Abstract
Recently, we identified procyanidin B2 3,3″-di-O-gallate (B2G2) as most active constituent of grape seed extract (GSE) for efficacy against prostate cancer (PCa). Isolating large quantities of B2G2 from total GSE is labor intensive and expensive, thereby limiting both efficacy and mechanistic studies with this novel anti-cancer agent. Accordingly, here we synthesized gram-scale quantities of B2G2, compared it with B2G2 isolated from GSE for possible equivalent biological activity, and conducted mechanistic studies. Both B2G2 preparations inhibited cell growth, decreased clonogenicity, and induced cell cycle arrest and apoptotic death, comparable to each other, in various human PCa cell lines. Mechanistic studies focusing on transcription factors involved in apoptotic and survival pathways revealed that B2G2 significantly inhibits NF-κB and AP1 transcriptional activity and nuclear translocation of Stat3 in PCa cell lines, irrespective of their functional androgen receptor status. B2G2 also decreased survivin expression which is regulated by NF-κB, AP1 and Stat3, and increased cleaved PARP level. In summary, we report B2G2 chemical synthesis at gram-quantity with equivalent biological efficacy against human PCa cell lines and same molecular targeting profiles at key transcription factors level. The synthetic B2G2 will stimulate more research on prostate and possibly other malignancies in preclinical models and clinical translation.
Keywords: Apoptosis, NF-κB, Stat3, AP1, chemoprevention, prostate cancer, B2G2
1. Introduction
Cancer is one of the foremost causes of non-natural deaths around the world. One form of cancer, prostate cancer (PCa) is the most frequently diagnosed malignancy and second leading cause of death in the US [1]. Statistical estimates for the year 2012 indicate that there would be 241,740 new cases of PCa and 28,170 associated deaths in the US alone [1]. Progression of PCa can take significant time to advance to a castration-resistant PCa, suggesting that a considerable time window is available for various prevention strategies to be employed for its prevention and control [2,3]. Grape seed extract (GSE) is a naturally-occurring dietary agent with significant potential for the prevention and intervention of various malignancies including PCa [4]. For example, several recent studies by us and others have shown the anti-cancer efficacy of GSE against colon, breast, lung, skin, prostate, and head & neck cancer in various in vitro and in vivo models [4–16]. GSE is a complex mixture of polyphenols, and our research group has isolated and characterized various active constituents of GSE, mainly bioactive procyanidins, using a combination of chromatographic separations and, thereafter screened the biological efficacy of the fractions [17–19]. One of the biologically active constitute of GSE, gallic acid, was shown by us to inhibit the growth of DU145 xenografts in nude mice and prostate tumor in TRAMP mice [20,21]. Furthermore, completed studies by us have recently identified that procyanidin B2 3,3″-di-O-gallate (B2G2) accounts for a major biological constituent of GSE, and causes growth inhibition, death and apoptosis induction in human PCa DU145 and LNCaP cells [18,19]. However, to overcome the limitations of isolating gram quantities of B2G2 from the crude mixture, procedures were adapted by us to synthesize multi gram-scale quantities of B2G2 for use in biological studies. The present study compares the anti-PCa efficacy of the GSE-isolated versus the synthetic B2G2. Our results revealed that B2G2, irrespective of these two sources, caused a comparable and significant inhibition of cell growth and proliferation, decreased clonogenic ability, and induced apoptotic death in a panel of human PCa cell lines.
Cell survival machinery is maintained by proper coordination between cell proliferation, differentiation and cell death; however, deregulation of these events is recognized as one of the underlying causes for the development and progression of cancer [22–24]. Recent studies have shown that transcription factors, namely nuclear factor-κB (NF-κB), activator protein1 (AP1) and signal transducer and activator of transcription3 (Stat3), are the major regulators of cellular survival, apoptotic machinery and inflammation; and unrestrained activity of these transcription factors is critical for the growth and progression of some cancers, including PCa [25–30]. Moreover, it is also recognized that persistent activation of NF-κB, AP1 and Stat3 signaling induces survivin expression and confers resistance to apoptosis in cancer cells [17,31,32]. Notably, survivin belongs to the inhibitor of apoptosis (IAPs) family and is a regulator of cell proliferation and cell viability in most human tumors [33]. Also, survivin inhibits apoptosis by binding specifically to the terminal effecter cell death proteases, caspase-3 and -7 [34,35]; this being one of the foremost reasons for the ineffectiveness of chemotherapeutic agents in inducing apoptosis in cancer cells including PCa [31,36]. Together, these transcription factors (NF-κB, AP1 and Stat3) along with survivin constitute a potential therapeutic target for the treatment of PCa. Therefore, in the present study, we also analyzed the effect of B2G2 on the transcription factors (NF-κB, AP1 and Stat3) and their target gene survivin in PCa cells.
2. Materials and methods
2.1. Cell lines and reagents
Human PCa PC3 and 22Rv1 cells were obtained from American Type Culture Collection (Manassas, VA), and C4-2B cells were purchased from ViroMed Laboratories (Minneapolis, MN). All these lines were obtained during 2008, and tested and authenticated by DNA profiling for polymorphic short tandem repeat markers at University of Colorado cDNA sequencing & Analysis Core in August 2010. RPMI1640 media, cell culture materials, and Annexin V-Vybrant apoptosis kit were from Invitrogen Corporation (Gaithersburg, MD). Antibodies for cleaved poly (ADP-ribosyl) polymerase (PARP), survivin, pStat3tyr 705, total Stat3 and anti-rabbit peroxidase-conjugated secondary antibody were from Cell Signaling (Danvers, MA). Specific oligonucleotides (NF-κB and AP1) and the gel shift assay system were from Promega Corp (Madison, WI). Propidium iodide (PI), dimethylsulfoxide (DMSO), β-actin antibody, gallic acid, (−)-epicatechin, 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ), 4-(dimethylamino)pyridine (DMAP), dicyclohexylcarbodiimide (DCC), hydrogen, palladium hydroxide on carbon (Pd(OH)2), benzyl bromide (BnBr), trimethylsilyl trifluoromethanesulfonate (TMSOTf), and 2-ethoxyethanol (EtOCH2CH2OH) were procured from Sigma-Aldrich (St. Louis, MO). Annexin V-Vybrant apoptosis kit was from Molecular Probes (Eugene, Oregon). Methanol (MeOH), dimethylformamide (DMF), sulfuric acid (H2SO4), potassium carbonate (K2CO3), sodium hydroxide (NaOH), dioxane, sodium hydride (NaH), methylene chloride (CH2Cl2), hexanes, chloroform, and ethyl acetate were purchased from Fisher Scientific (Pittsburgh, PA). Reactions were monitored via silica gel IB2-F thin layer chromatography (TLC) plates from J.T. Baker (Phillipsburg, NJ).
