Abstract
Buprenorphine is a partial mu opioid agonist and kappa opioid antagonist currently under development as a maintenance medication for heroin dependence. Because of concerns about illicit diversion of buprenorphine, a combination tablet containing buprenorphine and naloxone has been developed. The present study evaluated the reinforcing effects of intravenously-administered placebo, buprenorphine alone (BUP; 2, 8 mg), and the buprenorphine/naloxone combination (BUP/NX; 2 mg buprenorphine plus 0.5 mg naloxone, 8 mg buprenorphine plus 2 mg naloxone) in recently detoxified heroin abusers during a 6-week inpatient study. Participants (N=6) were detoxified from heroin over approximately 1 week immediately after admission. During the next 5 weeks, the reinforcing effects of placebo, BUP, and BUP/NX were evaluated. Participants first received a dose of drug and $20, and then were given the opportunity to self-administer either the dose or $20 during choice sessions. Progressive ratio break point values were significantly higher after active drug, compared to placebo, but they did not significantly differ as a function of dose or drug. In contrast, positive subjective ratings were higher after administration of BUP compared to BUP/NX, and these ratings increased in a dose-dependent fashion. BUP and the combination had few effects on performance. Relative to placebo, both BUP and BUP/NX decreased pupil diameter, but there were no significant differences in pupil diameter as a function of drug or dose. These results demonstrate that both BUP and BUP/NX served as reinforcers under these conditions, and that they may have similar abuse liability in recently detoxified individuals who abuse heroin.
Both Phase I and II clinical trials have demonstrated that buprenorphine is effective in treating opioid dependence (Bickel and Amass, 1995; Johnson et al., 2000). Despite its clear clinical utility, several lines of evidence suggest that buprenorphine has some abuse liability. Studies using non-human primates showed that buprenorphine serves as a reinforcer (Negus and Woods, 1995). For example, Winger and Woods (2001) showed that rates of responding maintained by buprenorphine were higher than placebo, but lower than rates of responding for the full mu agonists heroin and alfentanil (Winger and Woods, 2001). Another study demonstrated that buprenorphine was less reinforcing than heroin, but equivalent to methadone (Mello et al., 1988). Thus, buprenorphine is self-administered by laboratory animals, and under some conditions, is self-administered at rates comparable to full mu agonists.
In humans, two studies examined the reinforcing effects of buprenorphine in heroin users maintained on sublingual buprenorphine in outpatient treatment settings (Amass et al., 2000; Petry and Bickel, 1999). Petry and Bickel (1999) examined the reinforcing effects of sublingual buprenorphine. Participants, who were given the opportunity to choose between sublingual buprenorphine and money, almost exclusively self-administered buprenorphine when the alternative money value was low. When the alternative money value increased, buprenorphine self-administration decreased. These data are consistent with a number of other studies demonstrating that drug self-administration varies as a function of the magnitude of an alternative reinforcer (e.g., Comer et al., 1997, 1998; Heishman et al., 2000; Higgins et al., 1994; Stitzer et al., 1983; Vuchinich and Tucker, 1983). Amass et al. (2000) studied the reinforcing effects of intravenously-administered buprenorphine, the buprenorphine/naloxone combination, and hydromorphone in individuals maintained on the buprenorphine/naloxone combination. When given the opportunity to self-administer either money or drug, participants almost exclusively chose money. However, these individuals were currently in outpatient treatment for their heroin dependence. Given that illicit drug abstinence was a requirement for study participation, it may be that participants were also unlikely to self-administer opiates in the laboratory.
In both of the studies described above, the reinforcing effects of buprenorphine were evaluated in participants who were maintained on buprenorphine. Winger and Woods (2001) showed that buprenorphine was self-administered more than placebo in rhesus monkeys who were not morphine-dependent, but it did not serve as a reinforcer when the same monkeys were maintained on morphine. These data suggest that opioid dependence may be a critical variable in the reinforcing effects of buprenorphine. A study recently completed in our laboratory (Comer et al., 2002) showed that buprenorphine was self-administered above placebo levels in recently-detoxified, and therefore non-dependent, individuals who were not seeking treatment for their heroin use. The present study was designed to compare the reinforcing effects of intravenous buprenorphine alone and in combination with naloxone.
Partly in response to several epidemiological and case report studies worldwide demonstrating buprenorphine abuse (Baumevieille et al., 1997; O’Connor et al., 1988; Sakol et al., 1989; Singh et al., 1992), a combination tablet containing both buprenorphine and naloxone has been developed in an attempt to reduce illicit diversion of buprenorphine. The rationale behind the development of a buprenorphine/naloxone combination medication is that bioavailability of naloxone is poor after sublingual administration, but it is effective as an opioid antagonist after parenteral administration. Thus, naloxone should have little effect on buprenorphine’s therapeutic utility when the combination buprenorphine/naloxone tablet is ingested sublingually, but if the tablet is dissolved in water or saline and injected intravenously, naloxone will antagonize the effects of buprenorphine, thereby reducing its abuse potential. However, it is not clear how effective this strategy will be in reducing buprenorphine abuse: Although the reported misuse, as well as the street price, of the combination buprenorphine/naloxone tablet decreased relative to buprenorphine alone, it was still abused in some countries (see Robinson et al., 1993). Therefore, the present study sought to systematically characterize the reinforcing effects of the combination, relative to buprenorphine alone. Although the exact amount of buprenorphine and/or naloxone that can be obtained from a crushed up tablet is unknown, doses of 2 and 8 mg buprenorphine and the combination were used in the present study because these are the dosage forms of the sublingual tablets that will be available for clinical use. The primary hypothesis in this study was that progressive ratio break points would be higher following active buprenorphine, relative to placebo, and that break point values would be higher following buprenorphine alone, relative to the combination. Secondary hypotheses were that buprenorphine alone would increase subjective ratings, impair performance, and decrease pupil diameter, relative to placebo and the combination.
