SUMMARY
Autism spectrum disorder (ASD) may arise from increased ratio of excitatory to inhibitory neurotransmission in the brain. Many pharmacological treatments have been tested in ASD, but only limited success has been achieved. Here we report that BTBR T+ Itpr3tf/J (BTBR) mice, a model of idiopathic autism, have reduced spontaneous GABAergic neurotransmission. Treatment with low non-sedating/non-anxiolytic doses of benzodiazepines, which increase inhibitory neurotransmission through positive allosteric modulation of postsynaptic GABAA receptors, improved deficits in social interaction, repetitive behavior, and spatial learning. Moreover, negative allosteric modulation of GABAA receptors impaired social behavior in C57BL/6J and 129SvJ wild-type mice, suggesting reduced inhibitory neurotransmission may contribute to social and cognitive deficits. The dramatic behavioral improvement after low-dose benzodiazepine treatment was subunit-specific—the α2,3-subunit-selective positive allosteric modulator L-838,417 was effective, but the α1-subunit-selective drug zolpidem exacerbated social deficits. Impaired GABAergic neurotransmission may contribute to ASD, and α2,3-subunit-selective positive GABAA receptor modulation may be an effective treatment.
INTRODUCTION
Autism spectrum disorders (ASD) are developmental neuropsychiatric diseases with characteristic symptoms of impaired social interaction, stereotyped behaviors, and delayed language development (Abrahams and Geschwind, 2008; Geschwind, 2011). One hypothesis is that the core behavioral features of autism are caused by an imbalance between excitatory and inhibitory neurotransmission in the brain (Gatto and Broadie, 2010; Markram and Markram, 2010; Rubenstein and Merzenich, 2003). Recent work on mouse models of syndromic autism caused by monogenic mutations in MeCP2, Scn1a, Shank3, and Cntnap2 has shown that an increased ratio of excitatory to inhibitory neurotransmission in the brain may cause autistic-like behaviors (Auerbach et al., 2011; Chao et al., 2010; Han et al., 2012; Peca et al., 2011; Penagarikano et al., 2011), and optogenetic increase in excitation/inhibition ratio can also induce social interaction deficits (Yizhar et al., 2011). The increased ratio of excitatory to inhibitory neurotransmission in these models may arise by increased excitatory transmission, decreased inhibitory transmission, or both.
This emerging research implicates increased excitation to inhibition ratio in causing autistic-like behaviors in monogenic animal models of autism, but there is much less evidence for the significance of this mechanism in idiopathic models of autism. BTBR mice are a well-studied model of idiopathic autism (Defensor et al., 2011; McFarlane et al., 2008; Yang et al., 2012). However, the inherited genetic changes that led to autistic-like behaviors in these mice are incompletely known and still under active investigation (Jones-Davis et al., 2013). In the experiments presented here, we provide evidence from recordings of spontaneous synaptic transmission that BTBR mice have a reduced level of inhibitory neurotransmission mediated by GABAA receptors in the hippocampus compared to the control strain C57BL/6J, which may contribute to their autistic-like behaviors.
Activation of GABAA receptors by GABA is enhanced by benzodiazepines, which are used in treatment of epilepsy, anxiety, panic disorder, and insomnia (Rudolph and Knoflach, 2011). Moreover, genetic linkage of the GABAA receptor to autism has been widely reported (Li et al., 2012). However, GABAA receptors have not been recognized as a therapeutic target for ASDs because of their sedative activity. Our previous studies showed that low-dose clonazepam was effective in treatment of impaired social interaction and cognitive deficit in Scn1a+/− mice, a model of Dravet Syndrome with marked autistic-like behaviors (Han et al., 2012). We present evidence here that treatment with low doses of positive allosteric benzodiazepine modulators of GABAA receptors improves characteristic autistic-like behaviors in BTBR mice. Interestingly, negative allosteric modulation of GABAA receptors with benzodiazepines induces social interaction deficits in C57BL/6J and 129SvJ wild type (WT) mice, supporting a causal role for reduced inhibitory neurotransmission in some features of autism. Moreover, autistic-like behavioral impairments can be treated effectively in both BTBR and Scn1a+/− mice by enhancement of inhibitory neurotransmission with low doses of subunit-selective positive allosteric modulators of GABAA receptors containing α2 and/or α3 subunits. Together, our results support the hypothesis that reduced GABAergic inhibitory neurotransmission contributes to autism-associated behavioral and cognitive deficits and suggest that enhancement of GABAergic neurotransmission with next-generation subunit-specific pharmacological agents may be beneficial.
