Abstract
Rationale
Naltrexone, an opioid antagonist, is currently approved as a treatment for heroin dependence. However, naltrexone is generally not well accepted by patients, and medication non-compliance is a difficult obstacle to treatment. A sustained-release form of naltrexone may improve compliance.
Objective
The present study was designed to evaluate the time course, safety, and effectiveness of a depot formulation of naltrexone (Depotrex®).
Methods
Twelve heroin-dependent individuals participated in an 8-week inpatient study. After a 1-week detoxification period, six participants received 192 mg naltrexone base and six participants received 384 mg naltrexone base. For safety, the low dose of depot naltrexone was tested before the high dose. The effects of heroin (0, 6.25, 12.5, 18.75, 25 mg, IV) were evaluated for the next 6 weeks. One dose of heroin was tested per day on Mondays through Fridays, and the entire dose range was tested each week. Active heroin doses were administered in ascending order during the week, while placebo could be administered on any day. Subjective, performance, and physiological effects were measured both before and after heroin administration. The hypotheses were that depot naltrexone would antagonize the effects of heroin, and that the high dose of depot naltrexone would produce a more effective and longer-lasting antagonism than the low dose.
Results
The low and high doses of depot naltrexone antagonized heroin-induced subjective ratings for 3 and 5 weeks, respectively. Plasma levels of naltrexone remained above 1 ng/ml for approximately 3 and 4 weeks after administration of 192 mg and 384 mg naltrexone. Other than the initial discomfort associated with the injection of depot naltrexone, there were no untoward side-effects. Conclusions: These results suggest that this depot formulation of naltrexone provides a safe, effective, long-lasting antagonism of the effects of heroin.
Keywords: Heroin, Human, Naltrexone, Opioid, Subjective effect, Sustained-release, Depotrex
Introduction
Naltrexone, an orally effective opioid antagonist, was approved in 1984 by the Food and Drug Administration as a maintenance medication for the treatment of heroin dependence. Naltrexone potently antagonizes the effects of opioid agonists, while producing no agonist effects of its own (Jaffe and Martin 1990). Tolerance does not develop to naltrexone’s antagonist effects and the drug has few side effects, even after chronic administration of over 1 year (Kleber et al. 1985). Because of its ability to antagonize the effects of mu opioid agonists, its long duration of action, and its favorable pharmacokinetic and metabolic characteristics (Martin et al. 1966, 1973), naltrexone initially held great promise as a treatment for opioid dependence. The early rationale for using a pure antagonist was that once the individual was maintained on naltrexone, subsequent attempts to self-administer the illicit opioid would not produce euphoria (Wikler 1965; Martin et al. 1966) and the user would eventually discontinue opioid use altogether.
Although the use of naltrexone as a maintenance therapy for opioid abuse can be effective (Martin et al. 1973; O’Brien et al. 1975; Judson et al. 1981), it has been used most successfully with only a select subpopulation of highly motivated individuals. Because of the problems with medication non-compliance, naltrexone therapy has not lived up to its initial promise. This may be in part because opioid users are accustomed to self-administering potent reinforcers, and, by contrast, the complete absence of opioid-induced reinforcing effects may be unacceptable. Another factor that may contribute to noncompliance is that, unlike methadone, discontinuation of naltrexone maintenance has no adverse consequences (e.g. withdrawal effects). Furthermore, naltrexone itself may induce adverse neuropsychiatric and gastrointestinal effects, such as dysphoria, nausea, and abdominal pain (Hollister et al. 1981; Crowley et al. 1985; Oncken et al. 2001).
Sustained-release forms of naltrexone could increase compliance and ultimately improve treatment effectiveness (Martin and Sandquist 1974; Abrahams and Ronel 1975; Chiang et al. 1985a, 1985b). Chiang et al. (1985a, 1985b), for example, administered biodegradable beads containing a dose of 63 mg naltrexone to normal, healthy volunteers. Following an initial burst of release, this formulation yielded relatively constant plasma levels of naltrexone (0.3–0.5 ng/ml) for up to 1 month. However, when these investigators administered challenge doses of morphine (15 mg IM), the results were variable. In some participants, morphine was completely ineffective, while in others, morphine-like effects were observed. In addition, three of the five participants who completed the study developed tissue inflammation near the site of bead implantation (Chiang et al. 1985b). Although the adverse tissue reaction and the variable antagonist effectiveness of the naltrexone beads limited its clinical utility, the rationale behind the development of a sustained-release form of naltrexone was sound.
A new depot formulation of naltrexone (Depotrex®) has been developed that provides a stable, long-lasting elevation in plasma naltrexone levels with either no or minimal side-effects (Heishman et al. 1994; Alim et al. 1995; Kranzler et al. 1998). In an early tolerability study, Alim and colleagues (1995) reported blockade of the physiological and subjective effects of 10 mg intravenous (IV) morphine in cocaine-dependent participants who received 206 mg depot naltrexone; side-effects associated with naltrexone were minimal in these participants. Kranzler and colleagues (1998) further showed that 206 mg depot naltrexone significantly reduced the percentage of heavy drinking days in alcoholics. Adverse effects reported after depot naltrexone were comparable to those reported after oral naltrexone administration. Although this formulation of depot naltrexone appears to be safe and effective in treating alcohol dependence, it has not yet been tested with heroin. The purpose of the current study was 1) to determine whether the new formulation of depot naltrexone will antagonize the effects of heroin at doses comparable to those used on the streets today, and 2) to assess the duration of antagonist effect of 192 mg and 384 mg depot naltrexone. The hypothesis was that depot naltrexone would dose-dependently antagonize the effects of heroin.
Materials and methods
Participants
Fifteen heroin-dependent men, who were not seeking treatment for their drug use, began the 8-week protocol. Three participants left the study prior to depot naltrexone administration: one was discharged for aggressive behavior toward the staff, and two left for personal reasons. Twelve participants (eight non-Hispanic Caucasian, three Hispanic, and one African American) completed the study: six received 192 mg depot naltrexone, and six received 384 mg depot naltrexone (Table 1). The low dose of depot naltrexone was tested in the first six participants. The groups did not differ in age, years of heroin use, and amount of money spent on heroin per day. All participants had experience using heroin IV. One participant in the low-dose group and two in the high-dose group preferred to use heroin intranasally; all other participants preferred to use heroin IV. All participants were dependent on heroin at the start of the study, as verified by a naloxone challenge test (Wang 1974).
Table 1.
