Abstract
Rationale
Although buprenorphine is effective in treating opioid dependence, optimal maintenance doses of buprenorphine or the buprenorphine/naloxone combination have not yet been established.
Objective
The present study was designed to evaluate the effects of buprenorphine/naloxone maintenance (2/0.5, 8/2, 32/8 mg sublingual) on the reinforcing and subjective effects of heroin (0, 12.5, 25, 50, and 100 mg intranasal) in heroin-dependent individuals.
Methods
During test weeks, participants (N=7) first sampled a dose of heroin and $20. During subsequent choice sessions, participants could choose to self administer heroin and/or money. Participants responded under a modified progressive-ratio schedule (PR 50, …, 2800) during a 10-trial self-administration task.
Results
Heroin break point values and subjective responses were significantly lower under 8/2 and 32/8 mg buprenorphine/naloxone compared to 2/0.5 mg. The self-administration and subjective effects data for heroin in the presence of buprenorphine/naloxone were compared to a separate control group of recently detoxified participants (N=8) in order to obtain estimates for the apparent in vivo dissociation constant (KA), the efficacy estimate (tau), and the estimated fraction of receptors remaining after buprenorphine/naloxone treatment (q). The apparent in vivo dissociation constant for heroin ranged from 50–126 mg (KA) and the efficacy estimate ranged from 13–20 (tau). In addition, 2/0.5, 8/2 and 32/8 mg buprenorphine/naloxone dose-dependently reduced the receptor population by 74%, 83%, and 91%, respectively.
Conclusions
These data demonstrate that both 8/2 and 32/8 mg buprenorphine/naloxone were well tolerated and effective in reducing the reinforcing and subjective effects of heroin, relative to the 2/0.5 mg dose. The data also show for the first time in humans that it is possible to quantify the efficacy and affinity of heroin for mu opioid receptors and that 80–90% of mu receptors need to be inactivated in order to obtain significant reductions in heroin-induced effects. These results have important implications for future studies in which it will be possible to obtain estimates of relative affinity and efficacy of different agonists at mu opioid receptors.
Keywords: buprenorphine, buprenorphine/naloxone, heroin, opioids, progressive ratio, self administration, subjective effects, efficacy, affinity
Introduction
Because both heroin and prescription opioid dependence (Zacny et al., 2003) are significant problems in the U.S., the search for effective treatment medications for opioid dependence remains important. Several clinical trials have shown that buprenorphine is a safe and effective treatment medication for opioid dependence (e.g, Bickel et al., 1988a, 1999; Johnson et al., 1992, 2000; Ling et al., 1996; Strain et al., 1994). Other laboratory-based studies conducted in both humans and non-human primates have demonstrated that buprenorphine reduces the reinforcing effects of heroin (e.g., Mello et al., 1982, 1983, Mello and Mendelson, 1980; Winger and Woods, 1996). A previous study conducted in our laboratory demonstrated that 16 mg of the tablet formulation of buprenorphine produced surprisingly small reductions in intravenous heroin self-administration, relative to 8 mg buprenorphine (Comer et al., 2001). Clinical experience suggests that the initial target maintenance dose of the buprenorphine/naloxone combination tablet should be between 12/3 and 16/4 mg, with flexible dose titrations based on continued illicit opioid use (Johnson et al., 2003). However, relatively few laboratory studies have characterized the maximally effective maintenance dose of the combination buprenorphine tablet in reducing the reinforcing and subjective effects of heroin. The purpose of the present study was to extend our previous results with buprenorphine alone by examining the effects of the combination tablet, using a wider range of buprenorphine/naloxone maintenance doses (2/0.5, 8/2, and 32/8 mg).
In addition to standard procedures for evaluating the effectiveness of treatment medications for heroin dependence, we also estimated the apparent in vivo dissociation constant (KA) and relative efficacy values (tau) for heroin using the long-lasting antagonist buprenorphine/naloxone. Compounds such as clocinnamox (Comer et al., 1992), β-funaltrexamine (β-FNA) (Ward et al., 1982), and buprenorphine (Hambrook & Rance, 1976; Walker et al., 1995) produce a long-lasting or insurmountable antagonism of μ opioid receptors. Insurmountable antagonism of μ receptors reduces the number of receptors available for agonist interaction and therefore, eventually, the effects of an agonist. By comparing the effects of heroin in the presence and absence of buprenorphine/naloxone, KA, tau, and the fraction of receptors remaining for agonist interaction (q) may be estimated using the method of partial irreversible blockade described by Furchgott (1966) and modified by Black and Leff (1983). For many years, insurmountable antagonists have been indispensable pharmacological tools to determine relative efficacy for opioid agonists. For example, buprenorphine was used in previous antinociceptive studies to estimate the KA and efficacy of morphine in rhesus monkeys (Walker et al., 1995) and rodents (Raffa et al., 1982; Tallarida and Cowan, 1982). More recently, clocinnamox and β-FNA have been used to obtain affinity and efficacy estimates for heroin as well as fentanyl, alfentanil, etonitazene, etorphine, l-methadone, morphine, buprenorphine, and nalbuphine in a variety of behavioral assays in laboratory animals (Adams et al., 1990; Barrett et al., 2003; Negus et al., 2003; Pitts et al., 1998; Walker et al., 1995, 1998; Zernig et al., 1994). In the present study, we obtained estimates of KA and tau for an opioid agonist, heroin, in humans using buprenorphine/naloxone as a long-lasting, insurmountable antagonist.
In this study, affinity and efficacy estimates were obtained for the reinforcing and subjective effects of heroin. To our knowledge, this is the first experiment to estimate quantitatively these pharmacological variables for μ opioids in humans in vivo. These estimates are meaningful in that they allow us to compare our estimates for heroin to those previously obtained in laboratory animals. Furthermore, we can compare the relative efficacy requirements of several different behavioral measures produced by heroin. This approach provides order and predictability for drug effects across behavioral measures and permits a quantitative description of the relation between receptor events, agonist efficacy, and behavioral effects.
Methods
Participants
Twelve non-treatment-seeking, heroin-dependent individuals began the 6-week protocol. Seven participants (5 men, 2 women; 4 Hispanic, 2 African American, and 1 non-Hispanic Caucasian) completed the study. Of the 5 participants who did not complete the study, 2 discontinued for personal reasons and 3 were discharged for non-compliance with unit policies. The 7 volunteers who completed the study (aged 23 to 45 years; mean: 33 years) reported using heroin for an average of 9 years (range: 3 to 20 years). Participants reported spending an average of $54 per day (range: $40 to $80) on heroin. Opioid dependence was verified by a naloxone challenge test. All seven participants smoked tobacco cigarettes (6 to 20 cigarettes per day), three used cocaine (twice per week or less), five used alcohol (3 to 30 times per month [the participant who used alcohol every day drank one beer or less each evening after work]), and three used marijuana (once per month). Participants were not physically dependent on alcohol or illicit drugs other than heroin.
After an initial telephone interview, eligible participants received additional screening at the laboratory, which included completion of detailed questionnaires on drug use, general health and medical history, and a medical and psychological evaluation. Participants were told that they would be maintained on an opioid for the duration of the study, and that different doses of the maintenance medication would be tested. An electrocardiogram and Mantoux test or chest x-ray were also performed. Routine laboratory analyses included a blood chemistry panel, thyroid function test, syphilis screening and urinalysis. Urine drug toxicologies were performed repeatedly during screening.
