Abstract
Background
Low dose aspirin is used to prevent thromboembolic complications in dogs, but some animals are non-responsive to the anti-platelet effects of aspirin (‘aspirin resistance’).
Hypothesis/Objectives
That low dose aspirin would inhibit platelet function, decrease thromboxane synthesis, and alter platelet cyclooxygenase (COX) expression.
Animals
Twenty-four healthy dogs
Methods
A repeated measures study. Platelet function (PFA-100® closure time, collagen/epinephrine), platelet COX-1 and COX-2 expression, and urine 11-dehydro-thromboxane B2 (11-dTXB2) was evaluated prior to and during aspirin administration (1 mg/kg Q24 hours PO, 10 days). Based on prolongation of closure times after aspirin administration, dogs were divided into categories according to aspirin responsiveness: responders, non-responders, and inconsistent responders.
Results
Low dose aspirin increased closure times significantly (62% by Day 10, P<0.001), with an equal distribution among aspirin responsiveness categories, 8 dogs per group. Platelet COX-1 mean fluorescent intensity (MFI) increased significantly during treatment, 13% on Day 3 (range, −29.7%–136.1%) (P=0.047) and 72% on Day 10 (range, −0.37–210.36%) (P<0.001). Platelet COX-2 MFI increased significantly by 34% (range, −29.2–270.4%) on Day 3 (P = 0.003) and 74% (range, −19.7–226.2%) on Day 10 (P<0.001). Urinary 11-dTXB2 concentrations significantly (P=0.005, P<0.001) decreased at both time points. There was no difference between aspirin responsiveness and either platelet COX expression or thromboxane production.
Conclusions and Clinical Importance
Low dose aspirin consistently inhibits platelet function in approximately one third of healthy dogs, despite decreased thromboxane synthesis and increased platelet COX expression in most dogs. Pre-treatment COX isoform expression did not predict aspirin resistance.
Keywords: Canine, COX, thromboxane, aspirin resistance, PFA-100
Thromboembolic complications in dogs are associated with multiple diseases, including immune-mediated hemolytic anemia (IMHA), protein-losing nephropathies, and hyperadrenocorticism.1,2 Anti-platelet therapy plays an important role in the prevention of thromboembolic complications. One of the most commonly used anti-platelet medications, aspirin, is highly effective at inhibiting platelet function in dogs and humans.3–7 Anti-inflammatory doses of aspirin inhibit platelet function, decrease inflammation, and provide analgesia, but can cause important adverse effects.8 Lower doses of aspirin, in contrast, maintain the anti-platelet properties of the medication without adverse side effects, making low dose aspirin an appealing treatment for the prevention of thromboembolic complications. The administration of low doses of aspirin has been associated with an improved survival rate in dogs with IMHA.9
Unfortunately, despite preventative therapy, some dogs treated with aspirin develop thromboembolic complications. A similar phenomenon occurs in humans receiving low doses of aspirin, indicating that these patients are poorly responsive to the anti-platelet effects of aspirin (‘aspirin resistant’). The true incidence of aspirin resistance in humans is unknown, but estimates ranges, from 8–45% of human patients.3,10–12 Since dogs appear to inconsistently respond to low dose aspirin, it is possible that, similar to humans, some dogs are aspirin resistant. The incidence of aspirin resistance in dogs is, however, unknown. The exact mechanism for aspirin resistance in both humans and dogs is also unknown, but possible explanations include genetic and acquired differences in cyclooxygenase (COX) enzyme activity or thromboxane receptors, increased platelet reactivity to other platelet agonists, and alternative pathways of thromboxane generation.10
Once synthesized and released by activated platelets, thromboxane A2 (TXA2) triggers vasoconstriction, enhances platelet aggregation, and induces further platelet activation.5,13 The COX enzyme, which exists in two major isoforms, COX-1 and COX-2, is primarily responsible for platelet generation of thromboxane A2.14,15 Previously, circulating platelets were thought to contain only the COX-1 isoform,16–18 but recent studies have identified the COX-2 isoform in both human and canine platelets.15,17,19 The COX-1 isoform was originally believed to be the sole source of platelet TXA2 production,16 but platelet COX-2 could provide an alternate source of TXA2 production, offering a possible mechanism for aspirin resistance.
Aspirin functions as a permanent inhibitor of the COX enzyme via acetylation of a serine residue.10,20,21 Permanent inhibition of the COX enzyme prevents conversion of arachidonic acid to prostaglandin H2 and many subsequent prostaglandins, including TXA2 and prostacyclin. While platelet-derived TXA2 plays a critical role in vasoconstriction and promoting platelet aggregation, prostacyclin originating from the vascular endothelium has the opposite effect by causing vasodilation and inhibiting platelet aggregation. Low dose aspirin therapy, unlike higher anti-inflammatory doses, will alter platelet function without permanently inhibiting COX function in the vascular endothelium, allowing prostacyclin production to continue and contribute to the prevention of thrombus formation.5
High aspirin doses consistently inhibit platelet function6,7,19 and thromboxane production,19,22 while concurrently altering platelet COX-1 and COX-2 expression in dogs.19 Platelet COX enzyme expression in dogs receiving low dose aspirin has not been previously evaluated, but variations in COX isoform expression could provide an explanation for aspirin resistance. A better understanding of the relationship between platelet COX isoform expression and response to low doses of aspirin could potentially allow clinicians to predict which individuals will demonstrate aspirin resistance.
The objective of this study was to evaluate the effects of administration of a low dose of aspirin (1 mg/kg/day)a,8 on platelet function and COX expression in healthy dogs. The goals of our study were to determine whether low dose aspirin therapy would consistently affect platelet function and thromboxane production, and if not, whether pre-existing COX isoform expression or subsequent changes in isoform expression were associated with aspirin resistance. Our hypotheses were that low dose aspirin would inhibit platelet function, decrease platelet thromboxane synthesis, and alter platelet COX isoform expression.
Material and Methods
Study Population
Healthy client owned adult dogs were recruited from students, staff and faculty associated with the Mississippi State University College of Veterinary Medicine. Owner consent was obtained before initiation of the study. The dogs were not exposed to any medications or vaccines for at least two weeks prior to initiation of the study, and no non-aspirin medications were administered during the testing period. All drug dosing was based on the body weight obtained at the beginning of the study. Normal health status was established via physical examination, complete blood count, serum chemistry, urinalysis, heartworm testing and platelet function analysis using a point-of-care platelet function analyzer, the PFA-100®b (collagen/ADP cartridgec). Dogs were excluded from the study if they were found to have abnormal physical examination findings, abnormal complete blood count or serum chemistry results, heartworm or tick-borne disease, or platelet abnormalities based on either an abnormal platelet count or prolonged PFA-100® (collagen/ADP) closure time. Animal use was approved by the Mississippi State University Institutional Animal Care and Use Committee.
Study Design
Aspirind was administered orally to each dog at a dose of 1 mg/kg once daily for 10 days. Based on individual body weight, the aspirin dose was compounded by the Mississippi State University College of Veterinary Medicine Pharmacy. Blood and urine samples were collected from all study participants for platelet function evaluation (PFA-100® collagen/epinephrine cartridgee), measurement of platelet cyclooxygenase expression, and urinary thromboxane analysis on Day 0 (prior to initiating aspirin), and again on Days 3 and 10 during aspirin administration.