RESULTS
Reduced Inhibitory Neurotransmission in BTBR Mice
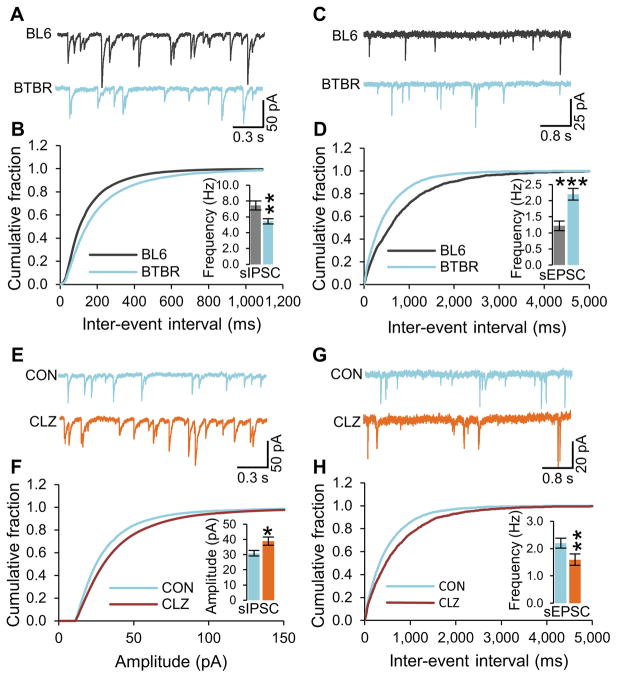
A challenge for research on BTBR mice is selection of an appropriate control mouse line for comparison, as different inbred strains differ in various behavioral and cognitive measures. Consistent with previous work, we chose to focus our study on differences in neurotransmission, behavior, and cognition between BTBR and C57BL/6J mice (see Supplementary Information for more discussion and references). To test the hypothesis that BTBR mice may have reduced inhibitory neurotransmission, we measured spontaneous excitatory and inhibitory postsynaptic currents in the CA1 region of hippocampal slices from age-matched (P21–25) BTBR and C57BL/6J mice. Although the amplitude of spontaneous inhibitory postsynaptic current (IPSC) was not altered in BTBR hippocampal slices compared to the C57BL/6J hippocampal slices (Figure S1A), the frequency of spontaneous IPSC was significantly reduced in BTBR hippocampal slices when compared with the C57BL/6J hippocampal slices (Figure 1A and 1B). In conjunction with decreased inhibitory neurotransmission, the amplitude and the frequency of spontaneous excitatory post-synaptic current (EPSC) were substantially increased in BTBR hippocampal slices when compared with C57BL/6J hippocampal slices (Figure 1C, 1D, and S1B). In control recordings of miniature postsynaptic currents, in which action potentials were blocked with tetrodotoxin (TTX), the amplitude and frequency of miniature IPSC and the frequency of miniature EPSC were unaltered (Figure S1E–S1G). However, the amplitude of miniature EPSCs was significantly increased in BTBR hippocampal slices when compared with C57BL/6J hippocampal slices (Figure S1H). Surprisingly, these studies reveal that BTBR mice have a deficit in inhibitory neurotransmission compared to the control strain C57BL/6J, which is caused by reduced frequency of inhibitory synaptic events without a corresponding decrease in postsynaptic response. This deficit in inhibitory neurotransmission is accompanied by a corresponding increase in excitatory neurotransmission. These results indicate that constitutively decreased inhibitory neurotransmission may be a contributing factor to the autistic-like behaviors in BTBR mice.
Figure 1. Reduced GABAergic neurotransmission in BTBR mice and enhancement by clonazepam.<.
br>Spontaneous IPSC (sIPSC) and sEPSC were recorded in the hippocampal slices from 3-week old male BTBR and C57Bl/6J mice. (A and B) Example traces of sIPSC (A) and cumulative plot and average values (inset) of sIPSC frequency in BTBR and C57BL/6J hippocampal CA1 neurons (B).
(C and D) Example traces of sEPSC (C) and cumulative plot and average values (inset) of sEPSC frequency in BTBR and C57BL/6J hippocampal CA1 neurons (D).
(E and F) Example traces of sIPSC (E) and cumulative plot and average value (inset) of sIPSC amplitude (F) in clonazepam and vehicle treated BTBR CA1 slices.
(G and H) Example traces of sEPSC (G) and cumulative plot and average value (inset) of sIPSC frequency (H) in clonazepam and vehicle treated BTBR CA1 slices.
CON, Control. CLZ, Clonazepam. All data shown are means ± s.e.m. from 15 – 19 recordings per strain. *P < 0.05, **P < 0.01, ***P < 0.001.
Increased GABAergic Inhibitory Neurotransmission in Response to Benzodiazepines
Attempts to reverse autistic-like traits by rebalancing the ratio of excitatory to inhibitory neurotransmission through pharmacological treatments that reduce excitatory neurotransmission have met with only partial success because of their limited efficacy and unwanted side effects in control groups (Berry-Kravis et al., 2012; Gandal et al., 2012; Henderson et al., 2012; Michalon et al., 2012; Yang et al., 2012). The results of Figure 1A–1D suggest that enhancing inhibitory neurotransmission might be effective. The GABAA receptor is a heteropentameric ligand-gated chloride channel that mediates the major inhibitory effects of GABAergic neurotransmission in the brain. Subsynaptic GABAA receptor subtypes are composed of two α, two β, and one γ subunit (Fritschy and Mohler, 1995). The action of GABA at these ionotropic receptors is increased through positive allosteric modulation by benzodiazepines, which are used to treat anxiety, insomnia, and epilepsy (Rudolph and Knoflach, 2011). In order to determine whether treatment with a benzodiazepine reverses the constitutively decreased GABAergic inhibitory signaling, we treated C57BL/6J and BTBR hippocampal slices with 0.5 μM clonazepam, a broad-acting, traditional benzodiazepine. These recordings revealed increased spontaneous IPSC amplitude (Figure 1E and 1F) and frequency (Figure S1C) in BTBR slices. In contrast, a significant increase of spontaneous IPSC amplitude (Figure S1I) but no change in IPSC frequency (Figure S1J) was observed in C57BL/6J slices. The increased GABAergic signaling after treatment with clonazepam led to a decrease in frequency of spontaneous EPSCs (Figure 1G and 1H), without change in amplitude in BTBR hippocampal slices (Figure S1D). Interestingly, the frequency of spontaneous EPSC was also decreased by clonazepam (Figure S1K), without change in amplitude (Figure S1L) in C57BL/6J slices. These data support the idea that low-dose clonazepam can reverse the underlying deficit in spontaneous GABAergic inhibitory neurotransmission in BTBR mice.