Participants were excluded from the study if they were pregnant or nursing, seeking drug treatment, or had a major Axis I psychiatric diagnosis other than opioid dependence. Those who had recent histories of violence or who were on parole/probation were excluded from the study. Volunteers were paid $25 per inpatient day and an additional $25 per day bonus if they completed the study. In addition, they could receive up to $40 per laboratory day during the experimental sessions. Participants signed consent forms describing the aims of the study, and the potential risks and benefits of participation. This study was approved by the Institutional Review Board of the New York State Psychiatric Institute.
Apparatus
During experimental sessions, participants were seated in a room equipped with Macintosh computers. All computer activities, vital signs and behaviors were continuously monitored by the experimenters in an adjacent control room via a continuous on-line computer network and vital signs monitors. Cardiovascular function was measured using a Sentry II Vital Signs Monitor (NBS Medical, Costa Mesa, CA). Arterial oxygen saturation (%SpO2) was measured using a Model 400 pulse oximeter (Palco Laboratories, Santa Cruz, CA).
General Procedures
The reinforcing effects of intranasal heroin (i.n.; placebo, 12.5, 25, 50, and 100 mg) were evaluated under three sublingual (s.l.) buprenorphine/naloxone maintenance dose conditions (2/0.5, 8/2, and 32/8 mg; Table 1). As demonstrated in a previous study conducted in our laboratory, intranasal heroin is approximately 4-fold less potent than intravenous heroin in producing reinforcing, subjective, and physiological effects (Comer et al., 1999). In general, participants report that they would be willing to pay $10–20 for the 50 mg dose of intranasal heroin, which corresponds well with data on the street value of heroin in New York City reported by the Drug Enforcement Administration [$6–35 per 100 mg heroin at 70% purity = $6–35 per 70 mg pure heroin; DEA, 2003]. All participants received all three doses of buprenorphine/naloxone. The order of buprenorphine/naloxone maintenance dose administration was counterbalanced across participants, who received each dose for 2 consecutive weeks (weeks 1–2, weeks 3–4, and weeks 5–6). During weeks 1, 3 and 5, participants were stabilized on a dose of buprenorphine/naloxone. Testing occurred during weeks 2, 4, and 6 on Monday through Friday morning and afternoon (i.e., 10 sessions per week, 2 sessions per day).
Table 1.
Representatitve experimental design*
| Week 1, 3, 5 | Stabilization on 2/0.5, 8/2 or 32/8 mg SL Buprenorphine/Naloxone | |||||
|---|---|---|---|---|---|---|
| Week 2, 4, 6 | AM Sample | 0 and $20 | 12.5 and $20 | 25 and $20 | 50 and $20 | 100 and $20 |
| PM Choice | 0 versus $20 | 12.5 versus $20 | 25 versus $20 | 50 versus $20 | 100 versus $20 | |
Buprenorphine/naloxone dose and heroin dose were administered in non-systematic fashion both within and between participants, with the exception that the highest dose of heroin was never tested first.
Experimental Sessions
During sample sessions, participants received drug and money. Subjective and performance effects were measured both before and repeatedly after drug administration (Table 2; see descriptions below). Choice sessions were similar in design to the sample session, except that participants completed a self-administration task (see below) after the first performance battery.
Table 2.
Experimental Sessions
| Sample Session | Self-Administration (Choice) Session | ||
|---|---|---|---|
| −40 | Begin Session | −80 | Begin Session |
| Baseline Vitals Signs (every 5 min) | Baseline Vitals Signs (every 5 min) | ||
| Withdrawal Symptom Questionnaires | Withdrawal Symptom Questionnaires | ||
| Performance Battery | Performance Battery | ||
| Subjective Effects Battery | Subjective Effects Battery | ||
| Pupil Photo | Pupil Photo | ||
| −40 | Self Administration Task | ||
| Subjective Effects Battery | |||
| 0 | Heroin/Money Administration | 0 | Heroin/Money Administration |
| 4 | Pupil Photo/Subjective Effects Battery | 4 | Subjective Effects Battery |
| 10 | Pupil Photo/Performance Battery | 10 | Performance Battery |
| 40 | Pupil Photo/Subjective Effects Battery | 40 | Subjective Effects Battery |
| 60 | Pupil Photo/Performance Battery/ Subjective Effects Battery | ||
Self Administration Task
Participants were told that they could work for all or part of the sampled dose of heroin or the sampled money amount ($20) by choosing the drug or money option each time a choice was available. Responses consisted of finger presses on a computer mouse. Standardized instructions were read to each participant explaining the self-administration task. Heroin and money were available under independent progressive ratio schedules, and participants were given 10 opportunities to choose between the two options. Ten percent of that day’s heroin dose or money value was available at each choice opportunity. Thus, if the dose of heroin for that day was 25 mg, at each opportunity participants could respond for 2.5 mg (10% of 25 mg) or $2 (10% of $20). Completion of the ratio requirement for each choice was accompanied by a visual stimulus on the computer screen. The response requirement for each of the two options increased independently such that the initial ratio requirement for each option was 50 responses. Thereafter, the ratio increased progressively each time the option was selected (100, 200, 400, 800, 1200, 1600, 2000, 2400, 2800). In order to receive all of the heroin or money available that day, participants were required to emit 11,550 responses within 40 min. Fewer total responses were required if choices were distributed between the two options. Although it required high rates of responding, participants were capable of completing 11,550 responses in the allotted time.
At the start of each self-administration task, two illustrations appeared on the computer screen: an empty balance scale and an empty bank. As each choice was completed, either the scale was incremented with a pile of powder or a dollar sign was added to the bank. Thus, participants could always see how many money and drug choices had been made. No reinforcers were delivered until after the entire task was completed. At that time, the participant received whatever he or she had chosen: money and/or drug.
Subjective Measures
Four questionnaires were used to assess subjective effects. The first questionnaire was a 26-item visual analog scale (VAS) designed to assess subjective and physiological effects. Participants rated each item on the VAS from “Not at all” (0 mm) to “Extremely” (100 mm), except for the “For this dose, I would pay” question, which ranged between $0 (0 mm) to $20 (100 mm). The second questionnaire was a 13-item opioid symptom checklist consisting of true/false questions designed to measure opioid effects (e.g., “My skin is itchy,” etc.; Foltin and Fischman, 1992; Fraser et al., 1961). The VAS and opioid symptom checklist together constituted the subjective-effects battery. The third questionnaire was the 16-item Subjective Opioid Withdrawal Scale (SOWS; Handelsman et al., 1987). Participants rated each item on a scale from 0 to 4, with 0 being “Not at all” and 4 being “Extremely” (e.g., “I have gooseflesh,” etc.). The fourth questionnaire was a 6-item Drug Effects Questionnaire (DEQ; Evans et al., 1995). Participants described drug effects by selecting among a series of possible answers ranging from 0 (“No effects at all”) to 4 (“Very strong (good, bad, etc.) effects”). Ratings of drug liking ranged between -4 (“Dislike very much”) to 4 (“Like very much”).
Task Battery
The task battery consisted of four tasks: a 3-min digit-symbol substitution task (McLeod et al., 1982), a 10-min divided attention task (Miller et al., 1988), a 10-min rapid information processing task (Wesnes & Warburton, 1983), and a 3-min repeated acquisition of response sequences task (Kelly et al., 1993).
Physiological Measures
A blood pressure cuff was attached to the non-dominant arm, and blood pressure was recorded automatically. Participants were also connected to a pulse oximeter via a soft sensor on a finger of the non-dominant hand, which monitored arterial blood oxygen saturation (%SpO2). For safety, supplemental oxygen (2 L/min) was provided via a nasal cannula during all experimental sessions. A specially-modified Polaroid camera with a close-up lens (2X magnification) was used to take pupil photographs. All photographs were taken under ambient lighting conditions. Horizontal and vertical measurements of pupil diameter were made using a calipers, and then these two measurements were averaged and divided by 2 to correct for the 2X magnification.
