Abstract
Aim—Rapid differentiation of mycobacterial species at the genomic level.
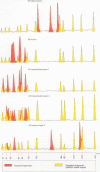
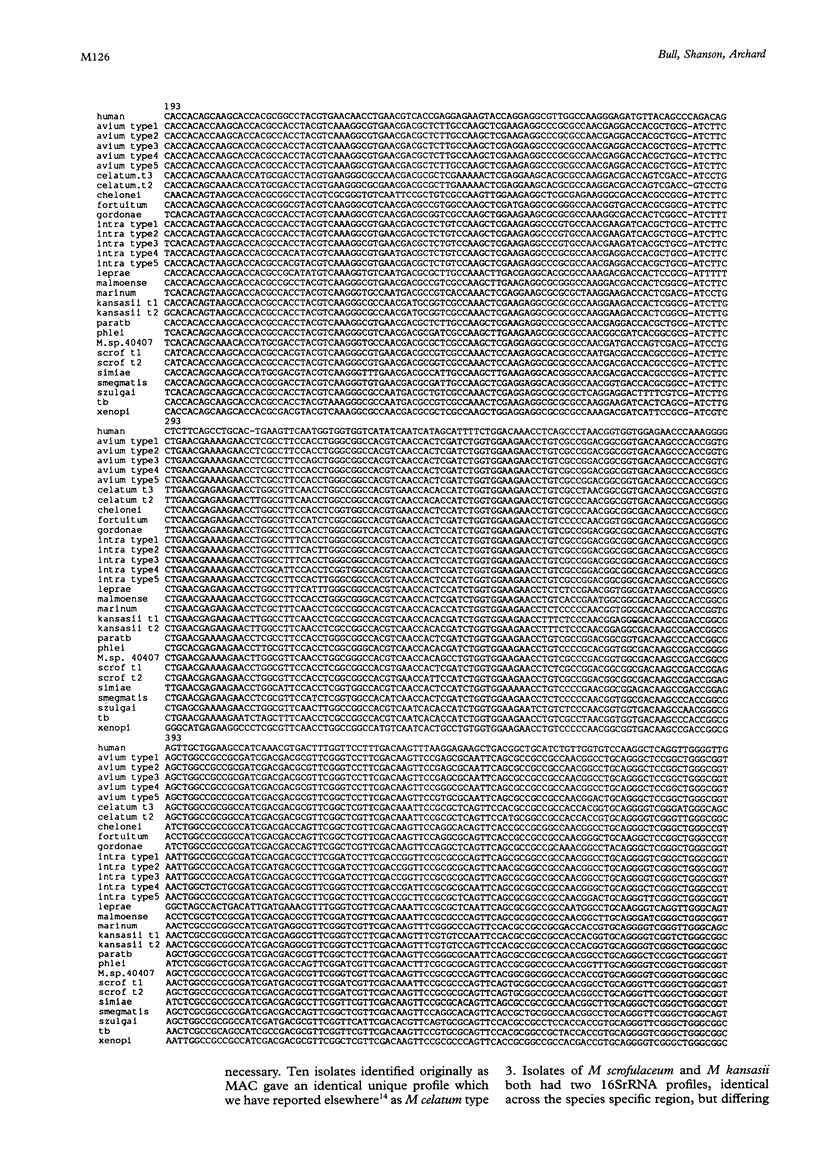
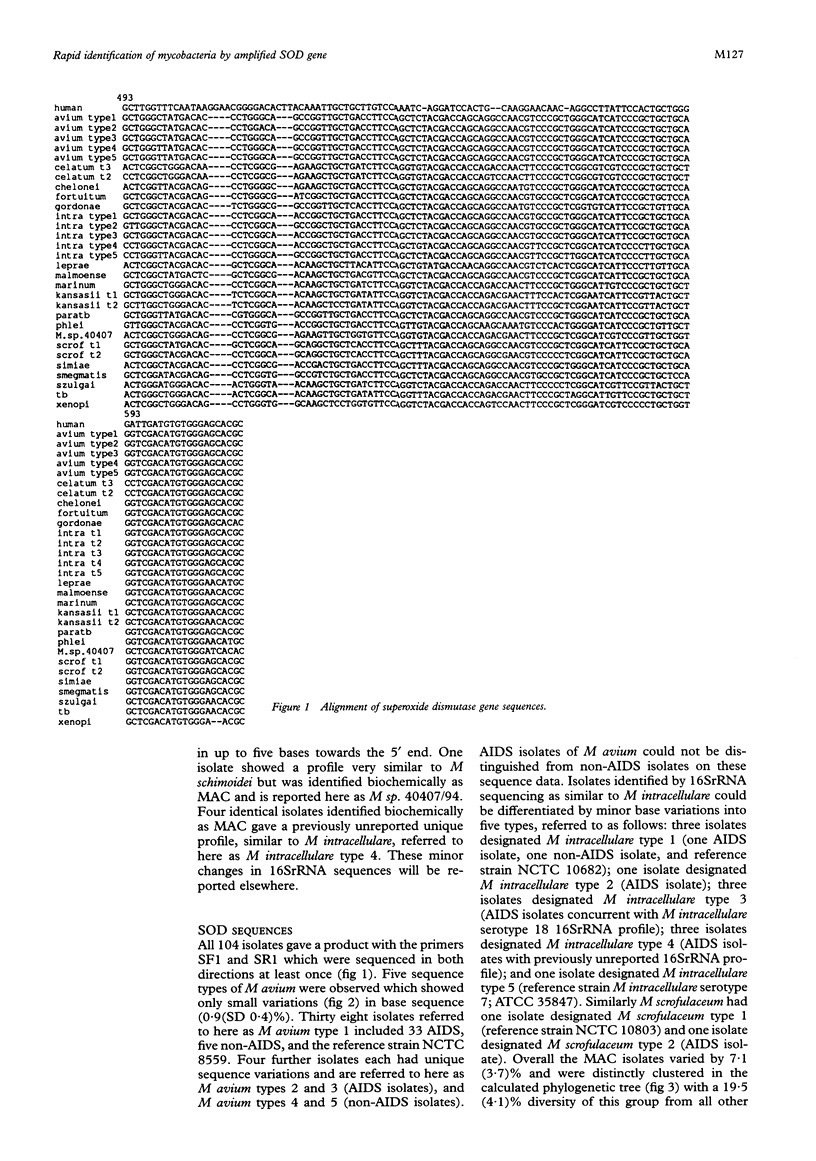