Improvement of Social Interaction by Treatment with Clonazepam
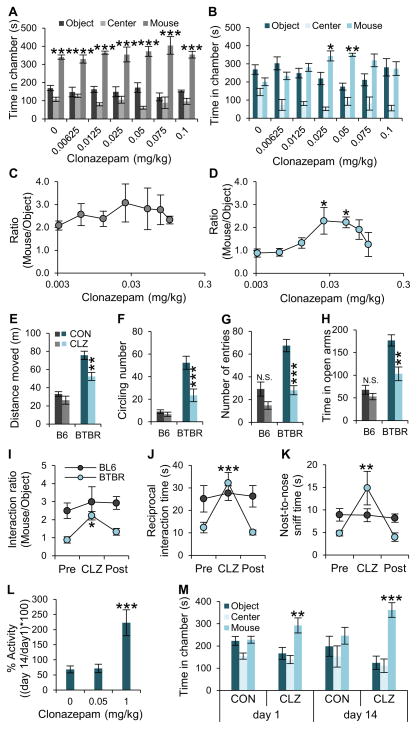
To test the behavioral effects of enhancing inhibitory neurotransmission in BTBR mice, we injected low non-sedating/non-anxiolytic doses of clonazepam intraperitoneally 30 min prior to behavioral tests. In the three-chamber social interaction test, acute clonazepam treatment had no effect on social interactions of C57BL/6J mice (Figure 2A and S2A) but increased social interactions in BTBR, with a maximal effect at 0.05 mg/kg (Figure 2B and S2B) and no sedation (Figure S2H). Measurements of the time of interaction of the test mouse with a stranger mouse vs. a novel object during three-chamber tests showed that the C57BL/6J mice are unaffected by any of the test doses (Figure 2C), whereas improvement of the social deficit in BTBR mice by clonazepam is strikingly dose-dependent (Figure 2D). Interestingly, the improved social interactions in BTBR mice was lost at higher doses of clonazepam (Figure 2B and 2D). Other behaviors in BTBR mice were also rescued by low-dose clonazepam. In the open field test, a single injection of 0.05 mg/kg clonazepam significantly reduced hyperactivity, measured as the total distance moved (Figure 2E), and stereotyped circling behavior, measured as the number of 360° rotations (Figure 2F). In contrast, these behaviors in C57BL/6J mice were unaffected by low-dose clonazepam. These low doses of clonazepam had little effect on anxiety-like behaviors of C57BL/6J mice, such as avoidance of the center of an open field or the open arms of an elevated plus maze (Figure 2G and 2H). However, compared to C57BL/6J, BTBR mice visited the center in the open field significantly more frequently and spent more time in open arms during the elevated-plus maze test under control conditions, as if they were less anxious than C57BL/6J mice, and these indicators of abnormally low anxiety in BTBR mice were changed toward the values for C57BL/6J mice after treatment with 0.05 mg/kg clonazepam (Figure 2G and 2H) without sedation (Figure S2I).
Figure 2. Effects of low-dose clonazepam on social interaction and cognitive deficits in BTBR mice.
(A–D) Age-matched male C57BL/6J (n = 6; each group) and BTBR mice (n = 9; each group) were treated with a single intraperitoneal dose of clonazepam at the indicated level and subjected to the three-chamber social interaction test. Test mice were not reused; different groups of mice were used for each dose of clonazepam treatment. (A, B) Time in chambers. (C, D) Ratio of time in mouse chamber to time in object chamber.
(E–G) The effects of low-dose clonazepam (CLZ; 0.05 mg/kg) on open field activity were measured in C57BL/6J (n = 8) and BTBR mice (n = 10).
(H) Time in open arms in elevated plus maze for BTBR mice (n = 10) and C57BL/6J mice (n = 10).
(I) In the three-chamber test, BTBR (blue) and C57BL/6J (black) mice were treated with vehicle (Pre, Post) or low-dose clonazepam (CLZ) 30 min before testing social interactions on Day 0 (Pre), Day 7 (CLZ), or Day 14 (Post). The ratio of interaction time with the stranger mouse or object is plotted.
(J and K) In the open-field reciprocal social interaction test, BTBR (blue) and C57BL/6J (black) mice were treated with vehicle (Pre, Post) or low-dose clonazepam (CLZ) 30 min before testing social interactions on Day 0 (Pre), Day 7 (CLZ), or Day 14 (Post). The ratio of interaction time with the stranger mouse vs. object is plotted for BTBR mice (n = 9) or C57BL/6J mice (n = 9) for total interaction time (J) and nose-to-nose interaction time (K).