Participant demographics. Numbers in parentheses represent+1 SEM
| 192 mg naltrexone | 384 mg naltrexone | |
|---|---|---|
| Age (average; years) | 33.8 (2.5) | 29.2 (3.2) |
| Years of heroin use (average) | 10.7 (2.5) | 9.1 (3.5) |
| Amount spent for heroin (average; $/day) | $39 (4) | $55 (12) |
| Tobacco cigarette use (range; no. per day) | 8–20 | 10–20 |
| Cocaine use (range; occasions/week) | 0–1 | 0–3 |
| Amphetamine use (range; occasions/week) | 0–1 | 0 |
| Marijuana use (range; occasions/week) | 0–1 | 0–3 |
| Alcohol use (range; occasions/week) | 0–1 | 0–3 |
| Sedative use (range; occasions/week) | 0–1 | 0–1 |
After an initial telephone interview, eligible participants completed detailed questionnaires on drug use, general health and medical history, and a medical and psychological evaluation in the laboratory. An electrocardiogram and Mantoux test or chest X-ray were also performed. Routine laboratory analyses included a blood chemistry panel, thyroid function test, syphilis and hepatitis (A, B, and C) screening, and urinalysis. Urine drug toxicologies (opioids, cocaine, benzodiazepines, cannabinoids, and amphetamines) were also performed using a radiative energy attenuation and fluorescence polarization immunoassay system (ADx System; Abbott Laboratories, Abbott Park, Ill., USA). Participants were told that they would be detoxified from heroin during the first week of the study, that they would receive one of two doses of a depot formulation of naltrexone, and that a range of IV heroin doses would be tested each week for the 6 weeks following depot naltrexone administration.
Participants were excluded from the study if they were seeking drug treatment, dependent on alcohol or illicit drugs other than opioids, or had a major Axis I psychiatric diagnosis other than opioid dependence. Those who had recent histories of violence or who were on parole/probation were excluded from the study. Participants were required to be physically healthy, and fully able to perform all study procedures. Although both men and women were screened for the study, none of the women met the eligibility requirements. Prior to admission, participants completed a training session, during which the study procedures were explained to them in detail. Volunteers were paid $25 per inpatient day and an additional $25 per day bonus if they completed the study. Participants signed consent forms describing the aims of the study, and the potential risks and benefits of participation. Free HIV testing and education were offered, and during the last week of the study, participants were offered referrals for treatment. This study was approved by the Institutional Review Board of the New York State Psychiatric Institute (NYSPI).
Apparatus
During experimental sessions, participants were seated in a room equipped with Macintosh computers. All computer activities, vital signs and behaviors were continuously monitored by the experimenters in an adjacent control room via a continuous on-line computer network, video cameras, and vital signs monitors (cardiovascular function was measured using a Sentry II Vital Signs Monitor, NBS Medical, Costa Mesa, Calif., USA; arterial oxygen saturation was measured using a pulse oximeter Model 400, Palco Laboratories, Santa Cruz, Calif., USA). Communication between the staff and participants was kept to a minimum during experimental sessions.
Detoxification procedures
Participants were admitted into the hospital, and detoxified during the first week after admission. Buprenorphine (8 mg sublingual tablet; National Institute on Drug Abuse, Rockville, Md., USA) was administered on the first 1–2 days after admission. Two days after the last buprenorphine dose, oral naltrexone (DuPont Pharma, Wilmington, Del., USA) was administered for 3 consecutive days (25, 50, and 50 mg per day) to ensure that participants were willing and able to tolerate its effects. Clonidine HCl (0.2 mg PO, every 6 h; Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, Conn., USA), ketorolac tromethamine (30 mg IM, every 6 h; Roche Laboratories, Nutley, N.J., USA), prochlorperazine (10 mg PO or IM, every 8 h; SmithKline Beecham Consumer Healthcare, Pittsburgh, Penn., USA) and clonazepam (2 mg PO, every 8 h; Roche Laboratories) were available, as needed, during the detoxification week. Thereafter, trazodone (50–100 mg PO, at bedtime; Warner Chilcott, Morris Plains, N.J., USA) was available if participants reported having trouble sleeping. Depot naltrexone was administered on a Monday morning, 2 days after the last oral naltrexone dose.
General procedures
The effects of IV heroin (placebo, 6.25, 12.5, 18.75, and 25 mg) were evaluated each week for 6 weeks following depot naltrexone administration. The entire dose range was tested each week, and one dose of heroin was tested each day on weekdays. For safety, active heroin doses were administered in ascending order within each week, with the exception that the day of placebo injection was varied across weeks. On the day that depot naltrexone was administered, placebo was tested during the experimental session.
Experimental sessions
During all sessions, participants completed computerized tasks and subjective-effects questionnaires. Heart rate and blood pressure were measured every 2 min, and blood oxygen saturation was monitored continuously with a pulse oximeter and recorded every minute during experimental sessions. Participants received breakfast between 0800 and 0900 and lunch between 1230 and 1330 hours. Experimental sessions occurred between 0930 and 1130 hours. Participants were not allowed to smoke tobacco cigarettes during experimental sessions.
Physiologic, subjective and performance effects were measured both before and after drug administration (see descriptions below). Heroin or placebo was administered only if vital signs were within safe limits (SpO2 >93%). A photograph was taken of the right pupil before and 4, 10, 20, 40 and 60 min after drug administration. The subjective-effects battery (see description below) was administered before and 4, 40 and 90 min after drug administration. The performance battery (see description below) was administered before and 10 and 60 min after drug administration. The Subjective Opioid Withdrawal Scale was administered before drug administration. The Drug Effects Questionnaire was administered 90 min after drug administration.
Subjective measures
Four questionnaires were used to assess subjective effects throughout the experimental sessions. The first questionnaire was a 26-item visual analog scale (VAS) designed to assess subjective and physiological effects (modified from Foltin and Fischman 1995). The first 18 lines were labeled with adjectives describing mood states (e.g., “I feel…:” “high”) and four additional lines, labeled with questions about the dose just received (i.e. “I liked the dose,” “For this dose, I would pay”). Participants also indicated, by making a mark along a 100 mm line, how much they “wanted” each of the following drugs: heroin, cocaine, alcohol, and tobacco. Participants rated each item on the VAS from “Not at all” (0 mm) to “Extremely” (100 mm), except for the “For this dose, I would pay” question, which ranged between $0 (0 mm) to $20 (100 mm). The second questionnaire was a 13-item opioid symptom checklist consisting of true/false questions designed to measure opioid effects (e.g. “My skin is itchy,” etc.; Fraser et al. 1961; Foltin and Fischman 1992). The VAS and opioid symptom checklist together constituted the subjective-effects battery. The third questionnaire was the 16-item Subjective Opioid Withdrawal Scale (SOWS; Handelsman et al. 1987). Participants rated each item on a scale from 0 to 4, with 0 being “Not at all” and 4 being “Extremely” (e.g. “I have gooseflesh,” etc.). The fourth questionnaire was a 6-item Drug Effects Questionnaire (DEQ; Evans et al. 1995). Participants described drug effects by selecting among a series of possible answers ranging from 0 (“No effects at all”) to 4 (“Very strong (good, bad, etc.) effects”). Ratings of drug liking ranged between –4 (“Dislike very much”) to 4 (“Like very much”).