Platelet Function Analysis
The PFA-100® has previously been evaluated for use in dogs6,7,23 as a commercial point-of-care platelet function analyzer. The PFA-100® is an in vitro platelet function analyzer that records the closure time, in seconds, needed to form a platelet plug after activation by platelet agonists. Blood was collected via jugular venipuncture with a 20 gauge needle directly into a 5 ml vacutainer tube containing 3.8% sodium citratef. Samples were well mixed, and 800 μl of whole blood was transferred into either a collagen/ADP cartridge (establishment of normal platelet function prior to the study) or collagen/epinephrine cartridge (main study) for analysis. All samples were analyzed within four hours of collection and were kept at room temperature until analysis. The cut-off time for the instrument is greater than 300 seconds, and results were considered to be prolonged if the closure time was greater than 300 seconds. All cartridges were stored at 4°C and warmed to room temperature before analysis.
Based on PFA-100® collagen/epinephrine closure times after aspirin administration, all dogs were divided into one of three groups: aspirin responders (prolonged closure times at Day 3 and 10), aspirin non-responders (no prolongation of closure times at either time point), and inconsistent aspirin responders (prolonged closure times on either Day 3 or 10, but not both). The initial Day 0 blood samples for platelet function analysis (collagen/epinephrine cartridge) were discarded due to an unanticipated instrument malfunction. Blood samples were obtained 4 weeks after completion of administration of aspirin to represent the baseline analysis for the PFA-100® collagen/epinephrine cartridge (all dogs included in the study had a baseline closure time of less than 300 seconds).
Flow Cytometry
Blood was collected via jugular venipuncture with a 20 gauge needle directly into a glass vacutainer tube containing 3.8% sodium citrate. Sample preparation was initiated within 1 hour of collection and completed within 4 hours.
COX-1 labeling
Platelet COX-1 expression was quantified by utilizing a previously described protocol.19 Briefly, 5 μL of whole blood was combined with 45 μL of FACS-PBS and incubated with a FITC-conjugated mouse anti-ovine-COX-1 monoclonal antibodyg. For platelet identification, samples were incubated with a mouse anti-human CD9:RPE monoclonal antibodyh. Samples were fixed for 10 minutes at 4°C in the dark with 1% paraformaldehydei.
COX-2 labeling
A previously reported protocol19 was used to quantify platelet COX-2 expression. Briefly, 5 μL of whole blood was added to 45 uL of FACS-PBS and fixed with 1% paraformaldehyde at 4°C in the dark. Samples were washed and then incubated in 0.3% Triton X-100j. Following another wash, samples were incubated with a FITC-conjugated mouse anti-human-COX-2 monoclonal antibodyk. Platelets were identified with a mouse anti-pig CD61-purified monoclonal antibodyl and goat anti-mouse IgG:RPE antibodym.
Samples were stored in the dark at 4°C prior to analysis. Isotype matched monoclonal antibodies were used for both COX antibodies. Analysis was performed with a flow cytometern with CellQuest Pro softwareo. Platelet populations were displayed on log forward-scatter versus log side-angle light scatter plots. Gates were adjusted to baseline platelet populations, and a total of 5,000 gated events were recorded for each labeling. Expression was quantified by the intensity of antibody fluorescence and expressed as mean fluorescence intensity (MFI). A histogram was created with MFI on the x-axis and events on the y-axis.
Urinary 11-dehydro-thromboxane B2 Analysis
Urinary 11-dehydro-thomboxane B2 (11-dTXB2) was measured using a multiplex analyzerp and a commercial competitive enzyme immunoassay kitq that has been previously validated for use with dog urine.24 Urine was collected either by free catch or cystocentesis and stored at −80°C until analysis. Prior to analysis, urine samples were thawed and the specific gravity was measured. The assay buffer was used to standardize each sample to a urine specific gravity (range, 1.002 to 1.014), which was the optimal working range for the analyzer. Each urine sample was analyzed in duplicate and reported in picograms per milliliter of urine. A correction factor was applied to account for the sample dilutions. A 96-well plate was prepared by adding 100 μL of the urine sample and 50 μL of both the 11-dTXB2 Phycoerythrin Tracer and the 11-dTXB2 XMAPR® beads to the appropriate well. Each plate was incubated at room temperature, in the dark and on an orbital shaker prior to analysis. The urine creatinine concentration of each sample was determined by a biochemistry analyzerr, and the urine 11-dTXB2 concentration was normalized to create a urinary 11-dTXB2 to creatinine ratio.24,25
Statistics
A power analysis was performed prior to study initiation, based on results from a previous study evaluating the effects of non-steroidal anti-inflammatory drugs on platelet function.26 The results of this analysis indicated that a sample size of 27 dogs would be needed to achieve a power value of 0.80 for detection of aspirin-induced changes in platelet function. A single population, repeated measures design was used in this study. After visual assessment of Q-Q plots and histograms, COX-2 MFI and percent of platelet expression appeared to be normally distributed; however, COX-1 MFI and percent of platelet expression, 11-dTXB2, and PFA-100® (collagen/epinephrine) closure times were not considered to be normally distributed. Consequently, nonparametric methods for analysis of repeated measures were utilized27,28 for all results. For each outcome, the data were ranked and then analysis of variance type statistics were obtained through PROC MIXED (SAS)s by using the ANOVAF option and the MIVQUE0 estimation method for the covariance parameters and a REPEATED statement specifying an unstructured covariance structure. With the cut-off time for the PFA-100® being greater than 300 seconds, all closure times that were considered to be prolonged (greater than 300 seconds) were analyzed with a closure time value of 300 seconds. Sample time was included in the model as a fixed effect. Differences in least square means were used for multiple comparisons of the three time points for the analyses; the simulation option within SAS was used for adjustment of p-values for the pair-wise comparisons. After classification into response groups based on PFA-100® collagen/epinephrine closure times, the effect of responder classification was assessed by the addition of response group and the time by response group interaction into the COX-1, COX-2, and 11-dTXB2 models. A p-value of less than 0.05 was considered to be significant for all analyses.
Results
Study Group Characteristics
A total of 25 dogs satisfied the inclusion criteria and participated in the study. There were 14 different breeds with Labrador Retrievers being the most commonly represented breed (n = 6). Other breeds included English Springer Spaniels (n = 2), Australian Shepherds (n = 2), Shar-Pei (n = 1), Pug (n = 1), Yorkshire Terrier (n = 1), Chesapeake Bay Retriever (n = 1), Chihuahua (n = 1), Boxer (n = 1), Border Collie (n = 1), German Shepherd (n = 1) and mixed breed dogs (n = 7). The study population consisted of 8 neutered males, 12 spayed females, and 5 intact females. The mean age was 3.7 years (range, 1–9 years old) and the mean body weight was 23.2 kg (range, 5.4–48.9 kg). One dog, a female intact Labrador Retriever, was excluded from the study after detection of a prolonged baseline PFA-100® collagen/epinephrine closure time. The remaining 24 dogs received aspirin for 10 days with no reported adverse effects.
Platelet Function Analysis
The median PFA-100® collagen/epinephrine closure time of all 24 dogs on Day 0 was 135 seconds (range, 72–248 seconds), while the median closure times of all dogs on Days 3 and 10 were 268 seconds (range, 91–300 seconds) and 260 seconds (range, 64–300 seconds), respectively, an increase of 70% from baseline values. When compared to Day 0, there was a significant increase in closure times at both Day 3 (P < 0.001) and Day 10 (P < 0.001). There was no difference in closure times between Days 3 and 10 (P = 0.991). There was an equal distribution among the three groups of aspirin responsiveness, with a total 8 dogs per group.
