Abstract
Localized deposits of amyloid structures are observed in various pathological conditions. One example of when local amyloidosis occurs is following repeated insulin injections in diabetic patients. The present study aimed to simulate the same condition in mice. To obtain the amyloid structures, regular insulin was incubated at 57°C for 24 h. The subsequently formed amyloid fibrils were analyzed using the Congo red absorbance test, as well as transmission electron microscopy images, and then injected into mice once per day for 21 consecutive days. Firm waxy masses were developed following this period, which were excised, prepared as thin sections and stained with hematoxylin and eosin, Congo red and Sudan black. Histological examination revealed that these masses contained adipose cells and connective tissue, in which amyloid deposition was visible. Thus, localized amyloidosis was obtained by the subcutaneous injection of insulin fibrils. The present results may be of further use in the development of models of amyloid tumors.
Keywords: amyloidosis, amyloid, insulin, mouse
Introduction
Amyloidosis refers to the extracellular accumulation of amyloid fibrils in various tissues and organs, which may result in the disruption of their function. Amyloid fibrils are a specific type of protein aggregate which, upon deposition in particular tissues, may cause serious illness that is often fatal when major organs are involved, or when the amyloidosis is systemic (1,2).
Amyloid tumors, in contrast to systemic amyloidosis, are localized deposits that are usually accompanied by mild clinical symptoms (3). The presence of these deposits has been reported in a number of anatomical sites, including the orbit, neck, oral cavity, breasts, heart, liver and nervous system (3,4). Common clinical conditions associated with amyloid tumors are long-term hemodialysis, chronic inflammation and infections, including tuberculosis and osteomyelitis. Occasionally, patients have been reported to have amyloid deposits in the soft tissue (5), bladder (6) or the respiratory (7) and gastrointestinal tracts (8) without clinical symptoms.
Diabetes mellitus is a highly prevalent illness and a number of diabetic patients require treatment with subcutaneous insulin injections. A previous study demonstrated that insulin injections are associated with local amyloidosis (9). Usually, these are case reports of patients who have detected an abnormal mass in the injection site (10–12). From these studies, it may be hypothesized that amyloidosis may be observed regardless of the location of the injection (13). For example, insulin amyloids have been observed in the shoulders (2), arm (14) and abdominal walls (14–16), and frequently in the areas surrounding the injection site. The formation of insulin fibrils occurs regardless of the source (14) and type (10,11) of insulin administered.
During the course of studying amyloid formation and its prevention, in vitro tests on the potential toxicity of amyloid fibrils and associated structures are usually performed on cells (17,18) or organelles (19). The subsequent step to this would be experiments on laboratory animals that are models of the specific amyloid-related disease (for example Alzheimer’s disease) (20–23). However, to the best of our knowledge, an animal model for local amyloidosis has not yet been presented. The method established in the present study may be used as a general representation of local amyloidosis, in a similar manner to the use of the in vitro fibril formation of model proteins, as an indicator of the behavior of pathogenic proteins.
Materials and methods
Animals
Eight male NMRI mice weighing 26–28 g (average weight 27 g) were obtained from the Pasteur Institute of Tehran (Tehran, Iran) and acclimatized to the new location for a week. All animals were housed under standard conditions with a 12 h dark/light cycle, 50% humidity, a temperature of 22±2°C and free access to water and food (standard pellet feed). The present study was approved by the Animal Ethics Committee of the Science and Research Branch at the Islamic Azad University (Tehran, Iran).
Amyloid preparation
Regular insulin (EXIR Pharmaceutical Co., Tehran, Iran) was diluted in 50 mM phosphate buffer (pH 7.4) to 0.5 mg/ml. It was incubated at 57°C for 24 h whilst being stirred by Teflon magnetic bars. To confirm the amyloid fibril formation of regular insulin, a Congo red absorbance assay was performed according to a previously described method (24). Images captured under a transmission electron microscopy (TEM; CEM 902A Zeiss microscope; Carl Zeiss, Jena, Germany) were also used as complementary proof. The Congo red kit was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Experimental groups
Eight mice were randomly divided into two groups (n=4). The first group (control) received daily injections of phosphate buffer (an insulin amyloid vehicle) for 21 consecutive days. The second group (experimental) received daily injections of amyloid fibrils (113 μl) subcutaneously for 21 consecutive days. All groups received their normal diet during the experimental period.
