Abstract
Aims—To investigate the expression of CD44 proteins in exfoliated urothelial cells and in tumour tissues from bladder cancer patients. A further objective was to evaluate the diagnostic potential of the changes observed in the expression of these proteins as a marker for non-invasive detection of bladder cancer.
Methods—Naturally voided urine specimens were collected from 47 patients with bladder cancer or severe urothelial dysplasia (n=3) and from a control group of 43 people with no evidence of neoplastic disease. Exfoliated urothelial cells floating in the urine were pelleted by centrifugation and lysed, and their constituent proteins extracted. The pattern of expression of CD44 proteins in each sample was examined by western blot analysis using a monoclonal antibody, Hermes 3, which recognises an epitope on the polypeptide backbone of the CD44 protein. Immunohistochemical studies were performed on neoplastic (n=10) and normal (n=4) bladder tissue specimens which were snap frozen in liquid nitrogen before examination with antibodies to CD44 gene products (CD44s and CD44v6).
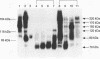
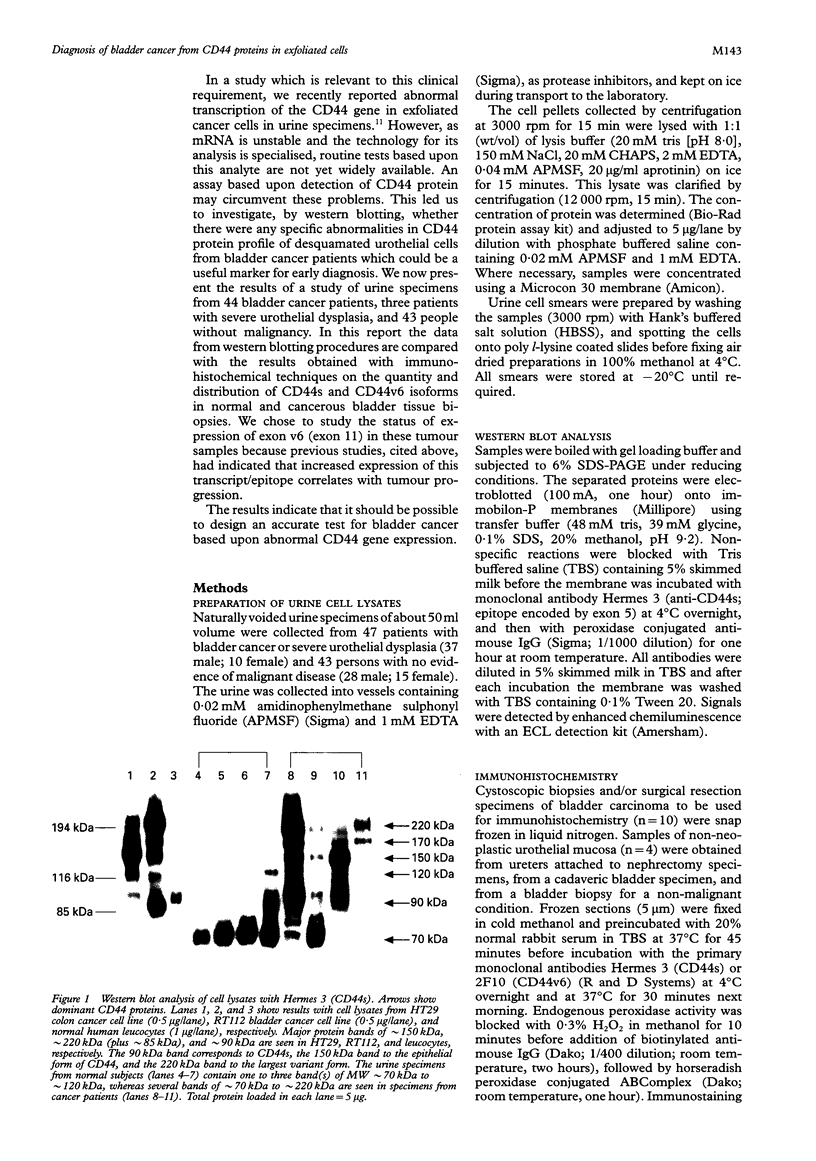
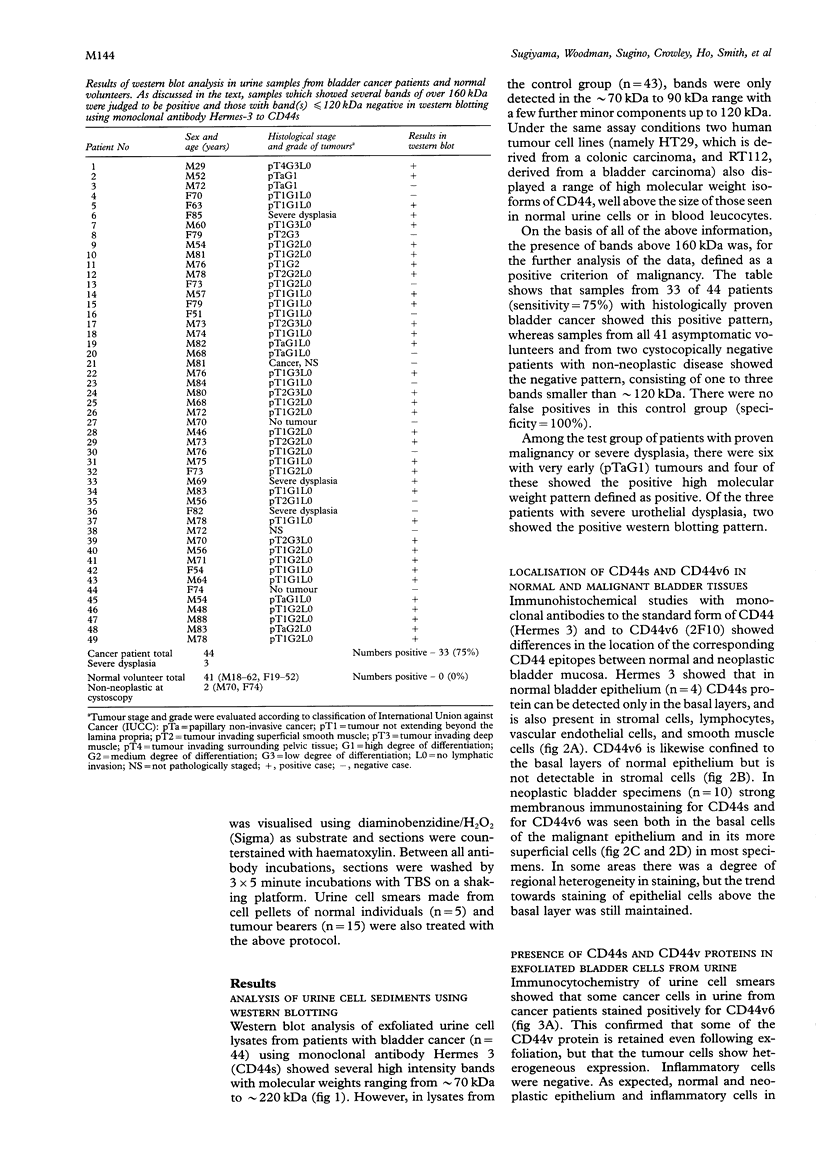
Results—Western blot analysis revealed several high molecular weight CD44 isoforms > 160 kDa in urine cell lysates from 75% of patients with histologically confirmed bladder cancer and in two of the three patients with severe dysplasia. Such patterns were not detected in the urine cell pellets from any persons in the control group. Immunohistochemical studies of the tissue distribution of CD44s and CD44v6 showed that the differentiation and maturation of the epithelial cells in the normal bladder mucosa is accompanied by a decrease in CD44 protein expression. However, carcinoma cells overexpress standard and variant CD44 isoforms and continue to do so as they proceed through the thickened epithelial layer to the luminal surface and after they are shed into the urine.
Conclusions—The abnormal expression of CD44 proteins in exfoliated cancer cells may be a useful marker for the noninvasive diagnosis of bladder cancer.
