Abstract
Although buprenorphine is used worldwide as a safe and effective maintenance medication for opioid dependence, some countries have reported a growing incidence of abuse of this medication. Buprenorphine is considered to have lower abuse potential because of its partial agonist profile, but no studies have directly compared the reinforcing effects of buprenorphine with those of full mu opioid agonists in humans. The present double-blind, placebo-controlled, inpatient study compared the reinforcing and subjective effects of intravenously administered buprenorphine (0.5, 2 and 8 mg) and methadone (5, 10, and 20 mg). Participants (N=6) were detoxified from heroin during the first 1-2 weeks after admission. During subsequent weeks, participants received a sample drug dose and $20 on Monday, and they could self-administer either the sampled dose or $20 during one choice session per day on Thursday and Friday. Participants responded under a modified progressive ratio schedule during each choice session. All active doses maintained higher progressive ratio break points (largest completed ratio) than placebo. There were no significant differences in break point values between buprenorphine and methadone or among the different doses of drug. However, several subjective ratings, including “Good Drug Effect,” “High,” and “Liking” dose-dependently increased after administration of buprenorphine and methadone. The peak ratings for these effects did not significantly differ for the two drugs. These results demonstrate that under these experimental conditions, buprenorphine and methadone were equally effective in producing reinforcing and subjective effects.
Introduction
Buprenorphine, a partial mu opioid agonist and kappa opioid antagonist, is used clinically to treat pain, and more recently, it is used as a safe and effective maintenance therapy for opioid dependence. The partial agonist profile of buprenorphine was defined by several studies conducted in both laboratory animals and humans showing that its effects are less robust than those of full mu agonists, particularly when the intensity of the stimulus (e.g., pain intensity) is high. For example, in a warm water tail withdrawal procedure, buprenorphine produces maximal analgesic effects when the water temperature is low, but not when it is high (Barrett et al., 2001; Morgan et al., 1999; Walker et al, 1995). Similarly, in a drug discrimination paradigm, buprenorphine consistently substitutes fully for other mu agonists when the training dose is low (Preston and Bigelow, 2000), but not when it is high (Picker et al., 1993). The partial agonist profile of buprenorphine is also revealed under conditions of opioid tolerance: the effects of buprenorphine are reduced more than other mu agonists in opioid tolerant animals. For example, in rats chronically treated with buprenorphine, the analgesic effects of buprenorphine itself were reduced to a greater extent than the analgesic effects of other mu agonists, such as etorphine or morphine (Walker and Young, 2001; see also Paronis and Holtzman, 1992). In rhesus monkeys trained to self administer the mu opioid agonist alfentanil, intravenously delivered buprenorphine maintained moderate rates of responding in non-dependent animals, but responding was virtually eliminated when the animals were chronically treated with morphine (Winger and Woods, 2001). These studies and others demonstrate that under some conditions, buprenorphine produces full effects, but under other conditions, it does not. Thus, the experimental conditions under which the effects of buprenorphine are measured are important to consider when evaluating its abuse liability.
In a study comparing the effects of sublingual buprenorphine (0.5-32 mg) and oral methadone (3.75-60 mg) in opioid abusing humans, Walsh and colleagues (1994, 1995) showed a “ceiling” on the effects produced by buprenorphine, but not methadone. An early study conducted by Jasinski et al. (1978) showed that subcutaneous buprenorphine (0.2-2 mg) produced dose-dependent increases in subjective effects that were comparable to those produced by morphine (15-40 mg) and methadone (30 mg). In that study, there was no evidence for a ceiling on buprenorphine’s effects, which may have been due to the relatively low doses of buprenorphine administered. A recent study by Umbricht et al. (2004) showed that high doses of intravenously administered buprenorphine (2-16 mg) produced dose-related increases in subjective effects, but due to large inter-subject variability in response, these effects were not significantly different from placebo. In a study conducted in rhesus monkeys trained to self-administer opioids under progressive ratio schedules, Mello and colleagues showed that progressive ratio break point values for buprenorphine and methadone were comparable and were relatively low, compared to heroin (Mello et al., 1988). No studies to date have directly compared both the reinforcing and subjective effects of buprenorphine with those of methadone, a full mu opioid agonist, in humans. The present study examined the abuse liability of high doses of intravenously delivered buprenorphine (0.5-8 mg) compared to methadone (5-20 mg) using a drug self-administration procedure that is designed to evaluate potential differences in reinforcing efficacy. The maximal doses were chosen to be those that would be safely tolerated in opioid-experienced, non-dependent individuals. For methadone, studies have shown a roughly 2:1 ratio in steady-state plasma concentrations after oral and intravenous administration, respectively (Dale et al., 2004). Clinical experience suggests that substantial toxicity, including death, may occur in non-opioid-dependent individuals receiving a starting dose of 30-40 mg methadone orally (Senay, 1999). Although a 30 mg subcutaneous dose of methadone has been administered safely to opioid-experienced, non-dependent individuals (Jasinski et al., 1978), we opted to test a maximum dose of 20 mg intravenous methadone to ensure safety. For buprenorphine, a maximal dose of 8 mg was also chosen for safety reasons. As described by Umbricht and colleagues (2004), a dose of 12 mg intravenous buprenorphine produced severe nausea and vomiting in an opioid-experienced, non-dependent individual. Our previous studies using 8 mg intravenous buprenorphine showed that this dose was well-tolerated, so we opted to use this maximum dose in the present study.
Methods
Participants
Six heroin-dependent individuals (5 men, 1 woman; 3 White, 2 Black, 1 Hispanic), who currently were not seeking treatment for their drug use, completed the 8-week protocol. Participants were 39.5 ± 2.8 years of age on average (range: 27 to 43 years), and reported using heroin for an average of 11.8 ± 3.7 years (range: 3 to 24 years). Volunteers weighed 65.8 ± 2.4 kg and were 1.7 ± 0.0 m tall. All participants had experience using heroin by the intravenous route, were currently physiologically dependent on it as verified by a naloxone challenge test prior to admission, and reported spending an average of $62 ± $14 per day on heroin (range: $20-$100). Heroin was the drug of choice for all participants. Six individuals smoked tobacco cigarettes (15 ± 3 cigarettes per day), two participants used cocaine (once per month and twice per week or less), three participants used alcohol (1 to 2 times per week or less), and one participant used marijuana (twice per month). Three additional male participants (2 White, 1 Hispanic) began the study but did not complete it. One participant was discharged early because he decided to seek treatment, one discontinued because he decided that he did not want to undergo detoxification, and one discontinued because he found the study procedures too boring.
After an initial telephone interview, eligible participants received additional screening, which included completing detailed questionnaires on drug use, general health and medical history, and a medical and psychological evaluation. Participants were told that they would receive opioids during the study, and that different doses would be tested. An electrocardiogram and Mantoux test or chest x-ray were also performed. Routine laboratory analyses included a hematology screen, blood chemistry panel, liver function tests, thyroid function tests, syphilis serology, and urinalysis. Urine drug toxicologies (opioids, benzoylecgonine, benzodiazepines, cannabinoids, and amphetamines) were also performed using a radiative energy attenuation and fluorescence polarization immunoassay system (ADx System, Abbott Laboratories, Abbott Park, IL).
Participants were excluded from the study if they were seeking drug treatment, dependent on alcohol or illicit drugs other than opioids, or had a major Axis I psychiatric diagnosis other than heroin dependence (e.g., bipolar disorder, schizophrenia, major depression). Those who had recent histories of violence or who were on parole/probation were excluded from the study. Participants were required to be physically healthy, and fully able to perform all study procedures.
Prior to admission, participants completed a training session, during which the study procedures were explained to them in detail. Volunteers were paid $25 per inpatient day and an additional $25 per day bonus if they completed the study. In addition, they could receive an additional $20 per experimental session. Participants signed consent forms describing the aims of the study, and the potential risks and benefits of participation. Participants were offered free human immunovirus testing, drug and risk reduction education, and referrals for treatment. This study was approved by the Institutional Review Board of the New York State Psychiatric Institute.