2.2. Synthesis of procyanidin B2 3,3″-di-O-gallate
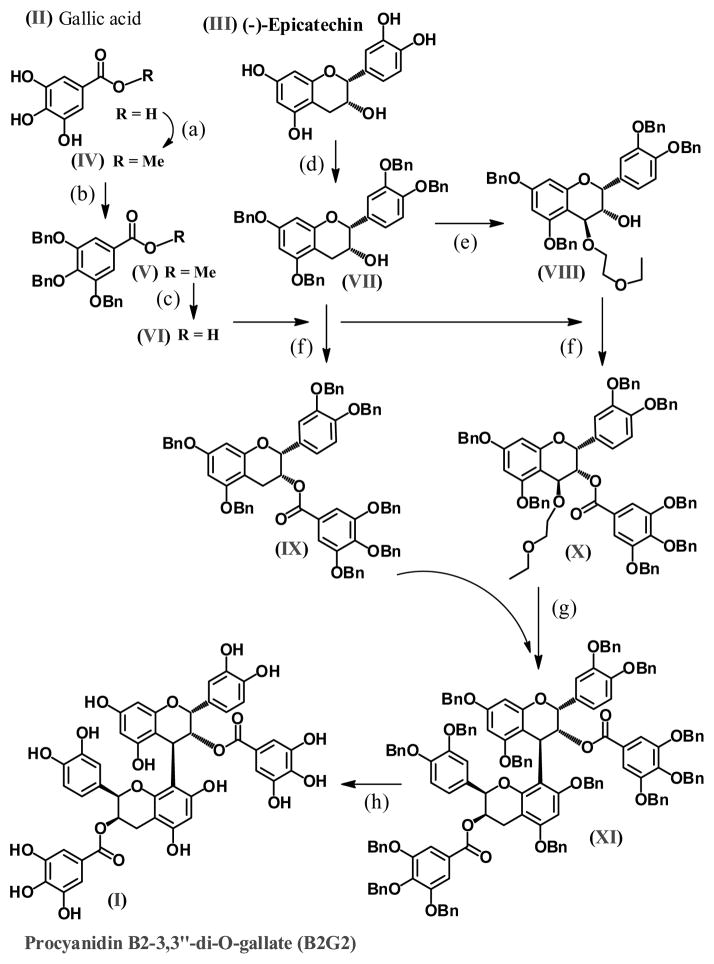
As summarized in figure 1, we have utilized previously published methods to conduct the synthesis of Procyanidin B2-3,3″-di-O-gallate (B2G2; I) [37–41]. We started with two commercially available materials, gallic acid (II) and (−)-epicatechin (III); nine steps were required to produce B2G2. Gallic acid (II) was converted to the corresponding methyl ester (IV) followed by phenolic protection via benzyl (-O-CH2-Phenyl; -OBn; V), and subsequently hydrolyzed to the corresponding protected acid (VI). As in the case of gallic acid, the phenolic hydroxyl groups in (−)-epicatechin were first tetra-protected to afford (VII) followed by DDQ carbon-4 oxidation of (VII) in the presence of 2-ethoxyethanol to produce (VIII). At this point, compounds (VII) and (VIII) were each coupled with the protected gallic acid (VI) to afford gallate compounds (IX) and (X), respectively. Compounds (IX) and (X) were then coupled via acid catalyzed condensation to produce the fully protected (14 phenolic groups) (OBn)14-procyanidin B2 di-O-gallate (XI). Lastly, compound (XI) was fully de-protected via hydrogenation to afford Procyanidin B2-3,3″-di-O-gallate (B2G2; I).
Figure 1. Chemical synthesis of procyanidin B2-3,3″-di-O-gallate.
(a) MeOH, H2SO4 Δ; (b) DMF, BnBr, K2CO3; (c); NaOH, MeOH, H2O, Dioxane, Δ (d) DMF, BnBr, NaH; (e) DDQ, EtOCH2CH2OH, DMAP, DCM; (f) DCC/DMAP; (g) TMSOTf, −78°C, CH2Cl2; (h) Pd(OH)2, H2.
2.3. Cell culture and treatments
PC3, 22Rv1 and C4-2B cells were cultured in RPMI1640 medium containing 10% fetal bovine serum and 1% penicillin-streptomycin under standard culture conditions. At 60–65% confluency, cells were treated for 6–72 h with desired doses of B2G2 (25–100 μM) in DMSO or with DMSO alone. Unless specified otherwise, the final concentration of DMSO in the culture medium during different treatments did not exceed 0.1% (v/v). Whole-cell/nuclear extracts were prepared as described previously [29,42].
2.4. Cell viability and clonogenic assay
Cells were plated at 5000 cells/cm2 density in 60 mm-dishes under the standard culture conditions. After 24 h, cells were treated with DMSO alone or different concentrations of B2G2 (25–100 μM). Cells were trypsinized, collected, and counted using a Trypan blue dye exclusion method to determine viable and dead cells after 24, 48, and 72 h of B2G2 treatments. To assess the clonogenic potential of the different cell lines (PC3, 22Rv1 and C4-2B), cells were seeded at a density of 1000 cells/well in 6-well plates. After being allowed to attach to the plates for 24 h these cells were then treated with B2G2 every 48 h. At the end of the 10th day, cells were washed with PBS and fixed in a mix of methanol:acetic acid (3:1) for 10 min and then stained with 0.1% crystal violet for 30 min. The plates were washed thrice with PBS and the colonies (≥50 or <50 cells/colony) were counted under inverted microscope.
2.5. Analysis for cell cycle distribution and apoptotic cell death
PCa cells were plated in 60 mm dishes to 50% confluency overnight, and subsequently treated with either DMSO alone or various doses of B2G2 for 24–48 h. After these treatments, cells were collected by brief trypsinization and washed with ice-cold PBS twice, and pellets were incubated in 0.5 ml of saponin/propidium iodide solution at 4°C for 24 h in dark as reported earlier. The percentage of cells in the different phases of the cell cycle was determined by FACS analysis [8]. Alternatively, cells were subjected to Annexin V and PI staining using Vybrant Apoptosis Assay Kit 2 following the step-by-step protocol provided by the manufacturer. Stained cells were analyzed by FACS analysis, utilizing the core service of the University of Colorado Cancer Center (Aurora, CO), in order to quantify the apoptotic cells.
2.6. Electrophoretic mobility shift assay (EMSA)
Consensus sequences of double stranded NF-κB oligonucleotide (5′-AGT TGA GGG GAC TTT CCC AGG C-3′ and 3′ TCA ACT CCC CTG AAA GGG TCC G-5′) or AP1 oligonucleotide (5′-CGC TTG ATG AGT CAG CCG GAA-3′ and 3′ GCG AAC TAC TCA GTC GGC CTT-5′) were end labeled with γ-32P-ATP (3,000 Ci/mmol at 10 m Ci/ml) as per manufacturer’s protocol (Promega Corp). Labeled probes were separated from free γ-32P-ATP using G-25 Sephadex column. Nuclear extract (10 μg) along with 5X gel shift binding buffer was incubated with 1–2 μl (20,000 cpm) of 32P-labeled NF-κB or AP1 probe for 20 min at 37°C. In super shift and competition assays(data not shown), nuclear extract was incubated with anti –p65/p50 and cjun/cfos antibodies for NF-κB and AP1, respectively, or unlabeled-oligonucleotide before adding labeled NF-κB or AP1 oligonucleotide. DNA retardation gel (6%) was used to resolve DNA-protein complexes followed by gel drying and autoradiography.