(L and M) To test tolerance to the effects of low-dose clonazepam on locomotor and social behaviors, BTBR mice (n = 10; each group) were treated with low-dose (0.05 mg/kg), and high-dose (1 mg/kg) clonazepam for 14 days. (L) Total distance moved during the open field test after drug treatment on Day 1 and Day 14 was measured, and % activity change was calculated by comparing the activity on Day 1 and Day 14 of treatment with the indicated doses of clonazepam. (M) Social interaction behavior was compared after treatment with low-dose clonazepam (n = 10 for each group). CON, Control. CLZ, Clonazepam. All data shown are means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
Three sets of identical three-chamber tests at one-week intervals with the same group of mice showed the reversibility of rescue of social interaction deficits in BTBR by 0.05 mg/kg clonazepam (Figure 2I–2K, and S1C–S1F). In the first trial, with no treatment, BTBR mice displayed characteristic social interaction deficits when compared with C57BL/6J mice for interaction ratio with a stranger mouse vs. an inanimate object (Figure 2I). In a second identical trial one week later, these social deficits were improved by treatment with 0.05 mg/kg clonazepam 30 min before testing in the same group of BTBR mice (Figure 2I). In the third trial one week later, injection of vehicle had no effect to rescue the social behavior in the same mice (Figure 2I). None of these treatments had any significant effect on C57BL/6J mice (Figure 2I). This reversible effect of clonazepam treatment was also observed in the open-field reciprocal social interaction test in an intra-group comparison setting in which the test mouse cannot escape from the social stimulus provided by the stranger mouse. During three identical sets of reciprocal social interaction tests, impaired reciprocal social interaction, and nose-to-nose contact in BTBR mice were significantly enhanced in the 0.05 mg/kg clonazepam treated group, whereas C57BL/6J mice were unaffected (Figure 2J and 2K). These data show that low-dose clonazepam increases social interaction in BTBR mice within a narrow effective dose range.
Long-term treatment with the standard high doses of benzodiazepines causes tolerance in humans (Bateson, 2002). Tolerance to the sedative effects of high-dose clonazepam begins on Day 7 and reaches maximum on Day 14 in mice (Galpern et al., 1991; Loscher et al., 1996). To test tolerance in this context in BTBR mice, 0.05 mg/kg clonazepam was injected intraperitoneally daily for 14 days. For untreated animals, the locomotor activity in an open field was 68 ± 11% of normal on Day 14 compared to Day 1 (Figure 2L), probably because the open field chamber is familiar from their experience on Day 1 and they do not explore it as extensively on Day 14. Treatment with 0.05 mg/kg clonazepam did not have any effect on locomotor activity and did not alter the ratio of activity on Days 1 and 14 (Figure 2L). In contrast, injection of 1 mg/kg clonazepam for 14 days caused significant tolerance, as indicated by the large increase in level of locomotor activity on Day 14 compared to Day 1 due to the repeated administration of the drug (222 ± 42%, Figure 2L). In the three-chamber social interaction test, 0.05 mg/kg clonazepam significantly increased social interactions on Day 1, and this effect was fully retained and even increased after 14 days of repeated treatment (Figure 2M). These data indicate that repeated treatment with 0.05 mg/kg dose of clonazepam does not elicit tolerance to its rescue of social interaction behavior in the time frame of development of tolerance for the sedative effects of the drug.
Amelioration of Cognitive Deficits by Treatment with Clonazepam
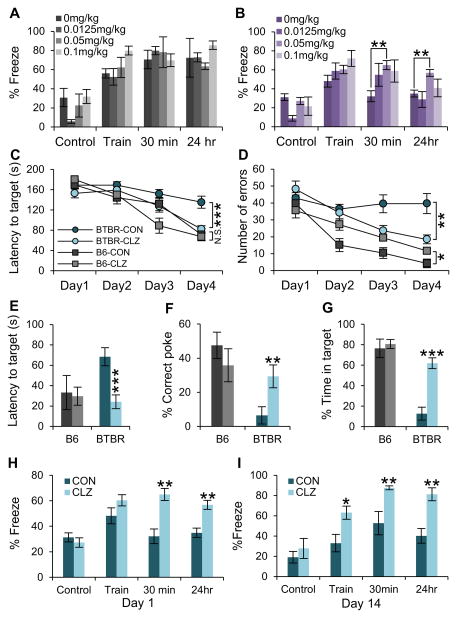
Cognitive problems are often associated with ASD (Zoghbi and Bear, 2012), and BTBR mice are known to have impaired fear memory (MacPherson et al., 2008). To test the effects of low-dose clonazepam on cognitive deficits, we performed context-dependent fear conditioning after treatment with increasing doses of clonazepam in both BTBR and C57BL/6J mice (Figure 3A and 3B). Short-term (30 min) and long-term (24 h) memory performance in fear conditioning to the spatial context in BTBR mice were improved by treatment with 0.05 mg/kg clonazepam, but no significant effects were observed after treatment with 0.0125mg/kg or 0.1 mg/kg clonazepam (Figure 3B and S3B). In contrast, no cognitive changes were observed in C57BL/6J mice at any dose (Figure 3A and S3A). To test spatial learning and memory in the absence of fear, we performed the Barnes circular maze test in which mice rapidly escape a brightly lit field by learning the location of a hole with a dark refuge at its periphery. BTBR mice failed to improve their performance during repeated training sessions, and this learning impairment was improved by clonazepam treatment (Figure 3C and 3D). In probe trials, in which mice search for a learned refuge that has been removed, spatial memory in BTBR mice was also increased by clonazepam treatment (Figure 3E–3G). In contrast, C57BL/6J mice displayed improved learning performance during repeated training sessions (Figure 3C and 3D), and normal spatial memory during the probe trial (Figure 3E–3G), regardless of clonazepam treatment. These data indicate that spatial learning in BTBR mice is substantially restored by treatment with low-dose clonazepam.