Histological processing
After 21 days, the waxy masses were excised. Tissue sections were embedded in paraffin and hematoxylin and eosin (H&E), as well as Congo red and Sudan black staining, were applied to each tissue block. A light microscope (Carl Zeiss AG, Oberkochen, Germany) was used to observe the tissue secion.
Results
Model development
The in vitro incubation of insulin under amyloidogenic conditions resulted in the formation of insulin fibrils. The shift observed in the absorption spectrum of Congo red (Fig. 1), along with the TEM images indicating the presence of distinct fibrils, were taken as validation of insulin amyloid formation. The pre-formed amyloids were subsequently injected into the mice.
Figure 1.
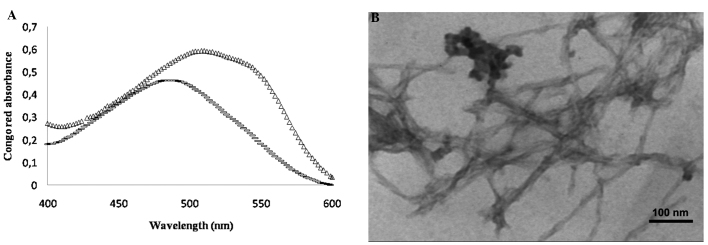
(A) Congo red absorbance spectrum for regular insulin (Δ) after 24 h and Congo red alone (−). (B) Transmission electron microscopy (TEM) image of regular insulin incubated at pH 7.4 and 37°C. The TEM image shows amyloid fibril formed from regular insulin.
Tumor formation
After 21 days, no abnormalities in appearance around the injection site were observed in the control group. An abnormal mass was detected in all mice in the experimental group. Two mice from the experimental group were randomly selected, and a biopsy was performed. The masses formed upon amyloid injection were waxy bodies of a white-yellow color and a size of ~10×10×2 mm3. The masses appeared similar to the areas of lipohypertrophy observed in human diabetic cases as previously reported (9,25).
Tissue staining and analysis
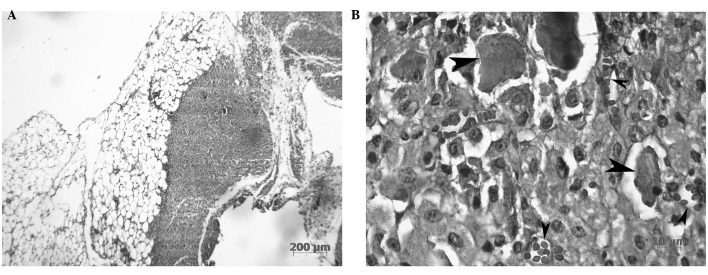
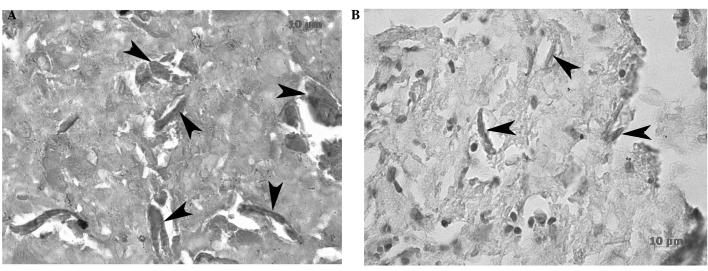
Upon microscopic investigation of the tissues, localized amyloid fibrils surrounded by connective tissue were detected following H&E staining (Fig. 2). Congo red staining was also performed (Fig. 3), since it is a specific stain for the detection of amyloid structures (24). Sudan black staining confirmed that the excised tissue included adipose tissue (Fig. 3). Based on these results, it was concluded that an amyloid tumor containing fibrillar deposits was formed at the injection site of the two mice.
Figure 2.
Light microscopic results of excised mass, revealing amorphous eosinophilic material in the hematoxylin and eosin (H&E) staining. Large and small arrows show amyloid deposits and red blood cells, respectively. (A) and (B) are ×10 and ×40 magnifications, respectively.
Figure 3.
Verification of amyloid deposition in the adipose cells (magnification, ×40). (A) The homogenous depositions are positive with Congo red dye. (B) The adipose cells that surround the amyloid fibril deposition are stained with Sudan black B.