Methods
Participants
Six heroin-dependent individuals (5M, 1F; 2 Hispanic, 2 White, and 2 Black), who currently were not seeking treatment for their drug use completed the 6-week protocol. Participants were 35.7 years old on average (range: 28 to 42 years), and reported using heroin for an average of 10.8 years (range: 4 to 20 years). All participants had experience using heroin by the intravenous route, were currently physiologically dependent on it as verified by a naloxone challenge test prior to admission, and reported spending an average of $55 per day on heroin (range: $40-$100). Heroin was the drug of choice for all participants. Six participants smoked tobacco cigarettes (10 cigarettes per day), two participants used cocaine (once per week or less), three participants used alcohol (1 to 2 times per week), one participant used marijuana (once per week or less), and two participants used sedatives (once per week or less). One additional participant completed the study, but because he only chose money throughout the study, his data were not included in the analysis. This participant stated that he needed to earn money to support his children.
After an initial telephone interview, eligible participants received additional screening, which included completing detailed questionnaires on drug use, general health and medical history, and a medical and psychological evaluation. Participants were told that they would receive opioids during the study, and that different doses would be tested. An electrocardiogram and Mantoux test or chest x-ray were also performed. Routine laboratory analyses included a hematology screen, blood chemistry panel, liver function tests, thyroid function tests, syphilis serology, and urinalysis. Urine drug toxicologies (opioids, benzoylecgonine, benzodiazepines, cannabinoids, and amphetamines) were also performed using a radiative energy attenuation and fluorescence polarization immunoassay system (ADx System, Abbott Laboratories, Abbott Park, IL).
Participants were excluded from the study if they were seeking drug treatment, dependent on alcohol or illicit drugs other than opioids, or had a major Axis I psychiatric diagnosis other than heroin dependence (e.g., bipolar disorder, schizophrenia, major depression). Those who had recent histories of violence or who were on parole/probation were excluded from the study. Participants were required to be physically healthy, and fully able to perform all study procedures.
Prior to admission, participants completed a training session, during which the study procedures were explained to them in detail. Volunteers were paid $25 per inpatient day and an additional $25 per day bonus if they completed the study. In addition, they could receive an additional $40 per day during some of the experimental sessions. Participants signed consent forms describing the aims of the study, and the potential risks and benefits of participation. Participants were offered free HIV testing, drug and risk reduction education, and referrals for treatment. This study was approved by the Institutional Review Board of the New York State Psychiatric Institute (NYSPI).
Apparatus
During experimental sessions, participants were seated in a room equipped with Macintosh computers. All computer activities, vital signs and behaviors were continuously monitored by the experimenters in an adjacent control room via a continuous on-line computer network, one-way mirror, and vital signs monitors (cardiovascular function was measured using a Sentry II Vital Signs Monitor, NBS Medical, Costa Mesa, CA; arterial oxygen saturation was measured using a pulse oximeter Model 400, Palco Laboratories, Santa Cruz, CA). Communication between the staff and participants was kept to a minimum during experimental sessions.
Detoxification Procedures
Participants were detoxified during the first week after their admission into the hospital. Buprenorphine (8 mg sublingual tablet; National Institute on Drug Abuse, Rockville, MD) was administered during the first 2 days after admission. Clonidine HCl (0.2 mg p.o., q. 6 h; Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT), ketorolac tromethamine (30 mg i.m., q. 6 h for up to 5 days; Roche Laboratories, Nutley, NJ), prochlorperazine (10 mg p.o. or i.m., q. 8 h; SmithKline Beecham Consumer Healthcare, Pittsburgh, PA) and clonazepam (2 mg p.o., q. 8 h; Roche Laboratories Inc., Nutley, NJ) were available, as needed. All of these medications were discontinued approximately 36 h prior to the first experimental session.
General Procedures
The reinforcing effects of intravenous placebo, BUP (2, 8 mg), and BUP/NX (2/0.5, 8/2 mg) were evaluated following detoxification. All doses were administered in nonsystematic order both within and between participants. On Mondays, a dose of drug and $20 was administered, and subjective, performance, and physiological effects were examined both before and repeatedly after drug administration. These measures were repeated on Tuesdays (24 h) and Wednesdays (48 h) in order to assess the time course of the drug administered on Monday. No drugs were administered on Tuesdays and Wednesdays. On Thursdays and Fridays, participants completed two choice sessions per day, for a total of four choice sessions. They could work to receive all, or part of the dose or $20. The total amount of drug and/or money chosen during the self-administration task was given as a bolus at the end of the task. Within each choice day, an interdose interval of 5 h was used because it mimics the typical pattern of heroin use reported by heroin-dependent individuals.
Experimental Sessions
During all sessions, participants completed computerized tasks and subjective-effects questionnaires. Heart rate and blood pressure were measured every 5 min, and blood oxygen saturation was monitored continuously with a pulse oximeter and recorded every minute during experimental sessions. Pupil photographs were taken repeatedly during the sessions. Participants were not allowed to smoke tobacco cigarettes during experimental sessions.
Sample Session
Physiological, subjective and performance effects were measured both before and repeatedly after drug administration (see descriptions below). Following baseline measures, drug and $20 were administered simultaneously at time 0 min, provided that oxygenation was sufficient (SpO2 > 93%). A photograph was taken of the right pupil before and 4, 10, 40, 60, 90, 120 and 180 min after drug administration. The subjective-effects battery (see description below) was administered before and 4, 40, 90, 150 and 210 min after drug administration. The performance battery (see description below) was administered before and 10, 60, 120, and 180 min after drug administration. The Subjective Opioid Withdrawal Scale (SOWS) was administered before and 180 min after drug administration. The Drug Effects Questionnaire (DEQ) was administered 4, 10, 60, 120, and 180 min after drug administration.
No Drug Sessions
Physiological, subjective and performance effects were measured 24 and 48 h after administration of drug to evaluate potential long-lasting agonist effects.
Choice Sessions
Choice sessions were similar in design to the sample session, except that participants completed a self-administration task (see below) after the baseline assessments. Participants were instructed to choose between tenths of $20 and the dose that they received during the sample session. A pupil photograph was taken before drug administration. The subjective-effects battery (see description below) was administered before and 4 and 40 min after drug administration. The performance battery was completed before, and 10 min after drug administration. The SOWS was completed before drug administration. The DEQ was completed before and 10 min after drug administration. Choice sessions were otherwise identical to the sample session.
