7. PIF1 Interacts with the bZIP Domain of HY5 in Yeast.
Supplemental Figure 8. Model Showing How PIFs and COP1-SPA Proteins Function Synergistically as Well as Independently to Repress Photomorphogenesis in the Dark.
Supplemental Table 1. Primer Sequences Used in Experiments Described in the Text.
Supplementary Material
Acknowledgments
We thank the members of the Huq laboratory for critical reading of the article, In-Cheol Jang for technical advice on the ubiquitylation assay, Peter H. Quail for sharing pif mutants, Ute Hoecker for sharing the spa123 mutant, Woo Taek Kim for sharing the His-AtUBC8 construct, and Hui Shen for sharing the MBP-COP1 construct. This work was supported by the National Institutes of Health (Grant GM47850 to X.W.D.), the National Science Foundation (Grant IOS-1120946), and the Human Frontier Science Program (Grant RGP0025/2013 to E.H.).
AUTHOR CONTRIBUTIONS
X.X., I.P., L.Z., Q.B., and E.H. designed experiments. X.X., I.P., L.Z., and Q.B. carried out experiments. X.H. and X.W.D. contributed new tools. X.X., I.P., L.Z., Q.B., and E.H. analyzed data. X.X., I.P., L.Z., and E.H. wrote the article.
Glossary
- bHLH
basic helix-loop-helix
- Col-0
Columbia-0
- APB
active phytochrome binding
- MS
Murashige and Skoog
- qRT-PCR
quantitative RT-PCR
- ORF
open reading frame
- PVDF
polyvinylidene difluoride
- co-IP
coimmunoprecipitation
Footnotes
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Al-Sady B., Ni W., Kircher S., Schäfer E., Quail P.H. (2006). Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 23: 439–446 [DOI] [PubMed] [Google Scholar]
- Ang L.-H., Chattopadhyay S., Wei N., Oyama T., Okada K., Batschauer A., Deng X.-W. (1998). Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol. Cell 1: 213–222 [DOI] [PubMed] [Google Scholar]
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. (1994). Saccharomyces cerevisiae In Current Protocols in Molecular Biology, Supplement. (New York: John Wiley & Sons), pp. 13.16.12–13.16.14. [Google Scholar]
- Bae G., Choi G. (2008). Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 59: 281–311 [DOI] [PubMed] [Google Scholar]
- Bauer D., Viczián A., Kircher S., Nobis T., Nitschke R., Kunkel T., Panigrahi K.C., Adám E., Fejes E., Schäfer E., Nagy F. (2004). Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16: 1433–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E., Denti S., Catena R., Rossetti G., Polo S., Gasparian S., Putignano S., Rogge L., Pardi R. (2003). Characterization of human constitutive photomorphogenesis protein 1, a RING finger ubiquitin ligase that interacts with Jun transcription factors and modulates their transcriptional activity. J. Biol. Chem. 278: 19682–1969012615916 [Google Scholar]
- Bu Q., Zhu L., Yu L., Dennis M., Lu X., Person M., Tobin E., Browning K., Huq E. (2011). Phosphorylation by CK2 enhances the rapid light-induced degradation of phytochrome interacting factor 1 in Arabidopsis. J. Biol. Chem. 286: 12066–12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillon A., Shen H., Huq E. (2007). Phytochrome interacting factors: Central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 12: 514–521 [DOI] [PubMed] [Google Scholar]
- Chen D., Xu G., Tang W., Jing Y., Ji Q., Fei Z., Lin R. (2013). Antagonistic basic helix-loop-helix/bZIP transcription factors form transcriptional modules that integrate light and reactive oxygen species signaling in Arabidopsis. Plant Cell 25: 1657–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.D., Huang X., Gusmaroli G., Terzaghi W., Lau O.S., Yanagawa Y., Zhang Y., Li J.G., Lee J.H., Zhu D.M., Deng X.W. (2010). Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA complexes to regulate photomorphogenesis and flowering time. Plant Cell 22: 108–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Chory J. (2011). Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 21: 664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Galvão R.M., Li M., Burger B., Bugea J., Bolado J., Chory J. (2010). Arabidopsis HEMERA/pTAC12 initiates photomorphogenesis by phytochromes. Cell 141: 1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X.-W., Matsui M., Wei N., Wagner D., Chu A.M., Feldmann K.A., Quail P.H. (1992). COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a G β homologous domain. Cell 71: 791–801 [DOI] [PubMed] [Google Scholar]
- Dornan D., Wertz I., Shimizu H., Arnott D., Frantz G.D., Dowd P., O’Rourke K., Koeppen H., Dixit V.M. (2004). The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature 429: 86–92 [DOI] [PubMed] [Google Scholar]
- Duek P.D., Fankhauser C. (2005). bHLH class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci. 10: 51–54 [DOI] [PubMed] [Google Scholar]