Apparatus
During experimental sessions, participants were seated in a room equipped with Macintosh computers. All computer activities, vital signs and behaviors were continuously monitored by the experimenters in an adjacent control room via a continuous on-line computer network, one-way mirror, and vital signs monitors (cardiovascular function was measured using a Sentry II Vital Signs Monitor, NBS Medical, Costa Mesa, CA; arterial oxygen saturation was measured using a pulse oximeter Model 400, Palco Laboratories, Santa Cruz, CA). Communication between the staff and participants was kept to a minimum during experimental sessions.
Detoxification Procedures
Participants were detoxified during the first one to two weeks after their admission into the hospital. Buprenorphine (2-8 mg sublingual tablet; National Institute on Drug Abuse, Rockville, MD) was administered during the first 2 days after admission. Clonidine HCl (0.2 mg p.o., q. 6 h; Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT), ketorolac tromethamine (30 mg i.m., q. 6 h for up to 5 days; Roche Laboratories, Nutley, NJ), prochlorperazine (10 mg p.o. or i.m., q. 8 h; SmithKline Beecham Consumer Healthcare, Pittsburgh, PA) and clonazepam (2 mg p.o., q. 8 h; Roche Laboratories Inc., Nutley, NJ) were available, as needed. All of these medications were discontinued approximately 36 h prior to the first experimental session.
General Procedures
The reinforcing effects of intravenously delivered placebo, buprenorphine (0.5, 2, 8 mg), and methadone (5, 10, 20 mg) were evaluated following detoxification. All doses were administered in nonsystematic order both within and between participants. On Mondays, a dose of drug and $20 was administered, and subjective, performance, and physiological effects were examined both before and repeatedly after drug administration. These measures were repeated on Tuesdays (24 h) and Wednesdays (48 h) in order to assess the time course of the drug administered on Monday. No drugs were administered on Tuesdays and Wednesdays. On Thursdays and Fridays, participants completed one choice session per day, for a total of two choice sessions. They could work to receive all, or part of the dose or $20. The total amount of drug and/or money chosen during the self-administration task was given as a bolus at the end of the task.
Experimental Sessions
During all sessions, participants completed computerized tasks and subjective-effects questionnaires. Heart rate and blood pressure were measured every 5 min, and blood oxygen saturation was monitored continuously with a pulse oximeter and recorded every minute during experimental sessions. Pupil photographs were taken repeatedly during the session. Participants were not allowed to smoke tobacco cigarettes during experimental sessions.
Sample Session
Physiological, subjective and performance effects were measured both before and repeatedly after drug administration. Following baseline measures, drug and $20 were administered simultaneously at time 0 min, provided that oxygenation was sufficient (SpO2 > 93%). A photograph was taken of the right pupil before and 4, 10, 40, 60, 90, 120 and 180 min after drug administration. A subjective-effects battery was administered before and 4, 40, 90, 150 and 210 min after drug administration. A performance battery was administered before and 10, 60, 120, and 180 min after drug administration. The Subjective Opioid Withdrawal Scale (SOWS) was administered before and 180 min after drug administration. The Drug Effects Questionnaire (DEQ) was administered 4, 10, 60, 120, and 180 min after drug administration.
No Drug Sessions
Physiological, subjective and performance effects were measured 24 and 48 h after administration of drug to evaluate potential long-lasting agonist effects.
Choice Sessions
Choice sessions were similar in design to the sample session, except that participants completed a self-administration task (see below) after the baseline assessments. Participants were instructed to choose between tenths of $20 and the dose that they received during the sample session. A pupil photograph was taken before drug administration. The subjective-effects battery was administered before, and 4 and 40 min after drug administration. The performance battery was completed before, and 10 min after drug administration. The SOWS was completed before drug administration. The DEQ was completed before and 10 min after drug administration. Choice sessions were otherwise identical to the sample session.
Self Administration Task
Participants were told that they could work for all or part of the sampled dose or the sampled money amount ($20) by choosing the drug or money option each time a choice was available. The alternative money value ($20) was chosen based on previous studies conducted in our laboratory (Comer et al., 1997, 1998) showing that the dose-response curve for heroin was the most lawful when this money value was used. Responses consisted of finger presses on a computer mouse. Standardized instructions were read to each participant explaining the self-administration task. Drug and money were available under independent progressive ratio schedules, and participants were given 10 opportunities (trials) to choose between the two options. Ten percent of that day’s dose or money value was available at each choice trial. Thus, if the dose for that day was 8 mg, at each opportunity participants could respond for 0.8 mg (10% of 8 mg) or $2 (10% of $20). Completion of the ratio requirement for each choice trial was accompanied by a visual stimulus on the computer screen. After a choice was made for one option, responding for the other option was not possible until the ratio was completed and another trial was initiated. The response requirement for each of the two options increased independently such that the initial ratio requirement for each option was 50 responses; the ratio increased progressively each time the option was selected (50, 100, 200, 400, 800, 1200, 1600, 2000, 2400, 2800). In order to receive all of the drug or money available that day, participants were required to emit 11,550 responses within 40 min. Fewer total responses were required if choices were distributed between the two options. These ratio values were chosen based on previous research conducted in our laboratory (e.g., Comer et al.,1999). Although required to sustain high rates of responding, participants were capable of completing 11,550 responses in the allotted time. For example, 4 of the 6 participants in the present study completed 11,550 responses on multiple occasions during the study.
At the start of each self-administration task, two illustrations appeared on the computer screen: an empty balance scale and an empty bank. As each choice trial was completed, either the scale was implemented with a pile of powder or a dollar sign was added to the bank. Thus, participants could always see how many money and drug choices had been made. At the end of the 40-min self-administration task, the participant received whatever he/she had chosen: money and/or drug.
Subjective Measures
Four questionnaires were used to assess subjective effects (see Comer et al., 1999 for details). The first questionnaire was a 26-item visual analog scale designed to assess subjective and physiological effects. The first eighteen lines were labeled with adjectives describing mood states (e.g., “I feel...:” “high”) and four additional lines, labeled with questions about the dose just received (e.g., “I liked the dose,” “For this dose, I would pay”). Participants also indicated, by making a mark along a 100 mm line, how much they “wanted” each of the following drugs: heroin, cocaine, alcohol, and tobacco. Participants rated each item on the visual analog scale from “Not at all” (0 mm) to “Extremely” (100 mm), except for the “For this dose, I would pay” question, which ranged between $0 (0 mm) and $20 (100 mm). The second questionnaire was a 13-item opioid symptom checklist consisting of true/false questions designed to measure opioid effects (e.g., “My skin is itchy”). The visual analog scale and opioid symptom checklist together constituted the subjective-effects battery. The third questionnaire was the 16-item Subjective Opioid Withdrawal Scale. Participants rated each item on a scale from 0 to 4, with 0 being “Not at all” and 4 being “Extremely” (e.g., “I have gooseflesh,” etc.). The fourth questionnaire was a 6-item Drug Effects Questionnaire. Participants described drug effects by selecting among a series of possible answers ranging from 0 (“No (good, bad, etc.) effect at all”) to 4 (“Very strong effects”). Ratings of drug liking ranged between −4 (“Dislike very much”) to 4 (“Like very much”).
Task Battery
The task battery consisted of four tasks: a 3-min digit-symbol substitution task, a 10-min divided attention task, a 10-min rapid information processing task, and a 3-min repeated acquisition of response sequences task (see Comer et al., 1999 for details). Briefly, the digit-symbol substitution task consisted of nine 3-row by 3-column squares (with one square blackened per row) displayed across the top of the computer screen (McLeod et al., 1982). A randomly generated number indicated which of the nine patterns should be emulated on a keypad by the participant on a particular trial. Participants were required to emulate as many patterns as possible by entering the patterns associated with randomly generated numbers appearing on the bottom of the screen. The divided attention task consisted of concurrent pursuit-tracking and vigilance components (Miller et al., 1988). Participants tracked a moving stimulus on the video screen using the mouse and also signaled when a small black square appeared at any of the four corners of the video screen. The distance between the cursor and the moving stimulus was measured, as was the speed of the moving stimulus (with greater accuracy, the stimulus moved at a faster rate). During the rapid information processing task, a series of digits was displayed rapidly on the computer screen (100 digits/min), and participants were instructed to press a key as quickly as possible after three consecutive odd or even digits (Wesnes and Warburton, 1983). During the repeated acquisition of response sequences task, four buttons were illuminated and participants were instructed to learn a 10-response sequence of button presses (Kelly et al., 1993). A position counter incremented by one each time a correct button was pressed, and remained unchanged whenever the participant responded on an incorrect button. A points counter increased by one each time the 10-response sequence was correctly completed. The sequence remained the same throughout the 3-min task, but a new, random sequence was generated every time the task occurred again. Participants were instructed to earn as many points as possible during the 3-min task by pressing the buttons in the correct sequence.