Drugs
Heroin HCl was provided by the National Institutes on Drug Abuse (Rockville, MD) and prepared by the Columbia-Presbyterian Medical Center research pharmacy. For nasal insufflation of heroin, lactose powder was used as the placebo and was added to each dose of heroin (12.5, 25, and 50 mg) to achieve a final weight of 100 mg. The highest dose (100 mg) contained only heroin. Each dose was placed in a small plastic cup, along with a short soda straw. Participants were instructed to insufflate the entire dose within a 30 sec period into either one or two nostrils. For safety, a catheter was placed in an antecubital vein and physiological saline solution was infused continuously during experimental sessions.
Buprenorphine/naloxone HCl tablets (2/0.5 mg or 8/2 mg per active tablet, 0/0 mg per placebo tablet) were provided by the National Institutes on Drug Abuse (Rockville, MD). Buprenorphine/naloxone dosing occurred at 8 p.m. each evening, approximately 15 hrs prior to heroin administration. Participants were instructed to hold the tablets under the tongue for 10 min, without swallowing. Compliance was verified midway through the dosing period by a mouth check. Upon admission to the hospital, participants were inducted directly onto either 2/0.5, 8/2 or 32/8 mg buprenorphine/naloxone after they showed symptoms of opioid withdrawal.
Supplemental medications available to all participants for the duration of the study included: Trazodone for insomnia, Mylanta® for stomach upset, acetaminophen and ibuprofen for muscle pain, Colace® and Milk of Magnesia® for constipation, and multi-vitamins with iron. During each stabilization period, the following medications were available: Clonidine HCl for piloerection, sweating, lacrimation, rhinorrhea, and hot/cold flashes, ketorolac tromethamine for muscle pain, prochlorperazine for nausea, and clonazepam for anxiety.
Morning urine samples were collected daily and one random sample per week was screened for the presence of other illicit substances, none of which was found in the participants’ urines.
Statistical Analyses
Repeated measures analyses of variance (ANOVAs) were performed for progressive-ratio break point values (the highest ratio that participants completed). The analyses were designed to answer two basic questions: 1) Does each heroin dose function as a reinforcer under each buprenorphine/naloxone maintenance condition? and 2) Do the reinforcing effects of heroin differ under 2/0.5, 8/2 and 32/8 mg buprenorphine/naloxone? Heroin and money break point values were analyzed as a function of buprenorphine/naloxone dose (2/0.5, 8/2 and 32/8 mg) and heroin dose (placebo, 12.5, 25, 50, and 100 mg i.n.). The following planned comparisons were performed to answer the two questions above: 1) Each active heroin dose was compared to placebo, and 2) break point values for each heroin dose were compared under 2/0.5, 8/2 and 32/8 mg buprenorphine/naloxone.
Repeated measures ANOVAs were also performed for average rate of responding and latency to respond during the self-administration task, pupil diameter, task performance, and subjective ratings during the sample session. Data were analyzed as a function of buprenorphine/naloxone dose and heroin dose, collapsing across time. Pulse oximeter data obtained during the morning sample session were averaged within participants, and analyzed as a function of buprenorphine/naloxone dose and heroin dose. Planned comparisons were similar to those described above (questions #1 and 2). Results were considered statistically significant at P<0.05, using Hunyh-Feldt corrections, where appropriate.
Quantitative Analyses
To determine meaningful and accurate calculations for KA, tau, and q, control dose effect curves for intranasal heroin (0, 12.5, and 50 mg) were obtained in a separate group of 8 non-opioid-dependent, recently detoxified heroin abusers (7 men, 1 woman; 4 Hispanic and 4 non-Hispanic Caucasian) who participated in a separate study designed to evaluate the reinforcing effects of heroin under maintenance on 0, 30, and 60 mg per day memantine. Data from the 0 mg memantine maintenance dose condition were used in the present study. All experimental procedures were identical for the two groups of participants, with the exception that the choice session was conducted on the day after the sample session, rather than on the afternoon of the same day as the sample session. Previous studies in our laboratory have demonstrated that there is no significant difference in the reinforcing effects of intranasal heroin when choice sessions occur during the afternoon or the morning following the sample session (Comer et al., 2001). Furthermore, the reinforcing and subjective effects of intranasal heroin are remarkably stable across studies (please see Comer et al., 1997, 1999), which further justifies the use of the control data in the present study.
The dose-effect curves for heroin alone and in the presence of 2/0.5, 8/2 and 32/8 mg buprenorphine/naloxone were analyzed according to the model proposed by Black and Leff (1983) and applied to behavioral data according to methods described extensively by Zernig et al. (1996). Tau, an operational definition of efficacy, is defined as the reciprocal of the fraction of receptors necessary to give a half-maximum response (Black and Leff, 1983). An estimate of the fraction of receptors available for agonist interaction after blockade with a given dose of buprenorphine/naloxone corresponds to q in Furchott’s model (1966). The q value is calculated by dividing the tau value obtained after inactivation of the receptors with the insurmountable antagonist by the tau value obtained under control conditions (see Zernig et al., 1996). For non-hyperbolic E/[A] curves, Black et al. (1985) propose a function of the form:
where E is the effect (progressive ratio break point, “Good Drug Effect,” “High,” or “Potent”), Em is the maximum attainable response, [A] is the agonist concentration, KA is the estimate for the apparent in vivo dissociation constant, and n is a slope factor of a transducer function relating receptor occupancy to the observed response. The semilogarithmic form of the above equation is extended by c, the baseline response, with tau represented as [(q)(taucontrol)]:
All dose-response curves obtained with a given agonist were simultaneously fitted to the above equation using the non-linear fitting program Efficalc© (Zernig and Issaevitch, 1995) and the general mathematical software package Mathematica Version 4.2.1 (Wolfram Research, Champaign, USA; Wolfram, 2002). By analyzing the family of dose-response curves simultaneously for a given effect (e.g., self administration) reasonable confidence limits for KA, tau, and q values could be obtained (Zernig et al., 1996). To analyze the progressive-ratio breakpoint self-administration data, the baseline variable in the Efficalc© program was offset by 200 to correct for the responding produced by saline. One-way, repeated measures analyses of variance (ANOVAs) and Tukey’s Multiple Comparison Test were performed to determine whether q values were significantly different after 2/0.5, 8/2 or 32/8 mg buprenorphine/naloxone (Prism v 4.02, GraphPad Software, San Diego, CA).
Results
Choice
Figure 1 shows mean progressive ratio break point values for heroin (left panel) and money (right panel) as a function of heroin dose and buprenorphine/naloxone maintenance dose. Planned contrasts for each active heroin dose compared to placebo under each maintenance dose condition (i.e., 12 contrasts) revealed that mean heroin break point values for 25, 50, and 100 mg heroin were significantly greater than placebo (all P<0.005). Maximal heroin break point values occurred at 100 mg heroin under the 2/0.5 mg buprenorphine/naloxone maintenance dose (2514 responses). In general, buprenorphine/naloxone produced a dose-related downward shift in the heroin dose-response curve. Heroin break point values were reduced by 24% and 32% under the 8/2 mg and 32/8 mg buprenorphine/naloxone maintenance doses, respectively, relative to the 2/0.5 mg dose. The main effect of buprenorphine/naloxone dose was statistically significant (F 1,2=5.5, P<0.02), and both 8/2 and 32/8 mg were significantly different from 2/0.5 mg (2/0.5 mg versus 8/2 mg: F 1,12=5.5, P<0.04; 2/0.5 mg versus 32/8 mg: F 1,12=10.2, P<0.008). Planned comparisons revealed that heroin break point values were significantly lower for the 25 mg and 50 mg doses of heroin when participants were maintained on 32/8 mg compared to 2/0.5 mg buprenorphine/naloxone (25 mg heroin: F 1,48=4.4, P<0.04; 50 mg heroin: F 1,48=5.3, P<0.03).