In the aspirin non-responder group, median closure time on Day 0 was 113 seconds (range, 91–203), while the median closure times on Days 3 and 10 were 159 seconds (range, 91–235) and 174 seconds (range, 92 – 219), respectively. In the incomplete aspirin responder group, median closure time on Day 0 was 135 seconds (range, 72–155). Four dogs had closure times of greater than 300 seconds on Day 3, and the other 4 dogs had closure times of greater than 300 seconds on Day 10. In the aspirin responder group, the median closure time on Day 0 was 167 seconds (range, 90 – 248). Median closure times during aspirin administration could not be calculated in the incomplete aspirin responder and the aspirin responder groups because, at each time point, a number of dogs exceeded the machine cut-off closure time.
Platelet COX-1 and COX-2 Expression
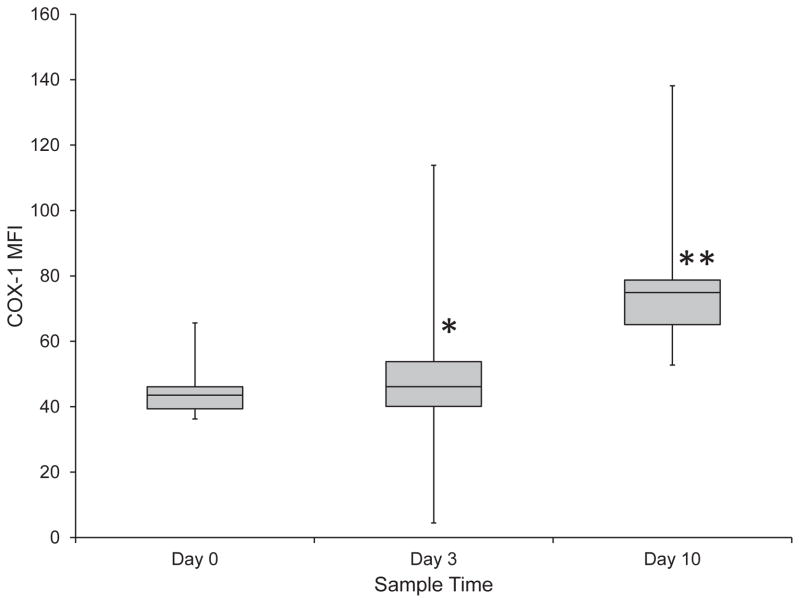
Platelet expression of COX-1 and COX-2 was measured prior to and at all tested time points during aspirin administration. There was a significant increase in platelet COX-1 expression from Day 0 to Day 3 with a median increase of 13% (range, −29.7%–136.1%) (P = 0.047) and from Day 0 to Day 10 with a median increase of 72% (range, −0.37–210.36%) (P < 0.001). (Figure 1) There was also a significant (P < 0.001) increase in COX-1 expression by 52% (range, −24.7–241.4%) from Day 3 to Day 10. There were no significant (P = 0.958) differences in COX-1 expression between the three groups of aspirin responsiveness. Prior to drug administration, the percentage of platelets that expressed COX-1 was 55% (range, 50.2–63%). During aspirin administration, there was no significant change in the percentage of platelets that expressed COX-1 on Day 3 compared to baseline, however, this percentage increased significantly (P < 0.001) to 65% (range, 57.2–77.8%) on Day 10. Additionally, there was a significant (P < 0.001) difference in the percentage of platelets that expressed COX-1 from Day 3 to Day 10. (Figure 2) There were no significant (P = 0.600) differences in the percentage of platelets that expressed COX-1 between the three groups of aspirin responsiveness.
Figure 1.
Platelet COX-1 expression during treatment of 24 healthy dogs with low dose aspirin (1 mg/kg PO every 24 hours for 10 days). There was a significant increase in COX-1 expression from Day 0 to Day 3 (P = 0.047) (*) and from Day 0 to Day 10 (P < 0.001) (**). There was also a significant (P < 0.001) increase between Day 3 and Day 10.
Figure 2.
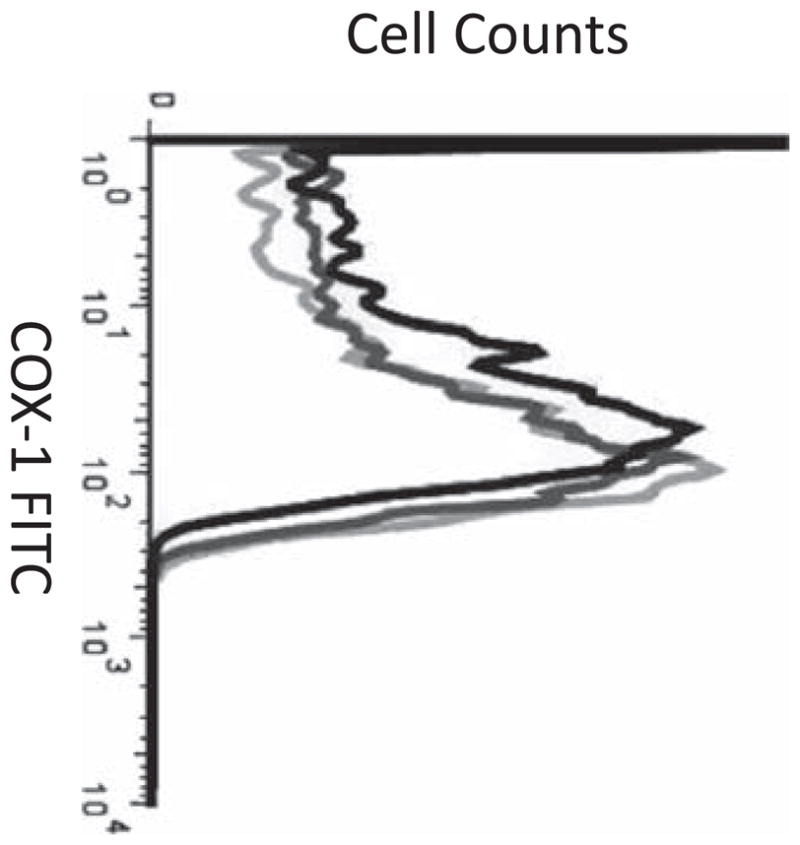
Representative flow cytometry histograms demonstrating platelet COX-1 expression on Day 0 (black line), Day 3 (dark gray line), and Day 10 (light gray line).