Physiological Measures
A blood pressure cuff was attached to the non-dominant arm, and blood pressure was recorded automatically every 5 min. Participants were also connected to a pulse oximeter via a soft sensor on a finger of the non-dominant hand, which continuously monitored arterial blood oxygen saturation (%SpO2). For safety, supplemental oxygen (2 L/min) was provided via a nasal cannula during all experimental sessions. If oxygen saturation decreased below 90%, breaths were prompted verbally by staff and the oxygen flow rate was increased. Average arterial oxygen saturation remained above 90% during all sessions. A specially-modified Polaroid camera with a close-up lens (2X magnification) was used to take pupil photographs. All photographs were taken under ambient lighting conditions. Horizontal and vertical measurements of pupil diameter were made using a calipers, and then these two measurements were averaged and divided by 2 to correct for the 2X magnification.
Drugs
Buprenorphine HCl for injection (4 mg/ml) was provided by the National Institute on Drug Abuse (Rockville, MD). Methadone HCl for injection (10 mg/ml) was obtained from Roxane Laboratories, Inc. (Columbus, OH). Naloxone HCl (1 mg/ml; Narcan®) for injection was obtained from DuPont Pharma (Wilmington, DE). Buprenorphine and methadone were diluted in 5% dextrose to produce each dose. Placebo (5% dextrose) or active drug was administered intravenously through a catheter over a 30-sec period in a total volume of 4 ml. Physiologic saline solution was infused continuously during experimental sessions, except during drug administration. Between 1 and 2 ml heparinized saline (10 units/ml) was flushed into the catheter four to eight times each day. All venous catheters were maintained as heparin locks and were removed within 36 h of insertion.
Supplemental medications available to all participants for the duration of the study included: Calcium carbonate (Mylanta®), acetaminophen, ibuprofen, docusate sodium (Colace®), magnesium hydroxide (Milk of Magnesia®), and multi-vitamins with iron. Trazodone HCl (Desyrel®, 50 mg p.o., at bedtime; Warner Chilcott, Morris Plains, NJ) was available if participants reported having trouble sleeping. Three participants used acetaminophen during the study (mean: 2 occasions; range: 1-4 occasions), three used ibuprofen (mean: 31 occasions; range: 19-51 occasions), and one used calcium carbonate on one occasion. All six participants used trazodone during the study (mean: 35 occasions; range: 4-60 occasions). All six participants also used multi-vitamins (mean: 53 occasions; range: 33-66 occasions).
Morning urine samples were collected daily and one random sample per week was screened for the presence of other illicit substances. No illicit substances, other than opioids, were found in the participants’ urines.
Statistical Analyses
Repeated measures analyses of variance with planned comparisons were performed to answer the following primary questions: 1) Do buprenorphine and methadone function as reinforcers? and 2) How do the reinforcing effects of buprenorphine compare to methadone? Specifically, to evaluate the reinforcing effects of buprenorphine and methadone, the progressive ratio break point values and amount of drug self administered in mg for each active dose were compared to placebo. To evaluate dose-related effects, the break point values for each active dose were compared to other active doses (e.g., 0.5 mg versus 2 mg buprenorphine, 0.5 mg versus 8 mg buprenorphine, and 2 mg versus 8 mg buprenorphine). To determine whether the effects of buprenorphine differed from those produced by methadone, the following comparisons were made: 0.5 mg buprenorphine versus 5 mg methadone, 2 mg buprenorphine versus 10 mg methadone, 8 mg buprenorphine versus 20 mg methadone. To assess changes in reinforcing effects across days, break point values and mg drug self administered across the two choice sessions were compared. To assess both peak and time course of effects produced by the sample dose, the subjective, physiological, and performance effects produced by each active dose was compared to placebo.
Drug and money break point values and mg self administered were analyzed as a function of dose (0 mg, 0.5 mg buprenorphine, 2 mg buprenorphine, 8 mg buprenorphine, 5 mg methadone, 10 mg methadone, 20 mg methadone) and choice session (1-2). Pupil diameter, task performance, and subjective ratings during the sample session were analyzed as a function of dose and time. SOWS data during the detoxification week were also analyzed using repeated measures analyses of variance. Only those comparisons with P values less than 0.05 were considered statistically significant. Because only one woman completed the study, it was not possible to examine potential sex differences in the present study.
ED50 values and 95% confidence limits for buprenorphine and methadone were calculated by log-linear regression analysis and analyses of variance. ED50 values were defined as the doses required to produce: a progressive ratio break point value of 2000 for the self-administration task; a 50 mm rating (good, high, liked, quality, potent) or a $10 rating (pay) for the visual analog scale; a value of 2.0 for the Drug Effects Questionnaire; 18000 pixels for tracking distance; 6.5 pixels/sec for maximum speed; and 1.5 for false alarms. Slopes of the dose-response curves were compared using parallel line assays (Finney, 1964). For each of the effects analyzed, the slopes of the buprenorphine and methadone dose-response curves were similar. Therefore, a potency ratio and 95% confidence limits could be determined using parallel line assays. A potency ratio is considered different if the 95% confidence limit does not include the value 1.0. The ED50 values, potency ratios and 95% confidence limits were determined using the statistical software for pharmacology, PharmToolsPro (v1.1.27, Philadelphia, PA).
Results
Self-administration
Figure 1 shows progressive ratio break point values for drug (left panel) and money (middle panel) as a function of dose for buprenorphine and methadone. Mean drug break point values for each active dose were significantly greater than for placebo (P < 0.001 for each comparison: 0 mg versus 0.5 mg buprenorphine [F(1,6) = 16.8]; 0 mg versus 2 mg buprenorphine [F(1,6) = 22.1]; 0 mg versus 8 mg buprenorphine [F(1,6) = 22.1]; 0 mg versus 5 mg methadone [F(1,6) = 16.8]; 0 mg versus 10 mg methadone [F(1,6) = 21.3]; 0 mg versus 20 mg methadone [F(1,6) = 30.9]). However, drug break point values did not significantly differ between the active doses, either within or between drug conditions. Drug break point values were not significantly different during the first and second choice sessions.
Figure 1.
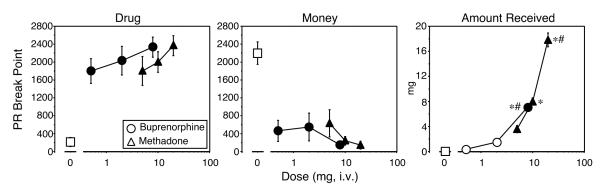
Progressive ratio break point values for drug (left panel) and money (middle panel) and amount of drug self-administered (right panel) as a function of dose and drug. Break point values could range between 0 and 2800. Data points represent the mean across 6 participants and 2 choice sessions. Error bars represent ± 1 standard error of the mean (S.E.M.). Filled symbols indicate significant differences from placebo (open squares); * indicates significant differences from the lowest active dose; # indicate significant differences from the intermediate active dose (P<0.05).
Mean break point values for money (Figure 1, middle panel) when active drug was available were correspondingly lower than when placebo was available (P < 0.001 for each dose comparison: 0 mg versus 0.5 mg buprenorphine [F(1,6) = 21.1]; 0 mg versus 2 mg buprenorphine [F(1,6) = 19.0]; 0 mg versus 8 mg buprenorphine [F(1,6) = 29.4]; 0 mg versus 5 mg methadone [F(1,6) = 17.2]; 0 mg versus 10 mg methadone [F(1,6) = 26.8]; 0 mg versus 20 mg methadone [F(1,6) = 29.5]). As with drug break point values, there were no statistically significant differences in money break point values between the active dose conditions. Money break point values were not significantly different during the first and second choice sessions.