Figure 1.
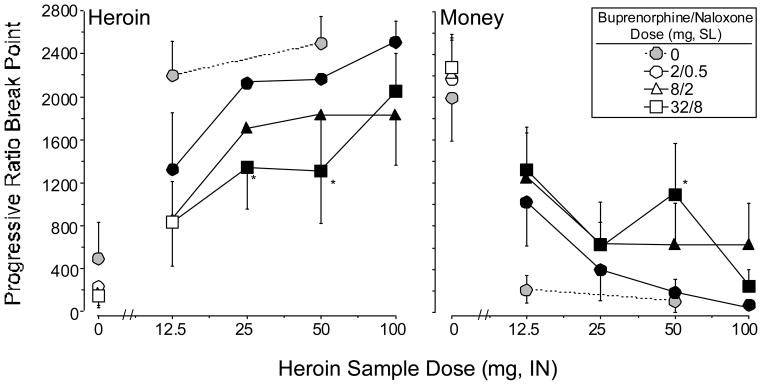
Progressive ratio break point values for heroin (left panel) and money (right panel) as a function of heroin dose and buprenorphine/naloxone maintenance dose. Data points connected with solid lines represent the average break point values across the 7 participants maintained on buprenorphine/naloxone. Data points connected with dashed lines represent the average break point values across the 8 participants maintained on placebo during a separate study. Break point values could range between 0 and 2800. Error bars represent ± 1 standard error of the mean (S.E.M.). * indicates a significant difference at that heroin dose from the 2/0.5 mg maintenance dose condition; filled symbols represent a significant difference from placebo heroin.
Mean money break point values for all of the active doses of heroin were significantly lower than 0 mg heroin under each maintenance dose condition (right panel). Maximal money break point values occurred at 0 mg heroin under all of the buprenorphine/naloxone maintenance doses (2171–2285 responses). There were no significant differences in break point values for money under the three maintenance dose conditions, although the effects of 32/8 mg buprenorphine/naloxone neared statistical significance (2/0.5 mg versus 32/8 mg: F 1,12=3.4, P<0.09). Planned comparisons revealed that money break point values were significantly higher for 50 mg heroin when participants were maintained on 32/8 mg compared to 2/0.5 mg buprenorphine/naloxone (50 mg heroin: F 1,48=6.7, P<0.01).
Average latency to respond (data not shown), which ranged between 0.41 and 0.88 sec, did not vary as a function of either buprenorphine/naloxone dose or heroin dose. Similarly, average rates of responding, which ranged between 5.9 and 6.2 responses/sec, also did not vary as a function of buprenorphine/naloxone dose or heroin dose.
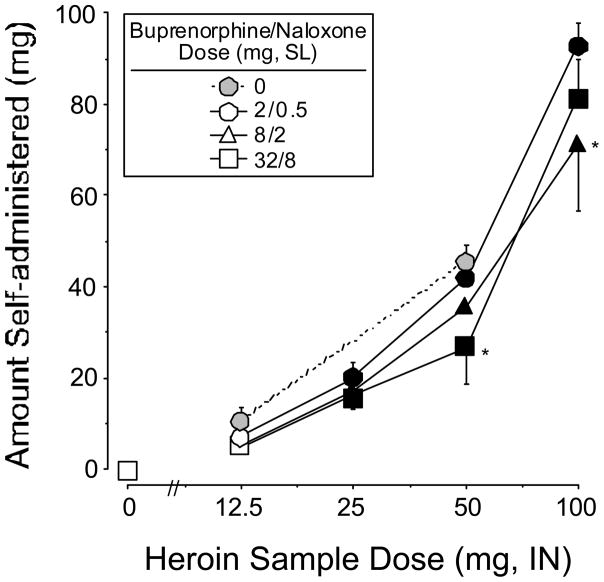
Figure 2 shows the average amount of drug self administered during choice sessions as a function of the sample heroin dose and buprenorphine/naloxone maintenance dose. As expected based on the progressive ratio break point data, the amount of drug self administered significantly increased in a dose-related fashion under all maintenance dose conditions. The amount of drug self administered was lower, but not significantly so, when participants were maintained on 8/2 mg (F 1,2=4.2, P<0.06) and 32/8 mg (F 1,2=4.2, P<0.06) buprenorphine/naloxone, compared to 2/0.5 mg. Planned contrasts revealed that the amount of drug self administered was significantly lower for the 50 mg dose of heroin when participants were maintained on 32/8 mg buprenorphine/naloxone compared to 2/0.5 mg buprenorphine/naloxone (50 mg heroin: F 1,48=5.6, P<0.02). In addition, the amount of drug self administered was significantly lower for the 100 mg dose of heroin when participants were maintained on 8/2 mg compared to 2/0.5 mg buprenorphine/naloxone (100 mg heroin: F 1,48=11.5, P<0.001).
Figure 2.
Amount of heroin self administered during choice sessions as a function of heroin sample dose and buprenorphine/naloxone maintenance dose. Data points represent the average amount of drug received across participants. Values could range between 0 and 100 mg. All else as in Figure 1.
Subjective Effects
Figure 3 shows selected mean visual analog scale ratings collected during the sample session as a function of heroin dose and buprenorphine/naloxone maintenance dose. In general, ratings of “Good Drug Effect,” “High,” and “Potent” were similar to the results obtained for heroin self-administration. Ratings of “Good Drug Effect” increased in a heroin dose-related manner under the 2/0.5 mg and 8/2 mg buprenorphine/naloxone maintenance conditions (Figure 3, left panel). Planned comparisons showed that ratings of “Good Drug Effect” under the 32/8 mg dose were significantly different from the 2/0.5 mg dose after administration of 25, 50, and 100 mg heroin. After administration of 25 mg heroin under the 32/8 mg buprenorphine/naloxone dose condition, ratings of “Good Drug Effect” were significantly greater than under the 2/0.5 mg buprenorphine/naloxone dose condition. This effect occurred for several of the ratings and was due to one participant who received this dose combination first. Similar, although less robust effects were obtained for ratings of “High” (Figure 3, middle panel), “Potent” (Figure 3, right panel), “Of High Quality,” “Liked the Choice,” “Mellow,” and amount the participants would be willing to pay for the dose (data not shown). Participants reported that they would pay more for the higher doses of heroin than the lower doses (main effect of heroin dose: F 1,4=5.0, P<0.004). Averaged across all time points during the session, participants reported that they would pay $4–5 for the 100 mg dose (peaks were approximately $7.50 for the 100 mg dose). Under the 32/8 mg buprenorphine/naloxone dose condition, participants reported that they would pay significantly less for 50 mg heroin (F 1,48=14.0, P<0.0005). Ratings of “I Want Heroin” were consistently high (50–70 mm on the 100 mm scale) under the 2/0.5 mg and 8/2 mg buprenorphine/naloxone doses, and did not consistently vary as a function of heroin dose. Under the 32/8 mg dose condition, ratings of “I Want Heroin” were significantly lower than under 2/0.5 or 8/2 mg [Pbo, 2/0.5 vs. 32/8 mg: F 1,32=3.9, P<0.05; 25 mg, 8/2 vs. 32/8 mg: F 1,32=5.0, P<0.03; 50 mg, 2/0.5 vs. 32/8 mg: F 1,32=5.7, P<0.02; 100 mg, 8/2 vs. 32/8 mg: F 1,32=4.2, P<0.05]. Ratings of “Talkative” (F 1,4=2.9, P<0.04), “Social” (F 1,4=4.7, P<0.006), “Stimulated” (F 1,4=2.8, P<0.05), “Sedated” (F 1,4=6.5, P<0.001), and “I Want Tobacco” (F 1,4=3.0, P<0.04) also increased in a heroin dose-related manner, but the effects of buprenorphine/naloxone on these measures were less consistently dose related. Baseline data collected prior to heroin administration revealed that subjective ratings were minimal under all buprenorphine/naloxone maintenance dose conditions, suggesting that buprenorphine/naloxone itself produced minimal opioid agonist effects under these conditions.