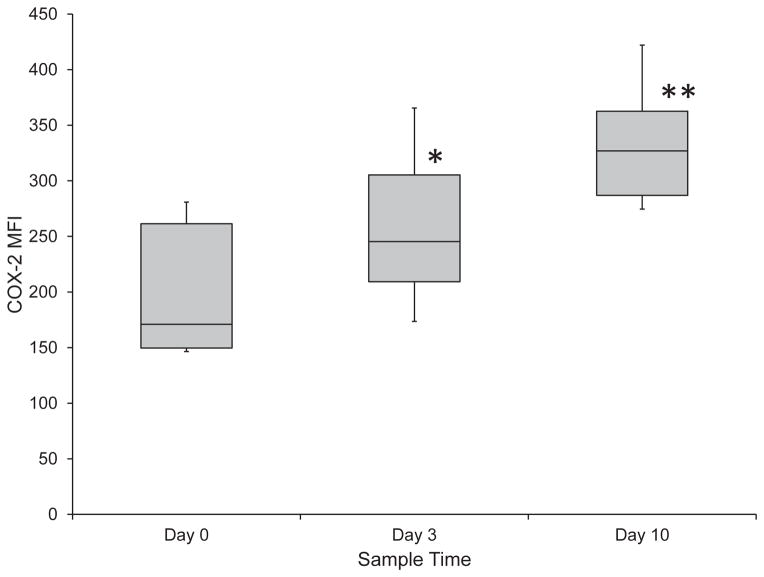
Platelet COX-2 expression was significantly (P = 0.003, P <0.001 respectively) increased from Day 0 to Day 3 by 34% (range, −29.2–270.4%) and from Day 0 to Day 10 by 74% (range, −19.7–226.2%). (Figure 3) COX-2 expression was also significantly (P = 0.014) increased between Days 3 and 10 by 21% (range, −27.3–117.2%). Similar to the COX-1 expression, there were no significant (P = 0.479) differences in COX-2 expression between the three groups of aspirin responsiveness. Prior to drug administration, the percentage of platelets that expressed COX-2 was 78% (range, 66.6–82.6%), while during aspirin administration, there was a significant (P < 0.002) increase in the percentage of platelets that expressed COX-2 on Day 3 (to 85%; range, 74.3–94%), but no significant (P = 0.896) difference from baseline on Day 10. There was a significant (P < 0.001) difference in the percentage of platelets that expressed COX-2 when Day 3 was compared to Day 10. (Figure 4) There were no significant (P = 0.213) differences in the percentage of platelets that expressed COX-2 between the three groups of aspirin responsiveness.
Figure 3.
Platelet COX-2 expression during treatment of 24 healthy dogs with low dose aspirin. There was a significant increase in COX-2 expression from Day 0 to Day 3 (P = 0.003) (*) and from Day 0 to Day 10 (P < 0.001) (**). There was also a significant (P = 0.014) increase between Day 3 and Day 10.
Figure 4.
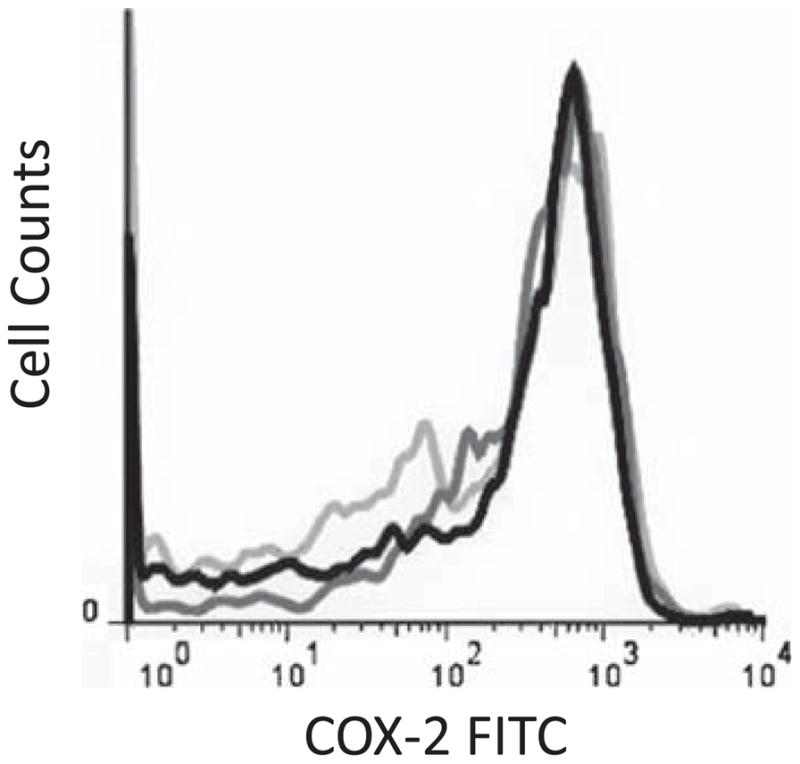
Representative flow cytometry histograms demonstrating platelet COX-2 expression on Day 0 (black line), Day 3 (dark gray line), and Day 10 (light gray line).
Urinary Thromboxane Concentration
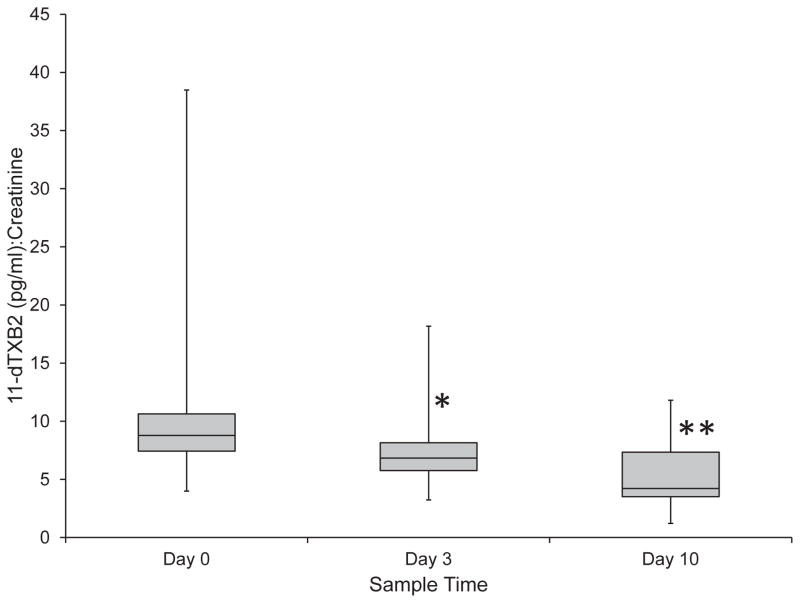
The median urine 11-dTXB2 to creatinine ratio on Day 0 was 8.8 (range, 4.0–38.5), while the median ratios for Day 3 and Day 10 were 7.5 (range, 3.2–18.2) and 5.2 (range, 1.2–11.8), respectively. There was a 29% decrease in the urine 11-dTXB2 to creatinine ratio between Day 0 and Day 3, while there was a 56% decrease between Day 0 and Day 10 and 35% decrease between Day 3 and Day 10. (Figure 5) Significant decreases in the urinary 11-dTXB2 to creatinine ratio occurred at all time points, from Day 0 to Day 3 (P = 0.005), from Day 0 to Day 10 (P < 0.001), and from Day 3 to Day 10 (P = 0.014). There were no significant (P = 0.885) differences in the urine 11-dTXB2 to creatinine ratio between the three groups of aspirin responsiveness.
Figure 5.
Median urinary 11-dTXB2 to creatinine ratios during treatment of 24 healthy dogs with low dose aspirin. There was a significant decrease in the urinary 11-dTXB2 to creatinine ratio from Day 0 to Day 3 (P = 0.005) (*) and from Day 0 to Day 10 (P < 0.001) (**). There was also a significant (P = 0.014) decrease between Day 3 and Day 10.
