Abstract
Vaccination of chimpanzees against hepatitis C virus (HCV) using T-cell based vaccines targeting non-structural proteins have not resulted in the same levels of control and clearance as those seen in animals re-exposed after HCV clearance. We hypothesized that the outcome of infection depends on the different subtypes of activated T-cells. We used multi-color flow cytometry to evaluate activation (CD38+/HLA-DR+) and proliferation (Ki67+/Bcl-2-low) profiles of CD4+ and CD8+ T-cells in peripheral blood before and after challenge in chimpanzees vaccinated using DNA/adenovirus, mock vaccinated and chimpanzees that had spontaneously cleared infection (rechallenged). The frequencies of activated or proliferating CD8+ T-cells peaked at 2 weeks post-challenge in the vaccinated and rechallenged animals, coinciding with reductions in viral titers. However, the magnitude of the responses did not correlate with outcome or sustained control of viral replication. In contrast, proliferation of the CD8+ T-cells co-expressing HLA-DR either with or without CD38 expression was significantly higher at challenge in animals that rapidly cleared HCV and remained so throughout the follow-up period.
Conclusion
Our data suggest that the appearance of proliferating HLA-DR+/CD8+ T-cells can be used as a predictor of a successfully primed memory immune response against HCV and as a marker of effective vaccination in clinical trials.
Keywords: immunology, infectious diseases, vaccine, efficacy, T-cell phenotypes
Worldwide >150 million individuals are chronically infected with hepatitis C virus (HCV) and ~3.2 million are infected in the USA alone (1). Studies show that 60–85% of acute infections become chronic and it has been determined that ~15–20% of all individuals that become infected with HCV progress to serious end-stage liver disease, such as cirrhosis or hepatocellular carcinoma (2). With ~18,000 new HCV infections occurring in the U.S. annually (3) the development of a vaccine against this virus is imperative.
Although HCV persists in the majority of cases, acute infections are spontaneously resolved in ~20–25% of humans (4) and ~40% of chimpanzees (5). Chimpanzees represent the only animal model available to study pathogenesis of this viral disease and replicate the features of HCV infection in humans, such as kinetics of viremia and persistent infection even in the presence of immune responses (6). Spontaneous clearance following primary infection with HCV has been associated with effective T-cell responses, the major characteristics of which have been shown to be strong proliferating capacity of HCV-specific T-cells (7, 8), higher frequencies of CD8+ T-cells expressing perforin in the liver (9) and early and multispecific IFNγ-production by CD4+ and CD8+ T-cells (10, 11). Due to this association, several chimpanzee prophylactic vaccine studies have focused on T-cell induction targeting non-structural proteins for prevention of chronic HCV infection. Although these T-cell-based studies confirm that vaccine-induced T-cell responses can lead to memory cells and early control of viral replication (5) there is no greater incidence of viral clearance in vaccinees compared to naïve animals when non-structural components are included in vaccines (5). In addition, we have previously shown that an ineffective T-cell vaccine can create greater pressure for viral mutation and therefore immune escape, which may lead to persistence of HCV (12). The failure of most T-cell vaccines to result in clearance of HCV is at odds with the knowledge that following spontaneous clearance and re-infection, viremia and liver disease are significantly reduced in both intensity and duration in chimpanzees (13, 14) and humans (4) and that this rapid clearance appears to be associated with faster T-cell activation (7, 14).
We wished to assess if vaccine-induced T-cell responses differ qualitatively from those induced by natural infection and if memory T-cells with specific phenotypes in immune-primed animals can be associated with rapid and successful clearance of HCV. The initiation of the immune response starts with activation of T-cell receptors which leads to a signal cascade, up-regulation of activation markers and cellular proliferation. Analyses of hepatic gene expression in chimpanzees have suggested that efficient T-cell activation or recruitment is critical for HCV clearance during primary infections (15). The surface markers CD38 and/or HLA-DR have been used as measures of T-cell activation in many studies of acute and chronic infections, including HCV (16, 17), and during studies of yellow fever and smallpox vaccinees (18). These two markers in combination with Ki-67 and Bcl2 have been suggested as a means to identify CD8+ effector T-cell responses after immunization or infection (18). Ki-67 is a marker of proliferation in T-cells (19) while reduced Bcl2 expression indicates susceptibility to apoptosis; resting T-cells express high levels of the protein (20). We analyzed the frequency and kinetics of these markers of activation and proliferation on CD4+ and CD8+ T-cells in chimpanzees that were previously infected with HCV and had spontaneously cleared infection, vaccinated and control chimpanzees before and after infection with HCV and correlated this data with rapid control of the virus or progression to persistent infection.
EXPERIMENTAL PROCEDURES
Animal Studies
The housing, maintenance, and care of chimpanzees (Pan troglodytes) were in compliance with all Federal regulations and policies governing animal care and use. All procedures were approved by the FDA and CDC Animal Care and Use Committees and the Interagency Animal Model Committee. Vaccinated, control and rechallenged animals are designated with V-, C- or R- respectively.
Vaccinated and Control Animals
Vaccinated animals (n=4) (V-1611; V-6407; V-91A025, V-0712) and control animals (n=2) (C-0257, C-0710) were inoculated with DNA/liposome complexes (8mg DNA/dose delivered intravenously (i/v)) 4 weeks apart and boosted 16 weeks later with adenovirus (3×1011 particles, intramuscularly (i/m)). Vaccinated animals received plasmids and recombinant adenovirus (rAd) expressing NS3/NS4A, NS5A and NS5B of HCV (12). Control animals received empty backbone vector and non-recombinant adenovirus (non-rAd). All chimpanzees received IL-12-expressing plasmid in immunoliposome complexes. All animals were challenged 20 weeks after Ad boost with 100 50% chimpanzee infectious doses (CID50) of clonal HCV i/v (21).
Rechallenged Chimpanzees
Three chimpanzees had been infected with HCV genotype 1a more than 8 years previously and spontaneously cleared (R-4x0300, R-4x0329, R-4x0352). The animals were re-challenged with 100 CID50 clonal HCV i/v (21).
Serum and PBMCs
Serum was collected weekly; whole blood was collected bi-weekly. Serum HCV RNA was quantified using Amplicor Monitor (Roche Molecular Systems, Pleasanton, CA) (limit of 600 IU/mL) or real-time PCR (limit 200 RNA copies/mL) (22). PBMCs were isolated using Lymphoprep solution (AXIS-SHIELD PoC AS) as described (21).
ELIspot assay
ELIspot analysis was performed as described (23) using pre-stimulation with peptide pools (5ug/mL) containing 30–45 overlapping peptides 18-mers overlapping by 11 amino-acids, NIH AIDS Reagent Program) representing HCV proteins.