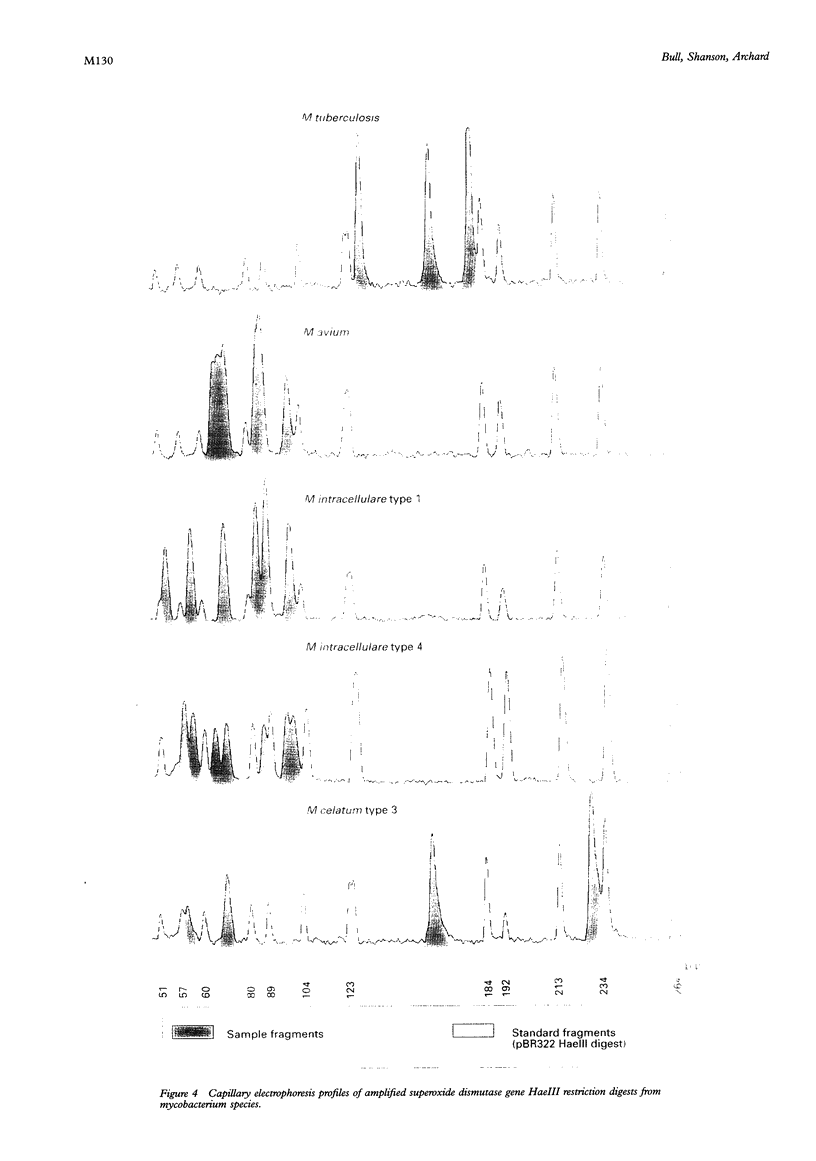
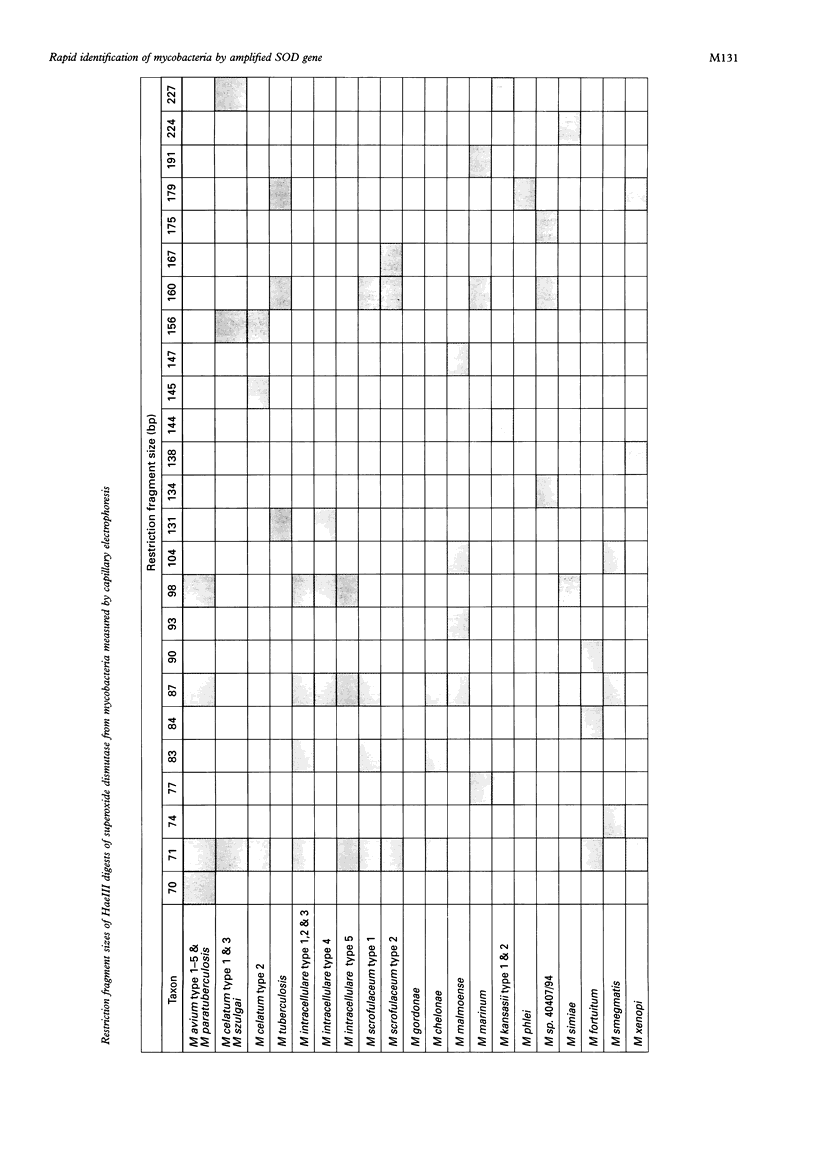
Methods—The manganese superoxide dismutase (SOD) gene (464 bp) and 16SrRNA (353 bp) from 104 isolates (18 species) of mycobacteria were amplified using polymerase chain reaction (PCR). Products were sequenced and a phenogram of SOD sequences derived. PCR products of SOD gene were digested with HaeIII, and restriction fragment profiles visualised using capillary electrophoresis.