2.7. Immunoblotting
Cells were treated as mentioned above and lysates were prepared and analyzed by immunoblotting as described previously [42]. Briefly, 50–60 μg protein per sample was denatured with 2X sample buffer and subjected to SDS-PAGE on 8 or 12% gel. Separated proteins were transferred onto membrane by Western blotting. Membranes were blocked with blocking buffer for 1 h at room temperature. After blocking, the membranes were probed with desired primary antibodies over night at 4°C followed by peroxidase-conjugated appropriate secondary antibody and visualized by ECL detection system. Some blots were multiplexed or stripped and reprobed with different antibodies including those for loading control.
2.8. Statistical analysis
Statistical significance of differences between control and treated samples were calculated by one-way ANOVA followed by a Bonferroni’s test using SigmaStat version 3.5 software (Jandel Scientific, San Rafael, CA), and both sided P values of ≤0.05 were considered significant. The data in all cases are representative of at least 3–4 independent studies with reproducible results.
3. Results
3.1. Comparative growth inhibitory effects of naturally isolated and synthetic B2G2 against human PCa cell lines
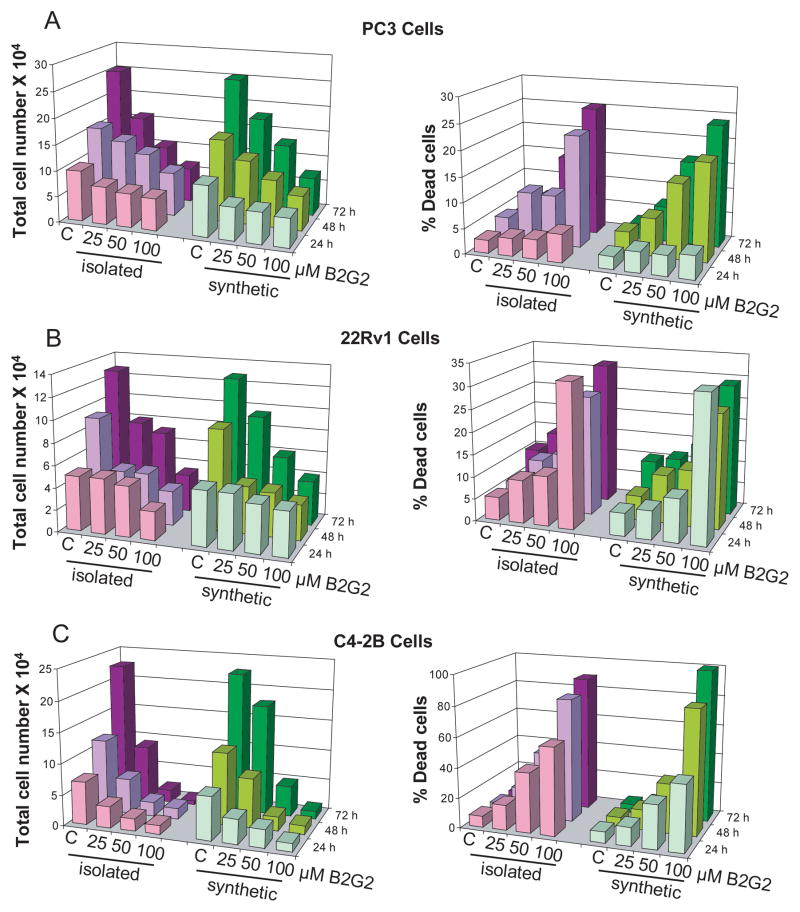
First, we compared the effect of the natural isolated B2G2 (B2G2-isolated) versus synthesized B2G2 (synthetic B2G2) on the growth of three different human PCa cell lines, differing in their status of androgen receptor (AR), namely PC3, 22Rv1 and C4-2B. Of these cells, PC3 is AR-negative/androgen independent and 22Rv1 is AR-positive/androgen independent, while C4-2B, derived from the bone metastasis of LNCaP variant cell line C4-2, is AR-positive/androgen independent. As shown in figure 2A, both B2G2-isolated and synthetic B2G2 inhibited the growth of PC3 cells in a concentration- and time-dependent manner. B2G2-isolated at 25, 50 and 100 μM doses resulted in 27–38% (P<0.001), 14–48% (P<0.001) and 37–74% (P<0.001) growth inhibition of PC3 cells after 24, 48 and 72 h of treatment, respectively (Fig. 2A, left panel). Concomitantly, there was an increase in the PC3 dead cell population following 48 and 72 h B2G2 treatments at 25, 50 and 100 μM doses which accounted for 10–22% (P<0.01) and 6–25% (P<0.001), respectively (Fig. 2A, right panel). With synthetic B2G2there was also a significant growth inhibition in PC3 cells ranging from 36–44% (P<0.001), 23–58% (P<0.001) and 29–71% (P<0.001) at 25, 50 and 100 μM concentration after 24, 48 and 72 h of treatment, respectively (Fig. 2A, left panel). Synthetic B2G2 also caused an increase in dead cell population [7–19% (P<0.01) and 7–24% (P<0.001)] of PC3 cells under identical treatment conditions at 48 and 72 h, respectively (Fig. 2A, right panel).
Figure 2. Comparative growth inhibitory effects of naturally isolated B2G2 and chemically synthesized B2G2 against human PCa cells.
B2G2-isolated and synthetic B2G2 inhibit growth and induce death in A) PC3, B) 22Rv1 and C) C4-2B cells in both dose- and time- dependent manner. Cells (1.05 x 105) were plated in 60 mm dishes, treated with DMSO (control) or different concentrations of B2G2, and after 24, 48 or 72 h, cells were harvested and counted as detailed in “Materials and methods”. C, control; B2G2, procyanidin B2 3,3″-di-O-gallate.
In the case of 22Rv1 cells, B2G2-isolated had no significant effect on the growth at 25–50 μM concentration for 24 h treatment time (Fig. 2B, left panel); however a strong effect was observed at 100 μM concentration (48%, P<0.001, Fig. 2B, left panel). Furthermore, growth inhibitory effect was observed at 25, 50 and 100 μM concentration by B2G2-isolated, accounting for 51–66% (P<0.001) and 38–73% (P<0.001) growth inhibition following 48 and 72 h treatment, respectively (Fig. 2B, left panel). Under similar experimental conditions, 25 μM dose did not cause any significant cell death; while the 50 and 100 μM B2G2-isolated doses caused strong 22Rv1 cell death [11–32% (P<0.01), 13–27% (P<0.01) and 16–32% (P<0.001)] at 24, 48 and 72 h, respectively (Fig 2B, right panel). Treatment with synthetic B2G2 also caused a strong growth inhibition at 25, 50 and 100 μM dose levels in the range of 53–65% (P<0.001) and 26–69% (P<0.001) following 48 and 72 h treatment time, respectively (Fig. 2B, left panel). Furthermore, almost similar effect of synthetic B2G2 compared to B2G2-isolated was observed on cell death in 22Rv1 cells accounting for 10–31% (P<0.001), 13–26% (P<0.001) and 16–32% (P<0.001) dead cell population following exposure to 50 and 100 μM doses of synthetic B2G2 for 24, 48 and 72h, respectively (Fig 2B, right panel).