FACS analysis
Surface and intracellular staining was performed according to standard protocols on previously frozen PBMCs using monoclonal antibodies (CD3–PE-TexRed (BeckmanCoulter, Brea, CA), CD3-Alexa-700, CD4-PacificBlue, CD8-APC-Cy7, HLA-DR-PE-Cy7, CD38–PerCP-Cy5.5, TNFα-APC, IFNγ-APC (Biolegend, San Diego, CA), Ki-67-FITC, Bcl-2-PE and LIVE/DEAD Stain Kit (Invitrogen, Frederick, MD). BD FACS Permeabilizing Solution (BectonDickinson) was used for intracellular staining. For detection of HCV-specific proliferating cells PBMCs were stimulated with peptide pools (5 ug/mL as for ELIspot) specific for NS3, NS5A and NS5B. Brefeldin (10ug/mL) (Sigma, St.Louis, MO) was added after 18 hours to arrest cytokine release and incubation was continued for a total of 24 hours before surface and intracellular staining. Data for FACS analyses were collected with LSR II and analyzed with FlowJo software 9.1.
Statistical analysis
Statistical analyses were performed using unpaired two-tailed t-test and Graphpad software. P value of <0.05 was considered significant.
RESULTS
DNA/Adenovirus vaccination and prior infection induce HCV-specific memory immune responses that modulate the kinetics of viral replication
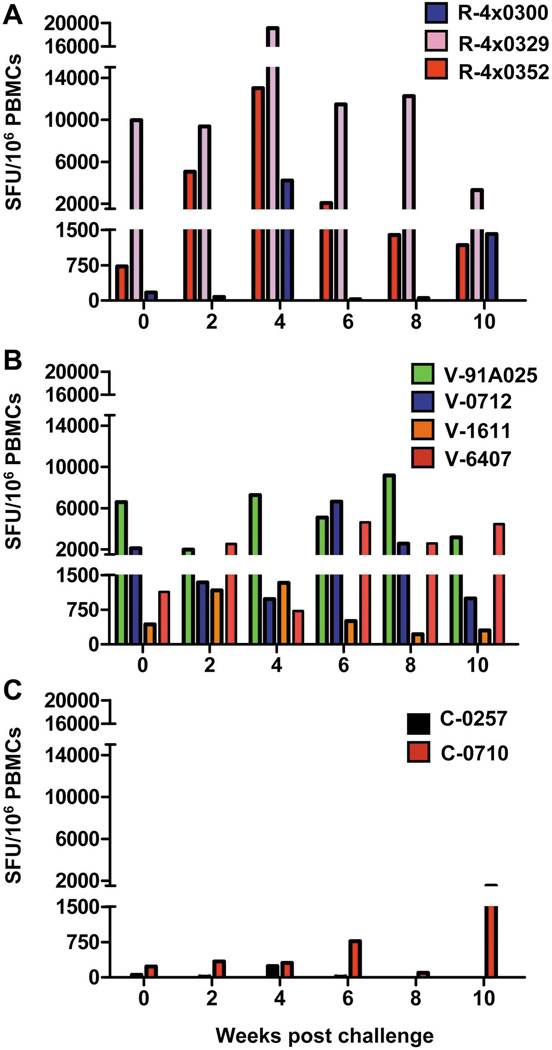
Table 1 summarizes treatment and outcomes for all animals included in this study. T-cell responses specific to multiple HCV antigens were assessed by ELIspot in all animals. At challenge all four vaccinated animals and two of the three rechallenged animals had levels of IFNγ-producing T-cells above the background levels detected in naïve animals (Figure 1). All rechallenged chimpanzees demonstrated increasing numbers of IFNγ-producing T-cells shortly after challenge (Figure 1A) with peak levels occurring at week 4. Specific responses were observed in the vaccinated chimpanzees post-vaccination and prior to challenge ((12) and data not shown) and anamnestic multi-specific responses were observed post-challenge (Figure 1B). As expected only low frequencies of IFNγ-producing cells were detected in the control animals post-challenge (Figure 1C).
Table 1.
Chimpanzees used in this study, treatment received and outcome of HCV challenge
| Treatment | Chimpanzee ID | Outcome* |
|---|---|---|
| Vaccinated | ||
|
Prime: Liposome complexed DNA expressing NS3/NS4A, NS5A, NS5B of HCV and IL-12 Boost: rAd expressing NS3/NS4A, NS5A, NS5B of HCV |
V-1611 | Chronic |
| V-6407 | Transiently controlled | |
| V-91A025 | Cleared at week 4 | |
| V-0712 | Transiently controlled | |
| Control | ||
|
Prime: Liposome complexed DNA backbone lasmid plus DNA expressing IL-12. Boost: Non-rAd |
C-0257 | Cleared at week 7 |
| C-0710 | Transiently controlled | |
| Rechallenged | ||
| Infected with HCV GT 1a and spontaneously leared the virus. | R-4X0300 | Cleared at week 4 |
| R-4X0329 | Cleared at week 3 | |
| R-4X0352 | Cleared at week 4 | |
Outcome following challenged with 100CID50 monoclonal HCV
Figure 1. HCV ELIspot data and RNA levels in rechallenged (n=3), vaccinated (n=4) and control (n=2) chimpanzees.
Frequency of IFNγ-producing HCV-specific T-cells post challenge from peripheral blood mononuclear cells (PBMCs) isolated from chimpanzees included in this study. (A) Rechallenged animals, blue bars: R-4x0300, pink bars: R-4x0329, red bars: R-4x0352. (B) Vaccinated animals, green bars: 91A025, blue bars: V-0712, orange bars: V-1611, red bars: V-6407. (C) Control animals, black bars: C-0257, red bars: C0710. Cumulative counts are shown for responses against peptide pools representing NS3, NS4A, NS4B, NS5A and NS5B. ELIspot data for V-1611, V-6407 and C-0257 have been previously reported (12) and are included here for clarity.
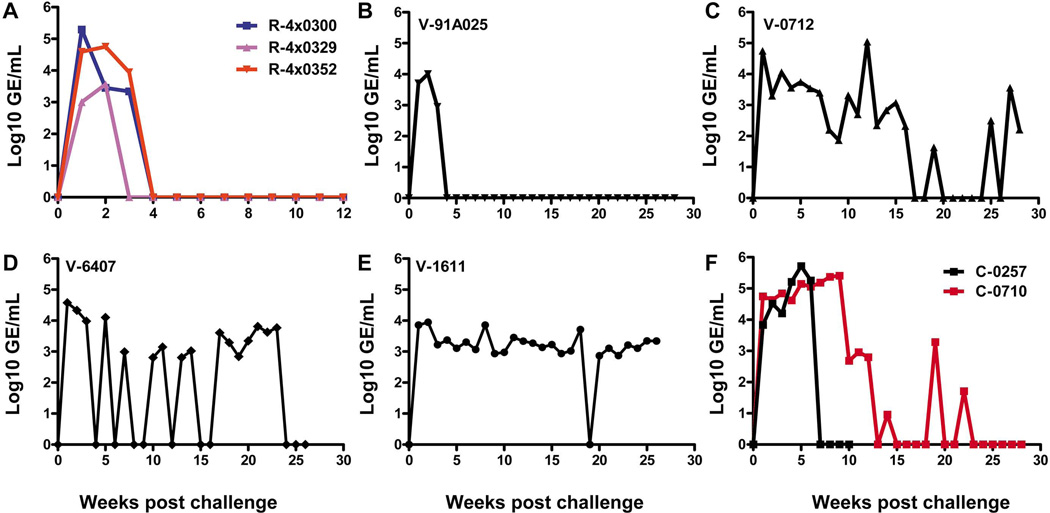
Consistent with our previous studies, the kinetics of HCV replication in the recovered chimpanzees after secondary infection were substantially different from the kinetics of HCV replication in naïve animals (5, 24). Peak titers in rechallenged animals occurred at weeks 1–2 and by week 4 the viral titers were below the level of detection in our quantitative assay (Figure 2A). Consistent with the presence of HCV-specific memory T-cell responses all vaccinated animals also rapidly controlled viral replication following challenge as evidenced by reductions in titers of 1–1.5 Log10 as early as 3 weeks (Figure 2B–E). These viral RNA kinetics are in contrast to exponential increases in virus titer in the control animals during the same period (Figure 2F). Although the viral kinetics and clearance patterns in the rechallenged animals were highly consistent the overall control and outcome of infection was different for each vaccinated animal. Only one vaccinated animal, V-91A025, cleared the infection rapidly (Figure 2B), the virus titer dropped below the level of quantification at week 4 and remained so throughout the 28 week follow-up period. Animal V-6407 also cleared HCV to non-quantifiable levels at week 4 (Figure 2D) but there followed intermittent RNA positivity in the serum for several months, indicating incomplete control of the virus. V-0712 was also able to control HCV replication transiently although reductions to non-quantifiable levels did not occur until week 17 (Figure 2C). The fourth animal (V-1611) developed a persistent infection with titers remaining at steady-state levels from week 3 onwards (Figure 2E).