Self Administration Task
Participants were told that they could work for all or part of the sampled dose or the sampled money amount ($20) by choosing the drug or money option each time a choice was available. Responses consisted of finger presses on a computer mouse. Standardized instructions were read to each participant explaining the self-administration task. Drug and money were available under independent progressive ratio schedules, and participants were given 10 opportunities (trials) to choose between the two options. Ten percent of that day’s dose or money value was available at each choice trial. Thus, if the dose for that day was 8 mg, at each opportunity participants could respond for 0.8 mg (10% of 8 mg) or $2 (10% of $20). Completion of the ratio requirement for each choice trial was accompanied by a visual stimulus on the computer screen. After a choice was made for one option, responding for the other option was not possible until the ratio was completed and another trial was initiated. The response requirement for each of the two options increased independently such that the initial ratio requirement for each option was 50 responses; the ratio increased progressively each time the option was selected (50, 100, 200, 400, 800, 1200, 1600, 2000, 2400, 2800). In order to receive all of the drug or money available that day, participants were required to emit 11,550 responses within 40 min. Fewer total responses were required if choices were distributed between the two options. These ratio values were chosen based on previous research conducted in our laboratory (e.g., Comer et al.,1999). Although it required sustained, high rates of responding, participants were capable of completing 11,550 responses in the allotted time.
At the start of each self-administration task, two illustrations appeared on the computer screen: an empty balance scale and an empty bank. As each choice trial was completed, either the scale was implemented with a pile of powder or a dollar sign was added to the bank. Thus, participants could always see how many money and drug choices had been made. At the end of the 40-min self-administration task, the participant received whatever he had chosen: money and/or drug.
Subjective Measures
Four questionnaires were used to assess subjective effects (see Comer et al., 1999 for details). The first questionnaire was a 26-item visual analog scale (VAS) designed to assess subjective and physiological effects. The first eighteen lines were labeled with adjectives describing mood states (e.g., “I feel…:” “high”) and four additional lines, labeled with questions about the dose just received (e.g., “I liked the dose,” “For this dose, I would pay”). Participants also indicated, by making a mark along a 100 mm line, how much they “wanted” each of the following drugs: heroin, cocaine, alcohol, and tobacco. Participants rated each item on the VAS from “Not at all” (0 mm) to “Extremely” (100 mm), except for the “For this dose, I would pay” question, which ranged between $0 (0 mm) and $20 (100 mm). The second questionnaire was a 13-item opioid symptom checklist consisting of true/false questions designed to measure opioid effects (e.g., “My skin is itchy”). The VAS and opioid symptom checklist together constituted the subjective-effects battery. The third questionnaire was the 16-item Subjective Opioid Withdrawal Scale (SOWS). Participants rated each item on a scale from 0 to 4, with 0 being “Not at all” and 4 being “Extremely” (e.g., “I have gooseflesh,” etc.). The fourth questionnaire was a 6-item Drug Effects Questionnaire (DEQ). Participants described drug effects by selecting among a series of possible answers ranging from 0 (“No (good, bad, etc.) effect at all”) to 4 (“Very strong effects”). Ratings of drug liking ranged between −4 (“Dislike very much”) to 4 (“Like very much”).
Task Battery
The task battery consisted of four tasks: a 3-min digit-symbol substitution task, a 10-min divided attention task, a 10-min rapid information processing task, and a 3-min repeated acquisition of response sequences task (see Comer et al., 1999 for details).
Physiological Measures
A blood pressure cuff was attached to the non-dominant arm, and blood pressure was recorded automatically every 5 min. However, because of a large number of missing data points from the blood pressure measurements, those data were not analyzed statistically. Participants were also connected to a pulse oximeter via a soft sensor on a finger of the non-dominant hand, which monitored arterial blood oxygen saturation (%SpO2). For safety, supplemental oxygen (2 L/min) was provided via a nasal cannula during all experimental sessions. If oxygen saturation decreased below 90%, breaths were prompted verbally by staff and the oxygen flow rate was increased. Average arterial oxygen saturation remained above 90% during all sessions. A specially-modified Polaroid camera with a close-up lens (2X magnification) was used to take pupil photographs. All photographs were taken under ambient lighting conditions. Horizontal and vertical measurements of pupil diameter were made using a calipers, and then these two measurements were averaged and divided by 2 to correct for the 2X magnification.
Drugs
Buprenorphine HCl for injection (4 mg/ml) was provided by the National Institute on Drug Abuse (Rockville, MD). Naloxone HCl (1 mg/ml; Narcan®) for injection was obtained from DuPont Pharma (Wilmington, DE). BUP and BUP/NX were diluted in 5% dextrose to produce each dose. Placebo (5% dextrose) or active drug was administered intravenously through a catheter over a 30-sec period in a total volume of 4 ml. Physiologic saline solution was infused continuously during experimental sessions, except during drug administration. Between 1 and 2 ml heparinized saline (10 units/ml) was flushed into the catheter four to eight times each day. All venous catheters were maintained as heparin locks and were removed within 36 h of insertion.
Supplemental medications available to all participants for the duration of the study included: Mylanta®, acetaminophen, ibuprofen, Colace®, Milk of Magnesia® and multi-vitamins with iron. Trazodone (50 mg p.o., at bedtime; Warner Chilcott, Morris Plains, NJ) was available if participants reported having trouble sleeping.
Morning urine samples were collected daily and one random sample per week was screened for the presence of other illicit substances. No illicit substances, other than opioids, were found in the participants’ urines.