Keywords: Bladder cancer
Keywords: exfoliated cancer cells
Keywords: CD44 protein
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbasi A. M., Chester K. A., Talbot I. C., Macpherson A. S., Boxer G., Forbes A., Malcolm A. D., Begent R. H. CD44 is associated with proliferation in normal and neoplastic human colorectal epithelial cells. Eur J Cancer. 1993;29A(14):1995–2002. doi: 10.1016/0959-8049(93)90461-n. [DOI] [PubMed] [Google Scholar]
- Dall P., Heider K. H., Hekele A., von Minckwitz G., Kaufmann M., Ponta H., Herrlich P. Surface protein expression and messenger RNA-splicing analysis of CD44 in uterine cervical cancer and normal cervical epithelium. Cancer Res. 1994 Jul 1;54(13):3337–3341. [PubMed] [Google Scholar]
- Fox S. B., Fawcett J., Jackson D. G., Collins I., Gatter K. C., Harris A. L., Gearing A., Simmons D. L. Normal human tissues, in addition to some tumors, express multiple different CD44 isoforms. Cancer Res. 1994 Aug 15;54(16):4539–4546. [PubMed] [Google Scholar]
- Guo Y. J., Liu G., Wang X., Jin D., Wu M., Ma J., Sy M. S. Potential use of soluble CD44 in serum as indicator of tumor burden and metastasis in patients with gastric or colon cancer. Cancer Res. 1994 Jan 15;54(2):422–426. [PubMed] [Google Scholar]
- Heider K. H., Dämmrich J., Skroch-Angel P., Müller-Hermelink H. K., Vollmers H. P., Herrlich P., Ponta H. Differential expression of CD44 splice variants in intestinal- and diffuse-type human gastric carcinomas and normal gastric mucosa. Cancer Res. 1993 Sep 15;53(18):4197–4203. [PubMed] [Google Scholar]
- Koss L. G., Deitch D., Ramanathan R., Sherman A. B. Diagnostic value of cytology of voided urine. Acta Cytol. 1985 Sep-Oct;29(5):810–816. [PubMed] [Google Scholar]
- Limas C., Bigler A., Bair R., Bernhart P., Reddy P. Proliferative activity of urothelial neoplasms: comparison of BrdU incorporation, Ki67 expression, and nucleolar organiser regions. J Clin Pathol. 1993 Feb;46(2):159–165. doi: 10.1136/jcp.46.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R., Terpe H. J., Stauder R., Marston W. L., Stark H., Günthert U. Expression and modulation of CD44 variant isoforms in humans. J Cell Biol. 1994 Jan;124(1-2):71–82. doi: 10.1083/jcb.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y., Hanbury D., Smith J., Tarin D. Non-invasive detection of malignancy by identification of unusual CD44 gene activity in exfoliated cancer cells. BMJ. 1994 Mar 5;308(6929):619–624. doi: 10.1136/bmj.308.6929.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y., Tarin D. Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet. 1992 Oct 31;340(8827):1053–1058. doi: 10.1016/0140-6736(92)93077-z. [DOI] [PubMed] [Google Scholar]
- Matzkin H., Moinuddin S. M., Soloway M. S. Value of urine cytology versus bladder washing in bladder cancer. Urology. 1992 Mar;39(3):201–203. doi: 10.1016/0090-4295(92)90288-8. [DOI] [PubMed] [Google Scholar]
- Mulder J. W., Kruyt P. M., Sewnath M., Oosting J., Seldenrijk C. A., Weidema W. F., Offerhaus G. J., Pals S. T. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1994 Nov 26;344(8935):1470–1472. doi: 10.1016/s0140-6736(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Screaton G. R., Bell M. V., Bell J. I., Jackson D. G. The identification of a new alternative exon with highly restricted tissue expression in transcripts encoding the mouse Pgp-1 (CD44) homing receptor. Comparison of all 10 variable exons between mouse, human, and rat. J Biol Chem. 1993 Jun 15;268(17):12235–12238. [PubMed] [Google Scholar]
- Screaton G. R., Bell M. V., Jackson D. G., Cornelis F. B., Gerth U., Bell J. I. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K. K., Ellis L. M., Saya H. Expression of CD44R1 adhesion molecule in colon carcinomas and metastases. Lancet. 1993 Mar 20;341(8847):725–726. doi: 10.1016/0140-6736(93)90490-8. [DOI] [PubMed] [Google Scholar]
- Tsujihashi H., Matsuda H., Uejima S., Akiyama T., Kurita T. Cell proliferation of human bladder tumors. J Urol. 1989 Oct;142(4):1113–1116. doi: 10.1016/s0022-5347(17)39008-0. [DOI] [PubMed] [Google Scholar]
- Tölg C., Hofmann M., Herrlich P., Ponta H. Splicing choice from ten variant exons establishes CD44 variability. Nucleic Acids Res. 1993 Mar 11;21(5):1225–1229. doi: 10.1093/nar/21.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielenga V. J., Heider K. H., Offerhaus G. J., Adolf G. R., van den Berg F. M., Ponta H., Herrlich P., Pals S. T. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993 Oct 15;53(20):4754–4756. [PubMed] [Google Scholar]