Discussion
More than 20 proteins and 24 protein precursors that are able to form amyloid fibrils in a comparable manner have been reported (26,27). In vivo, the deposition of these fibrils in the extracellular environment results in amyloidosis (28). Usually, soluble proteins become insoluble and are deposited as protein aggregates which then develop into amyloids (29,30). There are various types of amyloidosis which, in general, may be classified as primary (AL) and secondary (AA). AL amyloidosis is related to the deposition of immunoglobulin light chains. AA amyloidosis occurs with chronic disease, especially when an inflammatory process is present (2,12,31). The disease may influence several organs, or may be limited to a particular organ. Symptoms related to amyloidosis depend on the organ involved (32,33).
The skin may become involved with amyloidosis at various levels. Primary cutaneous amyloidosis occurs as nodular, macular and lichen (or papular) amyloidosis (33,34). In the latter two types, amyloid fibrils accumulate in the papillary dermis. An uncommon form of amyloidosis may affect the subcutis, dermis and vascular walls, causing local plasma cell dyscrasia (35,36). Nodular amyloidosis has a higher relapse rate compared with the other forms. The development of local cutaneous disease to a systemic form is rare, but has been reported for nodular amyloidosis (37,38). To the best of our knowledge, no specific therapeutic treatments currently exist for skin amyloidosis and surgical excision is the routine treatment. The method that has been reported in the present study may be expanded and used to test potential treatments for these conditions.
In diabetic patients, a cutaneous amyloid tumor may form at the site of insulin injection (11,25). Within the tumor, lipohypertrophy, which includes the amyloid fibrils, is usually present (11,25). The results of the current study are in accordance with this type of physiopathological finding. From 1983, incidences of amyloidosis in insulin injection sites have been reported in rats (11).
As previously mentioned, there are a limited number of published case reports about cutaneous amyloidosis caused by repeated subcutaneous insulin administration (2,15,14). A limited number of studies have associated cutaneous amyloidosis with non-human insulin, such as porcine insulin, and there are even fewer published studies investigating amyloidosis with human insulin (2,16).
One form of cutaneous amyloidosis is related to local subcutaneous injections of insulin, the incidence rate of which may be underestimated when considering the high prevalence of diabetes mellitus and insulin treatment. The simple method proposed in the present study may be useful in investigating the characteristics of local amyloidosis, as well in the search for potential treatments. The exact mechanism by which insulin-induced amyloidosis occurs remains largely unknown in the scientific literature. Notably, in previous studies, insulin itself was observed to have properties of lipohypertrophy, while the present study used injections of insulin amyloid fibrils and observed the same effect. It has been verified that long-term injection of insulin may result in lipohypertrophy, lipoatrophy and, rarely, infection (25). Further tests are required to ascertain whether insulin amyloid injection may cause the same effects. Another use for the method established in the current study may be to investigate the tendency of various insulin types to cause amyloidosis. Finally, irrespective of the amyloidosis type, the present method may be used to further study local amyloidosis.
Acknowledgements
The authors thank those who assisted in carrying out this study, particularly Dr Aidin Dilmaghanian for providing useful comments and for taking light-microscopic images, as well as Dr Farnaz Banakar and Ms. Raheleh Kheirbakhsh for their valuable assitance with regard to the in vivo experiment.
References
- 1.Husby G. Amyloidosis and rheumatoid arthritis. Clin Exp Rheumatol. 1985;3:173–180. [PubMed] [Google Scholar]
- 2.Sahoo S, Reeves W, DeMay RM. Amyloid tumor: a clinical and cytomorphologic study. Diagn Cytopathol. 2003;28:325–328. doi: 10.1002/dc.10296. [DOI] [PubMed] [Google Scholar]