Statistical Analyses
Repeated measures analyses of variance (ANOVAs) with planned comparisons were performed to answer the following questions: 1) Do BUP and BUP/NX function as a reinforcers? 2) Do the reinforcing effects of BUP and BUP/NX vary across choice sessions? and 3) What is the duration of action of BUP and BUP/NX’s subjective, performance, and physiological effects? Specifically: 1) To evaluate the reinforcing effects of BUP and BUP/NX, the progressive ratio break point value and number of choices for each active dose was compared to placebo. To evaluate dose-related effects, the break point value for 2 mg BUP was compared to 8 mg BUP, and the break point value for 2 mg BUP/NX was compared to 8 mg BUP/NX. To determine whether the effects of BUP differed from those produced by the combination, 2 mg BUP was compared to 2 mg BUP/NX and 8 mg BUP was compared to 8 mg BUP/NX. 2) To assess changes in reinforcing effects within and across days, break point values and number of choices across the four choice sessions were compared. 3) To measure the time course of effects produced by the sample dose, the subjective, physiological, and performance effects produced by each active dose was compared to placebo at each time point.
Drug and money break point values and choices were analyzed as a function of dose (0 mg, 2 mg BUP, 8 mg BUP, 2 mg BUP/NX, 8 mg BUP/NX) and choice session (1–4). Pupil diameter, task performance, and subjective ratings during the sample session were analyzed as a function of dose and time. SOWS data during the detoxification week were also analyzed using repeated measures ANOVAs. To control for type I errors, only those comparisons with P values less than 0.01 were considered statistically significant. Because only one female participant completed the study, it was not possible to examine potential sex differences in the present study.
Results
Choice
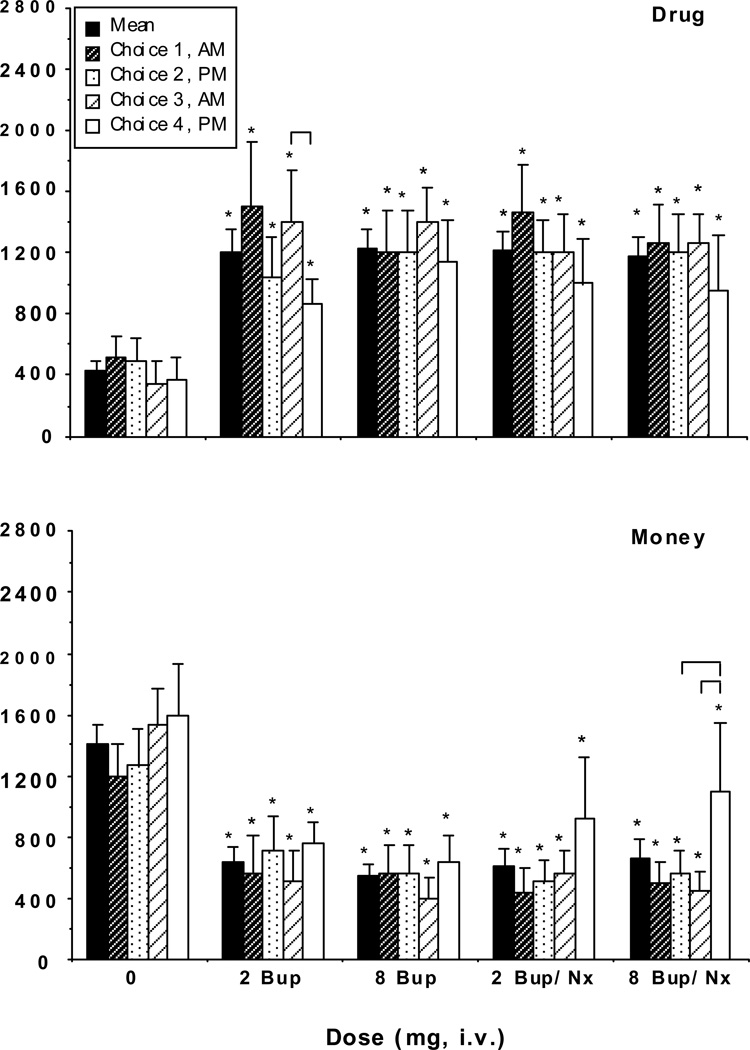
Figure 1 shows progressive ratio break point values for drug (top panel) and money (bottom panel) as a function of dose and choice session. Mean break point values for each dose of active drug were significantly greater than for placebo [2 mg BUP (F 1,20=8.72, P < 0.008); 8 mg BUP (F 1,20=9.50, P < 0.006); 2 mg BUP/NX (F 1,20=9.11, P < 0.007); 8 mg BUP/NX (F 1,20=8.07, P < 0.01)]. Break point values for drug at each choice session were also significantly different from the corresponding placebo break point value. However, break point values for 2 mg versus 8 mg BUP and 2 mg versus 8 mg BUP/NX did not significantly differ from each other. Similarly, break points for 2 mg BUP did not differ from 2 mg BUP/NX, and 8 mg BUP did not differ from 8 mg BUP/NX. Break point values for drug did not differ across the four choice sessions, with the exception that break point values were lower in the afternoons compared to the mornings after 2 mg BUP (Choice 1 versus 2: F 1,60=5.76, P < 0.02; Choice 3 versus 4: F 1,60=7.52, P < 0.008).
Figure 1.
Progressive ratio break point values for drug (top panel) and money (bottom panel) as a function of dose and choice session. Break point values could range between 0 and 2800. Data points represent the mean across 6 participants and 4 choice sessions (solid bars), and the mean across 6 participants during each of the 4 choice sessions. Error bars represent ± 1 standard error of the mean (S.E.M.). * indicates significant differences from placebo; brackets indicate significant differences between those two data points (P<0.01).
Mean break point values for money (Figure 1, bottom panel) when active drug was available were significantly lower than when placebo was available [2 mg BUP (F 1,20=9.26, P < 0.006); 8 mg BUP (F 1,20=11.74, P < 0.003); 2 mg BUP/NX (F 1,20=9.93, P < 0.005); 8 mg BUP/NX (F 1,20=8.86, P < 0.008)]. There were no statistically significant differences in break point values for money across the four choice sessions, with the exception of the fourth choice session when 8 mg BUP/NX was available (Choice 2 versus 4: F 1,60=9.93, P < 0.003; Choice 3 versus 4: F 1,60=14.75, P < 0.0003). Differences approached statistical significance between the fourth choice session and the other choice sessions when 2 mg BUP/NX was available (Choice 2 versus 4: F 1,60=5.59, P < 0.02; Choice 3 versus 4: F 1,60=4.28, P < 0.04).