Figure 3.
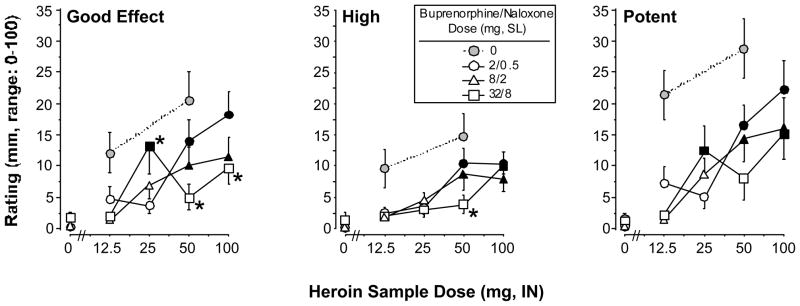
Selected VAS ratings during the sample session as a function of heroin dose and buprenorphine/naloxone maintenance dose. The VAS rating scale ranged between 0 and 100 mm. Data points represent mean ratings across time for each participant, which were then averaged across participants. All else as in Figure 1.
The pattern of results obtained from the opioid symptom checklist was similar to the VAS. Heroin produced dose-related increases in ratings of “High” (F 1,4=8.9, P < 0.0002), “Relaxed” (F 1,4=2.8, P < 0.05), and “Skin Itchy” (F 1,4=3.2, P < 0.03). Ratings of “High” (F 1,2=4.1, P<0.04), but not “Relaxed” or “Skin Itchy,” decreased in a buprenorphine/naloxone dose-related manner.
Heroin produced robust, dose-related increases in DEQ ratings of “Good Drug Effect” (F 1,4=11.8, P < 0.0001), drug liking (F 1,4=14.9, P < 0.0001), desire to take the drug again (F 1,4=6.8, P < 0.0008), strength of drug effect (F 1,4=11.7, P < 0.0001), and type of drug (participants rated heroin as being sedative-like; F 1,4=9.3, P < 0.0001). Although there were trends for buprenorphine/naloxone to decrease DEQ ratings of “Good Drug Effect” and drug liking, these effects were not statistically significant, and buprenorphine/naloxone had no effects on ratings of desire to take the drug again, strength of drug effect, and type of drug.
During the study, subjective ratings of opioid withdrawal as measured by total scores on the SOWS (maximum possible score = 64), were low and did not vary as a function of either heroin dose or buprenorphine/naloxone dose. Average ratings were below 10 across all of the heroin and buprenorphine/naloxone maintenance dose conditions. For comparison, peak total SOWS scores during the first week after admission ranged between 12 and 37 for six of the seven participants (one participant reported negligible withdrawal during the first week after admission). There was no evidence of buprenorphine/naloxone-precipitated withdrawal. During the first week after admission, all 7 participants used clonazepam (2–6 mg per day for 3–6 days) and clonidine (0.2–0.6 mg per day for 4–6 days). Three participants used acetaminophen (650–800 mg per day for 1 day), 4 participants used ibuprofen (800–1600 mg per day for 1–3 days), 6 participants used ketorolac (30–90 mg per day for 1–5 days), and 6 participants used trazodone (50–150 mg per night for 1–5 nights; please note that most participants used clonazepam initially and when it was discontinued, they switched to trazodone).
Performance Tasks
Heroin produced few effects on performance of any of the tasks, with the exception of the divided attention task. The number of missed targets was greater after most of the active doses of heroin (12.5 mg heroin: F 1,9=10.7, P < 0.002, 25 mg heroin: F 1,9=20.7, P < 0.0001, and 100 mg heroin: F 1,9=9.9, P < 0.003), and the number of correctly identified targets was lower after all of the active doses of heroin (12.5 mg heroin: F 1,9=10.0, P < 0.003, 25 mg heroin: F 1,9=19.4, P < 0.0001, and 100 mg heroin: F 1,9=10.0, P < 0.003). Relative to 2/0.5 mg, 8/2 mg buprenorphine/naloxone impaired performance of the divided attention task: the latency with which participants responded to a stimulus was longer under 8/2 mg buprenorphine/naloxone (100 mg heroin: F 1,9=5.2, P < 0.03), corresponding to a 350 msec difference between 2/0.5 and 8/2 mg buprenorphine/naloxone.
Physiological Effects
Buprenorphine/naloxone produced small, but dose-related decreases in pupil diameter (0.3 mm difference; Pbo, 2/0.5 vs. 32/8 mg: F 1,48=4.1, P < 0.05). Heroin also produced dose-related decreases in pupil diameter (0.4 mm difference; 0 mg compared to 100 mg heroin: F 1,4=6.5, P < 0.001). Although the main effect of buprenorphine/naloxone dose was not statistically significant, the buprenorphine/naloxone dose X heroin dose interaction was significant (F 1,8=2.8, P < 0.01). Heroin produced small, dose-related decreases in arterial oxygen saturation (0.5% difference; 0 mg compared to 100 mg heroin: P < 0.04; note that these values were obtained under supplemental oxygen conditions). The main effect of buprenorphine/naloxone dose on arterial oxygen saturation was not statistically significant. Heroin produced dose-related increases in diastolic pressure (3.6 mm Hg difference; 0 mg compared to 100 mg heroin: F 1,4=3.1, P < 0.04). There was a trend for a dose-related increase in heart rate (2.4 beats per minute difference; 0 mg compared to 100 mg heroin: F 1,4=2.5, P < 0.07). Across time, heart rate increased from 75.5 bpm at baseline to 77.2 bpm 10 min after administration of drug, and then declined to 68.4 bpm 60 min after administration of drug (main effect of time: F 1,6=14.1, P < 0.0001). Systolic pressure and mean arterial pressure were not significantly affected by heroin dose, and none of the cardiovascular measures was affected by buprenorphine/naloxone maintenance dose.
Quantitative Analyses
The data obtained for heroin alone (Figures 1 and 3) and in the presence of 2/0.5, 8/2 and 32/8 mg buprenorphine/naloxone were analyzed to obtain estimates for the in vivo dissociation constant, KA (which is inversely related to agonist affinity), efficacy (tau), and the fraction of receptors remaining after buprenorphine/naloxone treatment (q). Only those data (that is, the self-administration data and the VAS ratings of “Good Drug Effect,” “High,” and “Potent”) in which 2/0.5, 8/2 and 32/8 mg buprenorphine/naloxone dose-dependently shifted the heroin dose-response curve to the right and downward were amenable to this analysis (see Zernig et al., 1996). Analysis of the heroin dose-response curves indicated that buprenorphine/naloxone dose-dependently (F 2,3=9.08, P < 0.01) reduced the fraction of receptors available for agonist interaction (q) (Table 3). After a dose of 2/0.5 mg buprenorphine/naloxone treatment, 21 to 31% of the receptor population remained available for agonist interaction. After 8/2 and 32/8 mg buprenorphine/naloxone treatment, 11 to 22% and 6 to 12% of the receptor population remained available for agonist interaction, respectively. These data analyses indicate that 2/0.5, 8/2 and 32/8 mg buprenorphine/naloxone eliminated approximately 74%, 83%, and 91% of the receptor population under these dosing conditions. In addition, 32/8 mg buprenorphine/naloxone eliminated a significantly greater proportion of the receptor population than 2/0.5 mg (P<0.001) or 8/2 mg buprenorphine/naloxone (P<0.01).