Results—Novel SOD sequences were found for M szulgai, M marinum, M phlei, M smegmatis, M chelonei, M paratuberculosis, M malmoense, M intracellulare serotype 7, M intracellulare serotype 18, and M celatum types 1, 2, and 3. Phylogenetic analysis indicated that 18 of 19 species studied had 8-29% interspecies and <6% intraspecies sequence diversity in the SOD gene. No consistent differences were detected between AIDS and non-AIDS isolates. M paratuberculosis showed a unique SOD sequence with a 1·1% (SD 0·5%) diversity from M avium. Capillary electrophoresis profiles were able to differentiate 16 of 18 species within 24 hours.
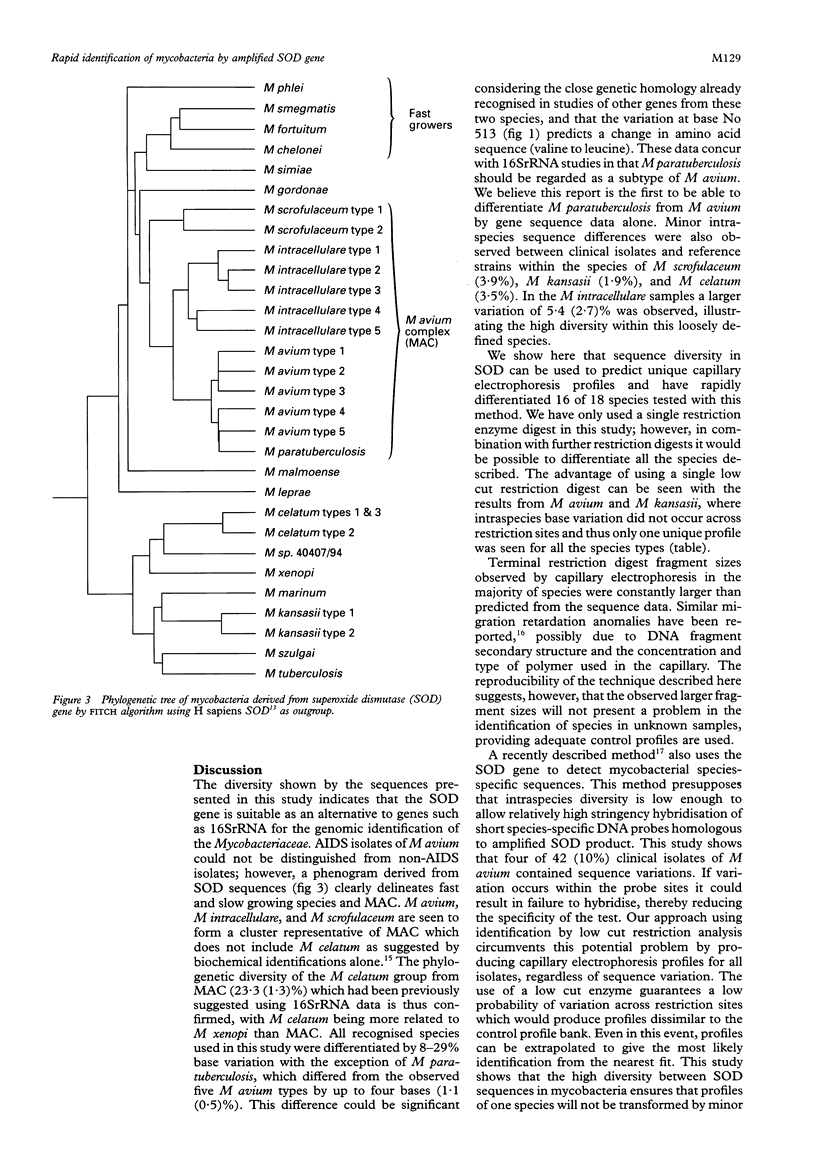
Conclusions—A phenogram of SOD sequences clearly delineated all mycobacterial species and showed two distinct clusters, fast growing species, and the M avium complex (MAC). Within the MAC, M avium (five types), M intracellulare (five types), M scrofulaceum (two types), and M paratuberculosis (one type) could be demonstrated. Phylogenetic diversity of M celatum from MAC, previously suggested by 16SrRNA data, was confirmed. This simple and rapid method for DNA extraction, in conjunction with capillary electrophoresis of SOD restriction fragments, allows rapid identification of mycobacterial isolates.