The number of drug and money choices (Table 1) showed a very similar pattern as the break point values. Participants chose the drug option on 3–4 of the trials when placebo was available, and approximately 5–7 of the trials when active drug was available. Money choices mirrored the drug choices in that participants chose the money option on 6–7 of the trials when placebo was available, and approximately 3–5 of the trials when active drug was available. Drug and money choices were significantly different when active drug was available than when placebo was available during each of the four choice sessions.
Table 1.
Mean (± S.E.M.) drug and money choices (maximum=10).
| Mean Choice | Choice | Choice | Choice | Choice | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Session 1–4 | Session 1 | Session 2 | Session 3 | Session 4 | ||||||
| Drug | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| Placebo | 3.50 | 0.31 | 4.00 | 0.52 | 3.83 | 0.60 | 3.17 | 0.60 | 3.00 | 0.82 |
| 2 mg Bup | 5.96 | 0.4a | 6.67 | 1.12a | 5.50 | 0.72a | 6.50 | 0.85a | 5.17 | 0.4a |
| 8 mg Bup | 6.08 | 0.31a | 6.00 | 0.68a | 6.00 | 0.68a | 6.50 | 0.56a | 5.83 | 0.7a |
| 2 mg Bup/Nx | 5.92 | 0.39a | 6.67 | 0.76a | 6.00 | 0.52a | 6.00 | 0.63a | 5.00 | 1.12a |
| 8 mg Bup/Nx | 5.75 | 0.4a | 6.17 | 0.6a | 6.00 | 0.63a | 6.17 | 0.48a | 4.67 | 1.31a,b |
| Mean Choice | Choice | Choice | Choice | Choice | ||||||
| Session 1–4 | Session 1 | Session 2 | Session 3 | Session 4 | ||||||
| Money | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| Placebo | 6.50 | 0.31 | 6.00 | 0.52 | 6.17 | 0.60 | 6.83 | 0.60 | 7.00 | 0.82 |
| 2 mg Bup | 4.04 | 0.4a | 3.33 | 1.12a | 4.50 | 0.72a | 3.50 | 0.85a | 4.83 | 0.4a |
| 8 mg Bup | 3.92 | 0.31a | 4.00 | 0.68a | 4.00 | 0.68a | 3.50 | 0.56a | 4.17 | 0.7a |
| 2 mg Bup/Nx | 4.08 | 0.39a | 3.33 | 0.76a | 4.00 | 0.52a | 4.00 | 0.63a | 5.00 | 1.12a |
| 8 mg Bup/Nx | 4.25 | 0.4a | 3.83 | 0.6a | 4.00 32 | 0.63a | 3.83 | 0.48a | 5.33 | 1.31a,b |
indicates a significant difference from placebo;
indicates a significant difference between Sessions 3 and 4 at that dose. (P<0.01)
Subjective Effects
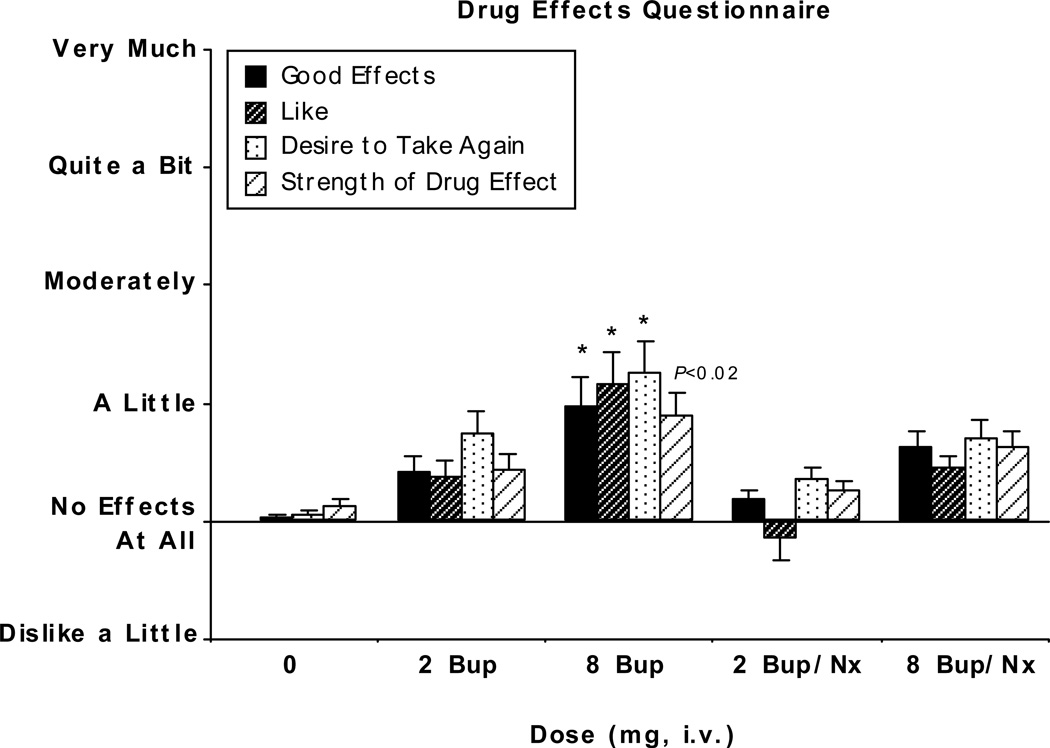
In contrast to the lack of a dose-response relationship for the reinforcing effects of BUP and BUP/NX, the subjective-effects ratings were clearly dose-related, particularly after administration of BUP alone. Figure 2 shows mean ratings on the Drug Effects Questionnaire: 8 mg BUP increased ratings of “Good Effects” (F 1,20=9.13, P < 0.006), drug “Liking” (F 1,20=8.85, P < 0.008), “Desire to Take the Drug Again” (F 1,20=8.06, P < 0.01), and “Strength of Drug Effect” (F 1,20=6.42, P < 0.02). In general, the drug effects were rated as being “mild to moderate” in magnitude. DEQ ratings after 2 mg BUP were intermediate between those produced by placebo and 8 mg BUP, but none of the ratings after 2 mg BUP were significantly different from placebo. Similarly, mean ratings after 2 mg and 8 mg BUP/NX were not significantly different from placebo. Ratings after 2 mg versus 8 mg BUP, 2 mg versus 8 mg BUP/NX, 2 mg BUP versus 2 mg BUP/NX, and 8 mg BUP versus 8 mg BUP/NX were not significantly different for any of the subjective measures on the DEQ.