Figure 3. Effects of low-dose clonazepam on context-dependent spatial learning and memory deficits in BTBR mice.
(A and B) Increasing doses (0, 0.0125, 0.05, and 0.1 mg/kg) of clonazepam were administered 30 min prior to context-dependent fear conditioning. (A) C57BL/6J (n=5–7). (B) BTBR (n = 5 – 7)
(C and D) Barnes circular maze. Spatial learning was measured for C57BL/6J and BTBR mice (n = 10 each) by measurement of the latency (C) and number of errors (D) for mice to find safety without and with treatment with 0.05 mg/kg clonazepam as indicated. Note that treatment with clonazepam significantly improved the performance of BTBR mice but, in contrast, significantly worsened the performance of C57BL/6J mice.
(E–G) On Day 5 of the Barnes maze test, a single injection of low-dose clonazepam was given 30 min prior to the trial for BTBR and C57BL/6J mice: (E), latency to target; (F), % correct pokes; (G), % time in target.
(H and I) BTBR mice (n = 10) were treated with 0.05 mg/kg clonazepam for 14 days. Contextual fear conditioning was performed 30 min after injection on Day 1 (H) and Day 14 (I).
CON, Control. CLZ, Clonazepam. All data shown are means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
To determine whether tolerance develops to clonazepam we performed context-dependent fear conditioning on Day 1 of clonazepam injection and Day 14 of daily treatment with 0.05 mg/kg clonazepam. Regardless of the treatment period, the context-dependent fear memory improved by clonazepam treatment in BTBR mice (Figure 3H and 3I; Figure S3C and S3D), suggesting that low-dose clonazepam treatment does not cause tolerance to its effects on cognitive deficit in BTBR mice.
Opposing Effects of Positive and Negative Allosteric Modulators of GABAA Receptors
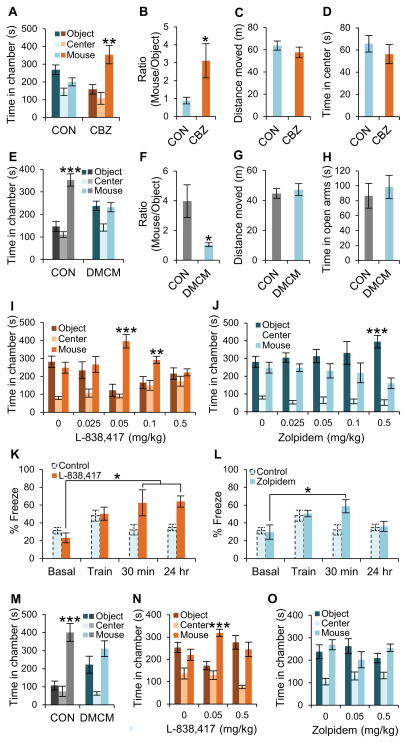
In order to test the effect of other classes of GABAA receptor modulators on social behavior, we treated BTBR mice with low-dose clobazam, an atypical benzodiazepine that is a positive allosteric modulator of GABAA receptors (Farrell, 1986). A single injection of 0.05 mg/kg clobazam 30 min before the three-chamber ameliorated social interaction deficits in BTBR mice (Figure 4A and 4B) without sedative (Figure 4C) or anxiolytic effects (Figure 4D). To further test the idea that the E/I balance is critical for normal social behaviors, we treated C57BL/6J and 129SvJ WT mice with low-dose DMCM, a negative allosteric modulator of the GABAA receptor function as assessed from IPSPs recorded in hippocampal slices and from behavioral tests (Rovira and Ben-Ari, 1993; Savic et al., 2006). In the three-chamber test, a single injection of a low non-convulsant/non-anxiogenic dose of DMCM (0.2 mg/kg) (Savic et al., 2004) 30 min prior to the test substantially reduced normal social interaction behavior in both C57BL/6J and 129SvJ mice (Figures 4E, 4F, 4M, and S4A). In contrast, the general locomotor behavior and anxiety levels were not altered by 0.2 mg/kg DMCM (Figure 4G and 4H). Our results with DMCM support the notion that impairment of GABAergic neurotransmission might contribute to autistic-like behaviors.
Figure 4. Effects of positive and negative GABAA receptor allosteric modulators on social behaviors and cognitive deficit.