The amount of drug self administered during choice sessions is also shown in Figure 1 (right panel). As expected, based on the progressive ratio break point values, participants self administered near maximal levels of the amount of drug available. Participants self administered significantly more drug when 8 mg buprenorphine was available, compared to placebo ([F(1,6) = 61.9], P < 0.0001) and compared to 0.5 mg ([F(1,6) = 55.7], P < 0.0001) and 2 mg buprenorphine ([F(1,6) = 38.2], P < 0.0001). Similarly, participants self administered significantly more drug when 5 mg ([F(1,6) = 15.9], P < 0.0004), 10 mg ([F(1,6) = 79.3], P < 0.0001), or 20 mg ([F(1,6) = 394.2], P < 0.0001) methadone was available compared to placebo. The amount of drug self administered when 20 mg methadone was available was significantly greater than when 5 mg ([F(1,6) = 251.7], P < 0.0001) or 10 mg ([F(1,6) = 119.9], P < 0.0001) methadone was available. Significantly more drug was also self-administered when 10 mg methadone was available compared to 5 mg methadone ([F(1,6) = 24.2], P < 0.0001).
Subjective Effects
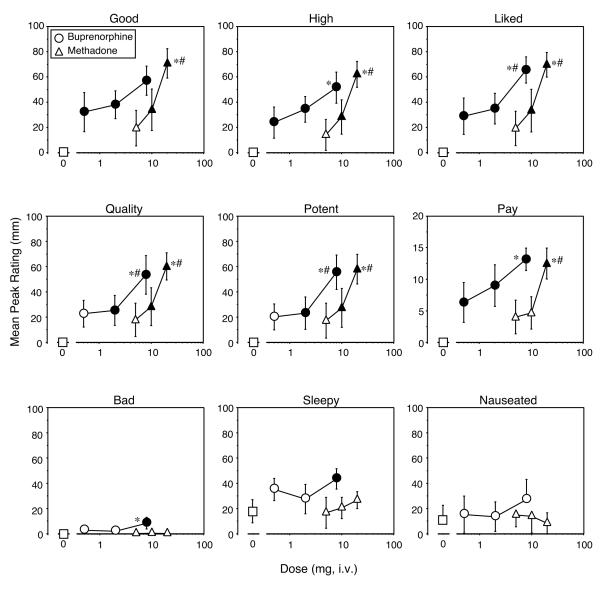
In contrast to the lack of a dose-response relationship for the reinforcing effects of buprenorphine and methadone, most of the subjective-effects ratings were more clearly dose-related. Figure 2 shows mean peak responses on visual analog scale ratings of “Good Drug Effect,” “High,” and drug liking (Figure 2, upper panels). For ratings of “Good Drug Effect,” all of the active doses of buprenorphine were significantly different from placebo (0 mg versus 0.5 mg buprenorphine [F(1,6) = 6.4], P < 0.02; 0 mg versus 2 mg buprenorphine [F(1,6) = 9.0], P < 0.005; 0 mg versus 8 mg buprenorphine [F(1,6) = 20.4], P < 0.0001) and there were no statistically significant differences between the doses, although the difference between 0.5 mg and 8 mg buprenorphine neared statistical significance ([F(1,6) = 3.9], P<0.06). A similar pattern was obtained for ratings of “High” (0 mg versus 0.5 mg buprenorphine [F(1,6) = 6.1], P < 0.02; 0 mg versus 2 mg buprenorphine [F(1,6) = 12.8], P < 0.001; 0 mg versus 8 mg buprenorphine [F(1,6) = 29.1], P < 0.0001), with the exception that the 8 mg dose of buprenorphine was significantly different from the 0.5 mg dose with ([F(1,6) = 8.5], P<0.007). For ratings of drug liking, the 8 mg buprenorphine dose was significantly different from both the 0.5 mg ([F(1,6) = 9.0], P<0.005) and 2 mg doses ([F(1,6) = 6.3], P<0.02). Similarly, ratings of drug quality (0 mg versus 2 mg buprenorphine [F(1,6) = 4.5], P < 0.04; 0 mg versus 8 mg buprenorphine [F(1,6) = 21.2], P < 0.0001; 0.5 mg versus 8 mg buprenorphine [F(1,6) = 7.5], P < 0.01; 2 mg versus 8 mg buprenorphine [F(1,6) = 6.2], P < 0.02), potency (0 mg versus 2 mg buprenorphine [F(1,6) = 4.1], P < 0.05; 0 mg versus 8 mg buprenorphine [F(1,6) = 23.8], P < 0.0001; 0.5 mg versus 8 mg buprenorphine [F(1,6) = 9.7], P < 0.004; 2 mg versus 8 mg buprenorphine [F(1,6) = 8.1], P < 0.008), and the amount of money participants were willing to pay for buprenorphine (0 mg versus 0.5 mg buprenorphine [F(1,6) = 5.6], P < 0.02; 0 mg versus 2 mg buprenorphine [F(1,6) = 11.4], P < 0.002; 0 mg versus 8 mg buprenorphine [F(1,6) = 24.3], P < 0.0001; 0.5 mg versus 8 mg buprenorphine [F(1,6) = 6.5], P < 0.02) were clearly dose related (Figure 2, middle panels).
Figure 2.
Selected Visual Analog scale ratings as a function of dose and drug. Data points represent mean peak ratings for the 6 participants across the sample and subsequent no drug sessions. Error bars represent ± 1 standard error of the mean (S.E.M.). All else as in Figure 1.
For methadone, ratings of “Good Drug Effect” (0 mg versus 10 mg methadone [F(1,6) = 7.2], P < 0.01; 0 mg versus 20 mg methadone [F(1,6) = 31.6], P < 0.0001; 5 mg versus 20 mg methadone [F(1,6) = 16.8], P < 0.0003; 10 mg versus 20 mg methadone [F(1,6) = 8.6], P < 0.006), “High” (0 mg versus 10 mg methadone [F(1,6) = 8.7], P < 0.006; 0 mg versus 20 mg methadone [F(1,6) = 42.2], P < 0.0001; 5 mg versus 20 mg methadone [F(1,6) = 25.6], P < 0.0001; 10 mg versus 20 mg methadone [F(1,6) = 12.6], P < 0.001), drug liking (0 mg versus 10 mg methadone [F(1,6) = 7.4], P < 0.01; 0 mg versus 20 mg methadone [F(1,6) = 32.5], P < 0.0001; 5 mg versus 20 mg methadone [F(1,6) = 17.2], P < 0.0003; 10 mg versus 20 mg methadone [F(1,6) = 8.9], P < 0.006), quality (0 mg versus 10 mg methadone [F(1,6) = 5.6], P < 0.02; 0 mg versus 20 mg methadone [F(1,6) = 27.0], P < 0.0001; 5 mg versus 20 mg methadone [F(1,6) = 14.1], P < 0.0008; 10 mg versus 20 mg methadone [F(1,6) = 7.9], P < 0.008), potency (0 mg versus 10 mg methadone [F(1,6) = 5.7], P < 0.02; 0 mg versus 20 mg methadone [F(1,6) = 26.0], P < 0.0001; 5 mg versus 20 mg methadone [F(1,6) = 13.0], P < 0.001; 10 mg versus 20 mg methadone [F(1,6) = 7.3], P < 0.01), and the amount of money participants were willing to pay for drug (0 mg versus 20 mg methadone [F(1,6) = 21.9], P < 0.0001; 5 mg versus 20 mg methadone [F(1,6) = 10.1], P < 0.003; 10 mg versus 20 mg methadone [F(1,6) = 8.6], P < 0.006) were all dose related. There were no significant differences between buprenorphine and methadone for any of the above ratings. The pattern of results obtained for ratings of “Mellow” and “Sedated” after administration of buprenorphine and methadone were virtually identical to “Good Drug Effect” (data not shown). Ratings of “Energetic” were significantly higher than placebo after administration of 10 mg ([F(1,6) = 5.4], P < 0.03) and 20 mg methadone ([F(1,6) = 6.2], P < 0.02), but not after any of the buprenorphine doses. The durations of action of buprenorphine and methadone for all of the subjective responses were similar (i.e., 8 mg buprenorphine was similar to 20 mg methadone, data not shown).