Task battery
The task battery consisted of four tasks: the first task was a 3-min digit-symbol substitution task, during which participants were required to emulate a series of patterns on a keypad (McLeod et al. 1982). The second task was a 10-min divided attention task, which consisted of concurrent pursuit-tracking and vigilance tasks (Miller et al. 1988). The third task was a 10-min rapid information processing task, during which a series of digits was displayed rapidly on the computer screen (100 digits/min), and participants were instructed to press a key as quickly as possible after three consecutive odd or even digits (Wesnes and Warburton 1983). The fourth task was a 3-min repeated acquisition of response sequences task, during which four buttons were illuminated, and participants were instructed to learn a ten-response sequence of button presses (Kelly et al. 1993).
Physiological measures
A blood pressure cuff was attached to the non-dominant arm, which recorded automatically every 2 min. Participants were also connected to a pulse oximeter via a soft sensor on a finger of the dominant hand, which monitored arterial blood oxygen saturation (%SpO2). For safety, supplemental oxygen (2 l/min) was provided via a nasal cannula during all experimental sessions. A specially modified Polaroid camera with a close-up lens (×2 magnification) was used to take pupil photographs. All photographs were taken under ambient lighting conditions. Horizontal and vertical measurements of pupil diameter were made using calipers, and then these two measurements were averaged and divided by 2 to correct for the ×2 magnification.
Blood was drawn 2 h, 1, 2, 3, 4, 5, 6, 8, 11, 15, 18, 22, 25, 29, 32, 36, and 39 days after administration of depot naltrexone, and immediately centrifuged at 3000 rpm for 15 min. Plasma was drawn off and stored at –20°C until it was shipped by overnight mail on dry ice for analyses of naltrexone and 6-β-naltrexol (Center for Human Toxicology, University of Utah, Salt Lake City, Utah, USA). Analyses were performed by solid phase extraction and negative ion chemical ionization gas chromatography/mass spectrometry, as described by Huang and colleagues (1997). The lower limit of detectability for both analytes was 0.1 ng/ml.
Blood was also drawn prior to, and at weekly intervals after administration of depot naltrexone for analyses of liver enzymes (AST, ALT, GGT).
Drugs
Depot naltrexone (Depotrex®) was manufactured by Biotek Inc. (Woburn, Mass., USA) and provided by the National Institute on Drug Abuse. Depotrex is a registered trademark of Biotek, Inc. Naltrexone microcapsules and placebo microspheres were packaged in sterile single-dose vials. After reconstituting in suspending medium, 2.4 ml of the suspension was injected. The active formulation contained drug equivalent to 192 mg naltrexone base. The placebo formulation contained the equivalent weight in polymer microspheres. Injections were administered subcutaneously into the buttocks (one injection per buttock), using an 18 gauge needle. For the low dose, participants received one placebo and one naltrexone injection (192 mg naltrexone base), and for the high dose, participants received two naltrexone injections (394 mg naltrexone base). For safety, the low dose of Depotrex was tested in the first six participants, and the high dose of Depotrex was tested in the next six participants.
Heroin HCl was provided by the National Institutes on Drug Abuse and prepared by the Columbia-Presbyterian Medical Center research pharmacy. A 25 mg/ml heroin concentration was prepared in a 5% dextrose solution to enhance stability. Dose calculations were based on the hydrochloride salt form. Heroin was stored in a freezer and used within 3 months of preparation. The stock solution was diluted in 5% dextrose to produce each dose. Placebo (5% dextrose solution) or heroin (6.25, 12.5, 18.75, and 25 mg) was administered intravenously over a 30-s period in a total volume of 2 ml. Heroin doses were administered in a double-blind fashion. Physiological saline solution was infused continuously during experimental sessions, except during drug administration. Between 1 and 2 ml heparinized saline (10 IU/ml) was flushed into the catheter four to eight times each day. All venous catheters were maintained as heplocks and were removed within 72 h of insertion.
Supplemental medications available to all participants for the duration of the study included: Mylanta, acetaminophen, ibuprofen, Colace, Milk of Magnesia and multi-vitamins with iron.
Morning urine samples were collected daily and one random sample per week was screened for the presence of other illicit substances. No illicit substances were found in the participants’ urine samples.
Statistical analyses
Repeated-measures analyses of variance (ANOVA) with planned comparisons were used to address the following questions: 1) What was the duration of antagonism of heroin’s effects? 2) Did the low and high doses of depot naltrexone differ in ability to antagonize the effects of heroin? In order to address the first question, the data for each group were analyzed separately as a function of week (1–6) and heroin dose (0, 6.25, 12.5, 18.75, 25 mg). Twenty-five planned comparisons were made: each week (2–6) was compared to week 1 for each dose (e.g. placebo-week 2 versus placebo-week 1, placebo-week 3 versus placebo-week 1, placebo-week 4 versus placebo-week 1, etc.) because it was likely that virtually complete antagonism would occur during week 1. In order to address the second question, an overall analysis was performed with one between-group factor (group) and two within-group factors (week, heroin dose): the main effect of group, and the week×group and dose×group effects were evaluated. Interaction effects were examined using post-hoc comparisons. Peak subjective ratings, peak performance effects, trough pupil diameter, liver enzyme levels, average arterial oxygen saturation, and plasma levels of naltrexone and 6-β-naltrexol were analyzed. Liver enzymes (AST, ALT, GGT) were also analyzed: each week post-depot naltrexone was compared to a pre-depot naltrexone baseline. Due to an excessive number of missing data points, the cardiovascular data were not analyzed. To control for type I errors, a modified Bonferroni test was used in that only those comparisons with P<0.01 were considered statistically significant.