- 3.Aono J, Yamagata K, Yoshida H. Local amyloidosis in the hard palate: a case report. Oral Maxillofac Surg. 2009;13:119–122. doi: 10.1007/s10006-009-0158-4. [DOI] [PubMed] [Google Scholar]
- 4.Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors. 4th edition. Mosby Elsevier; Philadelphia, PA, USA: 2001. [Google Scholar]
- 5.Krishnan J, Chu WS, Elrod JP, Frizzera G. Tumoral presentation of amyloidosis (amyloidomas) in soft tissues. A report of 14 cases. Am J Clin Pathol. 1993;100:135–144. doi: 10.1093/ajcp/100.2.135. [DOI] [PubMed] [Google Scholar]
- 6.Khan SM, Birch PJ, Bass PS, Williams JH, Theaker JM. Localized amyloidosis of the lower genitourinary tract: a clinicopathological and immunohistochemical study of nine cases. Histopathology. 1992;21:143–147. doi: 10.1111/j.1365-2559.1992.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 7.Ihling C, Weirich G, Gaa A, Schaefer H. Amyloid tumors of the lung - an immunocytoma? Pathol Res Pract. 1996;192:446–452. doi: 10.1016/s0344-0338(96)80006-9. [DOI] [PubMed] [Google Scholar]
- 8.Deans GT, Hale RJ, McMahon RF, Brough WA. Amyloid tumour of the colon. J Clin Pathol. 1995;48:592–593. doi: 10.1136/jcp.48.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sie MP, van der Wiel HE, Smedts FM, de Boer AC. Human recombinant insulin and amyloidosis: an unexpected association. Neth J Med. 2010;68:138–140. [PubMed] [Google Scholar]
- 10.Dische FE, Wernstedt C, Westermark GT, Westermark P, Pepys MB, Rennie JA, Gilbey SG, Watkins PJ. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158–161. doi: 10.1007/BF00276849. [DOI] [PubMed] [Google Scholar]
- 11.Störkel S, Schneider HM, Müntefering H, Kashiwagi S. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108–111. [PubMed] [Google Scholar]
- 12.Swift B. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabet Med. 2002;19:881–882. doi: 10.1046/j.1464-5491.2002.07581.x. [DOI] [PubMed] [Google Scholar]
- 13.Muzaffar M, Ahmad A. The mechanism of enhanced insulin amyloid fibril formation by NaCl is better explained by a conformational change model. PloS One. 2011;6:e27906. doi: 10.1371/journal.pone.0027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yumlu S, Barany R, Eriksson M, Röcken C. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40:1655–1660. doi: 10.1016/j.humpath.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Albert SG, Obadiah J, Parseghian SA, Yadira Hurley M, Mooradian AD. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374–376. doi: 10.1016/j.diabres.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Shikama Y, Kitazawa J, Yagihashi N, Uehara O, Murata Y, Yajima N, Wada R, Yagihashi S. Localized amyloidosis at the site of repeated insulin injection in a diabetic patient. Intern Med. 2010;49:397–401. doi: 10.2169/internalmedicine.49.2633. [DOI] [PubMed] [Google Scholar]
- 17.El-Agnaf OM, Jakes R, Curran MD, Middleton D, Ingenito R, Bianchi E, Pessi A, Neill D, Wallace A. Aggregates from mutant and wild-type α-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of β-sheet and amyloid-like filaments. FEBS letters. 1998;440:71–75. doi: 10.1016/s0014-5793(98)01418-5. [DOI] [PubMed] [Google Scholar]
- 18.Hertel C, Hauser N, Schubenel R, Seilheimer B, Kemp JA. β-amyloid-induced cell toxicity: enhancement of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-dependent cell death. J Neurochem. 1996;67:272–276. doi: 10.1046/j.1471-4159.1996.67010272.x. [DOI] [PubMed] [Google Scholar]
- 19.Meratan AA, Ghasemi A, Nemat-Gorgani M. Membrane integrity and amyloid cytotoxicity: a model study involving mitochondria and lysozyme fibrillation products. J Mol Biol. 2011;409:826–838. doi: 10.1016/j.jmb.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 20.Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996;706:181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto K, Yoshimi K, Tonohiro T, Yamada N, Oda T, Kaneko I. Co-injection of β-amyloid with ibotenic acid induces synergistic loss of rat hippocampal neurons. Neuroscience. 1998;84:479–487. doi: 10.1016/s0306-4522(97)00507-1. [DOI] [PubMed] [Google Scholar]