Discussion
Our study revealed that low dose aspirin, in contrast to administration of anti-inflammatory doses, does not inhibit platelet function in all dogs as measured using the PFA-100® collagen/epinephrine cartridge, indicating that aspirin resistance occurs in dogs as well as in humans. Chronic low dose aspirin administration will consistently inhibit thromboxane synthesis in dogs. The inhibition of thromboxane by low dose aspirin, however, does not appear to correlate with aspirin responsiveness as measured by the PFA-100®. With no differences in urinary thromboxane concentrations between aspirin responders, non-responders, and inconsistent responders, our study suggests that thromboxane synthesis that is independent of COX-1 activity, either through the COX-2 enzyme pathway or alternative pathways, is unlikely to be the mechanism for aspirin resistance in dogs.
One possible explanation for aspirin resistance in dogs could be variable responsiveness of platelets to thromboxane. Previous studies have suggested that about 70% of canine platelets are insensitive to thromboxane stimulation.29–31 The thromboxane insensitivity observed in some canine platelets could be due to impaired platelet thromboxane A2 receptor linked G proteins.31 If only 30% of dogs have platelets that are sensitive to the effects of thromboxane, this could explain why only one third of the animals in our study responded to low dose aspirin with a consistent increase in PFA-100 closure time. In those dogs that have platelets that are insensitive to thromboxane-induced activation, other agonists such as ADP or serotonin might play a more important role in platelet activation, and such dogs could therefore be relatively resistant to the inhibitory effects of low dose aspirin. A recent study of humans suggests that resistance to aspirin could be due to a COX independent pathway and that, in affected people, inhibition of platelet function is more readily achieved using an ADP antagonist.12 If platelet activation pathways are comparable in dogs and humans, ADP receptor antagonists could provide more reliable inhibition of platelet function than low dose aspirin, although additional studies are required to fully evaluate the effectiveness of this drug class in dogs.
We measured urine levels of the thromboxane metabolite 11-dehydro-thromboxane B2 to evaluate the effects of aspirin on thromboxane synthesis. Measurement of this metabolite has been used in previous studies as an indicator of aspirin-induced COX inhibition.32–34 After synthesis by platelets, thromboxane A2 has an immediate physiologic effect, and is then metabolized to thromboxane B2 and further metabolized into 11-dehydro-thromboxane B2 and 2,3-dinor-thromboxane B2 (2,3-dinorTXB2) before being eliminated in the urine.8,34,35 Concentrations of thromboxane metabolites in plasma or serum can be artifactually increased due to platelet activation during collection of blood and sample processing. The use of urine to measure thromboxane metabolites avoids the problem of in-vitro platelet activation associated with blood collection, and is therefore considered to be a more reliable indicator of platelet thromboxane synthesis.34,35
Although most dogs in this study had a persistent decrease in urine 11-dTXB2 to creatinine ratios after administration of low dose aspirin, and every dog had a decreased ratio for at least one time point, a few dogs demonstrated an increased ratio at a single time point. Similar inconsistent inhibition of urine 11-dTXB2 levels has previously been reported in dogs in a study using the same low dose of aspirin.8 This previous study also evaluated urinary 2,3-dinor-thromboxane B2 and demonstrated that, compared to 11-dTXB2, urine 2,3-dinorTXB2 levels were a more sensitive indicator of aspirin-induced thromboxane inhibition in dogs.8 However, although the urine 11-dTXB2 assay used in our study is less sensitive for detecting aspirin-induced thromboxane inhibition compared to the urine 2,3-dinorTXB2 assay, our assay was still sufficiently sensitive to detect a significant decrease in urine thromboxane metabolites at both measured time points after aspirin administration.
While cyclooxygenase function was inhibited by low dose aspirin as indicated by prolongation of PFA-100® collagen/epinephrine closure times and decreased urine 11-dTXB2 concentrations, platelet expression of both COX-1 and COX-2 progressively increased throughout the course of aspirin administration. The COX-1 enzyme isoform was originally thought to be strictly constitutively expressed, but recent studies have shown that COX-1 expression can be induced in certain tissues, including human megakaryoblasts during thrombocytopoiesis.15,36,37 Similar to the results of this study, anti-inflammatory doses of aspirin also cause increased platelet COX-1 expression, decreased thromboxane synthesis, and inhibition in platelet function.19 One possible explanation for increased platelet COX-1 expression could be a compensatory up-regulation of production of the COX-1 isoform by megakaryocytes in response to a reduction in platelet thromboxane A2 production. Platelet COX-2 expression also increased during administration of low dose aspirin, a finding that contrasts with a previous study in dogs treated with an anti-inflammatory dose of aspirin, in which platelet COX-2 expression decreased in most treated dogs.19 Since COX-2 expression is typically induced by inflammatory mediators,15,38,39 it is feasible that the anti-inflammatory effects of high doses of aspirin are sufficient to decrease COX-2 expression, whereas the low dose of aspirin used in our current study was insufficient to exert the same effect. Additionally, platelet COX-2 expression could have been up-regulated as a compensatory response to the inhibitory effects of aspirin on COX-1 function and subsequent thromboxane synthesis. Finally, the increase in platelet COX expression could have originated within the circulating platelets themselves, and could therefore be independent of effects on megakaryocytes. Platelets contain rough endoplasmic reticulum and polyribosomes, both of which allow platelets to biosynthesize protein from cytoplasmic mRNA. These intra-platelet organelles could provide a source of COX-1 and COX-2 mRNA that would enable platelets to increase cyclooxygenase expression.
Pre-treatment patient-to-patient variations in platelet COX-1 and COX-2 expression have been proposed as one potential mechanism for variable aspirin responsiveness. Our current study, however, did not demonstrate a difference in pre-treatment platelet COX-1 or COX-2 expression between the three categories of aspirin responsiveness. Our results suggest that differences in pre-treatment platelet COX expression do not play a major role in aspirin resistance in dogs, and indicate that pre-existing COX expression cannot be used to predict aspirin responsiveness
Platelet aggregometry has long been considered the gold standard methodology for the evaluation of platelet responsiveness to aspirin.7,40 Platelet aggregometry, however, requires specialized equipment and training, thus making it a less commonly utilized technology in a clinical setting. The PFA-100®, in contrast, is an instrument that is practical for routine clinical use, and prolongation of the PFA-100® closure time as measured with the collagen/epinephrine cartridge has become well established as a sensitive indicator of aspirin-induced platelet dysfunction in both humans and dogs.7 The PFA-100® closure times in our present study revealed that only one third of healthy dogs responded adequately and consistently to low dose aspirin therapy. If our study is an accurate representation of the canine population, the administration of a 1 mg/kg per day dose of aspirin may not be adequate to create a consistent and reliable preventive therapy for thromboembolic complications in a majority of dogs. Anti-inflammatory doses of aspirin (10 mg/kg bid), in contrast, have been shown to consistently inhibit platelet function in dogs.19 Commencement with low dose aspirin at 1 mg/kg per day and subsequent utilization of periodic measurement of platelet function via the PFA-100® to individually dose escalate ‘to effect’ could increase the population of aspirin responders.41 The aspirin responders in our study had inhibition of platelet function by Day 3 of therapy, suggesting that platelet function analysis after three days of aspirin should be adequate to detect whether a patient will respond to the current dose of aspirin, or require a higher dose. However, as the aspirin dose increases, so does the chance of developing adverse side effects. Furthermore, as aspirin dosage approaches anti-inflammatory doses, the likelihood of concurrent inhibition of prostacyclin production by the vascular endothelium increases, potentially negating the anti-platelet effects of aspirin.42,43
There were several limitations to our study. While our study population consisted of a variety of breeds and ages, we evaluated aspirin effects only in healthy dogs. Responsiveness to aspirin could vary in the presence of endogenous platelet activation, and a similar study in dogs with pro-inflammatory or prothrombotic diseases would be necessary to determine the incidence of aspirin resistance in sick patients. Additionally, our study used no methods to ensure that all dogs received the appropriate daily dose. Although it is unlikely, a lack of owner compliance might explain why some dogs either did not respond or inconsistently responded to the aspirin dose. Due to unexpected drop-out of several owners at the start of the study, and the exclusion of one dog because of a prolonged baseline PFA-100® collagen/epinephrine closure time, the study was completed with 24 dogs rather than the minimum of 27 dogs suggested by preceding power analysis. However, despite this slight reduction in the number of enrolled dogs, animal numbers were still sufficient to detect significant aspirin-induced changes in platelet function, platelet COX expression, and thromboxane production.