Results
Plasma drug levels
Figure 1 shows mean plasma levels of naltrexone (left panel) and 6-β-naltrexol (right panel) for each group as a function of time since the depot naltrexone injection. Two hours after administration of 192 mg and 384 mg depot naltrexone, plasma levels of naltrexone were 3.8 (±0.2) and 8.9 (±1.4) ng/ml. Plasma levels of 6-β-naltrexol were 8.5 (±0.3) and 17.4 (±1.3) ng/ml, respectively, 24 h after administration of 192 mg and 384 mg depot naltrexone. Across individual participants, plasma levels of naltrexone ranged between 3.1 and 4.5 ng/ml after administration of 192 mg depot naltrexone, and 5.6 and 14.2 ng/ml after administration of 384 mg depot naltrexone. After administration of 192 mg and 384 mg of depot naltrexone, plasma levels of naltrexone were less than 1 ng/ml on day 22 and 29, respectively. The group and group×day effects for naltrexone [group: F(1,10)= 48.5, P<0.0001; group×day: F(1,10)=8.6, P<0.0001] and 6-β-naltrexol [group: F(1,10)=33.8, P<0.0002; group× day: F(1,10)=8.3, P<0.0001] were significant.
Fig. 1.
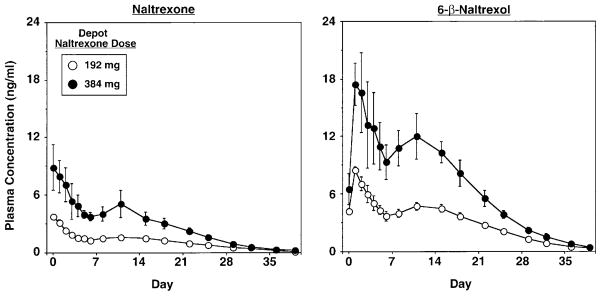
Mean plasma levels of naltrexone (left panel) and 6-β-naltrexol (right panel) as a function of depot naltrexone dose and days after administration of depot naltrexone. Data points represent the mean across 6 participants per group. Error bars represent±1 SEM
Subjective effects
Figure 2 shows mean peak visual analog scale ratings of “Good Drug Effect” for each group as a function of heroin dose and week. After low-dose depot naltrexone, ratings of “Good Drug Effect” significantly increased by week 4, relative to week 1, after administration of 18.75 mg [F(1,100)=6.4, P<0.01] and 25 mg heroin [F(1,100)=7.9, P<0.006]; ratings of “Good Drug Effect” significantly increased by week 5 after administration of 12.5 mg heroin [F(1,100)=8.4, P<0.004]. In the high-dose group, ratings of “Good Drug Effect” did not significantly increase until week 6, after 18.75 mg [F(1,100)=7.5, P<0.007] and 25 mg heroin [F(1,100)= 47.3, P<0.0001]. Both the week×group [F(5,50)=4.8, P<0.001] and dose×group [F(4,40)=4.4, P<0.005] effects were significant for ratings of “Good Drug Effect.” Several other VAS ratings showed a similar pattern including ratings of “High,” “Liking,” drug “Potency,” drug “Quality,” and how much they would be willing to pay for the dose (data not shown). The dose×group effect was significant [F(4,40)=4.2, P<0.006], and the week×group effect approached statistical significance [F(5,50)=2.9, P<0.02] for ratings of “High.” Although ratings tended to be higher in the low-dose group for VAS ratings of “Liking,” drug “Potency,” and drug “Quality,” the week×group and dose×group effects were not statistically significant for these items.
Fig. 2.
Mean peak VAS ratings of “Good Drug Effect” after administration of heroin (0–25 mg) as a function of depot naltrexone dose and study week (week 1: left panel; week 6: right panel). Maximum rating=100 mm. Data points represent mean peak ratings (n=6 per group). Error bars represent±1 SEM. * Indicates significant differences from week 1
VAS ratings of “I feel…” “Gooseflesh,” “Depressed,” “Muscle Pain,” “Anxious,” and “Restless” were elevated in both groups during the first week after receiving depot naltrexone, and were higher in the high-dose group (data not shown). The week×group effect was statistically significant for ratings of “Gooseflesh” [F(5,50)=3.4, P<0.01] and “Depressed” [F(5,50)=3.5, P<0.009], while the week×group effect for ratings of “Muscle Pain” (P<0.03), “Anxious” (P<0.04), and “Restless” (P<0.04) approached statistical significance. Ratings of “I Want Heroin,” which did not vary across study weeks or heroin doses, were significantly elevated in the high-dose group [main effect of group: F(1,10)=26.3, P<0.0004]. Ratings of “I Want Heroin” ranged between 26 and 37 in the low-dose group, and 86 and 95 in the high-dose group.
The pattern of results obtained from the opioid symptom checklist and DEQ (data not shown) were similar to the VAS ratings of “Good Drug Effect” (Fig. 2) in that total scores on the opioid symptom checklist and DEQ ratings of drug “Liking,” “Good Drug Effect,” strength of drug effect, and desire to take the drug again increased as a function of heroin dose and across study weeks. The week×group effect was statistically significant for the opioid symptom checklist [F(5,50)=3.2, P<0.01]. Although ratings tended to be higher in the low-dose group compared to the high-dose group, the week×group and dose×group effects were not statistically significant for any of the items on the DEQ.
Subjective ratings of opioid withdrawal, as measured by total scores on the SOWS, did not significantly differ between groups during detoxification, prior to administration of depot naltrexone. SOWS scores peaked on day 4 after admission: total SOWS scores on day 4 of withdrawal were 22.5 (±11.2) and 26.3 (±7.3), out of a maximum possible score of 64, after administration of 192 and 384 mg depot naltrexone, respectively. On the day prior to administration of depot naltrexone, SOWS scores were 11.0 (±8.2) and 18.7 (±9.0) in the low- and high-dose groups, respectively. Figure 3 shows total SOWS scores after administration of depot naltrexone for each group as a function of heroin dose and week. Total SOWS scores were significantly elevated during the first week in the high-dose group, but not in the low-dose group. By the second week after administration of depot naltrexone, SOWS scores did not differ between groups. The week× group interaction for total SOWS scores approached statistical significance [F(5,50)=2.5, P<0.04].
Fig. 3.
Mean total scores on the Subjective Opioid Withdrawal Scale (SOWS) after administration of heroin as a function of depot naltrexone dose and study week. Maximum score=64. All other details are as in Fig. 2
Performance tasks
Heroin minimally affected task performance, with the exception that performance of the divided attention task was significantly impaired: in the low-dose group, the latency to identify a target significantly increased by 1.7 s during week 5, relative to week 1, after administration of 25 mg heroin [F(1,100)=8.3, P<0.005]. In the high-dose group, the latency to identify a target significantly increased by 1.3 s during week 6, relative to week 1, after administration of 25 mg heroin [F(1,100)=30.9, P<0.0001]. Latency to identify a target did not significantly change across weeks after administration of placebo in either group. The week×group and dose×group effects were not statistically significant for latency to identify a target during the divided attention task.