Growth inhibitory effect of B2G2-isolated and synthetic B2G2 was also identical in C4-2B cells at the 25, 50 and 100 μM doses for 24, 48 and 72 h treatments. B2G2-isolated treatments at all the concentrations studied produced strong cell growth inhibitory effects [50–80% (P<0.001) 24 h, 51–86% (P<0.001) 48 h, 60–97% (P<0.001) 72 h)] in C4-2B cells (Fig. 2C, left panel). Interestingly, B2G2-isolated at 25, 50 and 100 μM dose caused strong cell death at all the time points studied, accounting for 17–58% (P<0.001, 24 h), 19–83% (P<0.001, 48 h) and 12–89% (P<0.001, 72 h) dead cell population in C4-2B cells (Fig. 2C, right panel). Treatment with synthetic B2G2 caused similar effects in C4-2B cells accounting for 43–81% (P<0.001), 32–88% (P<0.001) and 22–95% (P<0.001) growth inhibition at 25, 50 and 100 μM doses following 24, 48 and 72 h of treatment, respectively (Fig. 2C, left panel). Similarly, synthetic B2G2 also caused significant cell death in C4-2B cells accounting for 12–43% (P<0.001), 14–82% (P<0.001) and 8–100% (P<0.001) dead cell population by these doses and treatment times (Fig. 2C, right panel).
Taken together, the above results revealed that irrespective of the natural or synthetic source of B2G2, both displayed similar 1H-NMR and LC/MS profiles (data not shown), and there was a comparable inhibition in cell growth and induction of cellular death by this novel agent in all three PCa cell lines examined in the present study. Accordingly, we proceeded with synthetic B2G2 for additional efficacy and mechanistic studies in PCa cells.
3.2. B2G2 induces cell cycle arrest and apoptotic death in human PCa cell lines
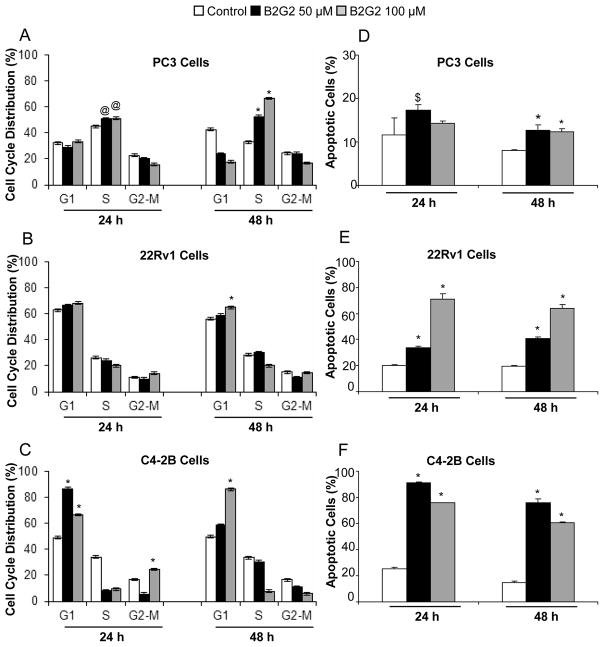
Since synthetic B2G2 (hereafter referred as B2G2) treatment caused a significant growth inhibitory effect in PC3, 22Rv1 and C4-2B cells, its effect on cell cycle progression in PCa cells was anticipated, and therefore a cell cycle distribution analysis was done. In PC3 cells, B2G2 (50–100 μM) treatment for 24–48 h caused a significant accumulation of cells at S phase (P<0.01-P<0.001; Fig. 3A). However, in 22Rv1 cells, G1 arrest was observed at 100 μM dose of B2G2 treatment for 48 h (P<0.001; Fig. 3B). In C4-2B cells, 50 μM B2G2 treatment for 24 h and 100 μM B2G2 for 24–48 h led significant accumulation of cells in G1 phase (P<0.001; Fig. 3C); while 50 μM B2G2 treatment for 48 h did not show significant change in cell cycle distribution (Fig. 3C). Overall, the observed cell cycle arrest by B2G2 possibly accounts for its cell growth inhibitory effect in PCa cells.
Figure 3. B2G2 induces cell cycle arrest and apoptotic death in human PCa cells.
A–C) PC3, 22Rv1 and C4-2B cells were treated with DMSO (control) or B2G2 for the mentioned time and dose. At the conclusion of the experiment, cells were collected by trypsinization, washed with PBS and cells pellet was incubated over night with saponin/propidium iodide solution at 4°C as detailed in the Materials and methods. The percentage of cells in different phases of the cell cycle was determined by FACS analysis. D–F) Alternatively PC3, 22Rv1 and C4-2B cells pellets were stained with Annexin V- propidium iodide. Stained cells were processed for FACS analysis. $, p<0.05; @, p<0.01; *, p<0.001 for differences with control group; B2G2, procyanidin B2 3,3″-di-O-gallate.
Furthermore, since B2G2 treatment also caused a strong death of PC3, 22Rv1 and C4-2B cells, we next examined whether it is an apoptotic death. Compared to controls, B2G2 treatment caused a marginal apoptotic cell death in PC3 cells (Fig. 3D); however, similar B2G2 doses caused a strong [34–71% (P<0.001) and 41–64% (P<0.001)] apoptotic death in 22Rv1 cells after 24 and 48 h treatment, respectively, compared to 20% apoptotic cells in vehicle controls (Fig. 3E). Unlike PC3 and 22Rv1 cells, a much stronger effect was observed in C4-2B cells under the same treatment conditions (Fig. 3F), accounting for 91–76% (P<0.001) and 76–60% (P<0.001) apoptotic death following 24 and 48 h treatment, respectively, at 50 and 100 μM B2G2 doses, compared to 25% and 15% apoptotic cells in controls at same time-points. The lower apoptotic cells death observed at higher doses (100 μM) of B2G2 in C4-2B and PC-3 cells could be attributed to undetectable dead cell debris of apoptotic/dead cells.