Table 3.
Proportion of receptors remaining (q values; ± 95%C.L.) after treatment with 2/0.5, 8/2 or 32/8 mg buprenorphine/naloxone
| 2/0.5 mg | 8/2 mg | 32/8 mg | |
|---|---|---|---|
| Heroin Self-administration | 0.21 (0.17–0.34) | 0.11 (0.086–0.17) | 0.060 (0.039–0.14) |
| Good Drug Effect | 0.31 (0.25–0.46) | 0.22 (0.17–0.27) | 0.096 (0.036–0.58) |
| High | 0.31 (0.21–0.41) | 0.20 (0.16–0.26) | 0.12 (0.020–0.67) |
| Potent | 0.21 (0.18–0.25) | 0.16 (0.14–0.19) | 0.090 (0.057–0.16) |
q values were significantly different overall F 2,3=9.08, p<0.01; 2/0.5 vs. 8/2 mg, p<0.01; 2/0.5 vs. 32/8 mg, p<0.001; 8/2 vs. 32/8 mg, p<0.01).
Apparent affinity (KA) and efficacy (tau) estimates for heroin were also determined from these data (Table 4). The apparent KA values for heroin ranged from 50–126 mg to produce self-administration and subjective effects. In general, the 95% C.L. for the apparent KA values were relatively small indicating the high agreement across the family of heroin dose-response curves within a given behavioral measure. The efficacy estimate (tau) ranged from 13.1–20.2.
Table 4.
Apparent affinity (KA) and efficacy (tau) estimates (± 95%C.L.) for heroin
| KA (log mg/kg) | KA (mg/kg) | tau | |
|---|---|---|---|
| Heroin Self-administration | 1.7 (1.6–1.8) | 50 | 20.2 (19.8–20.3) |
| Good Drug Effect | 2.1 (2.0–2.2) | 126 | 13.1 (12.9–13.2) |
| High | 2.1 (2.0–2.1) | 126 | 14.4 (13.9–14.5) |
| Potent | 1.9 (1.8–2.0) | 79 | 13.4 (13.3–13.5) |
Discussion
Numerous clinical and pre-clinical studies have demonstrated that buprenorphine reduces the effects of opioid agonists. However, the optimum doses and interdose intervals have yet to be determined. The data shown in the present study demonstrate that heroin self-administration decreased in a dose-related fashion under maintenance on the buprenorphine/naloxone combination. In our previous study, the effects of 16 mg buprenorphine alone produced relatively small reductions in heroin self-administration, compared to 8 mg buprenorphine (Comer et al., 2001). Because a wider range of doses was tested in the current study (2/0.5, 8/2, and 32/8 mg buprenorphine/naloxone), it was possible to detect greater reductions in heroin self-administration. For example, comparing the 2/0.5 mg and 32/8 mg buprenorphine/naloxone maintenance dose conditions, the break point values for 25 mg and 50 mg intranasally delivered heroin were reduced by 786 and 857 responses, respectively. In our previous study with buprenorphine alone, break point values for 12.5 mg intravenously delivered heroin were reduced by only 529 responses under the 16 mg buprenorphine maintenance dose condition, compared to 8 mg buprenorphine (Comer et al., 2001). The results presented here are similar to those reported in a previous study evaluating the effects of maintenance on buprenorphine alone (Greenwald et al., 2002). In that study, buprenorphine produced a dose-related reduction in the reinforcing effects of hydromorphone. Mello and colleagues (Mello and Mendelson, 1980; Mello et al., 1982) demonstrated that intravenous heroin self-administration also was antagonized by buprenorphine. In contrast to the present results, Mello and Mendelson (1980) showed that the reinforcing effects of heroin were almost completely eliminated by 8 mg buprenorphine. Two important procedural differences may account for this difference in results: buprenorphine was administered subcutaneously and a lower maximum dose of heroin was tested by Mello and Mendelson (1980). Nevertheless, all of these laboratory studies support the clinical data demonstrating the effectiveness of buprenorphine in treating opioid dependence. The present results extend those findings to the buprenorphine/naloxone combination and suggest that sublingual daily doses between 8 and 32 mg are well tolerated and effective in reducing the reinforcing effects of heroin.
The effects of buprenorphine/naloxone on the subjective effects of heroin were similar to but slightly less robust than its effects on heroin self-administration. For example, the effects of buprenorphine/naloxone on ratings of heroin potency, quality, and liking were not very robust. However, ratings of “Good Effect” were reduced in a robust manner by buprenorphine/naloxone (see Figure 3). This variability in the effects of buprenorphine on subjective ratings has been shown in previous studies (e.g., Bickel et al., 1988b; Comer et al., 2001).
Buprenorphine/naloxone itself produced minimal subjective effects, as measured by near-zero baseline ratings prior to heroin administration. These data are not entirely surprising, given the fact that participants were stabilized on buprenorphine/naloxone for at least one week prior to initiation of testing. In addition, buprenorphine/naloxone was administered approximately 15 h prior to laboratory sessions when agonist effects were likely at a minimum. A previous study conducted in non-opioid-dependent individuals showed that subjective ratings produced by 8 mg sublingual buprenorphine had returned to near-zero levels within 6–8 h after dosing (Nath et al., 1999). Furthermore, based on previous studies examining the antagonist effects of sublingual naloxone, it is unlikely that naloxone contributed in any significant way to the antagonist effects found in the present study (Chiang and Hawks, 2003; Harris et al., 2004). The 15-hr pretreatment interval also supports the view that the antagonist effects of naloxone, which are of short duration, were essentially insignificant.
Consistent with our previous studies, intranasal heroin produced few disruptions in performance in the present study (Comer et al., 1997, 1999), with the exception of performance on the divided attention task. On that task, 8/2 mg buprenorphine/naloxone produced small decrements in performance relative to 2/0.5 mg. Previous studies of acute administration of buprenorphine have reported that buprenorphine impairs psychomotor performance. For example, Zacny et al. (1997) showed that performance of the Digit Symbol Substitution task, as well as four other psychomotor tests, was impaired in a dose-related manner after acute, intravenous administration of buprenorphine. However, Mello et al. (1982) reported that during maintenance on buprenorphine, performance of an operant task (button presses on a manipulandum) was not impaired, relative to a placebo-maintained group. In the present study, the degree of impairment produced by sublingual buprenorphine/naloxone in combination with intranasal heroin was relatively small. A key difference between the results reported by Zacny and colleagues (1997) and those reported by other investigators (present results; Mello et al., 1982; Walsh et al., 1994) was that participants in the study conducted by Zacny and colleagues (1997) were normal, healthy volunteers with limited opioid use histories.