Figure 2.
Selected Drug Effects Questionnaire ratings as a function of dose. Data points represent mean ratings for the 6 participants across the sample and no drug sessions. Error bars represent ± 1 standard error of the mean (S.E.M.). * indicates significant differences from placebo (P<0.01).
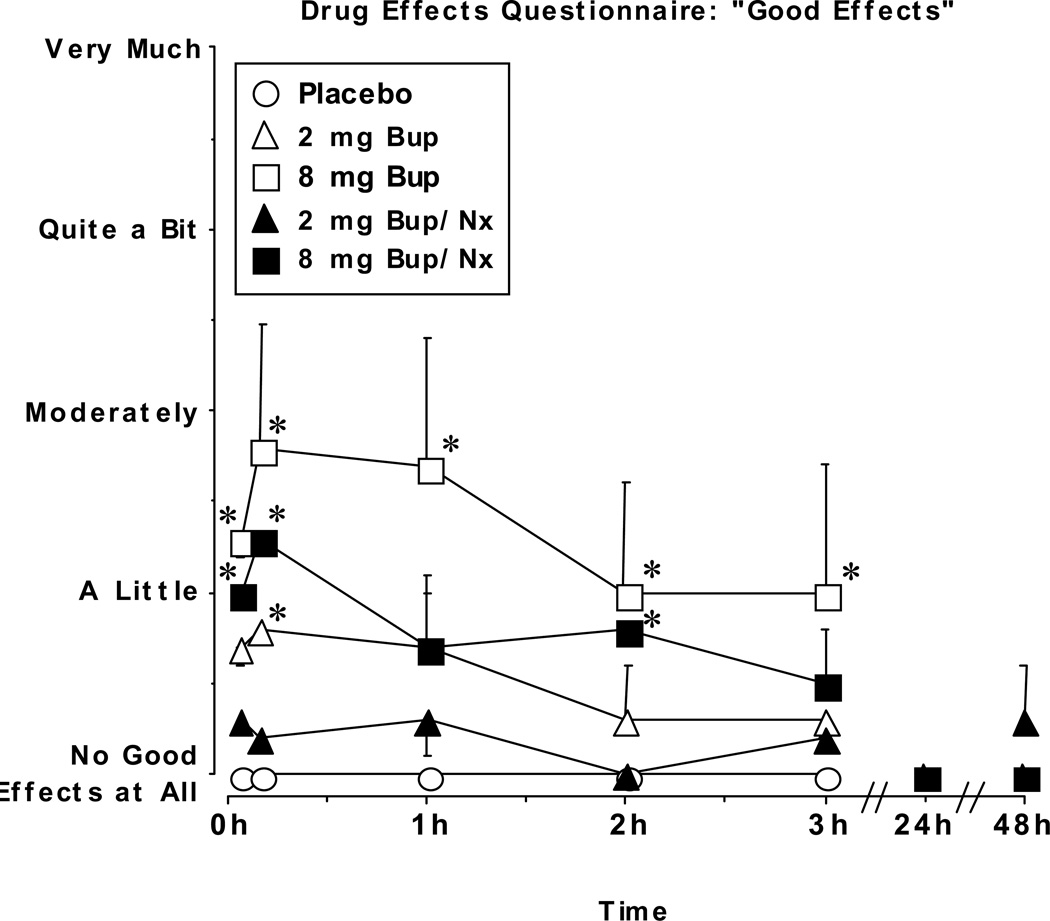
The time course of subjective ratings even more clearly differentiated the dose-response relationships for BUP alone and BUP/NX. Figure 3 shows ratings of “Good Effects” as a function of dose and time. Peak ratings after 2 mg BUP, 8 mg BUP, and 8 mg BUP/NX were significantly different from the corresponding time point after placebo administration, and tended to occur within the first hour after drug administration. The 2 mg BUP/NX dose did not produce ratings that were significantly different from placebo at any time point. For BUP alone, the peak rating was higher and the duration of effect was generally longer after administration of the 8 mg compared to the 2 mg dose. Ratings after 8 mg BUP/NX were slightly lower than after 8 mg BUP, and tended to dissipate more quickly. The time course of DEQ ratings for the other measures (drug “Liking,” “Desire to Take the Drug Again,” and “Strength of Drug Effect”) showed a similar pattern. All of the DEQ ratings returned to placebo levels 24 hr after administration of drug.
Figure 3.
Drug Effects Questionnaire ratings of “Good Effects” during the sample session and subsequent no drug sessions as a function of dose and time. Data points represent mean ratings for the 6 participants. Error bars represent ± 1 standard error of the mean (S.E.M.). * indicates significant differences from placebo at that time point (P<0.01).
The pattern of results obtained from the opioid symptom checklist and visual analog scales were somewhat similar to the DEQ. Sum scores on the opioid symptom checklist after 8 mg BUP compared to placebo approached statistical significance (F 1,20=5.71, P < 0.03). Visual analog scale ratings of drug “Liking,” “Potency,” and the amount of money participants were willing to pay for the drug were significantly greater after 8 mg BUP relative to placebo (Table 2), and changes in several other VAS ratings, such as “Good Effects” (F 1,20=5.39, P < 0.03), “High” (F 1,20=5.99, P < 0.02), “High Quality” (F 1,20=5.13, P < 0.03), “Mellow” (F 1,20=5.27, P < 0.03), “Sedated” (F 1,20=6.34, P < 0.02), “Sleepy” (F 1,20=5.12, P < 0.03), and “Stimulated” (F 1,20=5.56, P < 0.03) after administration of 8 mg BUP approached statistical significance. As with results on the DEQ, mean VAS ratings after 2 mg BUP and both doses of BUP/NX did not significantly differ from placebo. However, the effects of 2 mg versus 8 mg BUP were either significantly different or approached statistical significance for ratings of “High” (F 1,20=4.61, P < 0.04), “Potent” (F 1,20=4.36, P < 0.05), and the amount of money participants were willing to pay for the drug (F 1,20=9.60, P < 0.006). In addition, the effects of 8 mg BUP versus 8 mg BUP/NX were either significantly different or approached statistical significance for ratings of drug “Liking” (F 1,20=4.68, P < 0.04), “Sedated” (F 1,20=4.23, P < 0.05), and the amount of money participants were willing to pay for the drug (F 1,20=8.40, P < 0.009).