Another limitation of this study is the absence of Day 0 closure times for the PFA-100® collagen/epinephrine cartridge prior to aspirin administration. On Day 0, blood was collected from all enrolled animals for analysis, and then owners were sent home with the prescribed course of aspirin. Unfortunately, we then experienced an instrument malfunction, and, since most dogs had already received their first dose of aspirin, we could not repeat the testing. Instead, we elected to repeat ‘baseline’ samples 4 weeks after completion of aspirin therapy, which is supported by a 14 day washout period being adequate for closure times to return to normal in dogs after administration of various non-steroidal anti-inflammatory drugs.26 Similarly, after administration of an anti-inflammatory dose of aspirin (10 mg/kg BID for 10 days) to normal dogs, collagen/epinephrine cartridge closure times returned to normal in all dogs within 14 days of discontinuing the medication (unpublished data).19 Four weeks therefore should have been more than sufficient time after the completion of aspirin therapy to determine each dog’s normal platelet function.
In humans, the PFA-100® collagen/epinephrine cartridge is typically utilized to detect aspirin-induced platelet dysfunction, and the collagen/ADP cartridge is recommended to evaluate for congenital and acquired platelet function defects.44 In healthy dogs, collagen/epinephrine cartridges have been shown to generate a wider range of closure times, and in some studies,45 a higher prevalence of results that exceed machine cut-off values when compared to collagen/ADP cartridges. This has prompted some authors to suggest that the collagen/epinephrine cartridge might not be useful in dogs.45 However, in dogs as in humans, administration of anti-inflammatory doses of aspirin will consistently inhibit platelet function based on prolongation of closure times when using collagen/epinephrine cartridges, but not when collagen/ADP cartridges are utilized.7,19 Platelet aggregometry studies have similarly confirmed that anti-inflammatory doses of aspirin also do not inhibit ADP-induced platelet aggregation in dogs.46 Given that aspirin doses that are considerably greater than the dose of aspirin used in our study do not appear to inhibit ADP-induced platelet aggregation, we believe that it is highly unlikely that we would have been able to detect any inhibition of platelet function using collagen/ADP cartridges. Therefore, although collagen/epinephrine cartridge might provide greater variability in closure times in dogs compared to the collagen/ADP cartridge, we believe that in dogs, as in people, the collagen/epinephrine cartridge is a more sensitive indicator of aspirin-induced platelet dysfunction.
Determination of aspirin responsiveness in our study hinged on the results of a single test methodology, the PFA-100® using the collagen/epinephrine cartridge. Since platelet aggegrometry using agonists such as epinephrine also appears to be a sensitive indicator of aspirin-induced platelet dysfunction, future studies would be strengthened by adding aggregometry to augment the results of PFA-100® analysis. Further studies would also be strengthened by analyzing baseline and post-treatment PFA-100® closure times in duplicate or triplicate in order to better account for inherent test variability. In our study, aspirin responsiveness was defined as a PFA-100® collagen/epinephrine closure time that exceeded the cut-off time for the instrument (greater than 300 seconds). This definition has previously been established in studies of aspirin resistance in human patients.t47 An alternative approach to defining aspirin responsiveness would have been to calculate the ratio between baseline and post-aspirin closure times for each individual dog, and use an arbitrary change in ratio (for example, doubling) as evidence of treatment response. However, since the response after aspirin administration exceeded the instrument cut-off time in half of the samples analyzed, and we had no means of determining a quantitative closure time above this value, in our opinion using by default a post-treatment value of ‘300 seconds’ for all results that exceeded instrument cut-off times would have led to inaccurate ratio calculations in a large number of the samples analyzed.
In our original study group of 25 dogs tested with the collagen/epinephrine cartridge, only one dog had a closure time of greater than 300 seconds in the absence of aspirin. Based on previous studies conducted by the authors,19,26 this finding is not unexpected, since in our experience it is relatively uncommon for normal dogs to have collagen/epinephrine cartridge closure times that exceed the machine cut-off time of 300 seconds. Similar findings have been previously reported in other comparable studies in dogs.48 Other researchers, however, have reported a higher rate of prolonged collagen/epinephrine cartridge closure times in normal dogs: in one study, for example, 5 of 29 dogs tested had closure times of over 300 seconds.45 Variability in the proportion of normal dogs that exceed machine cut-off times may be breed-related. In one study of collagen/EPI cartridge closure times in four different dog breeds, closure times remained below cut-off times in three breeds, but exceeded 300 seconds in a few individual dogs of the fourth breed (Labrador Retrievers).7 Interestingly, the one normal dog in our study that had a baseline closure time of over 300 seconds was also a Labrador Retriever, although another 5 dogs of the same breed had normal closure times. Within the population of normal dogs investigated in our study, since baseline prolongations of collagen/EPI cartridge closure times are uncommon, the dramatic increase in the rate of closure times above the machine cut-off time of 300 seconds after commencement of aspirin (24 of 48 tests performed exceeded 300 seconds, compared to only 1 in 25 in the absence of the drug) is undoubtedly attributable to the effects of the drug rather than normal individual-to-individual variations in test results.
Our study demonstrates that low dose aspirin inhibits platelet thromboxane synthesis and increases platelet expression of both COX-1 and COX-2, while inhibition of platelet function is less consistently observed in individual dogs. Platelet COX expression before and during therapy was not related to aspirin responsiveness. Additional research is needed to better define the mechanism of aspirin resistance in dogs.
Acknowledgments
Funded by the Mississippi State University College of Veterinary Medicine Salsbury Research Grant, the WH Lindley Fund for Excellence, and the Dr. Hugh G. Ward Endowment
The authors thank Jodi Griswold and Angela Briggs for their assistance.
Abbreviations
- IMHA
immune mediated hemolytic anemia
- COX
cyclooxygenase
- TXA2
thromboxane A2
- PFA-100®
Platelet Function Analyzer - 100®
- ADP
adenosine diphosphate
- FACS-PBS
fluorescence-activated cell sorting – phosphate buffered saline
- FITC
fluorescein isothiocyanate
- RPE
R-phycoerythrin
- IgG
immunoglobulin G
- MFI
mean fluorescence iIntensity
- 11-dTXB2
11-dehydro-thromboxane B2
- ANOVA
analysis of variance
- 2, 3-dinorTXB2
2,3-dinor-thromboxane B2
Footnotes
Shearer L, Kruth SA, Wood D. Effects of aspirin and clopidogrel on platelet function in healthy dogs (abstr). J Vet Intern Med 2009; 23: 745.