In addition to positive subjective responses, 8 mg buprenorphine also produced small, but statistically significant, increases in ratings of “Bad Drug Effect” (0 mg versus 8 mg buprenorphine [F(1,6) = 8.3], P < 0.007) and “Sleepy” ([F(1,6) = 7.0], P < 0.01; Figure 2, lower panels). Peak ratings of “Bad Drug Effect” were significantly higher after 8 mg buprenorphine compared to 20 mg methadone ([F(1,6) = 8.3], P < 0.007). Ratings of “Nauseated” were also significantly higher after 8 mg buprenorphine compared to 20 mg methadone ([F(1,6) = 5.1], P < 0.03).
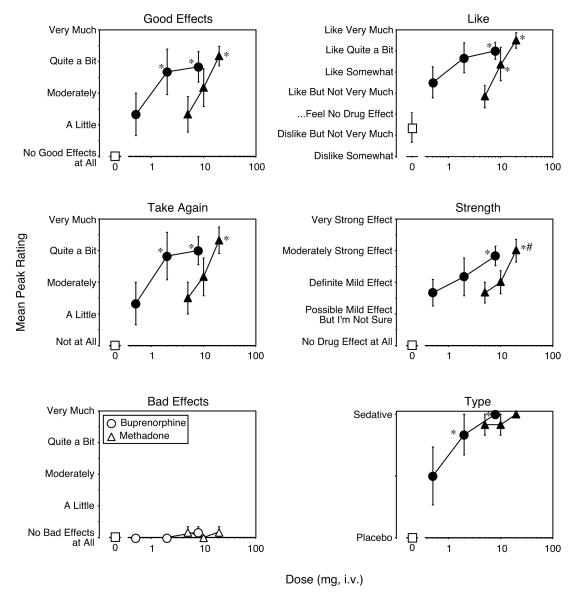
Drug effect questionnaire ratings of “Good Effects” (0 mg versus 0.5 mg buprenorphine [F(1,6) = 5.9], P < 0.02; 0 mg versus 2 mg buprenorphine [F(1,6) = 23.6], P < 0.0001; 0 mg versus 8 mg buprenorphine [F(1,6) = 26.6], P < 0.0001; 0.5 mg versus 2 mg buprenorphine [F(1,6) = 5.9], P < 0.02; 0.5 mg versus 8 mg buprenorphine [F(1,6) = 7.5], P < 0.01; 0 mg versus 5 mg methadone [F(1,6) = 5.9], P < 0.02; 0 mg versus 10 mg methadone [F(1,6) = 15.6], P < 0.0004; 0 mg versus 20 mg methadone [F(1,6) = 33.3], P < 0.0001; 5 mg versus 20 mg methadone [F(1,6) = 11.1], P < 0.002), drug liking (0 mg versus 0.5 mg buprenorphine [F(1,6) = 8.8], P < 0.006; 0 mg versus 2 mg buprenorphine [F(1,6) = 21.0], P < 0.0001; 0 mg versus 8 mg buprenorphine [F(1,6) = 25.4], P < 0.0001; 0.5 mg versus 8 mg buprenorphine [F(1,6) = 4.2], P < 0.05; 0 mg versus 5 mg methadone [F(1,6) = 4.2], P < 0.05; 0 mg versus 10 mg methadone [F(1,6) = 17.0], P < 0.0003; 0 mg versus 20 mg methadone [F(1,6) = 32.7], P < 0.0001; 5 mg versus 10 mg methadone [F(1,6) = 4.2], P < 0.05; 5 mg versus 20 mg methadone [F(1,6) = 13.4], P < 0.001), desire to take the drug again (0 mg versus 0.5 mg buprenorphine [F(1,6) = 4.9], P < 0.04; 0 mg versus 2 mg buprenorphine [F(1,6) = 22.0], P < 0.0001; 0 mg versus 8 mg buprenorphine [F(1,6) = 24.6], P < 0.0001; 0.5 mg versus 2 mg buprenorphine [F(1,6) = 6.2], P < 0.02; 0.5 mg versus 8 mg buprenorphine [F(1,6) = 7.6], P < 0.01; 0 mg versus 5 mg methadone [F(1,6) = 6.2], P < 0.02; 0 mg versus 10 mg methadone [F(1,6) = 12.8], P < 0.001; 0 mg versus 20 mg methadone [F(1,6) = 30.4], P < 0.0001; 5 mg versus 20 mg methadone [F(1,6) = 9.2], P < 0.005), and strength of drug effects (0 mg versus 0.5 mg buprenorphine [F(1,6) = 15.5], P < 0.0005; 0 mg versus 2 mg buprenorphine [F(1,6) = 26.2], P < 0.0001; 0 mg versus 8 mg buprenorphine [F(1,6) = 44.8], P < 0.0001; 0.5 mg versus 8 mg buprenorphine [F(1,6) = 7.6], P < 0.01; 0 mg versus 5 mg methadone [F(1,6) = 15.5], P < 0.0005; 0 mg versus 10 mg methadone [F(1,6) = 22.3], P < 0.0001; 0 mg versus 20 mg methadone [F(1,6) = 50.2], P < 0.0001; 5 mg versus 20 mg methadone [F(1,6) = 9.9], P < 0.004; 10 mg versus 20 mg methadone [F(1,6) = 5.6], P < 0.02), were increased in a dose-related manner for both buprenorphine and methadone, with no significant differences between the two drugs (Figure 3). Ratings were near maximal for all of these effects at the highest doses of buprenorphine and methadone tested. Ratings of “Bad Effect” were not significantly increased for either buprenorphine or methadone. For methadone, one of the six participants thought that 5 mg and 10 mg methadone were stimulants and the other five participants thought that these doses were sedatives (Figure 3, bottom right panel). All of the participants thought that 20 mg methadone was a sedative. For buprenorphine, 3 of the participants thought that 0.5 mg buprenorphine was placebo and 3 thought that it was a sedative. One of the participants thought that 2 mg buprenorphine was placebo and five thought that it was a sedative. All of the participants thought that 8 mg buprenorphine was a sedative.
Figure 3.
Drug Effects Questionnaire ratings as a function of dose and drug. Data points represent mean peak ratings for the 6 participants across the sample and subsequent no drug sessions. Error bars represent ± 1 standard error of the mean (S.E.M.). All else as in Figure 1.
Sum scores on the opioid symptom checklist increased in a dose-related fashion after administration of buprenorphine and methadone. Peak ratings after the high doses of each drug were approximately 5 (range: 0-12). Both 2 mg and 8 mg buprenorphine were significantly different from placebo, as was 20 mg methadone (0 mg versus 2 mg buprenorphine [F(1,6) = 8.3], P < 0.007; 0 mg versus 8 mg buprenorphine [F(1,6) = 16.0], P < 0.0004; 0 mg versus 20 mg methadone [F(1,6) = 17.8], P < 0.0002). Both 2 mg and 8 mg buprenorphine were also significantly different from 0.5 mg buprenorphine (0.5 mg versus 2 mg buprenorphine [F(1,6) = 4.9], P < 0.03; 0.5 mg versus 8 mg buprenorphine [F(1,6) = 11.1], P < 0.002). The 20 mg methadone dose was significantly different from the 5 mg and 10 mg doses (5 mg versus 20 mg methadone [F(1,6) = 12.6], P < 0.001; 10 mg versus 20 mg methadone [F(1,6) = 6.0], P < 0.02). There were no significant differences between buprenorphine and methadone.
Average subjective opioid withdrawal scores during the last five days of detoxification ranged between 16 and 21 (range: 0-64). Average peak SOWS scores during experimental sessions ranged between 7 and 11 and were not related to dose.
Performance Effects
Buprenorphine had few effects on performance, with the exception of small impairments in performance of the divided attention task. Relative to placebo, both 2 mg and 8 mg buprenorphine significantly increased the mean peak latency to respond correctly to a random distracter stimulus on the computer screen (0 mg versus 2 mg buprenorphine [F(1,6) = 4.4], P < 0.04; 0 mg versus 8 mg buprenorphine [F(1,6) = 8.1], P < 0.008). There were no statistically significant changes in latency to respond correctly to the stimulus after methadone administration, although there were trends for 20 mg methadone to impair performance on this task (0 mg versus 20 mg methadone [F(1,6) = 3.3], P<0.08). Across the two drugs tested, 2 mg buprenorphine produced significantly longer latencies to respond than 10 mg methadone ([F(1,6) = 5.0], P<0.03). The effects of methadone on task performance were not significantly different from placebo.