Keywords: Mycobacteria
Keywords: superoxide dismutase
Keywords: rapid identification
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bull T. J., Shanson D. C. Rapid misdiagnosis by Mycobacterium avium-intracellulare masquerading as tuberculosis in PCR/DNA probe tests. Lancet. 1992 Nov 28;340(8831):1360–1360. doi: 10.1016/0140-6736(92)92550-y. [DOI] [PubMed] [Google Scholar]
- Butler W. R., O'Connor S. P., Yakrus M. A., Smithwick R. W., Plikaytis B. B., Moss C. W., Floyd M. M., Woodley C. L., Kilburn J. O., Vadney F. S. Mycobacterium celatum sp. nov. Int J Syst Bacteriol. 1993 Jul;43(3):539–548. doi: 10.1099/00207713-43-3-539. [DOI] [PubMed] [Google Scholar]
- Crawford J. T. Development of rapid techniques for identification of M. avium infections. Res Microbiol. 1994 Mar-Apr;145(3):177–181. doi: 10.1016/0923-2508(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Crapo J. D. Isolation and characterization of complementary DNAs encoding human manganese-containing superoxide dismutase. FEBS Lett. 1988 Mar 14;229(2):256–260. doi: 10.1016/0014-5793(88)81136-0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A., Yajko D., Northfelt D., Charlebois E., Gary D., Brosgart C., Sanders C. A., Hadley W. K. Randomized, placebo-controlled trial of rifampin, ethambutol, and ciprofloxacin for AIDS patients with disseminated Mycobacterium avium complex infection. J Infect Dis. 1993 Jul;168(1):112–119. doi: 10.1093/infdis/168.1.112. [DOI] [PubMed] [Google Scholar]
- Masur H. Recommendations on prophylaxis and therapy for disseminated Mycobacterium avium complex disease in patients infected with the human immunodeficiency virus. Public Health Service Task Force on Prophylaxis and Therapy for Mycobacterium avium Complex. N Engl J Med. 1993 Sep 16;329(12):898–904. doi: 10.1056/NEJM199309163291228. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Barrow W. W. Cell envelope constituents and the multifaceted nature of Mycobacterium avium pathogenicity and drug resistance. Res Microbiol. 1994 Mar-Apr;145(3):243–261. doi: 10.1016/0923-2508(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Rogall T., Flohr T., Böttger E. C. Differentiation of Mycobacterium species by direct sequencing of amplified DNA. J Gen Microbiol. 1990 Sep;136(9):1915–1920. doi: 10.1099/00221287-136-9-1915. [DOI] [PubMed] [Google Scholar]
- Thangaraj H. S., Lamb F. I., Davis E. O., Colston M. J. Nucleotide and deduced amino acid sequence of Mycobacterium leprae manganese superoxide dismutase. Nucleic Acids Res. 1989 Oct 25;17(20):8378–8378. doi: 10.1093/nar/17.20.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaraj H. S., Lamb F. I., Davis E. O., Jenner P. J., Jeyakumar L. H., Colston M. J. Identification, sequencing, and expression of Mycobacterium leprae superoxide dismutase, a major antigen. Infect Immun. 1990 Jun;58(6):1937–1942. doi: 10.1128/iai.58.6.1937-1942.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Krichevsky M. I., Portyrata D., Jackson C. K. Diagnostic probability matrix for identification of slowly growing mycobacteria in clinical laboratories. J Clin Microbiol. 1984 Oct;20(4):722–729. doi: 10.1128/jcm.20.4.722-729.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz H. M. Capillary electrophoresis as a technique to analyze sequence-induced anomalously migrating DNA fragments. Nucleic Acids Res. 1994 Sep 25;22(19):4002–4008. doi: 10.1093/nar/22.19.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. J., Madej R., Persing D. H. The polymerase chain reaction: clinical applications. Adv Clin Chem. 1992;29:161–196. doi: 10.1016/s0065-2423(08)60224-3. [DOI] [PubMed] [Google Scholar]
- Zolg J. W., Philippi-Schulz S. The superoxide dismutase gene, a target for detection and identification of mycobacteria by PCR. J Clin Microbiol. 1994 Nov;32(11):2801–2812. doi: 10.1128/jcm.32.11.2801-2812.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet G. M., Schukkink R. A., van Gemen B., Schepers P., Klatser P. R. Nucleic acid sequence-based amplification (NASBA) for the identification of mycobacteria. J Gen Microbiol. 1993 Oct;139(10):2423–2429. doi: 10.1099/00221287-139-10-2423. [DOI] [PubMed] [Google Scholar]