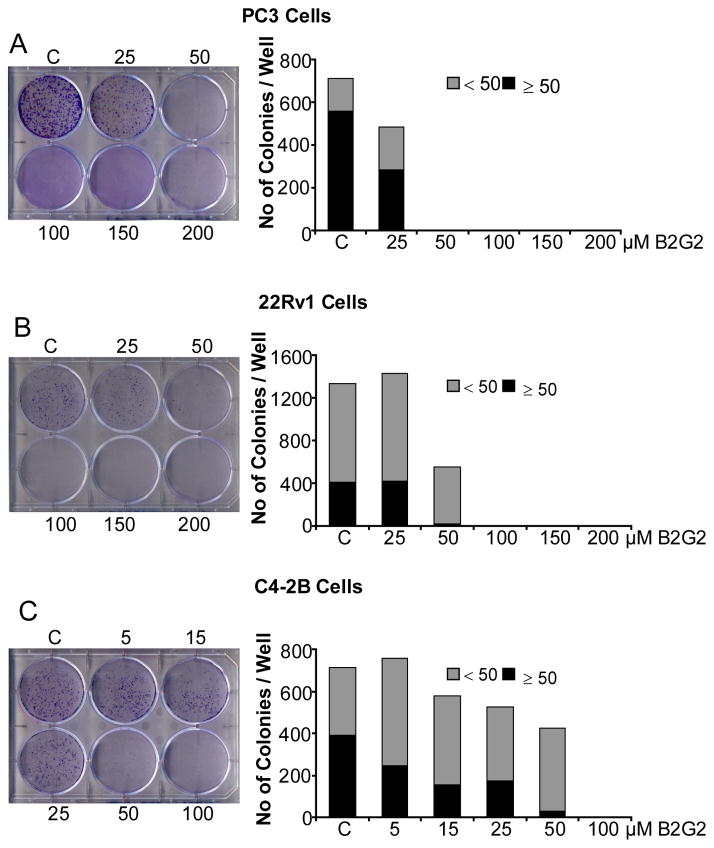
3.3. B2G2 inhibits the clonogenic potential of human PCa cell lines
Since we observed a strong reduction in cell number by B2G2 in PC3 cells but not apoptotic death, we next assessed the effect of this agent on clonogenicity of human PCa cells by counting the colonies with ≥ 50 cells. Treatment with 25 μM B2G2 every 48 h for 10 days significantly inhibited the colony formation by 50% (P<0.001), however; B2G2 doses (50–200 μM) completely inhibited colony formation in PC3 cells (Fig. 4A). The effect of 25 μM B2G2 was comparatively less in 22Rv1 cells than as observed in PC3 cells (5% inhibition, P<0.001, Fig. 4B); however, higher doses of B2G2 (50–200 μM) showed similar inhibitory effect on clonogenicity of 22Rv1 cells as in PC3 cells (100% inhibition, P<0.001, Fig. 4B). In the case of C4-2B cells, there was 37% (P<0.001), 60% (P<0.001), 56% and 93% (P<0.001) inhibition of colony formation at 5, 15, 25 and 50 μM B2G2, respectively (Fig. 4C). More importantly, at higher doses of B2G2 (100–200 μM; Fig. 4C) no colonies were observed.
Figure 4. B2G2 inhibits the clonogenic potential of human PCa cells.
A–C) PC3, 22Rv1 and C4-2B cells (_1 × 103) were plated in 6-well plates and 24 h later cells were treated with DMSO or different doses of B2G2. Fresh media with DMSO or B2G2 was added every 48 h. After 10 days of treatment, cells were processed as mentioned in “Materials and methods” and colonies were counted. The data shown are mean of 3 plates with similar treatment. Pictures were taken with a digital camera. C, control; B2G2, procyanidin B2 3,3″-di-O-gallate.
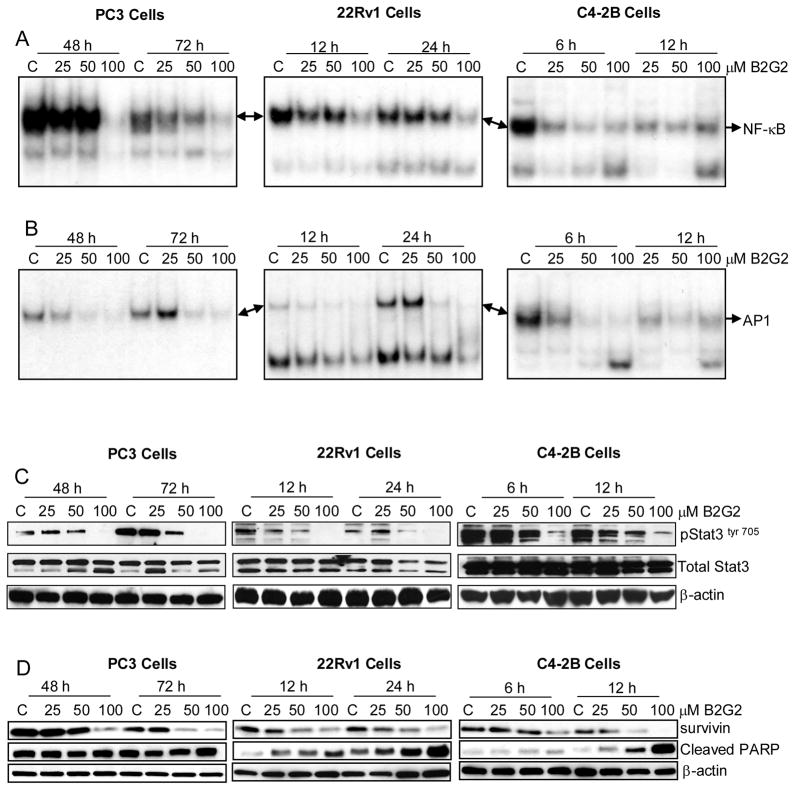
3.4. B2G2 inhibits the activation of various transcription factors, decreases survivin expression and induces cleaved PARP expression in different human PCa cell lines
Several studies have shown that the down modulation of either protein expression or a decrease in the activity of NF-κB, AP1 and Stat3 molecules causes both growth inhibition and apoptotic cell death in various cancer cell lines including PCa [6,29,43]. Accordingly, based on our findings showing strong growth inhibitory and apoptotic effect of B2G2 in a panel of human PCa cell lines, next we studied its effect on these transcription factors (NF-κB, AP1 and Stat3) in PCa cell lines. Based on the strong growth inhibitory/cell death effects of B2G2 at time points evaluated in earlier experiments in some of these cell lines, we assumed that for delineation of mechanistic events shorter exposure to B2G2 would provide a clear insight into the ongoing events. With that rationale, 25, 50, and 100 μM doses of B2G2 were used to treat PC3 cells for 48 and 72 h, 22Rv1 cells for 12 and 24 h, and C4-2B cells for 6 and 12 h. NF-κB transcriptional activity was evaluated in the nuclear lysates and results indicated that NF-κB activity was strongly inhibited at 100 μM B2G2 dose in PC3 (48–72 h) and 22Rv1 (12–24 h) cell lines; whereas in C4-2B cells, B2G2 (25–100 μM) treatment for 6 and 12 h strongly inhibited the NF-κB activation in a dose-dependent manner (Fig. 5A). Furthermore, EMSA assay was also performed for evaluating B2G2 effect on AP1 activity in these cells. As shown in figure 5B, B2G2 exerted a strong inhibitory effect on AP1-DNA binding at both 50 and 100 μM doses in PC3 (24–48 h), 22Rv1 (12–24 h) and C4-2B (6–12 h) cell lines. In case of C4-2B cells, 25 μM dose of B2G2 also significantly inhibited AP1-DNA binding, whereas in PC3 and 22Rv1 cells the AP1-DNA binding was not altered at this concentration (Fig. 5B). In other studies, the nuclear translocation of Stat3tyr 705 was inhibited in PC3 cells at 50 and 100 μM doses of B2G2 after 48 and 72 h of treatment. Similar inhibitory effects on the nuclear translocation of Stat3tyr 705 were also observed in 22Rv1 and C4-2B cells (Fig. 5C) after B2G2 treatment. Interestingly, 25 μM B2G2 did not show inhibitory effect on the nuclear expression of Stat3tyr 705 in all these three cell lines (Fig. 5C).