Figure 2. HCV RNA levels post challenge in rechallenged (n=3), vaccinated (n=4) and control (n=2) chimpanzees.
(A) Rechallenged animals, blue line: R-4x0300, pink line: R-4x0329, red line: R-4x0352 (B) V-91A025 (vaccinated) (C) V-0712 (vaccinated) (D) V-6407 (vaccinated) (E) V-1611 (vaccinated) (F) Control animals, black line: C-0257, red line: C0710. Values are represented on the Y axis as Log10 genome equivalents (GE)/mL. HCV challenge was carried out at week 0. HCV RNA levels for V-1611, V-6407 and C-0257 have been previously reported (12) and are included here for clarity.
Kinetics of T-cell activation and proliferation in immune-primed animals following challenge do not correlate with outcome
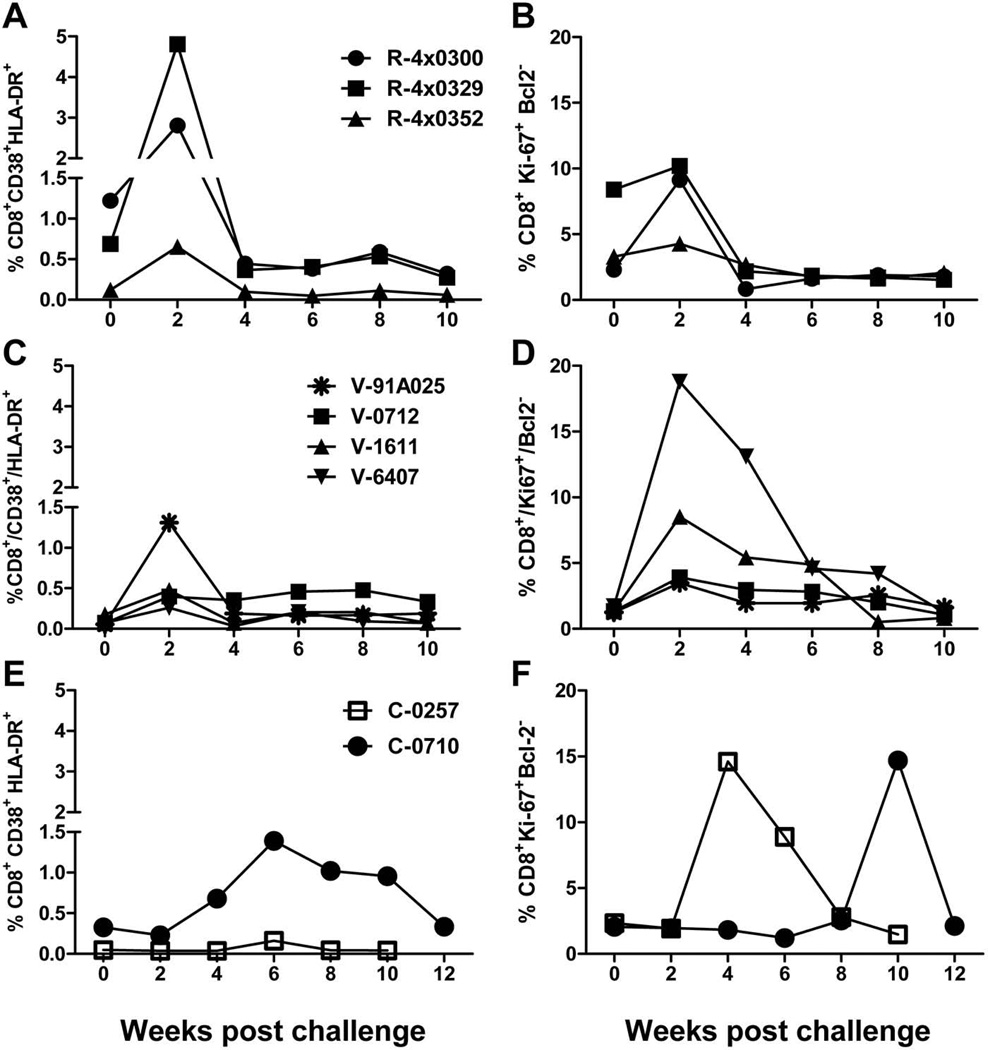
To study the kinetics of activation and proliferation of T-cells we monitored expression of CD38 and HLA-DR and of Ki-67 and Bcl-2 on CD4+ and CD8+ T-cells in all animals post-challenge. The kinetics and frequency of activated and proliferating CD8+ T-cells after HCV exposure in rechallenged chimpanzees was similar to that in vaccinated animals regardless of infection outcome with levels increasing immediately following challenge at week 2 (Figures 3A to 3D). The highest frequencies of activated CD8+ T-cells were detected in two rechallenged animals (R-4x300 and R-4x329) (Figure 3A) while R-4x0352 displayed levels similar to the vaccinated animals (Figures 3A and 3C). The highest level of proliferation among CD8+ T-cells from all the immune-primed animals was detected in V-6407, the animal that only transiently controlled the virus (Figures 3B and 3D). The animal that developed a persistent infection (V-1611) also demonstrated strong levels of proliferation among CD8+ T-cells (6.5-fold increase) similar to that in the rechallenged animals (Figure 3B).
Figure 3. Kinetics of activated or proliferating T-cells in rechallenged, vaccinated and control chimpanzees following challenge.
Frequency of total CD8+T-cells expressing activation or proliferation markers in rechallenged, vaccinated or control animals. A) % CD38+/HLA-DR+ CD8+ T-cells from rechallenged animals B) % Ki67+/Bcl-2-low CD8+ T-cells from rechallenged animals C) % CD38+/HLA-DR+ CD8+ T-cells from vaccinated animals D) % Ki67+/Bcl-2-low CD8+ T-cells from vaccinated animals. E) % CD38+/HLA-DR+ CD8+ T-cells from control animals F) % Ki67+/Bcl-2-low CD8+ T-cells from control animals. PBMCs were gated on CD3+/CD8+ cells.