Secondary analysis of the data as a function of drug and time revealed further changes in performance of the divided attention task (Figure 4). Tracking distance (distance between the cursor and the moving stimulus on the computer screen) was significantly longer after administration of 8 mg buprenorphine compared to placebo ([F(1,6) = 4.9], P<0.03; Figure 4, left panel). The difference in tracking distance between 0.5 mg and 8 mg buprenorphine approached statistical significance ([F(1,6) = 3.6], P<0.07), as did the difference between 5 mg and 20 mg methadone ([F(1,6) = 3.1], P<0.09). The maximum speed reached by the moving stimulus significantly decreased after administration of 2 mg and 8 mg buprenorphine, but not after administration of any of the methadone doses (0 mg versus 2 mg buprenorphine [F(1,6) = 9.7], P < 0.004; 0 mg versus 8 mg buprenorphine [F(1,6) = 8.7], P < 0.006; Figure 4, middle panel). And finally, the number of false alarms (responses made when the distracter stimulus did not actually appear) significantly increased after administration of 8 mg buprenorphine, relative to placebo ([F(1,6) = 4.4], P < 0.04; Figure 4, right panel).
Figure 4.
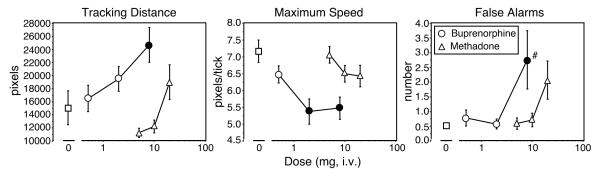
Performance on the Divided Attention Task as a function of dose and drug. Data points represent mean scores across time for the 6 participants during the sample session and subsequent no drug sessions. Tracking distance is the distance between the cursor and a moving stimulus on the computer screen (left panel), maximum speed is the highest speed with which the stimulus moves across the computer screen (the speed increases as the accuracy in tracking the moving stimulus increases; middle panel), and false alarms are the number of responses made when a distracter stimulus is not actually present. (right panel). Error bars represent ± 1 standard error of the mean (S.E.M.). All else as in Figure 1.
Physiological Effects
Compared to placebo, all of the active buprenorphine doses produced significant decreases in pupil diameter (0 mg versus 0.5 mg buprenorphine [F(1,6) = 4.1], P < 0.05; 0 mg versus 2 mg buprenorphine [F(1,6) = 16.6], P < 0.0003; 0 mg versus 8 mg buprenorphine [F(1,6) = 20.4], P < 0.0001). The miotic effects produced by both 2 mg and 8 mg buprenorphine were significantly different from 0.5 mg buprenorphine (0.5 mg versus 2 mg buprenorphine [F(1,6) = 4.2], P < 0.05; 0.5 mg versus 8 mg buprenorphine [F(1,6) = 6.3], P < 0.02), but there were no differences between 2 mg and 8 mg buprenorphine. The 20 mg dose of methadone produced miotic effects that were significantly different from those of placebo (F(1,6) = 7.1; P<0.01), 5 mg (F(1,6) = 12.0; P<0.002), and 10 mg (F(1,6) = 5.6; P<0.02). Comparing across drugs, significant differences were obtained for the miotic effects produced by 0.5 mg buprenorphine compared to 5 mg methadone (F(1,6) = 7.9; P<0.008) and 2 mg buprenorphine compared to 10 mg methadone (F(1,6) = 14.4; P<0.0007). However, there were no significant differences between 8 mg buprenorphine and 20 mg methadone.
Average arterial oxygen saturation was reduced in a dose-related manner by 2 mg (F(1,6) = 5.7; P<0.02, 96.2%) and 8 mg (F(1,6) = 8.3; P<0.007, 96.0%) buprenorphine, relative to placebo (97.0%). The effects of 0.5 mg buprenorphine (97.0%) were also significantly different from 2 mg (F(1,6) = 6.0; P<0.02) and 8 mg buprenorphine (F(1,6) = 8.6; P<0.006). The maximal decreases in arterial oxygen saturation occurred within 30 to 60 min after administration of 8 mg buprenorphine. Although methadone also produced slight decreases in arterial oxygen saturation, these changes were not statistically significant. Please note that arterial oxygen saturation was measured in the presence of supplemental oxygen, so the small reductions seen here were not entirely surprising.
For the cardiovascular measures, there were no significant changes in heart rate as a function of dose, although the main effect of time was significant (F(1,18) = 13.7; P<0.0001), with heart rate steadily decreasing throughout the session. Systolic pressure significantly increased after administration of 2 mg buprenorphine (F(1,6) = 5.3; P<0.03, mean: 116 mm Hg), 8 mg buprenorphine (F(1,6) = 8.7; P<0.006, mean: 118 mm Hg), and 20 mg methadone (F(1,6) = 4.1; P<0.05, mean: 115 mm Hg), relative to placebo (mean: 109 mm Hg). However, these changes were not clinically significant. Diastolic pressure did not significantly increase for any of the active doses, relative to placebo.
Potency Estimates
ED50 values and 95% confidence limits were determined for the reinforcing, subjective, and physiological effects of buprenorphine and methadone (Table 1). Potencies for buprenorphine and methadone to produce self-administration, subjective effects, and physiological effects ranged from 0.27-8.6 mg (32-fold range) and 6.1-20 mg (3.2-fold range), respectively. Buprenorphine was significantly more potent than methadone for all behavioral and physiological effects except ‘potent’ ratings on the visual analog scale and false alarms. For ‘potent’ ratings on the visual analog scale and false alarms, buprenorphine was 4.6 and 5.8 times more potent than methadone, respectively, but the confidence limits were large and included 1. In general, the potency ratios for buprenorphine relative to methadone were similar in the self-administration task (5.0-fold) and for subjective effects on the DEQ (4.4-5.5 fold) as well as ‘quality’ and ‘potent’ on the visual analog scale (4.5-4.6 fold). However, potency ratios between buprenorphine and methadone were higher for most of the subjective effects on the visual analog scales (8.5-12 fold) and the physiological effects (28-51 fold).
Table 1.
Potency estimates and comparisons for buprenorphine and methadone across behavioral measures.