Figure 5. B2G2 inhibits the activation of various transcription factors, decreases survivin expression, and induces cleaved PARP expression in different human PCa cell lines.
PC3, 22Rv1 and C4-2B cells were cultured as described in “Materials and methods”, and treated with either DMSO alone (control) or varying concentrations of B2G2. At the end of the treatments, both adherent and non-adherent cells were harvested and nuclear extracts/total cell lysates were prepared. A and B) Using nuclear extracts EMSA was performed for NF-κB and AP1 followed by drying of gels and autoradiography as detailed in “Materials and methods”. C and D) Nuclear extracts/total cell lysates were subjected to SDS-PAGE followed by Western immunoblotting. Membranes were probed with pStat3 (tyr 705), total Stat3, survivin and cleaved PARP antibodies followed by peroxidase-conjugated appropriate secondary antibody and visualized by ECL detection system. Equal protein loading was confirmed by stripping and re-probing the membranes with β-actin. C, Control; B2G2, procyanidin B2 3,3″-di-O-gallate.
To gain more insight into the molecular mechanism involved in the apoptotic cell death, we studied the effect of B2G2 on survivin expression and PARP cleavage, the established molecular markers for cell survival and apoptotic death, respectively. As shown in figure 5D, B2G2 strongly decreased survivin expression at 50–100 μM doses in PC3 (48–72 h), 22Rv1 (12–24 h) and C4-2B (6–12 h) cells, and under similar treatment conditions, this agent increased the expression of cleaved-PARP in all three PCa cell lines (Fig. 5D).
4. Discussion
PCa is the second leading cause of cancer-associated deaths among American men [1]. Growing evidence from epidemiologic surveys and case-control studies show that diet and lifestyle play an essential role in PCa development and progression [44,45]. It is also recognized in recent years that many nutrients and herbs have significant promise in controlling and managing PCa by slowing the progression and reducing recurrence via targeting survival and apoptotic machinery [4,44–46]. In this regard, GSE is a well-known dietary agent that has shown strong cancer chemopreventive and anti-cancer efficacy against various malignancies including PCa [4], and B2G2 accounts for the major biologically active constituent of GSE [17,18,47]. The isolated yield of B2G2 is only about 0.3% (w/w) of the intact GSE. Thus isolating gram quantities involves cumbersome labor intensive and expensive chromatographic techniques [17]. These limitations have hindered the advancement of this novel anti-cancer agent for both efficacy and mechanism studies. Thus to overcome the limitations of isolating gram quantities of B2G2 from the GSE mixture, we synthesized multi gram-scale quantities of B2G2 for use in biological studies. Thereafter, we first compared the anti-PCa efficacy of isolated versus synthetic B2G2, and our results revealed that B2G2, irrespective of its source, caused comparable and significant inhibition of cell growth and proliferation and induced cell cycle arrest along with apoptotic death in a panel of human PCa cell lines irrespective of their functional AR and p53 status. Notably, C4-2B (wild- type p53) and 22Rv1 (wild- type p53) cell lines were more sensitive to B2G2 treatment for apoptotic death, while PC3 cells (null- p53) showed less sensitivity. It has been known that accumulation of p53 plays an important role in regulating the apoptotic machinery [7]. Thus, we also assessed the role of p53 for driving the apoptotic death in C4-2B and 22Rv1 cells as these cells were more sensitive for the apoptotic death after B2G2 exposure and harbor wild-type p53. Results from these experiments showed that p53 protein expression was decreased in C4-2B and 22Rv1 cells after B2G2 treatment (data not shown), suggesting that p53 is possible not a major player in driving apoptosis in C4-2B and 22Rv1 cells.
Advanced age, family history and adoption of a westernized lifestyle increase the incidence of developing PCa [48]. This validates the idea that environmental factors are also involved in the pathogenesis of PCa in addition to hereditary factors. One such potential factor which has gained considerable attention is the development of chronic inflammation in the prostate [48,49], which is triggered either by infectious agents or exposure to dietary factors, corpora amylacea, hormonal changes and urine reflux, or by a combination thereof [49]. Epidemiological evidence for the role of inflammation in prostate cancer etiology stems from the protective function of non-steroidal anti-inflammatory drugs (NSAIDs) against PCa [48]; however, complications arise due to their side effects [50]. The inflammatory molecules that have been identified as potential mediators in the interplay between prostate inflammation and prostate carcinogenesis include transcription factors (NF-κB, Stat3, and AP1), multiple inflammatory cytokines (macrophage inhibitory cytokine, MCP1, IL6, TNFα, IL8, IL-1β), chemokine (CXCL12), and enzyme (COX-2) [48,49]. Among these, activated NF-κB, Stat3, and AP1 in tumors/tumor cells are known to activate a broad range of anti-apoptotic, pro-survival and inflammatory genes in response to inflammation [29,42,51]. This is one of the reasons for the ineffectiveness of chemotherapeutic agents in inducing apoptosis in PCa, suggesting that multiple molecular pathways may need to be modified in order to treat cancers that are resistant to pro-apoptotic therapies. In this regard, the results from the present study indicate that B2G2 strongly inhibits the activity of NF-κB and AP1 in PC3, 22Rv1 and C4-2B cells. Additional studies also showed that B2G2 strongly inhibits the nuclear translocation of Stat3tyr 705 in all the three cell lines. The inhibitory effect on the activity of these transcription factors by B2G2 could possibly be contributing (directly or indirectly) to the apoptosis induction or to anti-inflammatory effect in PCa cells. Furthermore, persistent activation of NF-κB and Stat3 signaling induces survivin expression, by their nuclear translocation and subsequent binding to the survivin promoter region, which confers resistance to apoptosis in cancer cells [31,32]. Survivin exhibits the most restricted expression in normal adult human or mouse tissues, whereas dramatic over expression of it has been seen in most of the common human cancers [52,53]. Notably, accumulating evidences show that the expression level of survivin contributes to prognosis, clinical outcome of tumors, susceptibility to anti-cancer drugs, and malignant behavior [54,55]. Survivin directly binds and inhibits certain caspases and therefore inhibits apoptosis induced by a variety of stimuli [34,35]. Thus, altering survivin expression is complex but important in PCa. In this regard, our results showing a decrease in survivin expression in PC3, 22Rv1 and C4-2B cells by B2G2 leads to the assumption that this decrease in survivin may in turn potentiate the apoptotic machinery induced by B2G2. Therefore, our observation of decreased activity of transcription factors (NF-κB, AP1 and Stat3), which are activated in human PCa, together with decreased expression of survivin, after B2G2 treatment suggest an important role of these molecules in B2G2 induced apoptosis in PCa cells. Whereas more studies are needed in the future to further assess and establish the mechanisms of anti-cancer activity of B2G2, this is the first study reporting the feasibility of B2G2 synthesis in large-quantity together with anti-cancer activity in a panel of human PCa cell lines supporting its efficacy studies in PCa mouse models.