(A and B) Effect of clobazam (0.05 mg/kg) on social interaction behavior of BTBR mice in the three-chamber test (n = 7 – 8).
(C and D) Effect of clobazam (0.05 mg/kg) on BTBR mice (n=7–8) in the open field test on total distance moved (C) and time spent in center (D).
(E and F) Effect of DMCM (0.2 mg/kg) on C57BL/6J mice (n=7–8) in the three-chamber test.
(G and H) Effect of DMCM (0.2 mg/kg) on overall exploratory behavior of C57BL/6J mice (n=8) in the open field test, measured as distance moved (G). Anxiety-like behavior of C57BL/6J mice (n=8) in the elevated plus maze test, measured as time in the open arms (H).
(I and J) Social interaction behavior of BTBR mice (n=6–8) in the 3-chamber test following treatment with the indicated doses of L838417 (I) or zolpidem (J). Test mice were not reused; different groups of mice were used for each dose.
(K and L) Contextual fear conditioning test of BTBR mice (n=5) following treatment with 0.05 mg/kg of L-838,417 (K) or zolpidem (L). Control data were replotted from Figure 2B.
(M) Effect of DMCM (0.2 mg/kg) on 129SvJ mice (n=8) in the three-chamber test.
(N and O) Effects of L838,417 (N) and zolpidem (O) on Scn1a+/− mice in the three-chamber test.
CON, Control. CBZ, Clobazam. CLZ, Clonazepam. All data shown are means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
Rescue by α2, α3-Specific Positive Allosteric Modulators of GABAA Receptors
Diversity of GABA receptor function is conferred by more than 20 different subunits, and receptors with different α subunits play distinct roles in the physiological and pharmacological actions of GABA and benzodiazepines (Fritschy and Mohler, 1995; Harmar et al., 2009; Rudolph and Knoflach, 2011; Rudolph and Mohler, 2004; Smith and Olsen, 1995). We tested the effects of subunit-selective positive allosteric modulators of GABAA receptors on social behavior in BTBR mice and C57BL/6J mice. A low dose of the α2,3 subunit-selective positive allosteric modulator L-838,417 (Low et al., 2000; Mathiasen et al., 2008) increased social interactions in BTBR mice, with maximal effective dose of 0.05 mg/kg, and the beneficial effect was lost when the dose increased (Figures 4I and S4E). In contrast, L-838,417 did not change the social interaction behavior of C57BL/6J mice (Figure S4I). Moreover, the α1 subunit-selective positive GABAA modulator zolpidem (Mathiasen et al., 2008; Sieghart, 1995) failed to show beneficial effects in BTBR mice and actually aggravated their social interaction deficit at high doses (Figures 4J and S4F). Interestingly, a high dose of zolpidem also impaired social behavior in C57BL/6J mice (Figure S4J). Total movement tended to increase at high doses of L-838,417 (Figure S4G; not significant), but significantly decreased at 0.5 mg/kg zolpidem (Figure S4H). These results indicate that different subtypes of GABAA receptors may have opposite roles in social behavior, with activation of GABAA receptors containing α2,3 subunits favoring and of GABAA receptors with α1 subunits reducing social interaction, respectively.
Subunit-selective GABAA receptor modulators may also have an important effect on cognitive behaviors. In the context-dependent fear conditioning test, treatment with 0.05 mg/kg L-838,417 improved short-term (30 min) and long-term (24 h) spatial memory in BTBR mice (Figure 4K), whereas 0.05 mg/kg zolpidem enhanced short-term memory, but not long-term memory (Figure 4L). These data show that α2,3 subunit-containing GABAA receptors may also be important for cognitive behaviors in BTBR mice. The bell-shaped dose-response curves observed for both L-838,417 and clonazepam may explain why high-dose benzodiazepine treatment for prevention of anxiety and seizures has not been reported to improve autistic traits in ASD patients. As illustrated in Figure 4N and 4O, treatment with low doses of L-838,417 also improves social interactions in the Scn1a+/− mice, a model of Dravet Syndrome with severe autistic-like behaviors (Han et al., 2012), within a narrow dose range. In contrast, similar treatment with zolpidem is not effective. Altogether, these experiments show that treatment with an α2,3-selective positive allosteric modulator of GABAA receptors is sufficient to rescue autistic-like behaviors and cognitive deficit in both a monogenic model of autism-spectrum disorder and the BTBR mouse model of idiopathic autism.
DISCUSSION
Our results on mouse models of autism support the hypothesis that social and cognitive in ASDs may be caused by an increased ratio of excitatory to inhibitory synaptic transmission (Gatto and Broadie, 2010; Han et al., 2012; Markram and Markram, 2010; Rubenstein and Merzenich, 2003). We found that autistic BTBR mice have constitutively reduced inhibitory neurotransmission in the hippocampus and that enhancement of their inhibitory neurotransmission with positive allosteric modulators of GABAA receptors improved autism-related traits. Conversely, we found that global pharmacological reduction of inhibitory neurotransmission by the negative allosteric modulator DMCM was sufficient to induce some autism-related behaviors in C57BL/6J and 129SvJ WT mice. These results are most consistent with the hypotheses that reduced inhibitory neurotransmission is sufficient to induce autistic-like behaviors in mice and that enhanced inhibitory neurotransmission can reverse autistic-like behaviors. However, even though the BTBR mouse has been widely used as an animal model of autism, it is not yet fully understood which genetic changes lead to its autistic-like behaviors (Jones-Davis et al., 2013) or whether similar genetic changes are among the large number of DNA polymorphisms that have been implicated in human autism. Similarly, even though treatment of C57BL/6J mice and 129SvJ mice with a negative allosteric modulator of GABA receptors, DMCM, induces specific autism-related behavioral impairments in these two mouse strains, it is not known whether this would be true for all mouse strains or whether decreasing the effectiveness of GABAergic inhibitory neurotransmission with DMCM or a related agent would cause any of the behavioral features of human autism.