PFA-100®, Siemens Healthcare Diagnostics, Deerfield, IL
PFA Collagen/ADP Test Cartridge, Siemens Healthcare Diagnostics, Duluth, GA
Aspirin, Major Pharmaceuticals, Livonia, MI
PFA Collagen/Epinephrine Test Cartridge, Siemens Healthcare Diagnostics, Duluth, GA
3.8% sodium citrate, Vacutainer tube, Becton Dickinson, Franklin Lakes, NJ
FITC-conjugated monoclonal COX-1, Clone CX111, Cayman Chemical Co, Ann Arbor, MI
Monoclonal anti-human CD9:RPE, Clone MM2/57, AbD Serotec, Raleigh, NC
Paraformaldehyde, Biolegend Inc., San Diego, CA
Triton X-100, Sigma-Aldrich, St. Louis, MO
FITC-conjugated monoclonal COX-2, Clone CX299, Cayman Chemical Co, Ann Arbor, MI
Monoclonal CD61-purified, Clone JM2E5, Accurate Chemical, Westbury, NY
Goat anti-mouse IgG:RPE, AbD Serotec, Raleigh, NC
FACSCalibur, Becton Dickinson, San Jose, CA
CellQuest software, Becton Dickinson, San Jose, CA
Luminex® 200 System xMAP Technology, Luminex Corporation, Austin, TX
Luminex® 11-dehydro Thromboxane B2 Kit, Cayman Chemical Co, Ann Arbor, MI
ACE Alera® Clinical Chemistry System, Alfa Wasserman, Inc., West Caldwell, NJ
SAS for Windows version 9.2, SAS Institute, INC., Cary, NC, 2008
Leone M, Mezzasoma, A, Davi G, Gresele P. Detection of a critical degree of platelet TxA2-production inhibition by the PFA100 in aspirin-treated subjects: Possible relevance for the monitoring of aspirin resistance. J Thromb Haemost 2005;3:P0964.
This work was performed at the College of Veterinary Medicine, Mississippi State University
Abstract presented in part at the 2011 ACVIM Forum, Denver, CO.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
References
- 1.Goggs R, Benigni L, Fuentes V, et al. Pulmonary thromboembolism. J Vet Emerg Crit Care. 2009;19:30–52. doi: 10.1111/j.1476-4431.2009.00388.x. [DOI] [PubMed] [Google Scholar]
- 2.Johnson L, Lappin M, Baker D. Pulmonary Thromboembolism in 29 Dogs: 1985–1995. J Vet Intern Med. 1999;13:338–345. doi: 10.1892/0891-6640(1999)013<0338:ptid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Patrono C, Dalen J, Fuster V, et al. Platelet-Active Drugs: The relationships among dose, effectiveness, and side effects. Chest. 2001;119:39S–63S. doi: 10.1378/chest.119.1_suppl.39s. [DOI] [PubMed] [Google Scholar]
- 4.Perneby C, Wallen N, Rooney C, et al. Dose- and time-dependent antiplatelet effects of aspirin. Thromb Haemost. 2006;95:652–658. [PubMed] [Google Scholar]
- 5.Rackear D, Feldman B, Farver T, et al. The effect of three different dosages of acetylsalicylic acid on canine platelet aggregation. J Am Anim Hosp Assoc. 1988;24(1):23–26. [Google Scholar]
- 6.Mischke R, Keidel A. Influence of platelet count, acetylsalicylic acid, von Willebrand’s Disease, coagulopathies, and haematocrit on results obtained using a platelet function analyser in dogs. Vet J. 2003;165:43–52. doi: 10.1016/s1090-0233(02)00169-7. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen L, Zois N, Pedersen H, et al. Platelet function in dogs: breed differences and effect of acetylsalicylic acid administration. Vet Clin Pathol. 2007;36:267–273. doi: 10.1111/j.1939-165x.2007.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoh C, Smith S, McMichael M, et al. Evaluation of effects of low-dose aspirin administration on urinary thromboxane metabolites in healthy dogs. Am J Vet Res. 2011;72:1038–1045. doi: 10.2460/ajvr.72.8.1038. [DOI] [PubMed] [Google Scholar]
- 9.Weinkle T, Center S, Randolph J, et al. Evaluation of prognostic factors, survival rates, and treatment protocols for immune-mediated hemolytic anemia in dogs: 151 cases (1993 – 2002) J Am Vet Med Assoc. 2005;226:1869–1880. doi: 10.2460/javma.2005.226.1869. [DOI] [PubMed] [Google Scholar]
- 10.Kour D, Tandon V, Kapoor B, et al. Aspirin Resistance. New Horiz. 2006;8:116–117. [Google Scholar]
- 11.Martin C, Talbert R. Aspirin Resistance: An evaluation of current evidence and measurement methods. Pharmacotherapy. 2005;25:942–953. doi: 10.1592/phco.2005.25.7.942. [DOI] [PubMed] [Google Scholar]
- 12.Frelinger A, Furman M, Linden M, et al. Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase-1 – and cyclooxygenase-2-independent pathway: A 700-Patient study of aspirin resistance. Circulation. 2006;113:2888–2896. doi: 10.1161/CIRCULATIONAHA.105.596627. [DOI] [PubMed] [Google Scholar]
- 13.Goodman T, Ferro A, Sharma P. Pharmacogenetics of aspirin resistance: a comprehensive systemic review. Br J Clin Pharmacol. 2008;66:222–232. doi: 10.1111/j.1365-2125.2008.03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka N, Sato T, Fujita H, et al. Constitutive expression and involvement of cyclooxygenase-2 in human megakaryocytopoiesis. Arterioscler Thromb Vasc Biol. 2004;24:607–612. doi: 10.1161/01.ATV.0000117181.68309.10. [DOI] [PubMed] [Google Scholar]
- 15.Rocca B, Secchiero P, Ciabattoni G, et al. Cyclooxygenase-2 expression is induced during human megakaryopoiesis and characterizes newly formed platelets. Proc Natl Acad Sci USA. 2002;99:7634–7639. doi: 10.1073/pnas.112202999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kay-Mugford P, Benn S, LaMarre J, et al. Cyclooxygenase expression in canine platelets and Mardin-Darby canine kidney cells. Am J Vet Res. 2000;61:1512–1516. doi: 10.2460/ajvr.2000.61.1512. [DOI] [PubMed] [Google Scholar]
- 17.Weber A, Przytulski B, Schumacher M, et al. Flow cytometry analysis of platelet cyclooxygenase-2 expression: induction of platelet cyclooxygenase-2 in patients undergoing coronary artery bypass grafting. Br J Haematol. 2002;117:424–426. doi: 10.1046/j.1365-2141.2002.03423.x. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y, Cheng Y, Fan J, et al. Differential impact of prostaglandin H synthase 1 knockdown on platelets and parturition. J Clin Invest. 2005;115:986–995. doi: 10.1172/JCI23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomason J, Lunsford K, Mullins K, et al. Platelet cyclooxygenase expression in normal dogs. J Vet Intern Med. 2011;25:1106–1112. doi: 10.1111/j.1939-1676.2011.00781.x. [DOI] [PubMed] [Google Scholar]
- 20.Guthikonda S, Lev E, Patel R, et al. Reticulated platelets and uninhibited COX-1 and COX-2 decrease the antiplatelet effects of aspirin. J Thromb Haemost. 2007;5:490–496. doi: 10.1111/j.1538-7836.2007.02387.x. [DOI] [PubMed] [Google Scholar]