As expected the kinetics of T-cell activation and proliferation in the control animals where the immune system had not been specifically primed was found to be very different from that in the vaccinated and rechallenged animals. Peak levels of activated CD8+ T-cells were detected at week 6 in both animals (Figure 3E). We also observed delayed proliferation of CD8+ cells, with the peaks at week 4 (C-0257) and week 10 (C-0710) (Figure 3F).
These data indicate that in immune-primed animals the kinetics of activated T-cells and the levels of proliferating CD8+ T-cells do not correlate with outcome. Analysis of the same markers on CD4+ T-cells did not reveal consistent increases in activated or proliferating cells post-challenge in the immune-primed animals (Figures S1A to S1D) and the increases when detected did not correlate with outcome. The kinetics of activated and proliferating CD4+ cells in the control animals followed the same pattern as that for CD8+ T-cells (Figure S1E and S1F).
Proliferating CD8+ Cells at Week 2 are HCV-Specific
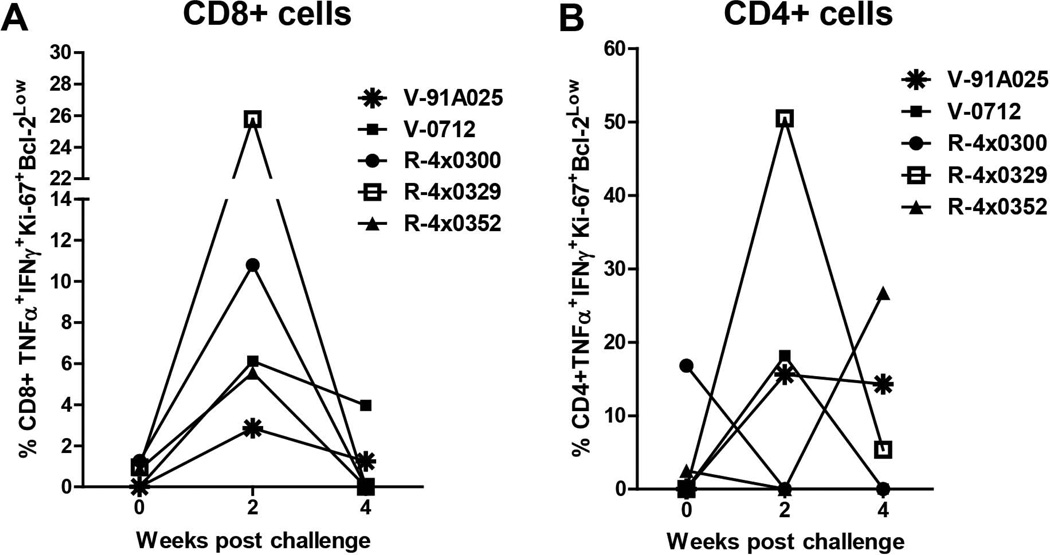
The markers of activation and proliferation applied in the previous analyses were not designed to detect HCV-specific cells. However, data for proliferating and activated cells in the control animals and the timing of the responses relative to infection suggested that the cell populations observed in the immune-primed groups were HCV-specific. To further support this we analyzed proliferation of CD8+ and CD4+ T-cells from V-91A025, V-0712, R-4x0300, R-4x0329 and R-4x0352 in response to stimulation with HCV-specific peptides. Insufficient cells from V-1611 and V-6407 were available for this analysis. CD8+ and CD4+ cells were stained for TNF-α and IFN-γ and for the proliferation markers Ki-67 and Bcl2. The population of CD8+ cells co-expressing all of these markers peaked at week 2 (Figure 4A), consistent with the proliferation and activation data obtained for unstimulated cells. The same population of CD4+ cells also followed a pattern similar to that observed for unstimulated cells (Figure 4B), indicating the presence of HCV-specific CD4+ cells in these animals that proliferate upon stimulation with antigen. No cytokine-positive cells were detected in the control animals during this same period (data not shown).
Figure 4. Frequency of HCV-specific proliferating CD8+ and CD4+ T-cells.
TNFα and IFNγ positive T-cells with the phenotype Ki67+ and Bcl-2-low post HCV challenge for animals V-91A025, V-0712, R-4x0300, R-4x0329 and R-4x0352. A) Frequency of TNF-α and IFN-γ positive CD8+ T-cells that are Ki-67+ and Bcl2low. B) Frequency of TNF-α and IFN-γ positive CD4+ T-cells that are Ki-67+ and Bcl2low. For detection of HCV-specific proliferating cells PBMCs were stimulated with HCV peptide pools representing NS3, NS5A, and NS5B. Brefeldin (10ug/mL) was added after 18 hours in order to arrest cytokine release and incubation was continued for a total of 24 hours. Stimulated cells were then stained with monoclonal antibodies specific to the following: CD3-Alexa 700, CD4-Pacific Blue, CD8-APC-Cy7, HLA-DR-PE-Cy7, CD38-PE-Cy5, TNFα-APC, IFNγ- APC, Ki-67-FITC, Bcl2-PE.