Table 2.
Mean (± S.E.M.) ratings on the Visual Analog Scales (VAS) after buprenorphine or buprenorphine/naloxone administration.
| Placebo | 2 mg Bup | 8 mg Bup | 2 mg Bup/Nx | 8 mg Bup/Nx | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Like | 0.98 | 0.22 | 7.31 | 2.35 | 21.56 | 4.76a | 3.25 | 0.84 | 5.60 | 1.86 |
| Potent | 0.83 | 0.21 | 5.38 | 1.88 | 19.27 | 4.38a | 2.98 | 0.81 | 6.31 | 2.01 |
| Would Pay | 0.19 | 0.01 | 0.50 | 0.17b | 3.06 | 0.72a | 0.75 | 0.24 | 0.67 | 0.25c |
indicates a significant difference from placebo;
indicates a significant difference between 2 and 8 mg buprenorphine or between 2 and 8 mg of the combination;
indicates a significant difference between buprenorphine alone and the combination at that dose. (P<0.01)
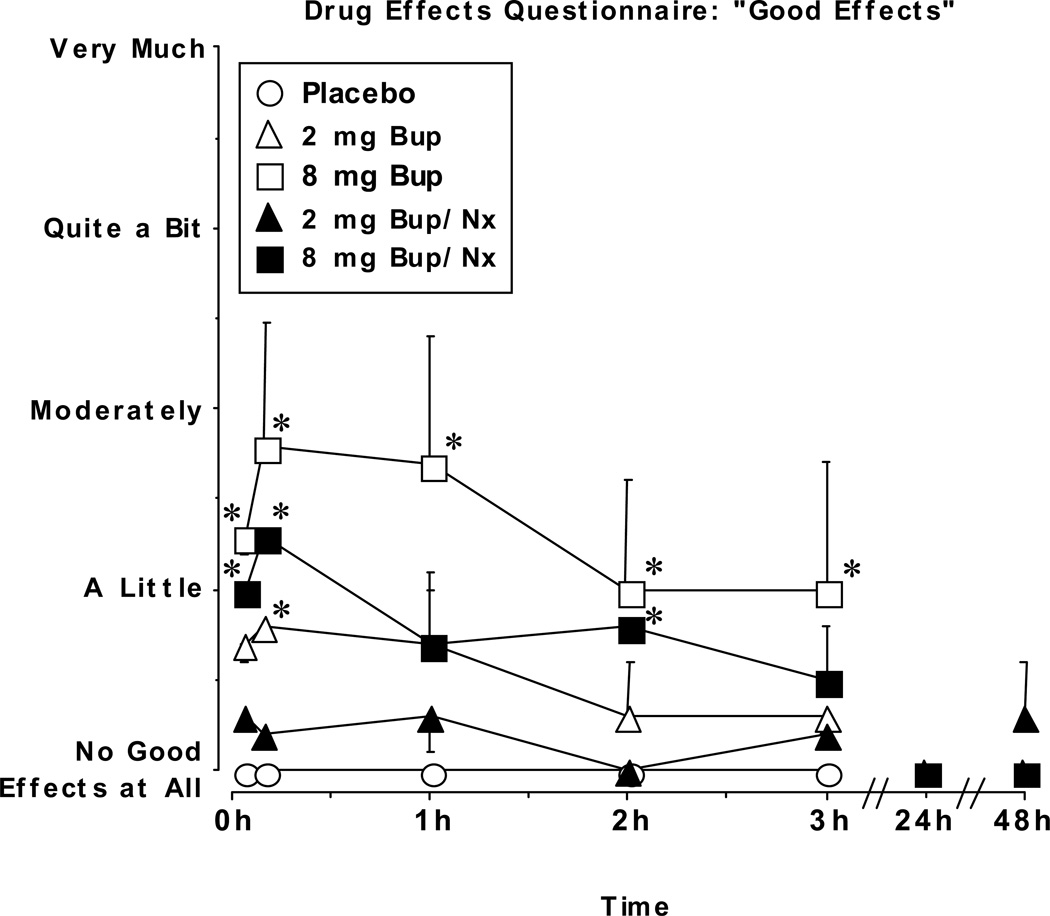
The time course of ratings on the VAS were similar to the DEQ. Figure 4 shows VAS ratings of “Good Effects” as a function of dose and time. Peak ratings after 2 mg and 8 mg BUP were significantly different from the corresponding time point after placebo administration, and tended to occur within the first hour after drug administration. In contrast to ratings of “Good Effect” on the DEQ, 8 mg BUP/NX did not produce ratings on the VAS that were significantly different from placebo at any time point. For BUP alone, the peak rating was higher and the duration of effect was longer after administration of the 8 mg compared to the 2 mg dose. The time course of VAS ratings for the other measures showed a similar pattern. All of the VAS ratings returned to placebo levels 24 hr after administration of drug. Participants reported that they would pay a maximum of $4 to $5 for 8 mg BUP.
Figure 4.
Visual analog scale ratings of “Good Effects” during the sample and no drug sessions as a function of dose and time. Data points represent mean ratings for the 6 participants. Error bars represent ± 1 standard error of the mean (S.E.M.). * indicates significant differences from placebo at that time point (P<0.01). BL, baseline.