Physiological effects
Figure 4 shows the effects of heroin on pupil diameter for each group as a function of heroin dose and week. During week 1, pupil diameter was large in both groups, consistent with the possibility that both groups were in mild withdrawal. After placebo administration during week 1, pupil diameter was 5.3 (±0.3) and 5.6 (±0.6) mm in the low- and high-dose groups, respectively. Pupil diameter was relatively stable under the placebo condition in both groups from weeks 2–6 (Fig. 4), although pupil diameter consistently remained larger in the high-dose group throughout the study. After administration of active doses of heroin, pupil diameter progressively decreased across study weeks in both groups. The week× group and dose×group effects were not significant for pupil diameter.
Fig. 4.
Mean trough pupil diameter after administration of heroin as a function of depot naltrexone dose and study week. All other details are as in Fig. 2
The average arterial oxygen saturation significantly decreased by 0.9% in both groups from week 1 to week 6, after administration of 25 mg heroin [low-dose group: F(1,100)=17.2, P<0.0001; high-dose group: F(1,100)=7.8, P<0.006]. However, these changes in oxygen saturation occurred in the presence of supplemental oxygen, and were not clinically significant.
Liver enzyme (ALT, AST, GGT) values at baseline were within the normal range for all participants, with the exception of one individual in the low-dose group whose GGT value (128 IU/l) slightly exceeded the normal range of 5–80 IU/l. Three individuals in the low-dose group tested positive for hepatitis, and four individuals in the high-dose group tested positive for hepatitis. In the low-dose group, average GGT levels significantly increased during weeks 3, 4, and 5, relative to baseline [Tables 2; week 3: F(1,30)=7.5, P<0.01; week 4: F(1,30)=18.6, P<0.0002; week 5: F(1,30)=9.6, P<0.004]. Both ALT [F(1,30)=7.5, P<0.01] and AST [F(1,30)=8.5, P<0.007] values in the low-dose group significantly increased during week 4, relative to baseline. Although these liver enzyme values following administration of the low dose were statistically significant, it is important to note that they were not clinically significant. The increases in liver enzyme values in the low-dose group were predominantly due to one hepatitis-negative individual: his baseline GGT value was 54 IU/l, which peaked at 226 IU/L during week 4, and returned to 54 IU/l by week 6. Liver enzyme levels did not significantly change across study weeks in the high-dose group. The non-significant, transient increase in ALT values during week 1 in the high-dose group was due to one individual who received several doses of ketorolac tromethamine during detoxification. His liver enzyme levels peaked at 630 IU/l during week 1, and returned to normal (32 IU/l) by week 3.
Table 2.
ALT, AST, and GGT values (IU/l) at baseline and for 6 weeks following administration of 192 mg or 384 mg depot naltrexone. Numbers in parentheses represent+1 SEM. Asterisks represent significant differences from baseline (P<0.01)
| Baseline | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | |
|---|---|---|---|---|---|---|---|
| 192 mg | |||||||
| ALT | 29.2 (7.2) | 54.7 (22.2) | 63.3 (26.5) | 65.3 (25.8) | 75.2 (31.0)* | 60.7 (21.4) | 34.3 (6.3) |
| AST | 19.7 (2.3) | 27.8 (6.9) | 31.2 (7.4) | 26.8 (6.2) | 34.7 (8.9)* | 31.5 (7.8) | 22.5 (3.1) |
| GGT | 42.3 (17.5) | 39.7 (14.6) | 46.8 (14.6) | 55.0 (18.4)* | 62.3 (20.7)* | 56.7 (18.0)* | 45.7 (16.1) |
| 384 mg | |||||||
| ALT | 24.3 (7.2) | 132.3 (99.8) | 51.8 (17.4) | 45.5 (16.0) | 50.0 (20.0) | 53.3 (24.2) | 49.0 (21.6) |
| AST | 21.5 (3.7) | 31.8 (5.9) | 22.8 (4.8) | 24.7 (5.5) | 29.5 (6.5) | 36.2 (11.8) | 31.0 (6.8) |
| GGT | 28.7 (9.7) | 55.0 (24.0) | 47.5 (16.4) | 40.0 (11.7) | 40.2 (12.1) | 39.0 (11.3) | 36.0 (11.3) |
Side-effects
Eleven of 12 participants reported pain during administration of the depot injections. These individuals reported no discomfort while standing, and only mild discomfort at the injection sites while seated, during the first 48–72 h after the injections. There was no evidence of induration, erythema, or irritation. One participant who inadvertently received one of the injections (placebo) intramuscularly reported pain on that side for the first 72–96 h after the injection.
Discussion
The depot formulation of naltrexone used in the current study provided a safe, effective, long-lasting antagonism of the effects of heroin. Across the time points measured, the highest naltrexone plasma levels attained after administration of 192 mg and 384 mg of depot naltrexone were 3.8 (±0.2) and 8.9 (±1.4) ng/ml, respectively. For comparison, Walsh et al. (1996) reported that daily administration of 50 mg oral naltrexone resulted in naltrexone plasma concentrations of approximately 30 ng/ml, while daily administration of 12.5 mg oral naltrexone resulted in naltrexone plasma concentrations of approximately 10 ng/ml (plasma samples were collected 30 min after administration of naltrexone). Therefore, the amount of drug found in plasma after depot naltrexone administration is lower than the amount found after a standard dose of naltrexone used clinically for treating heroin dependence (50 mg/day).
In the present study, antagonism of heroin’s effects occurred, despite negligible plasma levels of naltrexone. An early study conducted by Vereby and colleagues (Vereby et al. 1976) showed that 100 mg oral naltrexone did not completely antagonize the effects of 25 mg IV heroin when plasma levels of naltrexone fell below approximately 2 ng/ml. In contrast, Navaratnam and colleagues (Navaratnam et al. 1994) showed that after discontinuation of day 1-day 3-day 5 dosing with 100–100–150 mg oral naltrexone, antagonism of the effects of 25 mg IV heroin continued to occur when plasma levels of naltrexone were negligible. Schuh and colleagues (Schuh et al. 1999) also demonstrated that during a “wash-out period” after discontinuation of daily administration of 100 mg oral naltrexone, antagonism of the effects of up to 4 mg IM hydromorphone continued to occur when plasma levels of naltrexone were negligible. The data presented in the current study were more consistent with those of Navaratnam et al. (1994) and Schuh et al. (1999) in that 384 mg depot naltrexone antagonized heroin-induced ratings of “I Feel High” and “I Feel a Good Drug Effect” for up to 5 weeks, even though plasma levels of naltrexone were quite low (0.3±0.04 ng/ml). These data are not entirely surprising, given a previous study demonstrating that the percentage blockade of [11C]carfentanil binding in the brain of normal healthy volunteers at 48, 72, 120, and 168 h after administration of 50 mg oral naltrexone was 91, 80, 46, and 30%, respectively (Lee et al. 1988). Results from the Lee et al. (1988) study suggest that orally delivered naltrexone continues to inhibit brain opioid receptors in the absence of measurable plasma levels of naltrexone. In the present experiment, it is also possible that 6-β-naltrexol, the active metabolite of naltrexone, contributed to the continued antagonism of heroin’s effects, particularly because plasma levels of this metabolite were consistently higher than naltrexone throughout the study.