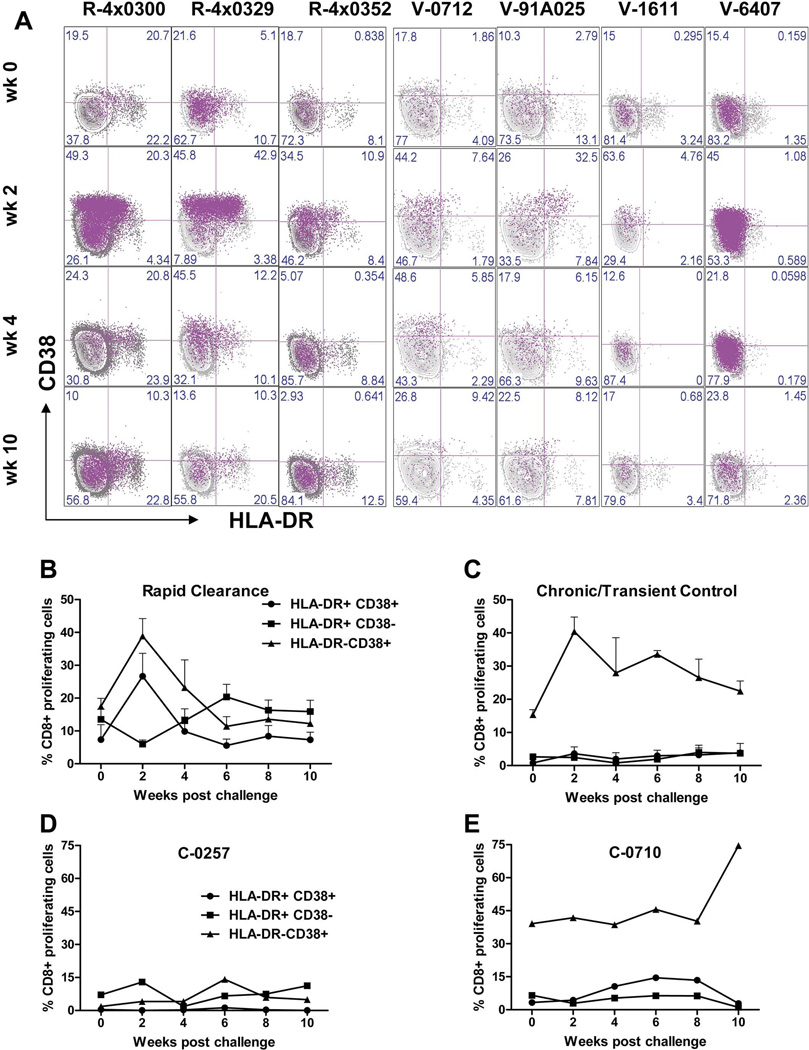
Proliferation of HLA-DR+/CD38+ CD8+ T-cells correlates with outcome
The patterns of activation and proliferation for CD8+ T-cells in the immune-primed animals were very similar after challenge but the outcomes were different. This suggests that increased frequency of activated or proliferating CD4+ or CD8+ T-cells is insufficient for clearance of the virus. We hypothesized that infection outcome may depend upon proliferation of specific cell subsets that contribute to immune responses controlling viral replication. To identify these subsets we compared the frequency of proliferating T-cells co-expressing combinations of CD38 and HLA-DR: CD38+/HLADR−, CD38−/HLADR+, CD38+/HLADR+ and CD38−/HLADR−. The methodology for this analysis is shown in Supplemental Figure S2. The dot plots in Figure 5A represent the overlay of proliferating CD8+ cells onto cells expressing CD38 and/or HLA-DR and in pink show expression of CD38 and HLA-DR on proliferating CD8+ cells. The data from the rechallenged animals reveals high proportions of proliferating cells that are CD38+/HLADR− or CD38+/HLA-DR+ at week 2 (3 left-hand columns). The data from the vaccinated animals reveals that only the animal that rapidly cleared HCV (V-91A025) has a pattern similar to the rechallenged animals i.e. increased frequency of CD38+/HLADR− and CD38+/HLADR+ proliferating CD8+ cells at week 2. The remaining vaccinated animals were found to have substantial increases in CD38+/HLADR− but not CD38+/HLADR+ cells.
Figure 5. Analysis of proliferating CD8+ T-cells co-expressing activation markers for immune primed animals.
A) Overlay dot plot data for vaccinated and rechallenged animals. Proliferating cells are colored pink and the distribution of those cells in each quadrant of the HLA-DR/CD38 analysis is shown. B) Mean frequencies of proliferating CD8+ T-cells (Ki67+/Bcl-2low) expressing HLA-DR and CD38 markers for all animals that rapidly cleared HCV (R-4x0300; R-4x0329; R-4x0352 and V-91A025) (n=4) C) Mean frequencies of proliferating CD8+ T-cells (Ki67+/Bcl-2low) expressing HLA-DR and CD38 markers for vaccinated animals that developed persistent infection or transiently controlled the virus (V-0712; V-1611 and V-6407) (n=3). D) Mean frequencies of proliferating CD8+ T-cells (Ki67+/Bcl-2low) expressing HLA-DR and CD38 markers for control animal C-0257 E) Mean frequencies of proliferating CD8+ T-cells (Ki67+/Bcl-2low) expressing HLA-DR and CD38 markers for control animal C-0710. Bars represent standard error of the mean. For clarity the plot for proliferating cells double negative for CD38 and HLA-DR (CD38−/HLA-DR−) has been omitted. These numbers are presented in Figure 6.
We then analyzed the above data from all animals based on infection outcome regardless of how the immune system was primed, This approach included V-91A025 in the same group as the re-challenged animals. Figures 5B and 5C show the kinetics of proliferating CD8+ T-cells co-expressing combinations of CD38 and HLA-DR. The increase in double-positive proliferating cells in addition to CD38+/HLADR− proliferating cells in the rapidly clearing group can be clearly seen at week 2. This increase coincided with a decline in frequency of HLADR+/CD38− proliferating cells, suggesting HLADR+ cells acquire CD38 upon exposure to HCV. Only elevations in CD38+/HLADR− proliferating cells were observed in vaccinated animals that failed to clear HCV rapidly (Figure 5C) with little or no increase in the frequencies of cells carrying other markers. Analysis of proliferating and activated CD8+ cells from the control animals revealed no patterns in cell markers similar to either of the immune-primed groups (Figure 5D and 5E and Supplemental Figure S3). This is expected given that these animals were naïve for HCV antigens at challenge.
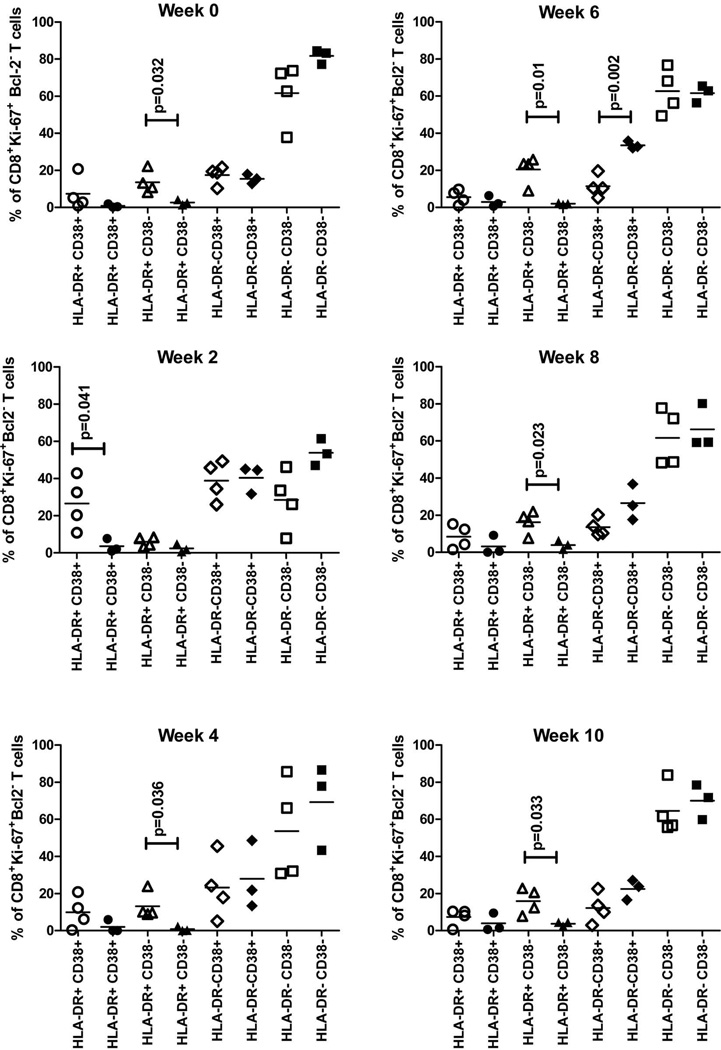
Statistical analysis further confirmed these differences between the immune-primed groups (Figure 6). When grouping the animals based on outcome all animals that rapidly cleared the virus had significantly higher frequencies of proliferating CD8+ T-cells expressing both HLA-DR and CD38 at week 2 (p=0.041) compared with vaccinated animals that did not rapidly control the virus (Figure 6, week 2). Post week 2, and prior to challenge (at week 0), clearance was associated with significantly higher levels of proliferating cells expressing HLA-DR alone (Figure 6). Interestingly, V-1611, V-6407 and V0712 displayed higher frequencies of proliferating cells expressing CD38 alone between weeks 6 and 10; that reached significance at week 6 (p=0.002) (Figure 6).