GABAA receptors with different subunit composition have different roles in synaptic transmission in hippocampal pyramidal neurons. Receptors containing α1 subunits mediate fast synaptic transmission at the synapses on distal dendrites, whereas receptors containing α2 subunits mediate fast synaptic transmission at synapses on the soma (Prenosil et al., 2006). Different inputs impinge on CA1 pyramidal neurons at these sites, providing a potential mechanism for understanding how specific modulation of these two receptor types with L-838,417 and zolpidem leads to differential effects on spatial learning and sedation. Because GABAA receptors containing α2 subunits have specific physiological roles (Prenosil et al., 2006) and drug actions on them do not induce tolerance (Vinkers et al., 2012), they provide an attractive molecular target for therapy of autism and other disorders with reduced GABAergic inhibitory neurotransmission.
Therapeutic approaches to treat autistic traits in animal studies or in clinical trials have primarily focused on reducing excitatory neurotransmission in glutamatergic synapses to rebalance E/I ratio in autistic brain (Michalon et al., 2012; Yang et al., 2012). However, autistic-like behaviors in ASD mouse models are only partially reversed by drugs that inhibit excitatory neurotransmission, and these drugs also have unwanted side effects on wild-type mice (Henderson et al., 2012; Michalon et al., 2012; Yang et al., 2012). To overcome these drawbacks, we focused on the opposing side, the GABAergic inhibitory transmission in autistic brain. Our results highlight the potential for therapy of autistic-like behaviors and cognitive deficit in ASD by low-dose treatment with subunit-selective benzodiazepines and other positive allosteric modulators of GABAA receptors. At low doses that do not induce sedative or anxiolytic effects, we found that clonazepam, clobazam, and L-838,417 all improved autistic-like behaviors and cognitive deficit in BTBR mice, supporting the hypothesis that α2,3 subunit-selective up-regulation of GABAergic neurotransmission could be an effective treatment for these core features of autism. Consistent with this conclusion, the α1 subunit-selective positive allosteric modulator zolpidem had opposite effects. It is possible that the biphasic dose-response relationship of the positive allosteric modulators reflects their actions on receptors containing α2,3 subunits at low doses and on receptors containing α1 subunits at higher doses.
Although tolerance develops during prolonged treatment of patients with high doses of traditional benzodiazepines, our experiments indicate that tolerance is not induced by treatment of mice with low doses of clonazepam for 14 days, and α2,3-selective positive allosteric modulators of GABAA receptors do not induce tolerance in rodents (Vinkers et al., 2012). Because of their broad availability and safety, benzodiazepines and other positive allosteric modulators of GABAA receptors administered at low non-sedating, non-anxiolytic doses that do not induce tolerance deserve consideration as a near-term strategy to improve the core social interaction deficits and repetitive behaviors in ASD. Consistent with this view, Astra-Zeneca and the National Institutes of Health have initiated clinical trials of the α2,3-selective positive allosteric modulator of GABAA receptors, AZD7325, for efficacy in autism (http://clinicaltrials.gov/show/NCT01966679).
EXPERIMENTAL PROCEDURES SUMMARY
Adult male mice 6–10 months old were used for all behavioral tests. All mice were singly housed at least 1 week before the behavioral tests. All experiments with animals were performed according to the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the University of Washington Institutional Animal Care and Use Committee. The Open-field Test, Elevated Plus Maze Test, Three-chamber Test, Reciprocal Interaction Test, Barnes Circular Maze Test, and Contextual Fear Conditioning Test were carried out as described previously (Han et al., 2012) and in Supplemental Information. As required for stable recordings of spontaneous synaptic activity, brain slices from 3–4 week-old mice were used for electrophysiological studies, which were carried out as described previously (Han et al., 2012). Drugs were administered and data were analyzed as described previously (Han et al., 2012) and in Supplemental Information. All data are shown as mean ± s.e.m. and analyzed using Student’s t-test, one-way ANOVA with Tukey’s post hoc comparison, and two-way ANOVA with Bonferroni’s post hoc comparison. All the statistical analyses were done using Prism 6 (GraphPad).
Supplementary Material
HIGHLIGHTS.