| Buprenorphine ED50, mg, i.v. (±95% C.L.) |
Methadone ED50, mg, i.v. (±95% C.L.) |
Potency Ratio (±95% C.L.) |
|
|---|---|---|---|
| Progressive Ratio | |||
| Progressive Ratio | 1.5 (0.0090-7.5) | 8.7 (5.5-20) | 5.0 (3.1-8.0) |
| Visual Analog Scale | |||
| Pay | 4.1 (0.070-6.9) | 17 (5.0-18) | 12 (6.1-20) |
| Good | 5.8 (4.3-8.6) | 14 (9.7-23) | 8.5 (7.3-9.6) |
| High | 7.4 (0.25-5.8) | 16 (13-23) | 8.7 (6.4-11) |
| Liked | 4.9 (3.4-7.2) | 14 (9.5-26) | 8.8 (7.6-10) |
| Quality | 8.6 (0.63-5.9) | 16 (5.4-17) | 4.5 (1.2-18) |
| Potent | 7.6a | 17 (5.4-17) | 4.6 (0.32-55) |
| Drug Effects Questionnaire | |||
| Good Effects | 1.2 (0.65-6.4) | 8.5 (5.7-12) | 5.5 (1.9-15) |
| Like | 0.98 (0.65-7.7) | 6.1 (2.3-23) | 4.4 (1.6-12) |
| Take Again | 1.1 (0.65-6.4) | 7.8 (5.8-21) | 5.3 (1.6-17) |
| Strength | 1.2 (0.73-19) | 7.9 (5.7-18) | 5.0 (2.2-11) |
| Physiological Effects | |||
| Tracking | 0.94 (0.78-8.7) | 20 (5.6-17) | 28 (6.2-131) |
| Maximum Speed | 0.27 (0.67-6.3) | 14 (5.6-18) | 51 (1.7-1500) |
| False Alarms | 2.4 (0.64-6.2) | 14 (5.7-19) | 5.8a |
Confidence limits could not be determined for the value
Discussion
Similar to findings from our previous studies (Comer and Collins, 2002; Comer et al., 2002), the present results demonstrate that intravenously administered buprenorphine served as a reinforcer in recently detoxified, non-treatment-seeking heroin abusers. The maximum reinforcing effects of buprenorphine and methadone did not differ from each other, nor did they differ across any of the active doses tested. Drug self administration occurred at some doses (e.g., 5 mg methadone) despite the presence of only minimal subjective responses. Similar results were obtained in our previous study after administration of the buprenorphine/naloxone combination (Comer and Collins, 2002). Other studies have also shown that subjective effects do not necessarily predict self-administration behavior (Comer et al., 1997, 1998; Heishman et al., 2000; Lamb et al., 1991). One potential variable that may have contributed to the pattern of self-administration in the current study was the presence of mild, residual withdrawal symptoms during testing. As noted above, subjective ratings of withdrawal were still slightly elevated during testing. Some participants reported during a debriefing session after study completion that they sometimes self-administered drug, even though they did not experience any subjective effects, because they noticed that they slept better that night. It is possible that the drugs were serving as both positive and negative reinforcers under the present experimental conditions (i.e., for their euphoric effects and to alleviate withdrawal). An additional complication for comparing subjective responses and self-administration may be efficacy requirement across different subjective effect measures. For example, in the present study, the potency differences between buprenorphine and methadone were generally greater on the visual analog scales than the DEQ, which may reflect the quantal nature of the DEQ compared to the continuous measure of subjective responses obtained from the visual analog scales. As in previous studies, the present results demonstrate that subjective responses and drug taking behavior are not always directly correlated. Alternatively, the subjective responses and drug taking behavioral measures may require different degrees of relative efficacy for agonists to produce maximum or near-maximum effects as reflected in the potency ratio differences for buprenorphine and methadone in the present study.
Results from the present study are consistent with previous research conducted in non-human primates demonstrating that buprenorphine serves as a reinforcer, and they provide additional evidence that the reinforcing effects of buprenorphine do not significantly differ from those of methadone. It is important to emphasize, however, that the present study was conducted in recently detoxified individuals. Previous studies conducted in laboratory animals have demonstrated that the reinforcing effects of buprenorphine are reduced to a greater extent than those of other mu opioid agonists in animals treated chronically with morphine. For example, under control conditions, alfentanil, heroin, morphine, buprenorphine, and nalbuphine all served as reinforcers (the rates of responding for these drugs were significantly greater than for placebo; Winger & Woods, 2001). However, when the animals were treated chronically with morphine, the potency of alfentanil was unchanged and the potencies of heroin and morphine were reduced only slightly. In contrast, both the potency and reinforcing effectiveness of buprenorphine and nalbuphine were reduced substantially in monkeys treated chronically with morphine. Similar results have been obtained for the analgesic and discriminative stimulus effects of buprenorphine relative to other mu opioid agonists (e.g., Barrett et al., 2001; Paronis & Holtzman, 1992; Pitts et al., 1998; Walker et al., 1998; Walker & Young, 2001, 2002; Young et al., 1991). The data collected in laboratory animals were consistent with those collected in human research volunteers: Hydromorphone produced robust subjective and physiological responses in methadone-maintained volunteers, while the effects of a wide range of buprenorphine doses were reduced substantially (Strain et al., 1992, 1995). Under some conditions, buprenorphine precipitated withdrawal symptoms in methadone-maintained participants (Strain et al., 1995; Walsh et al., 1995). Taken together, these results suggest that under non-dependent conditions, buprenorphine has moderate to robust reinforcing effects, but in opioid-dependent individuals, the reinforcing effects of buprenorphine may be minimal. However, in opioid-dependent individuals, we would predict that the potency ratios for buprenorphine and methadone would increase across all measures in direct relation to the degree of dependence. In other words, greater opioid-dependence will be associated with a greater reduction in potency for buprenorphine compared to methadone.
The pattern of subjective responses after intravenous administration of buprenorphine and methadone in the present study was similar to that reported after subcutaneous administration of buprenorphine and methadone (Jasinski et al., 1978). In the present study, higher doses of buprenorphine were tested and there were hints of a ceiling effect on several of the subjective responses, particularly on the drug effects questionnaire. While there were few overall differences in the subjective effects produced by buprenorphine and methadone, there was some evidence for subtle differences between the two drugs in their potency to produce different subjective effects. For example, buprenorphine produced small, but statistically significant, increases in ratings of “Bad Drug Effects” and ratings of nausea were significantly higher for 8 mg buprenorphine compared to 20 mg methadone.
The subjective effects found in the present study and those reported by Jasinski and colleagues (1978) differ somewhat from those reported recently by Umbricht and colleagues (2004), in which high doses of intravenously delivered buprenorphine (0, 2, 4, 8, 12, and 16 mg) were administered to non-dependent opioid abusers. Due to high variability in subjective responses among participants in that study, there were few statistically significant differences between active drug and placebo. One effect that did show significant changes as a function of dose was “Drug Effect.” However, at the highest dose tested (16 mg), the majority of participants reported that the effects were “weaker” than for the other doses (Umbricht et al., 2004). Similarly, an inverted U-shaped dose-response curve for buprenorphine has been reported for other effects, such as analgesia (e.g., Cowan et al., 1977; Dum and Herz, 1981; Walker et al., 1995), and is thought to reflect its increasing antagonist effects at these higher doses. In one other study, a relatively flat dose-response curve for the subjective effects of intravenously delivered buprenorphine was found (Pickworth et al., 1993). In the present study, there was little evidence for an inverted U-shaped dose-response curve. The reason for the inverted, flat, or dose-dependent effects of buprenorphine among the published studies may be due to the different behavioral measures examined. Future experiments examining the relative efficacy requirements of the different behavioral measures may help clarify the differences among the studies for buprenorphine.
Buprenorphine produced few effects on performance, which is consistent with other studies showing either mild (Comer and Collins, 2002; Comer et al., 2002; Pickworth et al., 1993) or no performance-impairing effects (Walsh et al., 1994) in opioid-experienced individuals. In relatively opioid-naïve individuals, performance of the digit symbol substitution task, as well as four other psychomotor tests, was impaired in a dose-related manner after acute, intravenous administration of buprenorphine (Zacny et al., 1997). In the present study, the degree of impairment produced by intravenous buprenorphine was also dose-related, but it was relatively small and was limited to the divided attention task. In contrast to buprenorphine, methadone did not significantly alter performance on any of the tasks. Why buprenorphine produced slightly greater effects on task performance is unclear.
Similar to several previous studies, buprenorphine produced few changes in arterial oxygen saturation or in cardiovascular measures (e.g., Umbricht et al., 2004; Walsh et al., 1994), even after high intravenous doses. These data serve to further confirm the clinical safety of buprenorphine, particularly at high doses given intravenously, which is the route by which illicit buprenorphine use often occurs.
In conclusion, the present results confirmed our hypothesis that buprenorphine would serve as a reinforcer under these laboratory conditions. The fact that the maximum break point values did not differ between buprenorphine and methadone was consistent with several previous studies conducted in laboratory animals showing that under some conditions, buprenorphine produces effects that are indistinguishable from other mu opioid agonists with high efficacy. The fact that both buprenorphine and methadone were more potent in producing reinforcing effects than in producing some subjective and physiological effects was also not entirely surprising. This finding is again consistent with studies conducted in laboratory animals showing that the potency of a particular drug can vary significantly across different dependent measures and this effect is greater for lower efficacy agonists than higher efficacy agonists. For example, in the present study, the buprenorphine ED50 values across the different measures spanned a 30-fold potency range whereas methadone ED50 values only spanned a 3-fold potency range. Whether the reinforcing effects of buprenorphine are suppressed to a greater extent than those of other mu opioid agonists in opioid-dependent individuals is not currently known. Future studies will examine the reinforcing effects of buprenorphine compared to a range of other mu opioid agonists in morphine-maintained individuals in order to examine their relative efficacies.