Figure 6. Percentage of proliferating cells expressing HLADR and CD38 markers pre and post challenge for all immune-primed animals (rechallenged and vaccinated).
Data is divided based upon outcome of infection, animals that rapidly cleared HCV are shown as open symbols (R-4x0300; R-4x0329; R-4x0352 and V-91A025), vaccinated animals that developed persistent infection or transiently controlled the virus are shown as closed symbols (V-0712; V-1611 and V-6407). Circles: HLA-DR+/CD38+; Triangle: HLA-DR+/CD38−; Diamonds: HLA-DR−/CD38+; Squares: HLADR−/CD38−.
Similar analyses of CD4+ cells revealed increases only in proliferating CD38+/HLADR− cells at week 2 with no distinctions between the groups (Figure S4A and S4B). In the control animals there were no such increases in CD4+ cells at early time points post-challenge (Figure S4C and S4D).
Frequency of HLA-DR-positive CD8+ cells post-vaccination correlates with outcome
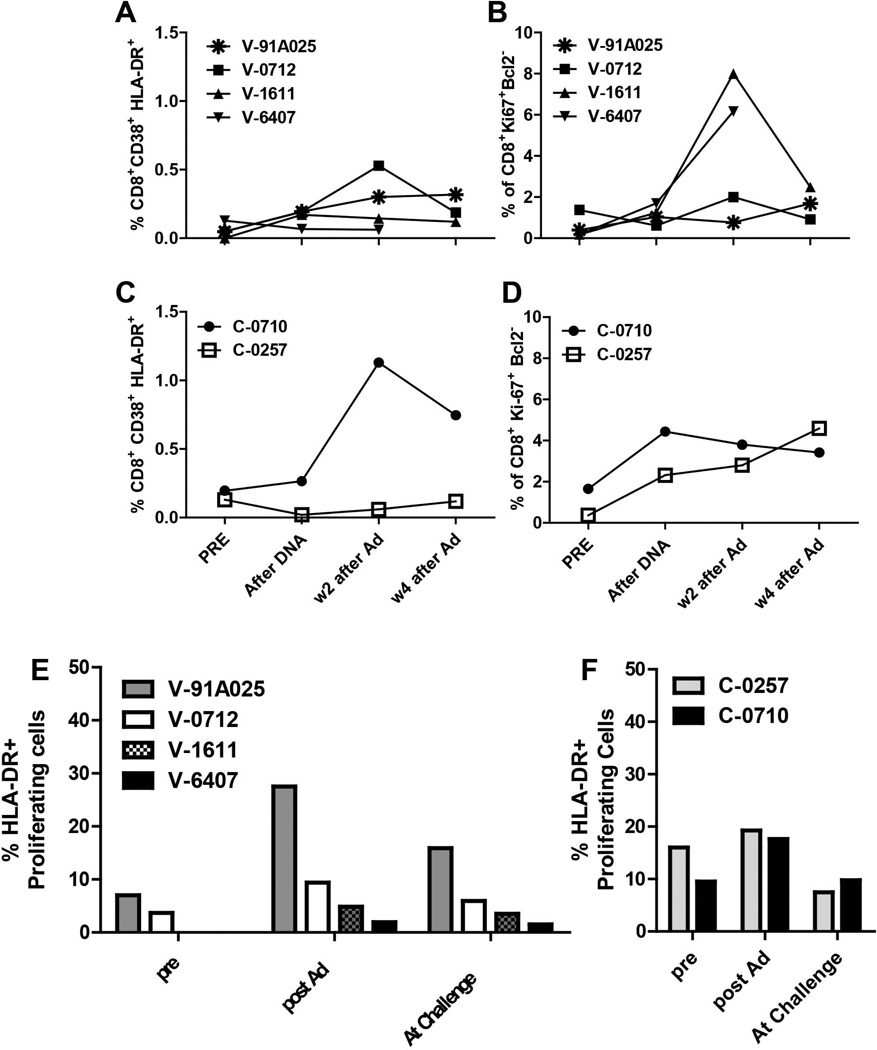
We then asked whether the presence of activation and proliferation markers can predict effectiveness of an HCV vaccine. DNA vaccination did not affect either activation or proliferation of CD8+ T-cells (Figure 7A and 7B). Boosting with rAd induced transient increases in the frequency of activated or proliferating CD8+ T-cells (Figure 7A and 7B); however, the levels did not correlate with the subsequent outcome of challenge. The control animals received non-recombinant DNA vector and were boosted with non-rAd. Although the Ad vector used is non-replicating, there is still adenovirus gene expression which can lead to T-cell stimulation. Therefore as the markers for activation and proliferation were not HCV-specific some increases in cells expressing these markers were seen in these animals (Figure 7C and 7D). No specific patterns of activation and proliferation were obtained for CD4+ cells that correlated with outcome in vaccinated animals during the vaccination phase (data not shown).
Figure 7. Kinetics of activated or proliferating T-cells in vaccinated and control chimpanzees during immunization and prior to challenge with HCV.
A) % CD38+/HLA-DR+ CD8+ T-cells from vaccinated animals B) % Ki67+/Bcl-2-low CD8+ T-cells from vaccinated animals. C) % CD38+/HLA-DR+ CD8+ T-cells from control animals D) % Ki67+/Bcl-2-low CD8+ T-cells from control animals. E) Frequencies of proliferating CD8+ T-cells expressing HLA-DR either alone or co-expressed with the marker CD38 for vaccinated animals post vaccination and pre-challenge. F) Frequencies of proliferating CD8+ T-cells expressing HLA-DR either alone or co-expressed with the marker CD38 for control animals post mock vaccination and pre challenge. PBMCs were gated on CD3+/CD8+ cells or CD3+/CD4+ cells.
Interestingly, when we analyzed the proportion of proliferating CD8+ cells that also expressed activation markers during the vaccine phase we found that the highest frequencies of proliferating cells expressing either HLA-DR alone or HLA-DR in combination with CD38 were again detected in the animal that rapidly cleared HCV (91A025) (Figure 7E). The same CD8+ T-cell subpopulations were also observed in both control animals (Figure 7F) but these levels were not substantially boosted following non-rAd inoculation compared to pre-study levels. Although the numbers of vaccinated animals in this study is small these data suggest that the levels of proliferating CD8+ T-cells co-expressing markers of activation could be used as an indicator of vaccine success in clinical trials.