The physiological effects of buprenorphine/naloxone were also consistent with previous studies. Both buprenorphine/naloxone and heroin produced dose-related decreases in pupil diameter. Furthermore, in the present study, both 8/2 and 32/8 mg buprenorphine/naloxone antagonized the miotic effects of heroin, which is consistent with Walsh and colleagues (1995). In the present study, heroin produced very small decreases in arterial oxygen saturation that were generally not affected by buprenorphine/naloxone, which is not surprising, given that participants received supplemental oxygen during all experimental sessions.
To begin to elucidate the relationship of underlying receptor events to the subjective and reinforcing effects of heroin in humans, the method of partial insurmountable blockade was used in the present study. The apparent affinity and efficacy values for heroin were calculated using progressive ratio break point values and subjective ratings as the dependent variables. The apparent KA estimates for heroin obtained in the present study were remarkably similar to each other across the different behavioral measures (range 50–126 mg). The exact relation of these apparent KA estimates determined in human subjects to those obtained in animal subjects is presently unknown due to the differences in dose measurement units. For example, apparent KA estimates for morphine range from 11–68 mg/kg in laboratory animals (Barrett et al., 2003; Pitts et al., 1998; Walker et al., 1995; Walker et al., 1998; Zernig et al., 1994, 1995). Interestingly, the efficacy estimates for heroin in the present study (range 13–20) were comparable to those obtained for morphine in these same rodent and monkey studies (range 9–38). Correspondingly, tau estimates for heroin and morphine to produce antinociception in rhesus monkeys were analogous to each other (Negus et al., 2003) as well as to the estimates obtained in the present study. These data suggest that the behavioral effects produced by heroin and morphine in these studies were mediated by pharmacologically related populations of mu opioid receptors. These efficacy estimates are important for drug classification because relative efficacy can only be determined in a functional assay. Furthermore, these results demonstrate that similar apparent agonist affinity and efficacy estimates for heroin (morphine) are calculated whether buprenorphine/naloxone combination (present study), buprenorphine alone (Tallarida and Cowan, 1982; Walker et al., 1995), or clocinnamox (Barrett et al., 2003; Pitts et al., 1998; Walker et al., 1998; Zernig et al., 1994) is used as the insurmountable antagonist. The data collected in the present study provide support for the validity and generalizability of the data collected in laboratory animals.
The method of partial insurmountable blockade can also be used to determine the proportion of receptors eliminated by a given dose of insurmountable antagonist. In the present study, 2/0.5, 8/2 and 32/8 mg buprenorphine/naloxone dose-dependently eliminated the receptors available for agonist interaction by approximately 74%, 83%, and 91%. A previous study by Greenwald et al. (2003) showed similar reductions in whole-brain mu opioid receptor availability (calculated as changes in binding potential) under maintenance on sublingual buprenorphine as measured with positron emission tomography and [11C] carfentanil. Although 32/8 mg buprenorphine/naloxone eliminated 91% of the receptor population in the present study, heroin continued to maintain self-administration and to produce subjective effects, albeit at reduced levels. One potential concern when comparing the reinforcing and subjective effects of heroin is that the subjective effects were measured immediately following heroin administration while the progressive ratio break point data were collected in the absence of heroin. However, the fact that participants responded on a progressive ratio schedule for heroin doses that they sampled in the beginning of the day, in an earlier session, in the presence of the different doses of buprenorphine/naloxone, indicates that buprenorphine/naloxone was reducing the reinforcing efficacy of heroin to the degree measured in this study. These data indicate that self-administration and subjective effects can be produced with very few opioid receptors available and therefore speaks to the efficacy of heroin as a reinforcer. Similar data were collected in rats self-administering heroin and pretreated with β-FNA (Martin et al., 1995; 1998).
Overall, however, caution is warranted when applying quantitative analyses to in vivo data. First, differences between in vivo efficacy or affinity estimates are rarely considered relevant using these particular quantitative methods unless the differences are 10-fold or greater (Zernig et al., 1996). Therefore, the 2–3 fold differences among the KA and tau values for heroin across the different behavioral measures are not distinguishable especially considering the variability that accompanies behavioral data. Second, the behavioral effects of drugs are modified by a number of non-pharmacological factors that undoubtedly may alter the estimates of KA, tau, and q. However, the quantitative analysis used in the present study has the advantage of simultaneously analyzing all dose-response curves together for a given effect, increasing the confidence in the estimated values. In our behavioral studies, we make the assumption that the non-pharmacological or even the pharmacokinetic factors are similar across all testing conditions. These null methods should limit the influence of outside variables that might dramatically alter the estimated values.
In conclusion, results of the present study demonstrate that both 8/2 and 32/8 mg of the tablet formulation of buprenorphine/naloxone significantly decreased the behavioral effects of intranasal heroin, relative to 2/0.5 mg buprenorphine/naloxone. Although the reductions in the effects of heroin were dose-related overall, the lack of statistically significant differences between the effects produced by 8/2 and 32/8 mg buprenorphine/naloxone suggests that there may be only small improvements in clinical effectiveness with increases in buprenorphine/naloxone dose within the range tested. The present study also demonstrates that it is possible to obtain apparent affinity and efficacy estimates in humans using buprenorphine. The quantitative analyses will be particularly useful in future studies for examining the relative efficacies of different opioid agonists in humans. A convergence of apparent KA, tau, and q values across behavioral effects, species, and laboratories will further define the reliability and validity of such estimates using in vivo preparations as well as improve our understanding of the relationship of underlying receptor events and observed behavior.
Acknowledgments
The assistance of Michael R. Donovan, Mabel Torres, and Drs. Evaristo Akerele, Adam Bisaga, and Maria Sullivan is gratefully acknowledged. This research was supported by grant DA09236.
References
- Barrett AC, Smith ES, Picker MJ. Use of irreversible antagonists to determine the relative efficacy of mu opioids in a pigeon drug discrimination procedure: Comparison of beta-funaltrexamine and clocinnamox. J Pharmacol Exp Ther. 2003:1061–1070. doi: 10.1124/jpet.102.047068. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Crean JP, Badger GJ. Buprenorphine dosing every 1, 2, or 3 days in opioid-dependent patients. Psychopharmacology. 1999;146:111–118. doi: 10.1007/s002130051096. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial with buprenorphine: Comparison with methadone in the detoxification of heroin addicts. Clin Pharmacol Ther. 1988a;43:72–78. doi: 10.1038/clpt.1988.13. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: Dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther. 1988b;247:47–53. [PubMed] [Google Scholar]
- Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Black JW, Leff P, Shankley NP, Wood J. An operational model of pharmacological agonism: the effects of E/[A] curve shape on agonist dissociation constant estimation. Br J Pharmacol. 1985;84:561–571. doi: 10.1111/j.1476-5381.1985.tb12941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CN, Hawks RL. Pharmacokinetics of the combination tablet of buprenorphine and naloxone. Drug Alcohol Depend. 2003;70:S39–S47. doi: 10.1016/s0376-8716(03)00058-9. [DOI] [PubMed] [Google Scholar]
- Comer SD, Burke TF, Lewis JW, Woods JH. Clocinnamox: A novel, systemically-active, irreversible opioid antagonist. J Pharmacol Exp Ther. 1992;262:1051–1056. [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol. 1997;8:667–690. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Buprenorphine sublingual tablets: Effects on IV heroin self-administration by humans. Psychopharmacology. 2001;154:28–37. doi: 10.1007/s002130000623. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, Fischman MW. Comparison of intranasal and intravenous heroin self-administration by morphine-maintained humans. Psychopharmacology. 1999;143:327–338. doi: 10.1007/s002130050956. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration Illegal Drug Price and Purity Drug Intelligence Report. 2003 Apr; http://www.usdoj.gov:80/dea/pubs/intel/02058/02058.html.