- 22.Piermartiri TC, Vandresen-Filho S, de Araújo Herculano B, Martins WC, Dal’agnolo D, Stroeh E, Carqueja CL, Boeck CR, Tasca CI. Atorvastatin prevents hippocampal cell death due to quinolinic acid-induced seizures in mice by increasing Akt phosphorylation and glutamate uptake. Neurotox Res. 2009;16:106–115. doi: 10.1007/s12640-009-9057-6. [DOI] [PubMed] [Google Scholar]
- 23.Prediger RD, Franco JL, Pandolfo P, Medeiros R, Duarte FS, Di Giunta G, Figueiredo CP, Farina M, Calixto JB, Takahashi RN, Dafre AL. Differential susceptibility following β-amyloid peptide-(1–40) administration in C57BL/6 and Swiss albino mice: Evidence for a dissociation between cognitive deficits and the glutathione system response. Behav Brain Res. 2007;177:205–213. doi: 10.1016/j.bbr.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Klunk WE, Pettegrew J, Abraham DJ. Quantitative evaluation of congo red binding to amyloid-like proteins with a β-pleated sheet conformation. J Histochem Cytochem. 1989;37:1273–1281. doi: 10.1177/37.8.2666510. [DOI] [PubMed] [Google Scholar]
- 25.Wallymahmed ME, Littler P, Clegg C, Haqqani MT, Macfarlane IA. Nodules of fibrocollagenous scar tissue induced by subcutaneous insulin injections: a cause of poor diabetic control. Postgrad Med J. 2004;80:732–733. doi: 10.1136/pgmj.2004.019547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westermark P, Araki S, Benson MD, Cohen AS, Frangione B, Masters CL, Saraiva MJ, Sipe JD, Husby G, Kyle RA, Selkoe D. Part 1: Amyloid; Nomenclature of amyloid fibril proteins: Report from the meeting of the International Nomenclature Committee on Amyloidosis; August 8–9, 1998; 1999. pp. 63–66. [DOI] [PubMed] [Google Scholar]
- 27.Westermark P, Benson MD, Buxbaum JN, Cohen AS, Frangione B, Ikeda S, Masters CL, Merlini G, Saraiva MJ, Sipe JD. Amyloid fibril protein nomenclature - 2002. Amyloid. 2002;9:197–200. doi: 10.3109/13506120209114823. [DOI] [PubMed] [Google Scholar]
- 28.Cortés A. Primary cutaneous amyloidosis. Dermatologica. 1969;139:109–114. doi: 10.1159/000253900. [DOI] [PubMed] [Google Scholar]
- 29.Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 8th edition. Saunders Elsevier; Philadelphia, PA, USA: 2010. [Google Scholar]
- 30.Rooban T, Saraswathi T, Al Zainab FH, Devi U, Eligabeth J, Ranganathan K. A light microscopic study of fibrosis involving muscle in oral submucous fibrosis. Indian J Dent Res. 2005;16:131–134. doi: 10.4103/0970-9290.29909. [DOI] [PubMed] [Google Scholar]
- 31.Lasagna-Reeves CA, Clos AL, Midoro-Hiriuti T, Goldblum RM, Jackson GR, Kayed R. Inhaled insulin forms toxic pulmonary amyloid aggregates. Endocrinology. 2010;151:4717–4724. doi: 10.1210/en.2010-0457. [DOI] [PubMed] [Google Scholar]
- 32.Hazenberg BP, van Gameren II, Bijzet J, Jager PL, van Rijswijk MH. Diagnostic and therapeutic approach of systemic amyloidosis. Neth J Med. 2004;62:121–128. [PubMed] [Google Scholar]
- 33.Yamamoto T. Amyloidosis in the skin. In: Güvenç IA, editor. Amyloidosis - an Insight to Disease of Systems and Novel Therapies. InTech; Rijecka, Croatia: 2011. pp. 91–104. [Google Scholar]
- 34.Touart DM, Sau P. Cutaneous deposition diseases. Part I. J Am Acad Dermatol. 1998;39:149–171. doi: 10.1016/s0190-9622(98)70069-6. [DOI] [PubMed] [Google Scholar]
- 35.Moon AO, Calamia KT, Walsh JS. Nodular amyloidosis: review and long-term follow-up of 16 cases. Arch Dermatol. 2003;139:1157–1159. doi: 10.1001/archderm.139.9.1157. [DOI] [PubMed] [Google Scholar]
- 36.Truhan AP, Garden JM, Roenigk HH., Jr Nodular primary localized cutaneous amyloidosis: immunohistochemical evaluation and treatment with the carbon dioxide laser. J Am Acad Dermatol. 1986;14:1058–1062. doi: 10.1016/s0190-9622(86)70132-1. [DOI] [PubMed] [Google Scholar]
- 37.Steciuk A, Dompmartin A, Troussard X, Verneuil L, Macro M, Comoz F, Leroy D. Cutaneous amyloidosis and possible association with systemic amyloidosis. Int J Dermatol. 2002;41:127–132. doi: 10.1046/j.1365-4362.2002.01411.x. [DOI] [PubMed] [Google Scholar]
- 38.Woollons A, Black MM. Nodular localized primary cutaneous amyloidosis: a long-term follow-up study. Br J Dermatol. 2001;145:105–109. doi: 10.1046/j.1365-2133.2001.04291.x. [DOI] [PubMed] [Google Scholar]