DISCUSSION
There have been numerous analyses to determine immune responses that impact clearance of HCV during the acute phase of primary infections (25–27) but there are very few that have directly assessed memory T-cell phenotypes that may be important for successful vaccination. Park et al. recently reported that successful vaccination in chimpanzees was associated with higher levels of CD127 on HCV-specific T-cells and lower levels of PD-1 (28) compared to mock vaccinated animals during the primary acute phase of infection. Our studies differ in that successful immune priming, either through vaccination or following clearance of natural infection, has been compared with unsuccessful immune priming. In all immune-primed animals included in this study virus replication was initially controlled by memory responses but the long-term outcome was different. We performed ex-vivo analysis of T-cells in order to avoid the possibility of changes in T-cell markers or phenotypes following in vitro stimulation and compared the frequency of T-cell subsets expressing activation and proliferation markers between two groups of HCV-infected chimpanzees that were immune-primed by vaccination or natural infection.
We found that all vaccinated animals developed HCV-specific T-cell responses as assessed by ELIspot. Amongst this group the highest number of cells at challenge was detected in the animal that cleared virus by week 4 (V-91A025) and this animal consistently maintained the highest levels throughout the follow-up period. The lowest preexisting level of IFNγ-producing T-cells was detected in the animal that developed persistent infection (V-1611). This would suggest that the strength of the ELIspot response correlates with clearance. However, when the immune-primed animals (rechallenged and vaccinated) were analyzed together we observed that R-4x0300 had lower ELIspot responses than any of the vaccinated animals at challenge and throughout most of the follow-up period, indicating that the magnitude of ELIspot responses cannot be taken as an indicator of rapid or long-term clearance.
In this study we concluded that the frequency and kinetics of activated or proliferating T-cell induction cannot predict efficacy of immune priming against HCV and outcome of infection. However, when we evaluated the frequency of proliferating CD8+ T-cells co-expressing combinations of CD38 and HLA-DR a significantly higher frequency of proliferating HLA-DR+/CD38− CD8+ T-cells (p=0.032) was detected at challenge in all animals that rapidly cleared virus compared with animals that developed chronic infection or transiently controlled HCV (Figures 5 and 6). This high frequency of HLA-DR+ proliferating cells, either with or without the expression of CD38, was not observed at any time post-challenge in the vaccinated animals that were unable to rapidly clear HCV (Figure 5 and 6). Only increased frequencies of CD38+ proliferating cells were observed in this group with the levels significantly higher (p=0.002) at week 6. This observation is consistent with data from other studies demonstrating that CD38+ HCV-specific CD8+ T-cells correlate with viral replication not HCV clearance during acute phase infections and that viral control is associated with the loss of the CD38 marker by CD8+ T-cells (26). A recent study suggested that CD8+ T-cells expressing high levels of CD38 represent regulatory T-cells with suppressor function (29).
The fact that the kinetics of T-cells expressing these markers after challenge was very different from those in the control animals and that the increases in frequency coincided with reductions in viral titers provides strong support to the notion that these represent HCV-specific memory cells. We demonstrated peak levels of HCV-specific Ki67+/Bcl2low cells at week 2 post-infection in immune-primed animals, further supporting this conclusion. We were unable to detect HCV-specific proliferating cells at weeks 0 and 4 in most of the animals included in this experiment despite high frequencies of HCV-specific cells detected by ELIspot (Figure 1). This is could be due to the shorter stimulation time used for the FACS analysis (24 hours versus 36–48 hours for ELIspot) and also due to low levels of proliferating cells at these time points in all animals (Figure 3) making the levels of antigen-specific proliferating cells too low to detect in FACS analysis. We were also unable to detect HLA-DR/CD38 subsets within this group which could be explained by an absence of IFNγ production by CD38+/CD8+ T-cells. It was previously demonstrated that during acute HCV infection activated CD8+/CD38+ T-cells do not produce IFNγ (26). Several previous studies also exclude the possibility that the cell populations we observed are non-specific. It was shown that even though bystander activation and proliferation occur at the initiation of an immune response (30) these cells do not contribute significantly to the pool of specific cells (18, 31, 32). Bystander activation occurs before antigen-specific responses and these non-specifically activated cells are subjected to apoptosis (33). Also, antigen-induced proliferation results in increased frequencies of T-cells, unlike bystander proliferation that does not lead to cell expansion (32). In order to unequivocally exclude bystander activity we would need to visualize HCV-specific cells ex-vivo using tetramers, which were unavailable for the animals included in these studies. It is possible that activation and proliferation of CD8+ T-cells is a surrogate of either more robust immune responses or differences in CD4+ T-cells that were inapparent from the markers we applied. Previous studies in rechallenged chimpanzees have shown that CD4+ cells are essential to prevent persistence of HCV in immune-primed animals (34). We showed CD4+ T-cell responses to antigen stimulation (Figure 4B) and increased frequencies post-challenge of CD4+/CD38+ proliferating cells (Figure S4), demonstrating the presence of memory CD4+ cells in all immune-primed animals. Although it can be concluded that the different outcomes were not due to the complete absence of CD4+ memory responses there could be more complex differences in CD4+ T-cell functionality that result in differences in CD8+ T-cell proliferation and activation. Additional studies would be needed to address these issues but these possibilities do not negate the importance or usefulness of the markers we have identified.
The analysis of proliferating T-cells from vaccinated animals pre-challenge revealed a higher frequency of HLA-DR-positive cells in V-91A025. The small numbers of animals available made statistical comparisons impossible but this data together with the data from the recovered/rechallenged animals suggests these CD8+ T-cell phenotypes can represent an indicator of successful immune priming that will lead to rapid clearance of HCV upon exposure to virus.
Overall, we found that immune-primed animals that rapidly cleared HCV had distinctly different memory CD8+ T-cell phenotypes post-challenge compared to animals that did not rapidly control viral replication. The application of activation and proliferation markers alone was insufficient to predict outcome when applied either before or after challenge. Additionally, HCV-specific T-cell frequency at challenge as measured by ELIspot did not correlate with post-challenge outcome. These data provide a new approach to applying T-cell markers during clinical trials for HCV that may be useful for assessing the potential of vaccines to induce functional memory T-cells and result in effective clearance of HCV.
Supplementary Material
Acknowledgements
We would like to thank the CDC veterinary staff for excellent support and care during the primate experiments at CDC. We also thank Dr. Marina Zaitseva for critical reading of the manuscript. The findings and conclusions in this article should not be construed to represent any FDA determination or policy.
Financial Support:
This work was supported by CDC and FDA intramural research funds. The project was supported in part by the appointment of Hongying Duan to the Research Participation Program at CBER administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
Abbreviations
- HCV
Hepatitis C virus
- PBMCs
Peripheral blood mononuclear cells
- SFU
Spot forming units
- IFNγ
Interferon gamma
- TNFα
Tumor necrosis factor alpha
- rAd
Recombinant adenovirus
- Ad
Adenovirus
- non-rAd
Non-recombinant adenovirus
- i/v
Intravenously
- i/m
Intramuscularly
- CID50
50% chimpanzee infectious doses
- GE
genome equivalents
- FACS
Fluorescence-activated cell sorting
Contributor Information
Iryna Zubkova, Email: Iryna.Zubkova@fda.hhs.gov.
Hongying Duan, Email: Hongying.Duan@fda.hhs.gov.
Frances Wells, Email: Frances.Wells@fda.hhs.gov.
Howard Mostowski, Email: Howard.Mostowski@fda.hhs.gov.