In the present study, both doses of depot naltrexone initially antagonized the positive subjective effects of heroin, consistent with naltrexone’s well-established effectiveness in blocking the subjective effects of opioid agonists (e.g. Altman et al. 1976; Mello et al. 1980; Preston and Bigelow 1993; Schuh et al. 1999). Although the present study clearly demonstrated a long-lasting antagonism of the subjective effects of up to 25 mg IV heroin, it did not address the question of what dose of heroin would effectively override the blockade during the first few weeks after administration of depot naltrexone. In previous studies in our laboratory, participants who were maintained on morphine reported that 25 mg heroin was roughly equivalent to one $10 “street bag” of heroin. Given that participants typically use between one and 2 bags of heroin per occasion, the effects of higher doses of heroin during the early weeks of the study would have provided useful information. However, because the present experiment was a late phase 1 study, and we did not have information regarding the duration of antagonism of opioid effects, we chose not to test higher heroin doses because of safety concerns. In addition, because of uncertainty about the effects of 25 mg heroin in non-dependent individuals, we chose to administer active heroin doses in ascending order in the present study. Therefore, order effects could have influenced responses on our outcome measures. Future studies will evaluate higher doses of heroin in the early weeks following administration of depot naltrexone, and heroin doses will be administered in non-systematic order.
One interesting finding in the present study was a dramatic difference in ratings of “I Want Heroin” in the low-dose group, relative to the high-dose group. Participants in the low-dose group had consistently lower scores for this item across study weeks and heroin doses. The differences in wanting heroin were perhaps related to the possibility that participants in the high-dose group were experiencing greater withdrawal. Consistent with this notion is the fact that throughout the study, pupil diameter was larger in the high-dose group, compared to the low-dose group. However, one argument against this possibility is that subjective ratings of withdrawal were negligible, and did not differ between groups from study weeks 2–6. In clinical settings, it is generally accepted that craving for heroin is reduced during the early weeks after treatment is initiated, perhaps due to a perception that heroin is unavailable under antagonist maintenance (e.g. Mirin et al. 1976; Hollister et al. 1977; Sideroff et al. 1978; Judson et al. 1981). However, it is interesting to note that in one clinical study comparing 60 and 120 mg thrice-weekly administration of oral naltrexone, craving for heroin increased in both groups during weeks in which heroin was used, but the increase in craving was significantly greater in the 120 mg group (Judson et al. 1981). As in the present study, Judson et al. (1981) had no clear explanation for why craving was greater in the group maintained on the higher dose of naltrexone. Future studies are needed to evaluate more systematically the effects of depot naltrexone on craving for heroin. A possible theory may relate to the effects of the higher dose of naltrexone on endogenous ligands, such as endorphin.
Another issue that warrants attention is the effect of depot naltrexone on liver enzymes. The package insert for oral naltrexone warns of an increased risk of hepatocellular injury. Given the high rate of hepatitis in intravenous drug abusers, combined with the possibility that naltrexone itself may produce hepatocellular injury, we carefully monitored liver enzymes throughout the study, and only admitted participants whose liver enzymes were within the laboratory normal range at baseline. Under these conditions, depot naltrexone did not produce clinically significant increases in liver enzyme levels. These results were consistent with several studies demonstrating a lack of effect of naltrexone on liver functioning, even after daily administration of high doses of naltrexone (100–350 mg; Brahen et al. 1988; Sax et al. 1994; Marrazzi et al. 1997). In fact, liver enzyme levels actually decreased in alcoholics who were treated with 50 mg per day of oral naltrexone, presumably due to the fact that they were drinking less alcohol (Volpicelli et al. 1997). Similarly, a previous study of depot naltrexone in the treatment of alcoholics showed that liver enzyme values decreased (Kranzler et al. 1998). Therefore, it appears that the formulation of naltrexone used in the present study has minimal effects on liver functioning, which is not entirely unexpected, given the fact that plasma naltrexone levels in the present study were equivalent to a relatively low dose of oral naltrexone.
There were few adverse events, other than an initial discomfort associated with the actual injections of depot naltrexone and mild soreness at the injection site for 2–3 days following the injections. Kranzler et al. (1998), who tested a single injection of the same formulation of depot naltrexone used in the present study, also noted that participants reported pain upon administration of depot naltrexone. In addition, these investigators reported that in 13 of 15 participants, an area of induration occurred at the injection site, which resolved over a period of 2.8 weeks (Kranzler et al. 1988). Induration was not found in the present study. Other negative side-effects typically reported after naltrexone administration are predominantly related to mood or gastrointestinal complaints (e.g. Hollister et al. 1981; Greenstein et al. 1984; Crowley et al. 1985; Shufman et al. 1994; Oncken et al. 2001), but these effects were not observed in the present study.
In addition to the discomfort associated with the injection itself, it is possible that depot naltrexone itself exacerbated withdrawal discomfort. The elevated SOWS scores in the high-dose group shown in Fig. 3 seem to provide support for this notion. However, this possibility is unlikely for a number of reasons. First, on the day prior to administration of 384 mg depot naltrexone, mean SOWS scores were 18.7 (±9.0), and on the day that depot naltrexone was administered, mean scores were only slightly higher at 20.8 (±4.9). Therefore, depot naltrexone did not appear to exacerbate withdrawal in the high-dose group. SOWS scores in the low-dose group were also not different immediately before and after administration of depot naltrexone. Second, up to 50 mg oral naltrexone was administered for several consecutive days prior to administration of depot naltrexone. Given that 384 mg depot naltrexone produced blood levels of naltrexone comparable to approximately 12.5 mg oral naltrexone, it is unlikely that depot naltrexone would have exacerbated withdrawal. And finally, during a debriefing session at the end of the study, we specifically asked each participant whether he felt withdrawal symptoms after receiving the injections of depot naltrexone, and none claimed to experience any symptoms. Therefore, it is likely that the elevated SOWS scores in the high-dose group during the first week after administration of depot naltrexone reflects lingering baseline differences in withdrawal, rather than any direct effects of depot naltrexone. In a treatment setting where oral naltrexone may not be given prior to depot naltrexone administration, exacerbation of withdrawal could be avoided by lengthening the duration of the detoxification, and/or making ancillary medications, such as clonidine and benzodiazepines, available following administration of depot naltrexone.