- Franklin K.A., Quail P.H. (2010). Phytochrome functions in Arabidopsis development. J. Exp. Bot. 61: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão R.M., Li M., Kothadia S.M., Haskel J.D., Decker P.V., Van Buskirk E.K., Chen M. (2012). Photoactivated phytochromes interact with HEMERA and promote its accumulation to establish photomorphogenesis in Arabidopsis. Genes Dev. 26: 1851–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke C.S., Gohda K., Osterlund M.T., Oyama T., Okada K., Deng X.W. (2000). HY5 stability and activity in Arabidopsis is regulated by phosphorylation in its COP1 binding domain. EMBO J. 19: 4997–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques R., Jang I.-C., Chua N.-H. (2009). Regulated proteolysis in light-related signaling pathways. Curr. Opin. Plant Biol. 12: 49–56 [DOI] [PubMed] [Google Scholar]
- Hoecker U. (2005). Regulated proteolysis in light signaling. Curr. Opin. Plant Biol. 8: 469–476 [DOI] [PubMed] [Google Scholar]
- Hoecker U., Tepperman J.M., Quail P.H. (1999). SPA1, a WD-repeat protein specific to phytochrome A signal transduction. Science 284: 496–499 [DOI] [PubMed] [Google Scholar]
- Hornitschek P., Kohnen M.V., Lorrain S., Rougemont J., Ljung K., López-Vidriero I., Franco-Zorrilla J.M., Solano R., Trevisan M., Pradervand S., Xenarios I., Fankhauser C. (2012). Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 71: 699–711 [DOI] [PubMed] [Google Scholar]
- Huq E., Quail P.H. (2002). PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 21: 2441–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq, E., and Quail, P.H. (2005). Phytochrome signaling. In Handbook of Photosensory Receptors, W.R. Briggs and J.L. Spudich, eds (Weinheim, Germany: Wiley-VCH), pp. 151–170. [Google Scholar]
- Huq E., Al-Sady B., Hudson M., Kim C., Apel K., Quail P.H. (2004). Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305: 1937–1941 [DOI] [PubMed] [Google Scholar]
- Jang I.-C., Henriques R., Seo H.S., Nagatani A., Chua N.-H. (2010). Arabidopsis PHYTOCHROME INTERACTING FACTOR proteins promote phytochrome B polyubiquitination by COP1 E3 ligase in the nucleus. Plant Cell 22: 2370–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I.C., Yang J.Y., Seo H.S., Chua N.H. (2005). HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev. 19: 593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon W.B., et al. (2005). High-throughput purification and quality assurance of Arabidopsis thaliana proteins for eukaryotic structural genomics. J. Struct. Funct. Genomics 6: 143–147 [DOI] [PubMed] [Google Scholar]
- Jiao Y., Lau O.S., Deng X.W. (2007). Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 8: 217–230 [DOI] [PubMed] [Google Scholar]
- Lau O.S., Deng X.W. (2012). The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 17: 584–593 [DOI] [PubMed] [Google Scholar]
- Laubinger S., Fittinghoff K., Hoecker U. (2004). The SPA quartet: A family of WD-repeat proteins with a central role in suppression of photomorphogenesis in Arabidopsis. Plant Cell 16: 2293–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.K., Cho S.K., Son O., Xu Z., Hwang I., Kim W.T. (2009). Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell 21: 622–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E. (2014). PIFs: Systems integrators in plant development. Plant Cell 26: 56–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Quail P.H. (2011). PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E., Al-Sady B., Carle C., Storer A., Alonso J.M., Ecker J.R., Quail P.H. (2008a). The Arabidopsis phytochrome-interacting factor PIF7, together with PIF3 and PIF4, regulates responses to prolonged red light by modulating phyB levels. Plant Cell 20: 337–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E., Oka Y., Liu T., Carle C., Castillon A., Huq E., Quail P.H. (2008b). Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 18: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Tepperman J.M., Monte E., Calderon R.H., Liu T.L., Quail P.H. (2009). Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell 21: 3535–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García J.F., Huq E., Quail P.H. (2000). Direct targeting of light signals to a promoter element-bound transcription factor. Science 288: 859–863 [DOI] [PubMed] [Google Scholar]
- Mathews S., Sharrock R.A. (1997). Phytochrome gene diversity. Plant Cell Environ. 20: 666–671 [Google Scholar]
- McNellis T.W., von Arnim A.G., Araki T., Komeda Y., Miséra S., Deng X.W. (1994). Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell 6: 487–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani A. (2004). Light-regulated nuclear localization of phytochromes. Curr. Opin. Plant Biol. 7: 708–711 [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Kurose T., Hino T., Tanaka K., Kawamukai M., Niwa Y., Toyooka K., Matsuoka K., Jinbo T., Kimura T. (2007). Development of series of Gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104: 34–41 [DOI] [PubMed] [Google Scholar]
- Oh E., Kang H., Yamaguchi S., Park J., Lee D., Kamiya Y., Choi G. (2009). Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell 21: 403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Kim J., Park E., Kim J.I., Kang C., Choi G. (2004). PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16: 3045–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund M.T., Deng X.W. (1998). Multiple photoreceptors mediate the light-induced reduction of GUS-COP1 from Arabidopsis hypocotyl nuclei. Plant J. 16: 201–208 [DOI] [PubMed] [Google Scholar]