Acknowledgments
This work was supported by the R01 grant CA91883 from the National Cancer Institute. The research utilized services of the Medicinal Chemistry Core facility (MFW) housed within the Department of Pharmaceutical Sciences. In part, the MCC has been funded via Colorado Clinical and Translational Sciences Institute grant 8UL1TR000154-05 from National Center for Research Resources at the National Institutes of Health (NCRR/NIH).
Abbreviations
- PCa
prostate cancer
- AR
androgen receptor
- PARP
poly (ADP-ribosyl) polymerase
- IAPs
inhibitor of apoptosis proteins
- NF-κB
nuclear factor-κB
- AP1
activator protein 1
- Stat3
signal transducer and activator of transcription
- B2G2
procyanidin B2 3,3″-di-O-gallate
- GSE
grape seed extract
- EMSA
Electrophoretic Mobility Shift Assay
Footnotes
Conflict of Interest Disclosures: The authors declare that there are no conflicts to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Abdulla M, Gruber P. Role of diet modification in cancer prevention. Biofactors. 2000;12:45–51. doi: 10.1002/biof.5520120108. [DOI] [PubMed] [Google Scholar]
- 3.Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer. 2006;13:751–778. doi: 10.1677/erc.1.01126. [DOI] [PubMed] [Google Scholar]
- 4.Kaur M, Agarwal C, Agarwal R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J Nutr. 2009;139:1806S–1812S. doi: 10.3945/jn.109.106864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal C, Singh RP, Agarwal R. Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis. 2002;23:1869–1876. doi: 10.1093/carcin/23.11.1869. [DOI] [PubMed] [Google Scholar]
- 6.Dhanalakshmi S, Agarwal R, Agarwal C. Inhibition of NF-kappaB pathway in grape seed extract-induced apoptotic death of human prostate carcinoma DU145 cells. Int J Oncol. 2003;23:721–727. [PubMed] [Google Scholar]
- 7.Kaur M, Agarwal R, Agarwal C. Grape seed extract induces anoikis and caspase-mediated apoptosis in human prostate carcinoma LNCaP cells: possible role of ataxia telangiectasia mutated-p53 activation. Mol Cancer Ther. 2006;5:1265–1274. doi: 10.1158/1535-7163.MCT-06-0014. [DOI] [PubMed] [Google Scholar]
- 8.Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res. 2006;12:6194–6202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- 9.Raina K, Singh RP, Agarwal R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. 2007;67:5976–5982. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]
- 10.Sharma G, Tyagi AK, Singh RP, Chan DC, Agarwal R. Synergistic anti-cancer effects of grape seed extract and conventional cytotoxic agent doxorubicin against human breast carcinoma cells. Breast Cancer Res Treat. 2004;85:1–12. doi: 10.1023/B:BREA.0000020991.55659.59. [DOI] [PubMed] [Google Scholar]
- 11.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 12.Song X, Siriwardhana N, Rathore K, Lin D, Wang HC. Grape seed proanthocyanidin suppression of breast cell carcinogenesis induced by chronic exposure to combined 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and benzo[a]pyrene. Mol Carcinog. 2010;49:450–463. doi: 10.1002/mc.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyagi A, Agarwal R, Agarwal C. Grape seed extract inhibits EGF-induced and constitutively active mitogenic signaling but activates JNK in human prostate carcinoma DU145 cells: possible role in antiproliferation and apoptosis. Oncogene. 2003;22:1302–1316. doi: 10.1038/sj.onc.1206265. [DOI] [PubMed] [Google Scholar]
- 14.Velmurugan B, Singh RP, Agarwal R, Agarwal C. Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats. Mol Carcinog. 2010;49:641–652. doi: 10.1002/mc.20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velmurugan B, Singh RP, Kaul N, Agarwal R, Agarwal C. Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice. Neoplasia. 2010;12:95–102. doi: 10.1593/neo.91718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Wang J, Chen Y, Agarwal R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotion protocol and identification of procyanidin B5-3′-gallate as the most effective antioxidant constituent. Carcinogenesis. 1999;20:1737–1745. doi: 10.1093/carcin/20.9.1737. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal C, Veluri R, Kaur M, Chou SC, Thompson JA, et al. Fractionation of high molecular weight tannins in grape seed extract and identification of procyanidin B2-3,3′-di-O-gallate as a major active constituent causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2007;28:1478–1484. doi: 10.1093/carcin/bgm045. [DOI] [PubMed] [Google Scholar]
- 18.Chou SC, Kaur M, Thompson JA, Agarwal R, Agarwal C. Influence of gallate esterification on the activity of procyanidin B2 in androgen-dependent human prostate carcinoma LNCaP cells. Pharm Res. 2010;27:619–627. doi: 10.1007/s11095-009-0037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veluri R, Singh RP, Liu Z, Thompson JA, Agarwal R, et al. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2006;27:1445–1453. doi: 10.1093/carcin/bgi347. [DOI] [PubMed] [Google Scholar]
- 20.Kaur M, Velmurugan B, Rajamanickam S, Agarwal R, Agarwal C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm Res. 2009;26:2133–2140. doi: 10.1007/s11095-009-9926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raina K, Rajamanickam S, Deep G, Singh M, Agarwal R, et al. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in TRAMP mice. Mol Cancer Ther. 2008;7:1258–1267. doi: 10.1158/1535-7163.MCT-07-2220. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal R. Cell signaling and regulators of cell cycle as molecular targets for prostate cancer prevention by dietary agents. Biochem Pharmacol. 2000;60:1051–1059. doi: 10.1016/s0006-2952(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 23.Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;15:657–664. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilmore TD. Introduction to NF-kappaB. players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 26.Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, et al. Transcription factor Stat3 stimulates metastatic behavior of human prostate cancer cells in vivo, whereas Stat5b has a preferential role in the promotion of prostate cancer cell viability and tumor growth. Am J Pathol. 2010;176:1959–1972. doi: 10.2353/ajpath.2010.090653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu A, Bray TM, Ho E. Anti-inflammatory activity of soy and tea in prostate cancer prevention. Exp Biol Med (Maywood) 2012;235:659–667. doi: 10.1258/ebm.2010.009335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouyang X, Jessen WJ, Al-Ahmadie H, Serio AM, Lin Y, et al. Activator protein-1 transcription factors are associated with progression and recurrence of prostate cancer. Cancer Res. 2008;68:2132–2144. doi: 10.1158/0008-5472.CAN-07-6055. [DOI] [PubMed] [Google Scholar]