In sum, the data presented in the current study demonstrate that this formulation of naltrexone produced a long-lasting antagonism of the effects of intravenous heroin, with minimal side-effects. Given that the primary difficulty associated with naltrexone maintenance in opioid abusers is medication compliance (Kosten and Kleber 1984; Fram et al. 1989; Preston et al. 1999), a formulation of naltrexone that requires only once-a-month administration has important and exciting treatment implications. Future studies in our laboratory will evaluate the clinical utility of depot naltrexone in the treatment of heroin dependence.
Acknowledgments
The authors would like to thank Michael R. Donovan, RN, Ronnie M. Shapiro, RN, Evaristo Akerele, MD, Adam Bisaga, MD, David Pierce, MD, Eric Rubin, MD, PhD and Maria Sullivan, MD, PhD for medical assistance, and Laura Andima, BS, Kevin Walsh, BS, and Christy Aberg, BA for technical assistance with this study. This research was supported by grant DA09236.
Contributor Information
Sandra D. Comer, Email: sdc10@columbia.edu, Division on Substance Abuse, New York State Psychiatric Institute and Department of Psychiatry, College of Physicians and Surgeons of Columbia University, 1051 Riverside Drive, Unit 120, New York, NY 10032, USA, Tel.: +1-212-5435981, Fax: +1-212-5435991
Eric D. Collins, Division on Substance Abuse, New York State Psychiatric Institute and Department of Psychiatry, College of Physicians and Surgeons of Columbia University, 1051 Riverside Drive, Unit 120, New York, NY 10032, USA
Herbert D. Kleber, Division on Substance Abuse, New York State Psychiatric Institute and Department of Psychiatry, College of Physicians and Surgeons of Columbia University, 1051 Riverside Drive, Unit 120, New York, NY 10032, USA
Elie S. Nuwayser, Biotek Inc., 21-C Olympia Avenue, Woburn, MA 01801, USA
James H. Kerrigan, Biotek Inc., 21-C Olympia Avenue, Woburn, MA 01801, USA
Marian W. Fischman, Division on Substance Abuse, New York State Psychiatric Institute and Department of Psychiatry, College of Physicians and Surgeons of Columbia University, 1051 Riverside Drive, Unit 120, New York, NY 10032, USA
References
- Abrahams RA, Ronel SH. Biocompatible implants for the sustained zero-order release of narcotic antagonists. J Biomed Mater Res. 1975;9:355–366. doi: 10.1002/jbm.820090310. [DOI] [PubMed] [Google Scholar]
- Alim TN, Tai B, Chiang CN, Green T, Rosse RB, Lindquist T, Deutsch SI. Tolerability study of a depot form of naltrexone substance abusers. In: Harris LS, editor. Problems of drug dependence 1994. Vol. 2. US Government Printing Office; Washington, D.C: 1995. p. 253. NIDA Research Monograph No. 153 (NIH Publ. No. 95-3883) [Google Scholar]
- Altman JL, Meyer RE, Mirin SM, McNamee HB. Opiate antagonists and the modification of heroin self-administration behavior in man: an experimental study. Int J Addict. 1976;11:485–499. doi: 10.3109/10826087609056165. [DOI] [PubMed] [Google Scholar]
- Brahen LS, Capone TJ, Capone DM. Naltrexone: Lack of effect on hepatic enzymes. J Clin Pharmacol. 1988;28:64–70. doi: 10.1002/j.1552-4604.1988.tb03102.x. [DOI] [PubMed] [Google Scholar]
- Chiang CN, Hollister LE, Gillespie HK, Foltz RL. Clinical evaluation of a naltrexone sustained-release preparation. Drug Alcohol Depend. 1985a;16:1–8. doi: 10.1016/0376-8716(85)90076-6. [DOI] [PubMed] [Google Scholar]
- Chiang CN, Kishimoto A, Barnett G, Hollister LE. Implantable narcotic antagonists: a possible new treatment for narcotic addiction. Psychopharmacol Bull. 1985b;21:672–675. [PubMed] [Google Scholar]
- Crowley TJ, Wagner JE, Zerbe G, Macdonald M. Naltrexone-induced dysphoria in former opioid addicts. Am J Psychiatry. 1985;142:1081–1084. doi: 10.1176/ajp.142.9.1081. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW, Levin FR, Fischman MW. Behavioral and subjective effects of DN-2327 (pazinaclone) and alprazolam in normal volunteers. Behav Pharmacol. 1995;6:176–186. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. The cardiovascular and subjective effects of intravenous cocaine and morphine combinations in humans. J Pharmacol Exp Ther. 1992;261:623–632. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Interaction of buprenorphine with cocaine-morphine combinations. Exp Clin Psychopharmacol. 1995;3:261–269. [Google Scholar]
- Fram DH, Marmo J, Holden R. Naltrexone treatment – the problem of patient acceptance. J Subst Abuse Treat. 1989;6:119–122. doi: 10.1016/0740-5472(89)90039-1. [DOI] [PubMed] [Google Scholar]
- Fraser HF, Van Horn GD, Martin WR, Wolbach AB, Isbell H. Methods for evaluating addiction liability. (A) “Attitude” of opiate addicts toward opiate-like drugs, (B) A short-term “direct” addiction test. J Pharmacol Exp Ther. 1961;133:371–387. [PubMed] [Google Scholar]
- Greenstein RA, Arndt IC, McLellan AT, O’Brien CP, Evans B. Naltrexone: a clinical perspective. J Clin Psychiatry. 1984;45:25–28. [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Francis-Wood A, Keenan RM, Chiang CN, Terrill JB, Tai B, Henningfield JE. Safety and pharmacokinetics of a new formulation of naltrexone. In: Harris LS, editor. Problems of drug dependence 1993. Vol. 2. US Government Printing Office; Washington, D.C: 1994. p. 82. NIDA Research Monograph No. 141 (NIH Publ. No. 94-3749) [Google Scholar]
- Hollister LE, Schwin RL, Kapser P. Naltrexone treatment of opiate-dependent persons. Drug Alcohol Depend. 1977;2:203–209. doi: 10.1016/0376-8716(77)90027-8. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Johnson K, Boukhabza D, Gillespie HK. Aversive effects of naltrexone in subjects not dependent on opiates. Drug Alcohol Depend. 1981;8:37–41. doi: 10.1016/0376-8716(81)90084-3. [DOI] [PubMed] [Google Scholar]