- Osterlund M.T., Hardtke C.S., Wei N., Deng X.W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466 [DOI] [PubMed] [Google Scholar]
- Oyama T., Shimura Y., Okada K. (1997). The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 11: 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacín M., Legris M., Casal J.J. (2013). COP1 re-accumulates in the nucleus under shade. Plant J. 75: 631–641 [DOI] [PubMed] [Google Scholar]
- Paik I., Yang S., Choi G. (2012). Phytochrome regulates translation of mRNA in the cytosol. Proc. Natl. Acad. Sci. USA 109: 1335–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail P.H. (2000). Phytochrome-interacting factors. Semin. Cell Dev. Biol. 11: 457–466 [DOI] [PubMed] [Google Scholar]
- Ryan P.E., Kales S.C., Yadavalli R., Nau M.M., Zhang H., Lipkowitz S. (2012). Cbl-c ubiquitin ligase activity is increased via the interaction of its RING finger domain with a LIM domain of the paxillin homolog, Hic 5. PLoS ONE 7: e49428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y., Sullivan J.A., Wang H.Y., Yang J.P., Shen Y.P., Rubio V., Ma L.G., Hoecker U., Deng X.W. (2003). The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 17: 2642–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, E., and Nagy, F. (2006). Photomorphogenesis in Plants and Bacteria. (Dordrecht, The Netherlands: Springer). [Google Scholar]
- Schmidt R., Mohr H. (1981). Time-dependent changes in the responsiveness to light of phytochrome-mediated anthocyanin synthesis. Plant Cell Environ. 4: 433–437 [Google Scholar]
- Seo H.S., Watanabe E., Tokutomi S., Nagatani A., Chua N.-H. (2004). Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev. 18: 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H.S., Yang J.Y., Ishikawa M., Bolle C., Ballesteros M.L., Chua N.H. (2003). LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 423: 995–999 [DOI] [PubMed] [Google Scholar]
- Shen H., Moon J., Huq E. (2005). PIF1 is regulated by light-mediated degradation through the ubiquitin-26S proteasome pathway to optimize photomorphogenesis of seedlings in Arabidopsis. Plant J. 44: 1023–1035 [DOI] [PubMed] [Google Scholar]
- Shen H., Zhu L., Castillon A., Majee M., Downie B., Huq E. (2008). Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell 20: 1586–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Khanna R., Carle C.M., Quail P.H. (2007). Phytochrome induces rapid PIF5 phosphorylation and degradation in response to red-light activation. Plant Physiol. 145: 1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Kim K., Kang H., Zulfugarov I.S., Bae G., Lee C.H., Lee D., Choi G. (2009). Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 106: 7660–7665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian C., Kim B.H., Lyssenko N.N., Xu X., Johnson C.H., von Arnim A.G. (2004). The Arabidopsis repressor of light signaling, COP1, is regulated by nuclear exclusion: Mutational analysis by bioluminescence resonance energy transfer. Proc. Natl. Acad. Sci. USA 101: 6798–6802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui G., Affar B., Shi Y., Brignone C., Wall N.R., Yin P., Donohoe M., Luke M.P., Calvo D., Grossman S.R., Shi Y. (2004). Yin Yang 1 is a negative regulator of p53. Cell 117: 859–872 [DOI] [PubMed] [Google Scholar]
- Toledo-Ortiz G., Huq E., Rodríguez-Concepción M. (2010). Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 107: 11626–11631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Pao G.M., Chen H.W., Verma I.M., Hunter T. (2003). Enhancement of BRCA1 E3 ubiquitin ligase activity through direct interaction with the BARD1 protein. J. Biol. Chem. 278: 5255–5263 [DOI] [PubMed] [Google Scholar]
- Yang J.P., Lin R.C., Sullivan J., Hoecker U., Liu B.L., Xu L., Deng X.W., Wang H.Y. (2005). Light regulates COP1-mediated degradation of HFR1, a transcription factor essential for light signaling in Arabidopsis. Plant Cell 17: 804–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C., Deng X.W. (2005). COP1—From plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 15: 618–625 [DOI] [PubMed] [Google Scholar]
- Zhang H.Y., He H., Wang X.C., Wang X.F., Yang X.Z., Li L., Deng X.W. (2011). Genome-wide mapping of the HY5-mediated gene networks in Arabidopsis that involve both transcriptional and post-transcriptional regulation. Plant J. 65: 346–358 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Mayba O., Pfeiffer A., Shi H., Tepperman J.M., Speed T.P., Quail P.H. (2013). A quartet of PIF bHLH factors provides a transcriptionally centered signaling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in Arabidopsis. PLoS Genet. 9: e1003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D.M., Maier A., Lee J.-H., Laubinger S., Saijo Y., Wang H.Y., Qu L.-J., Hoecker U., Deng X.W. (2008). Biochemical characterization of Arabidopsis complexes containing CONSTITUTIVELY PHOTOMORPHOGENIC1 and SUPPRESSOR OF PHYA proteins in light control of plant development. Plant Cell 20: 2307–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.