- Evans SM, Foltin RW, Levin FR, Fischman MW. Behavioral and subjective effects of DN-2327 (pazinaclone) and alprazolam in normal volunteers. Behav Pharmacol. 1995;6:176–186. [PubMed] [Google Scholar]
- Furchgott RF. The use of β-haloalkylamines in the differentiation of receptors and in the determination of dissociation constants of receptor-agonist complexes. Adv Drug Res. 1966;3:21–56. [Google Scholar]
- Greenwald MK, Johanson C-E, Moody DE, Woods JH, Kilbourn MR, Koeppe RA, Schuster CR, Zubieta J-K. Effects of buprenorphine maintenance dose on m-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacol. 2003;28:2000–2009. doi: 10.1038/sj.npp.1300251. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Schuh KJ, Hopper JA, Schuster CR, Johanson C-E. Effects of buprenorphine sublingual tablet maintenance on opioid drug-seeking behavior by humans. Psychopharmacology. 2002;160:344–352. doi: 10.1007/s00213-001-0975-0. [DOI] [PubMed] [Google Scholar]
- Hambrook J, Rance M. The interaction of buprenorphine with the opiate receptor: Lipophylicity as a determining factor in drug-receptor kinetics. In: Kosterlitz H, editor. Opiates and Endogenous Peptides. North-Holland; Amsterdam: 1976. pp. 295–301. [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Harris DS, Mendelson JE, Lin ET, Upton RA, Jones RT. Pharmacokinetics and subjective effects of sublingual buprenorphine, alone or in combination with naloxone: lack of dose proportionality. Clin Pharmcokinet. 2004;43(5):329–40. doi: 10.2165/00003088-200443050-00005. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. New Engl J Med. 2000;343(18):1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. J Amer Med Assoc. 1992;267(20):2750–2755. [PubMed] [Google Scholar]
- Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug and Alcohol Depend. 2003;70:S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Performance-based testing for drugs of abuse: Dose and time profiles of marijuana, amphetamine, alcohol, and diazepam. J Anal Toxicol. 1993;17:264–272. doi: 10.1093/jat/17.5.264. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. Pharmacologic Analysis of Drug-Receptor Interaction. 3. Lippincott Williams & Wilkins; 1997. [Google Scholar]
- Ling W, Wesson DR, Charuvastra C, Klett CJ. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Arch Gen Psychiatry. 1996;53:401–407. doi: 10.1001/archpsyc.1996.01830050035005. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Dworkin SI, Smith JE. Alkylation of mu opioid receptors by beta-funaltrexamine in vivo: comparison of the effects on in situ binding and heroin self-administration in rats. J Pharmacol Ther Exp. 1995;272:1135–1140. [PubMed] [Google Scholar]
- Martin TJ, DeMontis MG, Kim SA, Sizemore GM, Dworkin SI, Smith JE. Effects of beta-funaltrexamine on dose-effect curves for heroin self-administration in rats: comparison with alteration os [3H]DAMGO binding to rat brain sections. Drug Alcohol Depend. 1998;52:135–147. doi: 10.1016/s0376-8716(98)00082-9. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the Digit Symbol Substitution Test (DSST) Behav Res Meth Instr. 1982;14:463–466. [Google Scholar]
- Mello NK, Bree MP, Mendelson JH. Comparison of buprenorphine and methadone effects on opiate self-administration in primates. J Pharmacol Exp Ther. 1983;225:378–386. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;207:657–659. doi: 10.1126/science.7352279. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC. Buprenorphine effects on human heroin self-administration: An operant analysis. J Pharmacol Exp Ther. 1982;223:30–39. [PubMed] [Google Scholar]
- Miller TP, Taylor JL, Tinklenberg JR. A comparison of assessment techniques measuring the effects of methylphenidate, secobarbital, diazepam and diphenhydramine in abstinent alcoholics. Neuropsychobiology. 1988;19:90–96. doi: 10.1159/000118441. [DOI] [PubMed] [Google Scholar]
- Nath RP, Upton RA, Everhart ET, Cheung P, Shwonek P, Jones RT, Mendelson JE. Buprenorphine pharmacokinetics: Relative bioavailability of sublingual tablet and liquid formulations. J Clin Pharmacol. 1999;39:619–623. doi: 10.1177/00912709922008236. [DOI] [PubMed] [Google Scholar]
- Negus SS, Brandt MR, Gatch MB, Mello NK. Effects of heroin and its metabolites on schedule-controlled responding and thermal nociception in rhesus monkeys: Sensitivity to antagonism by quadazocine, naltrindole and beta-funaltrexamine. Drug Alcohol Depend. 2003;70(1):17–27. doi: 10.1016/s0376-8716(02)00331-9. [DOI] [PubMed] [Google Scholar]
- Pitts RC, Allen RA, Walker EA, Dykstra LA. Clocinnamox antagonism of the antinociceptive effects of mu opioids in squirrel monkeys. J Pharmacol Exp Ther. 1998;285:1197–1206. [PubMed] [Google Scholar]
- Raffa RB, Porreca F, Cowan A, Tallarida RJ. Morphine-receptor dissociation constant and the stimulus effect relation for inhibition of gastrointestinal transit in the rat. Eur J Pharmacol. 1982;79:11–16. doi: 10.1016/0014-2999(82)90569-6. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Comparison of buprenorphine and methadone in the treatment of opioid dependence. Am J Psychiatry. 1994;151:1025–1030. doi: 10.1176/ajp.151.7.1025. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. The Dose-Response Relationship in Pharmacology. Springer-Verlag; 1979. [Google Scholar]
- Tallarida RJ, Cowan A. The affinity of morphine for its pharmacologic receptor in vivo. J Pharmacol Exp Ther. 1982;222:198–201. [PubMed] [Google Scholar]
- Walker EA, Zernig G, Woods JH. Buprenorphine antagonism of mu opioids in the rhesus monkey tail-withdrawal procedure. J Pharmacol Exp Ther. 1995;273:1345–1352. [PubMed] [Google Scholar]
- Walker EA, Zernig G, Young AM. In vivo apparent affinity and efficacy estimates for μ opiates in a rat tail-withdrawal assay. Psychopharmacology. 1998;136:15–23. doi: 10.1007/s002130050534. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: Partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–372. [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9:223–229. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]
- Winger G, Woods JH. Effects of buprenorphine on behaviour maintained by heroin and alfentanil in rhesus monkeys. Behav Pharmacol. 1996;7(2):155–159. [PubMed] [Google Scholar]
- Zacny JP, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: Position statement. Drug and Alc Depend. 2003;69:215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Conley K, Galinkin J. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J Pharmacol Exp Ther. 1997;282:1187–1197. [PubMed] [Google Scholar]
- Zernig G, Butelman ER, Lewis JW, Walker EA, Woods JH. In vivo determination of mu opioid receptor turnover in rhesus monkeys after irreversible blockade with clocinnamox. J Pharmacol Exp Ther. 1994;269:57–65. [PubMed] [Google Scholar]
- Zernig G, Issaevitch T. Software for the calculation of agonist efficacy and apparent in vivo affinity by simultaneous analysis of several dose-response curves. Wolfram Research; Champaign, Ill: 1995. [Google Scholar]
- Zernig G, Issaevitch T, Woods JH. Calculation of agonist efficacy, apparent affinity, and receptor population changes after administratin of insurmountable antagonists: comparison of different analytical approaches. J Pharmacol Toxicol Meth. 1996;35:223–237. doi: 10.1016/1056-8719(96)00053-6. [DOI] [PubMed] [Google Scholar]