- Huang W, Moody DE, Foltz RL, Walsh SL. Determination of naltrexone and 6-β-naltrexol in plasma by solid-phase extraction and gas chromatography-negative ion chemical ionization-mass spectrometry. J Anal Toxicol. 1997;21:252–257. doi: 10.1093/jat/21.4.252. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Martin WR. Opioid analgesics and antagonists. In: Goodman Gilman A, Rall TW, Nies AS, Taylor P, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. Pergamon Press; New York: 1990. pp. 485–521. [Google Scholar]
- Judson BA, Carney TM, Goldstein A. Naltrexone treatment of heroin addiction: efficacy and safety in a double-blind dosage comparison. Drug Alcohol Depend. 1981;7:325–346. doi: 10.1016/0376-8716(81)90049-1. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Performance-based testing for drugs of abuse: dose and time profiles of marijuana, amphetamine, alcohol, and diazepam. J Anal Toxicol. 1993;17:264–272. doi: 10.1093/jat/17.5.264. [DOI] [PubMed] [Google Scholar]
- Kleber HD, Kosten TR, Gaspari J, Topazian M. Nontolerance to the opioid antagonism of naltrexone. Biol Psychiatry. 1985;20:66–72. doi: 10.1016/0006-3223(85)90136-2. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kleber HD. Strategies to improve compliance with narcotic antagonists. Am J Drug Alcohol Abuse. 1984;10:249–266. doi: 10.3109/00952998409002784. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Modesto-Lowe V, Nuwayser ES. Sustained-release naltrexone for alcoholism treatment: a preliminary study. Alcohol Clin Exp Res. 1998;22:1074–1079. [PubMed] [Google Scholar]
- Lee MD, Wagner HN, Tanada S, Frost JJ, Bice AN, Dannals RF. Duration of occupancy of opiate receptors by naltrexone. J Nucl Med. 1988;29:1207–1211. [PubMed] [Google Scholar]
- Marrazzi MA, Wroblewski JM, Kinzie J, Luby ED. High-dose naltrexone and liver function safety. Am J Addict. 1997;6:21–29. doi: 10.3109/10550499708993159. [DOI] [PubMed] [Google Scholar]
- Martin W, Sandquist VL. A sustained release depot for narcotic antagonists. Arch Gen Psychiatry. 1974;30:31–33. doi: 10.1001/archpsyc.1974.01760070019003. [DOI] [PubMed] [Google Scholar]
- Martin WR, Gorodetzsky CW, McClane TK. An experimental study in the treatment of narcotic addicts with cyclazocine. Clin Pharmacol Ther. 1966;7:455–465. doi: 10.1002/cpt196674455. [DOI] [PubMed] [Google Scholar]
- Martin WR, Jasinski DR, Mansky PA. Naltrexone, an antagonist for the treatment of heroin dependence. Arch Gen Psychiatry. 1973;28:784–791. doi: 10.1001/archpsyc.1973.01750360022003. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the Digit Symbol Substitution Test (DSST) Behav Res Meth Instr. 1982;14:463–466. [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC, Sellers MS. Operant analysis of human heroin self-administration and the effects of naltrexone. J Pharmacol Exp Ther. 1981;216:45–54. [PubMed] [Google Scholar]
- Mirin SM, Meyer RE, McNamee HB. Psychopathology, craving, and mood during heroin acquisition: an experimental study. Int J Addict. 1976;11:525–544. doi: 10.3109/10826087609056168. [DOI] [PubMed] [Google Scholar]
- Miller TP, Taylor JL, Tinklenberg JR. A comparison of assessment techniques measuring the effects of methylphenidate, secobarbital, diazepam, and diphenhydramine in abstinent alcoholics. Neuropsychobiology. 1988;19:90–96. doi: 10.1159/000118441. [DOI] [PubMed] [Google Scholar]
- Navaratnam V, Jamaludin A, Raman N, Mohamed M, Mansor SM. Determination of naltrexone dosage for narcotic agonist blockade in detoxified Asian addicts. Drug Alcohol Depend. 1994;34:231–236. doi: 10.1016/0376-8716(94)90161-9. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Greenstein RA, Mintz J, Woody GE. Clinical experience with naltrexone. Am J Drug Alcohol Abuse. 1975;2:365–377. doi: 10.3109/00952997509005662. [DOI] [PubMed] [Google Scholar]
- Oncken C, Kirk JV, Kranzler HR. Adverse effects of oral naltrexone: analysis of data from two clinical trials. Psychopharmacology. 2001;154:397–402. doi: 10.1007/s002130000666. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Differential antagonism of hydromorphone and pentazocine effects in human volunteers. J Pharmacol Exp Ther. 1993;264:813–823. [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht An, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend. 1999;54:127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Sax DS, Kornetsky C, Kim A. Lack of hepatotoxicity with naltrexone treatment. J Clin Pharmacol. 1994;34:898–901. doi: 10.1002/j.1552-4604.1994.tb04002.x. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Walsh SL, Stitzer ML. Onset, magnitude and duration of opioid blockade produced by buprenorphine and naltrexone in humans. Psychopharmacology. 1999;145:162–174. doi: 10.1007/s002130051045. [DOI] [PubMed] [Google Scholar]
- Shufman EN, Porat S, Witztum E, Gandacu D, Bar-Hamburger R, Ginath Y. The efficacy of naltrexone in preventing re-abuse of heroin after detoxification. Biol Psychiatry. 1994;35:935–945. doi: 10.1016/0006-3223(94)91240-8. [DOI] [PubMed] [Google Scholar]
- Sideroff SI, Charuvastra VS, Jarvik ME. Craving in heroin addicts maintained on the opiate antagonist naltrexone. Am J Drug Alcohol Abuse. 1978;5:415–423. doi: 10.3109/00952997809007017. [DOI] [PubMed] [Google Scholar]
- Vereby K, Volavka J, Mule SJ, Resnick RB. Naltrexone: disposition, metabolism, and effects after acute and chronic dosing. Clin Pharmacol Ther. 1976;20:315–329. doi: 10.1002/cpt1976203315. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence. Arch Gen Psychiatry. 1997;54:737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. J Pharmacol Exp Ther. 1996;279:524–538. [PubMed] [Google Scholar]
- Wang RIH, Wiesen RL, Lamid S, Roh BL. Rating the presence and severity of opiate dependence. Clin Pharmacol Ther. 1974;16:653–658. doi: 10.1002/cpt1974164653. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9:223–229. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]
- Wikler A. Conditioning factors in opiate addiction and relapse. In: Wilner DI, Kassenbaum GG, editors. Narcotics. McGraw-Hill; New York: 1965. pp. 85–100. [Google Scholar]