- 29.Raina K, Agarwal C, Agarwal R. Effect of silibinin in human colorectal cancer cells: Targeting the activation of NF-kappaB signaling. Mol Carcinog. 2011 doi: 10.1002/mc.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun M, Liu C, Nadiminty N, Lou W, Zhu Y, et al. Inhibition of Stat3 activation by sanguinarine suppresses prostate cancer cell growth and invasion. Prostate. 2012;72:82–89. doi: 10.1002/pros.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 32.Wang K, Brems JJ, Gamelli RL, Holterman AX. Survivin signaling is regulated through nuclear factor-kappa B pathway during glycochenodeoxycholate-induced hepatocyte apoptosis. Biochim Biophys Acta. 2010;1803:1368–1375. doi: 10.1016/j.bbamcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Rodel F, Sprenger T, Kaina B, Liersch T, Rodel C, et al. Survivin as a Prognostic/Predictive Marker and Molecular Target in Cancer Therapy. Curr Med Chem. 2012;19:3679–3688. doi: 10.2174/092986712801661040. [DOI] [PubMed] [Google Scholar]
- 34.Rubio N, Garcia-Segura LM, Arevalo MA. Survivin prevents apoptosis by binding to caspase-3 in astrocytes infected with the BeAn strain of Theiler’s murine encephalomyelitis virus. J Neurovirol. 2012;18:354–363. doi: 10.1007/s13365-012-0112-3. [DOI] [PubMed] [Google Scholar]
- 35.Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, et al. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117–1123. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- 36.Nomura T, Yamasaki M, Nomura Y, Mimata H. Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cells. Oncol Rep. 2005;14:993–997. [PubMed] [Google Scholar]
- 37.Saito A, Emoto M, Tanaka A, Doi Y, Shoji K, et al. Stereoselective synthesis of procyanidin B3-3-O-gallate and 3,3″-di-O-gallate, and their abilities as antioxidant and DNA polymerase inhibitor. Tetrahedron. 2004;60:12043–12049. [Google Scholar]
- 38.Saito A, Nakajima N, Tanaka A, Ubukata M. Synthetic studies of proanthocyanidins. Part 2: Stereoselective gram-scale synthesis of procyanidin-B3. Tetrahedron. 2002;58:7829–7837. doi: 10.1271/bbb.66.1764. [DOI] [PubMed] [Google Scholar]
- 39.Tüeckmantel W, Kozikowski AP, Romanczyk LJJ. Studies in Polyphenol Chemistry and Bioactivity. 1. Preparation of Building Blocks from (+)-Catechin. Procyanidin Formation. Synthesis of the Cancer Cell Growth Inhibitor, 3-O-Galloyl-(2R,3R)-epicatechin-4b,8-[3-O-galloyl-(2R,3R)-epicatechin] Journal of the American Chemical Society. 1999;121:12073–12081. [Google Scholar]
- 40.Saito A, Mizushina Y, Ikawa H, Yoshida H, Doi Y, et al. Systematic synthesis of galloyl-substituted procyanidin B1 and B2, and their ability of DPPH radical scavenging activity and inhibitory activity of DNA polymerases. Bioorganic & Medicinal Chemistry. 2005;13:2759–2771. doi: 10.1016/j.bmc.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 41.Viton F, Landreau C, Rustidge D, Robert F, Williamson G, et al. First Total Synthesis of 14C-Labeled Procyanidin B2 – A Milestone Toward Understanding Cocoa Polyphenol Metabolism. Eur J Org Chem. 2008;36:6069–6078. [Google Scholar]
- 42.Tyagi A, Agarwal C, Dwyer-Nield LD, Singh RP, Malkinson AM, et al. Silibinin modulates TNF-alpha and IFN-gamma mediated signaling to regulate COX2 and iNOS expression in tumorigenic mouse lung epithelial LM2 cells. Mol Carcinog. 2011 doi: 10.1002/mc.20851. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal C, Tyagi A, Kaur M, Agarwal R. Silibinin inhibits constitutive activation of Stat3, and causes caspase activation and apoptotic death of human prostate carcinoma DU145 cells. Carcinogenesis. 2007;28:1463–1470. doi: 10.1093/carcin/bgm042. [DOI] [PubMed] [Google Scholar]
- 44.Brand TC, Canby-Hagino ED, Pratap Kumar A, Ghosh R, Leach RJ, et al. Chemoprevention of prostate cancer. Hematol Oncol Clin North Am. 2006;20:831–843. doi: 10.1016/j.hoc.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Klein EA. Chemoprevention of prostate cancer. Annu Rev Med. 2006;57:49–63. doi: 10.1146/annurev.med.57.121304.131435. [DOI] [PubMed] [Google Scholar]
- 46.Bhatt RS, Bubley GJ. The challenge of herbal therapies for prostate cancer. Clin Cancer Res. 2008;14:7581–7582. doi: 10.1158/1078-0432.CCR-08-2316. [DOI] [PubMed] [Google Scholar]
- 47.Shrestha SP, Thompson JA, Wempe MF, Gu M, Agarwal R, et al. Glucuronidation and methylation of procyanidin dimers b2 and 3,3″-di-o-galloyl-b2 and corresponding monomers epicatechin and 3-o-galloyl-epicatechin in mouse liver. Pharm Res. 2012;29:856–865. doi: 10.1007/s11095-011-0614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60:199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.James ND, Sydes MR, Mason MD, Clarke NW, Anderson J, et al. Celecoxib plus hormone therapy versus hormone therapy alone for hormone-sensitive prostate cancer: first results from the STAMPEDE multiarm, multistage, randomised controlled trial. Lancet Oncol. 2012;13:549–558. doi: 10.1016/S1470-2045(12)70088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 53.Reed JC. The Survivin saga goes in vivo. J Clin Invest. 2001;108:965–969. doi: 10.1172/JCI14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyachi K, Sasaki K, Onodera S, Taguchi T, Nagamachi M, et al. Correlation between survivin mRNA expression and lymph node metastasis in gastric cancer. Gastric Cancer. 2003;6:217–224. doi: 10.1007/s10120-003-0255-2. [DOI] [PubMed] [Google Scholar]
- 55.Paydas S, Tanriverdi K, Yavuz S, Disel U, Sahin B, et al. Survivin and aven: two distinct antiapoptotic signals in acute leukemias. Ann Oncol. 2003;14:1045–1050. doi: 10.1093/annonc/mdg277. [DOI] [PubMed] [Google Scholar]