BTBR mice have reduced spontaneous GABAergic inhibitory transmission
Non-sedating doses of benzodiazepines improved autism-related deficits in BTBR mice
Impairment of GABAergic transmission reduced social interaction in wild-type mice
Behavioral rescue by low-dose benzodiazepine is GABAA receptor α2,3-subunit-specific
Acknowledgments
Research reported in this publication was supported by the Simons Foundation, the National Institute of Child Health and Human Development under award number P30HD02274, and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R01NS25704. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson AN. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des. 2002;8:5–21. doi: 10.2174/1381612023396681. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis EM, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, Mu Y, Nguyen DV, Gonzalez-Heydrich J, Wang PP, et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci Transl Med. 2012;4:152ra127. doi: 10.1126/scitranslmed.3004214. [DOI] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defensor EB, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav Brain Res. 2011;217:302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell K. Benzodiazepines in the treatment of children with epilepsy. Epilepsia. 1986;27(Suppl 1):S45–52. doi: 10.1111/j.1528-1157.1986.tb05733.x. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Galpern WR, Lumpkin M, Greenblatt DJ, Shader RI, Miller LG. Chronic benzodiazepine administration. VII. Behavioral tolerance and withdrawal and receptor alterations associated with clonazepam administration. Psychopharmacology (Berl) 1991;104:225–230. doi: 10.1007/BF02244183. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Sisti J, Klook K, Ortinski PI, Leitman V, Liang Y, Thieu T, Anderson R, Pierce RC, Jonak G, et al. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl Psychiatry. 2012;2:e142. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto CL, Broadie K. Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Front Synaptic Neurosci. 2010;2:4. doi: 10.3389/fnsyn.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15:409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Hills RA, Rosser EM, Jones M, Buneman OP, Dunbar DR, Greenhill SD, Hale VA, Sharman JL, Bonner TI, et al. IUPHAR-DB: the IUPHAR database of G protein-coupled receptors and ion channels. Nucleic Acids Res. 2009;37:D680–685. doi: 10.1093/nar/gkn728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C, Wijetunge L, Kinoshita MN, Shumway M, Hammond RS, Postma FR, Brynczka C, Rush R, Thomas A, Paylor R, et al. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABA(B) receptors with arbaclofen. Sci Transl Med. 2012;4:152ra128. doi: 10.1126/scitranslmed.3004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Davis DM, Yang M, Rider E, Osbun NC, da Gente GJ, Li J, Katz AM, Weber MD, Sen S, Crawley J, Sherr EH. Quantitative trait loci for interhemispheric commissure development and social behaviors in the BTBR T(+) tf/J mouse model of autism. PLoS One. 2013;8:e61829. doi: 10.1371/journal.pone.0061829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zou H, Brown WT. Genes associated with autism spectrum disorder. Brain Res Bull. 2012;88:543–552. doi: 10.1016/j.brainresbull.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Loscher W, Rundfeldt C, Honack D, Ebert U. Long-term studies on anticonvulsant tolerance and withdrawal characteristics of benzodiazepine receptor ligands in different seizure models in mice. I. Comparison of diazepam, clonazepam, clobazam and abecarnil. J Pharmacol Exp Ther. 1996;279:561–572. [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- MacPherson P, McGaffigan R, Wahlsten D, Nguyen PV. Impaired fear memory, altered object memory and modified hippocampal synaptic plasticity in split-brain mice. Brain Res. 2008;1210:179–188. doi: 10.1016/j.brainres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Markram K, Markram H. The intense world theory - a unifying theory of the neurobiology of autism. Front Hum Neurosci. 2010;4:224. doi: 10.3389/fnhum.2010.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiasen LS, Mirza NR, Rodgers RJ. Strain- and model-dependent effects of chlordiazepoxide, L-838,417 and zolpidem on anxiety-like behaviours in laboratory mice. Pharmacol Biochem Behav. 2008;90:19–36. doi: 10.1016/j.pbb.2008.01.014. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, Jaeschke G, Bear MF, Lindemann L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74:49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- Rovira C, Ben-Ari Y. Developmental study of benzodiazepine effects on monosynaptic GABAA-mediated IPSPs of rat hippocampal neurons. J Neurophysiol. 1993;70:1076–1085. doi: 10.1152/jn.1993.70.3.1076. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Savic MM, Obradovic DI, Ugresic ND, Cook JM, Yin W, Bokonjic DR. Bidirectional effects of benzodiazepine binding site ligands in the elevated plus-maze: differential antagonism by flumazenil and beta-CCt. Pharmacol Biochem Behav. 2004;79:279–290. doi: 10.1016/j.pbb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Savic MM, Obradovic DI, Ugresic ND, Cook JM, Yin W, Van Linn M, Bokonjic DR. Benzodiazepine site inverse agonists and locomotor activity in rats: bimodal and biphasic influence. Pharmacol Biochem Behav. 2006;84:35–42. doi: 10.1016/j.pbb.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Smith GB, Olsen RW. Functional domains of GABAA receptors. Trends Pharmacol Sci. 1995;16:162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, van Oorschot R, Nielsen EO, Cook JM, Hansen HH, Groenink L, Olivier B, Mirza NR. GABA(A) receptor α subunits differentially contribute to diazepam tolerance after chronic treatment. PLoS One. 2012;7:e43054. doi: 10.1371/journal.pone.0043054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Abrams DN, Zhang JY, Weber MD, Katz AM, Clarke AM, Silverman JL, Crawley JN. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol Behav. 2012;107:649–662. doi: 10.1016/j.physbeh.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.