Esther Chang, Email: change@georgetown.edu.
Kathleen Pirollo, Email: pirollok@georgetown.edu.
Kris Krawczynski, Email: kzk1@cdc.gov.
Robert Lanford, Email: rlanford@sfbr.org.
Marian Major, Email: marian.major@fda.hhs.gov.
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006 May;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000 Feb;132(4):296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 3.CDC. Estimates of disease burden from viral hepatitis. Atlanta, GA: US Department of Health and Human Services, CDC; 2007. Available at http://wwwcdcgov/hepatitis/PDFs/disease_burdenpdf. [Google Scholar]
- 4.Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010 Jan;138(1):315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahari H, Feinstone SM, Major ME. Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology. 2010 Sep;139(3):965–974. doi: 10.1053/j.gastro.2010.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grakoui A, Hanson HL, Rice CM. Bad time for Bonzo? Experimental models of hepatitis C virus infection, replication, and pathogenesis. Hepatology. 2001 Mar;33(3):489–495. doi: 10.1053/jhep.2001.23041. [DOI] [PubMed] [Google Scholar]
- 7.Nascimbeni M, Mizukoshi E, Bosmann M, Major ME, Mihalik K, Rice CM, Feinstone SM, et al. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J Virol. 2003 Apr;77(8):4781–4793. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shata MT, Anthony DD, Carlson NL, Andrus L, Brotman B, Tricoche N, McCormack P, et al. Characterization of the immune response against hepatitis C infection in recovered, and chronically infected chimpanzees. J Viral Hepat. 2002 Nov;9(6):400–410. doi: 10.1046/j.1365-2893.2002.00373.x. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe H, Wells F, Major ME. Clearance of hepatitis C in chimpanzees is associated with intrahepatic T-cell perforin expression during the late acute phase. J Viral Hepat. 2010 Apr;17(4):245–253. doi: 10.1111/j.1365-2893.2009.01172.x. [DOI] [PubMed] [Google Scholar]
- 10.Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A. 2002 Nov;99(24):15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spada E, Mele A, Berton A, Ruggeri L, Ferrigno L, Garbuglia AR, Perrone MP, et al. Multispecific T cell response and negative HCV RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut. 2004 Nov;53(11):1673–1681. doi: 10.1136/gut.2003.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zubkova I, Choi YH, Chang E, Pirollo K, Uren T, Watanabe H, Wells F, et al. T-cell vaccines that elicit effective immune responses against HCV in chimpanzees may create greater immune pressure for viral mutation. Vaccine. 2009 Apr;27(19):2594–2602. doi: 10.1016/j.vaccine.2009.02.045. [DOI] [PubMed] [Google Scholar]
- 13.Major ME, Mihalik K, Puig M, Rehermann B, Nascimbeni M, Rice CM, Feinstone SM. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J Virol. 2002 Jul;76(13):6586–6595. doi: 10.1128/JVI.76.13.6586-6595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassett SE, Guerra B, Brasky K, Miskovsky E, Houghton M, Klimpel GR, Lanford RE. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001 Jun;33(6):1479–1487. doi: 10.1053/jhep.2001.24371. [DOI] [PubMed] [Google Scholar]
- 15.Su AI, Pezacki JP, Wodicka L, Brideau AD, Supekova L, Thimme R, Wieland S, et al. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A. 2002 Nov;99(24):15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000 May;191(9):1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002 Apr;8(4):379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 18.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008 May;28(5):710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Endl E, Kausch I, Baack M, Knippers R, Gerdes J, Scholzen T. The expression of Ki-67, MCM3, and p27 defines distinct subsets of proliferating, resting, and differentiated cells. J Pathol. 2001 Nov;195(4):457–462. doi: 10.1002/path.978. [DOI] [PubMed] [Google Scholar]
- 20.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000 Apr;164(8):3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 21.Puig M, Major ME, Mihalik K, Yu MY, Feinstone SM. Immunization of chimpanzees with an envelope protein-based vaccine enhances specific humoral and cellular immune responses that delay hepatitis C virus infection. Vaccine. 2004;22:991–1000. doi: 10.1016/j.vaccine.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Puig M, Mihalik K, Yu MY, Feinstone SM, Major ME. Sensitivity and reproducibility of HCV quantitation in chimpanzee sera using TaqMan real-time PCR assay. J Virol Methods. 2002 Sep;105(2):253–263. doi: 10.1016/s0166-0934(02)00119-2. [DOI] [PubMed] [Google Scholar]
- 23.Puig M, Mihalik K, Tilton JC, Williams O, Merchlinsky M, Connors M, Feinstone SM, et al. CD4+ immune escape and subsequent T-cell failure following chimpanzee immunization against hepatitis C virus. Hepatology. 2006 Sep;44(3):736–745. doi: 10.1002/hep.21319. [DOI] [PubMed] [Google Scholar]
- 24.Major ME, Mihalik K, Fernandez J, Seidman J, Kleiner D, Kolykhalov AA, Rice CM, et al. Long-term follow-up of chimpanzees inoculated with the first infectious clone for hepatitis C virus. J Virol. 1999 Apr;73(4):3317–3325. doi: 10.1128/jvi.73.4.3317-3325.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smyk-Pearson S, Tester IA, Klarquist J, Palmer BE, Pawlotsky JM, Golden-Mason L, Rosen HR. Spontaneous recovery in acute human hepatitis C virus infection: functional T-cell thresholds and relative importance of CD4 help. J Virol. 2008 Feb;82(4):1827–1837. doi: 10.1128/JVI.01581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001 Nov;194(10):1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006 Nov;80(22):11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SH, Shin EC, Capone S, Caggiari L, De RV, Nicosia A, Folgori A, et al. Successful vaccination induces multifunctional memory T-cell precursors associated with early control of hepatitis C virus. Gastroenterology. 2012 Oct;143(4):1048–1060. doi: 10.1053/j.gastro.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bahri R, Bollinger A, Bollinger T, Orinska Z, Bulfone-Paus S. Ectonucleotidase CD38 demarcates regulatory, memory-like CD8+ T cells with IFN-gamma-mediated suppressor activities. PLoS One. 2012;7(9):e45234. doi: 10.1371/journal.pone.0045234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doisne JM, Urrutia A, Lacabaratz-Porret C, Goujard C, Meyer L, Chaix ML, Sinet M, et al. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J Immunol. 2004 Aug;173(4):2410–2418. doi: 10.4049/jimmunol.173.4.2410. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed R, Akondy RS. Insights into human CD8(+) T-cell memory using the yellow fever and smallpox vaccines. Immunol Cell Biol. 2011 Mar;89(3):340–345. doi: 10.1038/icb.2010.155. [DOI] [PubMed] [Google Scholar]
- 32.Kim SK, Brehm MA, Welsh RM, Selin LK. Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation. J Immunol. 2002 Jul;169(1):90–98. doi: 10.4049/jimmunol.169.1.90. [DOI] [PubMed] [Google Scholar]
- 33.McNally JM, Zarozinski CC, Lin MY, Brehm MA, Chen HD, Welsh RM. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J Virol. 2001 Jul;75(13):5965–5976. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003 Oct;302(5645):659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.