Acknowledgments
We wish to express our deep appreciation to the late Dr. Marian Fischman for her invaluable guidance and support during the planning of these studies. In addition, the assistance of Christy Aberg, Berena Cabarcas, Mabel Torres, Michael R. Donovan, Ronnie M. Shapiro, and Drs. Anthony Tranguch, Vladimir Ginsburg, and Adam Bisaga is also gratefully acknowledged.
This research was supported by grant DA10909.
Nonstandard abbreviations
- DEQ
Drug Effects Questionnaire
- ED50
dose producing a 50% response
- SOWS
Subjective Opioid Withdrawal Scale
- SpO2
arterial blood oxygen saturation
- S.E.M.
Standard Error of the Mean
References
- Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ. Importance of sex and relative efficacy at the mu opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacol. 2001;158:154–164. doi: 10.1007/s002130100821. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol. 1997;8:677–690. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J Pharmacol Exp Ther. 2002;303:695–703. doi: 10.1124/jpet.102.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Intravenous buprenorphine self-administration by detoxified heroin abusers. J Pharmacol Exp Ther. 2002;301:266–276. doi: 10.1124/jpet.301.1.266. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, Fischman MW. Comparison of intravenous and intranasal heroin self-administration by morphine-maintained humans. Psychopharmacology (Berl) 1999;143:327–338. doi: 10.1007/s002130050956. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Wilson ST, Donovan MR, Foltin RW, Fischman MW. Effects of an alternative reinforcer on i.v. heroin self-administration by humans. Eur J Pharmacol. 1998;345:13–26. doi: 10.1016/s0014-2999(97)01572-0. [DOI] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, MacFarlane I. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977;60:537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale O, Sheffels P, Kharasch ED. Bioavailabilities of rectal and oral methadone in healthy subjects. Br J Clin Pharmacol. 2004;58:156–162. doi: 10.1111/j.1365-2125.2004.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum JE, Herz A. In vivo receptor binding of the opiate partial agonist buprenorphine, correlated with its agonistic and antagonistic actions. Br J Pharmac. 1981;74:627–633. doi: 10.1111/j.1476-5381.1981.tb10473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney DJ. Statistical Methods in Biological Assay. 2nd ed Charles Griffin and Co. Ltd.; London: 1964. [Google Scholar]
- Heishman SJ, Schuh KJ, Schuster CR, Henningfield JE, Goldberg SR. Reinforcing and subjective effects of morphine in human opioid abusers: Effect of dose and alternative reinforcer. Psychopharmacology. 2000;148:272–280. doi: 10.1007/s002130050051. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: A potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Performance-based testing for drugs of abuse: Dose and time profiles of marijuana, amphetamine, alcohol, and diazepam. J Anal Toxicol. 1993;17:264–272. doi: 10.1093/jat/17.5.264. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, Henningfield JE, Goldberg SR. The reinforcing and subjective effects of morphine in post-addicts: A dose-response study. J Pharmacol Exp Ther. 1991;259:1165–1173. [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the Digit Symbol Substitution Test (DSST) Behav Res Meth Instr. 1982;14:463–466. [Google Scholar]
- Mello NK, Lukas SE, Bree MP, Mendelson JH. Progressive ratio performance maintained by buprenorphine, heroin and methadone in Macaque monkeys. Drug Alcohol Dependence. 1988;21(2):81–97. doi: 10.1016/0376-8716(88)90053-1. [DOI] [PubMed] [Google Scholar]
- Miller TP, Taylor JL, Tinklenberg JR. A comparison of assessment techniques measuring the effects of methylphenidate, secobarbital, diazepam and diphenhydramine in abstinent alcoholics. Neuropsychobiology. 1988;19:90–96. doi: 10.1159/000118441. [DOI] [PubMed] [Google Scholar]
- Morgan D, Cook CD, Smith MA, Picker MJ. An examination of the interactions between the antinociceptive effects of morphine and various mu-opioids: the role of intrinsic efficacy and stimulus intensity. Anesth Analg. 1999;88(2):407–413. doi: 10.1097/00000539-199902000-00035. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Holtzman SG. Development of tolerance to the analgesic activity of mu agonists after continuous infusion of morphine, meperidine or fentanyl in rats. J Pharmacol Exp Ther. 1992;262:1–9. [PubMed] [Google Scholar]
- Picker MJ, Yarbrough J, Hughes CE, Smith MA, Morgan D, Dykstra LA. Agonist and antagonist effects of mixed action opioids in the pigeon drug discrimination procedure: Influence of training dose, intrinsic efficacy and interanimal differences. J Pharmacol Exp Ther. 1993;266(2):756–767. [PubMed] [Google Scholar]
- Pickworth WB, Johnson RE, Holicky BA, Cone EJ. Subjective and physiologic effects of intravenous buprenorphine in humans. Clin Pharmacol Ther. 1993;53(5):570–576. doi: 10.1038/clpt.1993.72. [DOI] [PubMed] [Google Scholar]
- Pitts RC, Allen RM, Walker EA, Dykstra LA. Clocinnamox antagonism of the antinociceptive effects of mu opioids in squirrel monkeys. J Pharmacol Exp Ther. 1998;285:1197–1206. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Effects of agonist-antagonist opioids in humans trained in a hydromorphone/not hydromorphone discrimination. J Pharmacol Exp Ther. 2000;295:114–124. [PubMed] [Google Scholar]
- Senay EC. Opioids: Methadone maintenance. In: Galanter M, Kleber HD, editors. Textbook of Substance Abuse Treatment. 2nd edition American Psychiatric Press, Inc.; Washington DC: 1999. pp. 271–279. [Google Scholar]
- Strain EC, Preston KL, Liebson IA, Bigelow GE. Acute effects of buprenorphine, hydromorphone and naloxone in methadone-maintained volunteers. J Pharmacol Exp Ther. 1992;261(3):985–993. [PubMed] [Google Scholar]
- Strain EC, Preston KL, Liebson IA, Bigelow GE. Buprenorphine effects in methadone-maintained volunteers: Effects at two hours after methadone. J Pharmacol Exp Ther. 1995;272:628–638. [PubMed] [Google Scholar]
- Umbricht A, Huestis MA, Cone EJ, Preston KL. Effects of high-dose intravenous buprenorphine in experienced opioid abusers. J Clin Psychopharmacol. 2004;24(5):479–487. doi: 10.1097/01.jcp.0000138766.15858.c6. [DOI] [PubMed] [Google Scholar]
- Walker EA, Young AM. Differential tolerance to antinociceptive effects of mu opioids during repeated treatment with etonitazene, morphine, or buprenorphine in rats. Psychopharmacol. 2001;154:131–142. doi: 10.1007/s002130000620. [DOI] [PubMed] [Google Scholar]
- Walker EA, Young AM. Clocinnamox distinguishes opioid agonists according to relative efficacy in normal and morphine-treated rats trained to discriminate morphine. J Pharmacol Exp Ther. 2002;302:101–110. doi: 10.1124/jpet.302.1.101. [DOI] [PubMed] [Google Scholar]
- Walker EA, Zernig G, Woods JH. Buprenorphine antagonism of mu opioids in the rhesus monkey tail-withdrawal procedure. J Pharmacol Exp Ther. 1995;273:1345–1352. [PubMed] [Google Scholar]
- Walker EA, Zernig G, Young AM. In vivo apparent affinity and efficacy estimates for mu opiates in a rat tail-withdrawal assay. Psychopharmacol. 1998;136:15–23. doi: 10.1007/s002130050534. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: Partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–372. [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9:223–229. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]
- Winger G, Woods JH. The effects of chronic morphine on behavior reinforced by several opioids or by cocaine in rhesus monkeys. Drug Alcohol Depend. 2001;62:181–189. doi: 10.1016/s0376-8716(00)00166-6. [DOI] [PubMed] [Google Scholar]
- Young AM, Kapitsopoulos G, Makhay MM. Tolerance to morphine-like stimulus effects of mu opioid agonists. J Pharmacol Exp Ther. 1991;257(2):795–805. [PubMed] [Google Scholar]
- Zacny JP, Conley K, Galinkin J. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J Pharmacol Exp Ther. 1997;282:1187–1197. [PubMed] [Google Scholar]