- 21.Alagha A, Moman E, Adamo M, et al. Design, synthesis and evaluation of aspirin analogues having an additional carboxylate substituent for antithrombotic activity. Bioorg Med Chem Lett. 2009;19:4213–4216. doi: 10.1016/j.bmcl.2009.05.120. [DOI] [PubMed] [Google Scholar]
- 22.Brainard B, Meredith C, Callan MB, et al. Changes in platelet function, hemostasis, and prostaglandin expression after treatment with nonsteroidal anti-inflammatory drugs with various cyclooxygenase selectivities in dogs. Am J Vet Res. 2007;68:251–257. doi: 10.2460/ajvr.68.3.251. [DOI] [PubMed] [Google Scholar]
- 23.Morales F, Couto C, Iazbik M. Effects of 2 concentrations of sodium citrate on coagulation test results, von Willebrand Factor concentration, and platelet function in dogs. J Vet Intern Med. 2007;21:472–475. doi: 10.1892/0891-6640(2007)21[472:eocosc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Baltzer W, McMichael M, Ruaux C, et al. Measurement of urinary 11-dehydro-thromboxane B2 excretion in dogs with gastric dilatation-volvulus. Am J Vet Res. 2006;67:78–83. doi: 10.2460/ajvr.67.1.78. [DOI] [PubMed] [Google Scholar]
- 25.McConnell J, Cheryk L, Durocher A, et al. Urinary 11-dehydro-thromboxane B2 and coagulation activation markers measured within 24 h of human acute ischemic stroke. Neurosci Lett. 2001;313:88–92. doi: 10.1016/s0304-3940(01)02260-1. [DOI] [PubMed] [Google Scholar]
- 26.Mullins K, Thomason J, Lunsford K, et al. Effects of Carprofen, Meloxicam and Deracoxib on Platelet Function in Dogs. Vet Anaesth Analg. 2012;39:206–217. doi: 10.1111/j.1467-2995.2011.00684.x. [DOI] [PubMed] [Google Scholar]
- 27.Brunner E, Puri M. Nonparametric methods in factorial designs. Statistical Papers. 2001;42:1–52. [Google Scholar]
- 28.Shah DA, Madden LV. Nonparametric analysis of ordinal data in designed factorial experiments. Phytopathology. 2004;94:33–43. doi: 10.1094/PHYTO.2004.94.1.33. [DOI] [PubMed] [Google Scholar]
- 29.Johnson GJ, Leis LA, Rao GHR, White JG. Arachidonate-induced platelet aggregation in the dog. Thromb Res. 1979;14:147–154. doi: 10.1016/0049-3848(79)90033-1. [DOI] [PubMed] [Google Scholar]
- 30.Johnson GJ, Leis LA, King RA. Thromboxane responsiveness of dog platelets in inherited as an autosomal recessive trait. Thromb Haemostasis. 1991;65:578–580. [PubMed] [Google Scholar]
- 31.Johnson GJ, Leis LA, Dunlop PC. Thromboxane-insensitivive dog platelets have impaired activation of phospholipase C due to receptor-linked G protein dysfunction. J Clin Invest. 1993;92:2469–2479. doi: 10.1172/JCI116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones CJ, Budsberg SC. Physiologic characteristics and clinical importance of the cyclooxygenase isoforms in dogs and cats. J Am Vet Med Assoc. 2000;217:721–729. doi: 10.2460/javma.2000.217.721. [DOI] [PubMed] [Google Scholar]
- 33.Reilly IAG, FitzGerald GA. Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugs. Blood. 1987;69:180–186. [PubMed] [Google Scholar]
- 34.Perneby C, Granstrom E, Beck O, et al. Optimization of an enzyme immunoassay for 11-dehydro-thromboxane B(2) in urine: comparison with GC-MS. Thromb Res. 1999;96:427–436. doi: 10.1016/s0049-3848(99)00126-7. [DOI] [PubMed] [Google Scholar]
- 35.Catella F, Healy D, Lawson, et al. 11-Dehydrothromboxane B2: A quantitative index of thromboxane A2 formation in the human circulation. Proc Natl Acad Sci USA. 1986;83:5861–5865. doi: 10.1073/pnas.83.16.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhandari P, Bateman A, Mehta R, et al. Mucosal expression of cyclooxygenase isoforms 1 and 2 is increased with worsening damage to the gastric mucosa. Histopathology. 2005;46:280–286. doi: 10.1111/j.1365-2559.2005.02053.x. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann U, Banas B, Kruger B, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human renal allograft rejection – a prospective study. Transpl Int. 2006;19:203–212. doi: 10.1111/j.1432-2277.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 38.Bergh M, Budsberg S. The Coxib NSAIDs: Potential clinical and pharmacologic importance in veterinary medicine. J Vet Intern Med. 2005;19:633–643. doi: 10.1892/0891-6640(2005)19[633:tcnpca]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Mroske C, Plant M, Franks D, et al. Characterization of prostaglandin endoperoxide H synthase-1 enzyme expression during differentiation of the megakaryocytic cell line MEG-01. Exp Hematol. 2000;28:411–421. doi: 10.1016/s0301-472x(00)00125-9. [DOI] [PubMed] [Google Scholar]
- 40.Lordkipanidzé M, Pharand C, Schampaert E, et al. Evaluation of the platelet count drop method for assessment of platelet function in comparison with “gold standard” light transmission aggregometry. Thromb Res. 2009;124(4):418–422. doi: 10.1016/j.thromres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.FitzGerald G, Oates J, Hawiger J, et al. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983;71:676–688. doi: 10.1172/JCI110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McAdam B, Catella-Lawson F, Mardini I, et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: The human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kundu SK, Heilmann EJ, Sio R, et al. Description of an in vitro platelet function analyzer - PFA-100. Semin Thromb Hemost. 1995;21:106–112. doi: 10.1055/s-0032-1313612. [DOI] [PubMed] [Google Scholar]
- 45.Callan M, Giger U. Assessment of a point-of-care instrument for identification of primary hemostatic disorders in dogs. Am J Vet Res. 2001;62:652–658. doi: 10.2460/ajvr.2001.62.652. [DOI] [PubMed] [Google Scholar]
- 46.Boudreaux M, Dillon A, Ravis W, et al. Effects of treatment with aspirin or aspirin/dipyridamole combination in heartworm-negative, heartworm-infected, and embolized heartworm-infected dogs. Am J Vet Res. 1991;52(12):1992–1999. [PubMed] [Google Scholar]
- 47.Crescente M, Mezzasoma A, Del Pinto M, et al. Incomplete inhibition of platelet function as assessed by the platelet function analyzer (PFA-100) identifies a subset of cardiovascular patients with high residual platelet response while on aspirin. Platelets. 2011;22(3):179–187. doi: 10.3109/09537104.2010.543710. [DOI] [PubMed] [Google Scholar]
- 48.Keidel A, Mischke R. Clinical evaluation of platelet function analyzer PFA-100 in dogs. Berl Munch Tierarztl Wochenschr. 1998;111(11–12):452–456. [PubMed] [Google Scholar]