Subjective ratings of opioid withdrawal as measured by total scores on the SOWS (maximum possible score = 64) were relatively low. Mean total scores ranged between 17.7 and 11 during detoxification. Total SOWS scores throughout the study remained low (mean range: 0.8–6.8), and did not vary as a function of dose.
Performance Effects
Buprenorphine had few effects on performance, with the exception of impairments in performance of the digit-symbol substitution task. The number of patterns attempted significantly decreased after active drug administration, relative to placebo. The number of patterns attempted was significantly lower for 8 mg BUP, compared to placebo at the 10-, 60-, and 180-min assessment points (10-min: F 1,120=6.29, P < 0.01; 60-min: F 1,120=9.64, P < 0.002; 180-min: F 1,120=8.68, P < 0.004). The number of patterns attempted was significantly lower for 2 mg BUP only at the 120-min assessment point (F 1,120=6.29, P < 0.01). The number of patterns attempted was also significantly lower for 8 mg BUP/NX at the 10-min assessment point (F 1,120=7.77, P < 0.006), and for 2 mg BUP/NX at the 120- and 180-min assessment points (120-min: F 1,120=6.29, P < 0.01; 180-min: F 1,120=7.77, P < 0.006).
Physiological Effects
Figure 5 shows pupil diameter as a function of dose and time. Compared to placebo, all of the active drug doses produced significant decreases in pupil diameter [2 mg BUP (F 1,20=8.55, P < 0.008); 8 mg BUP (F 1,20=19.62, P < 0.0003); 2 mg BUP/NX (F 1,20=8.49, P < 0.009); 8 mg BUP/NX (F 1,20=18.66, P < 0.0003)]. However, there were no significant differences in pupil diameter as a function of active drug or dose. Across time, 8 mg BUP generally produced greater decreases in pupil diameter than 2 mg BUP, and 8 mg BUP/NX generally produced greater decreases in pupil diameter than 2 mg BUP/NX. The maximal decreases in pupil diameter tended to occur within the first 10–60 min after administration of BUP alone, and within the first 1–2 hr after administration of BUP/NX. Post-hoc comparisons revealed that within the first hour after drug administration, the differences in pupil diameter between 8 mg BUP and 8 mg BUP/NX approached statistical significance (4 min: F 1,200=4.60, P < 0.03; 10 min: F 1,200=2.99, P < 0.085). The duration of miosis was longer after 8 mg BUP and 8 mg BUP/NX: pupil diameter returned to placebo levels 24 hr after administration of 2 mg BUP and 2 mg BUP/NX, but it did not return to placebo levels until 72 hr after administration of 8 mg BUP and 8 mg BUP/NX.
Figure 5.
Pupil diameter during the sample and no drug sessions as a function of buprenorphine dose and time. See Figure 4 legend for additional details. Asterisks were aligned vertically for some data points to improve clarity.
Discussion
Similar to findings from our previous study (Comer et al., 2002), the present results demonstrate that intravenously-administered buprenorphine served as a reinforcer in non-dependent, non-treatment seeking heroin abusers. However, the break point values for 2 and 8 mg buprenorphine (1200 ± 156 and 1233 ± 125, respectively) in the present study were lower than in our previous study (2267 ± 246 and 2067 ± 217, respectively). This discrepancy may be due to potential long-lasting antagonist effects of buprenorphine (Kishioka et al., 2000; Schuh et al., 1999; Walker et al., 1995). Although our previous study showed that 2 and 8 mg i.v. buprenorphine did not appear to antagonize heroin’s subjective and physiological effects when heroin was administered 3 and 5 days following buprenorphine (Comer et al., 2002), the ability of buprenorphine to antagonize heroin’s reinforcing effects was not examined. Therefore, it is possible that buprenorphine’s antagonist effects may have contributed to the lower break point values obtained in the present study.
Contrary to expectations, the break point values in the present study for the combination did not differ from buprenorphine alone. Only two other studies have evaluated the effects of the buprenorphine/naloxone combination in non-opioid-dependent individuals who abuse heroin (Strain et al., 2000; Weinhold et al., 1992). Weinhold et al. (1992) demonstrated that intramuscular administration of the buprenorphine/naloxone combination reduced subjective and physiological (miosis, respiratory depression) effects, relative to buprenorphine alone, suggesting that the combination would have lower abuse liability. A more recent study comparing the effects of sublingual buprenorphine with sublingual buprenorphine/naloxone combinations in non-dependent heroin abusers showed that while peak effects for some subjective measures were lower for the combination relative to buprenorphine alone, these differences were not statistically significant (Strain et al., 2000). Both studies suggested that the buprenorphine/naloxone combination would have abuse potential in non-dependent heroin abusers, but the authors differed in their conclusions regarding the abuse liability of buprenorphine alone relative to the combination. The present results corroborate the data described by Weinhold et al. (1992) in that significant differences between buprenorphine alone and the combination were found for some subjective measures.
Despite the differences in the subjective effects data in the present study, progressive ratio break point values did not vary as a function of dose or active drug. Previous studies have shown that subjective effects do not necessarily predict self-administration behavior (Comer et al., 1997, 1998; Heishman et al., 2000; Lamb et al., 1991). For example, Lamb et al. (1991) reported that a dose of morphine that did not produce measurable subjective effects was still reliably self-administered in non-dependent individuals with a history of heroin abuse. Conversely, in a study examining the effects of the magnitude of alternative reinforcers on heroin self-administration, progressive ratio break points for drug were reduced when a larger, compared to a smaller amount of money was concurrently available, even though subjective ratings did not differ among the different money conditions (Comer et al., 1997). Therefore, as in previous studies, the present results demonstrate that subjective effects do not always predict reinforcing effects.
Across the four choice sessions, progressive ratio break point values for drug generally were not significantly different. However, as in our previous study evaluating the reinforcing effects of buprenorphine (Comer et al., 2002), break point values for 2 mg buprenorphine were higher during the morning sessions, compared to the afternoon sessions. It is unclear why 2 mg buprenorphine was self-administered more in the morning than the afternoon, while no significant difference existed in self-administration of 8 mg buprenorphine in the morning versus the afternoon. Break point values for money were generally inversely related to break point values for drug. Across the four choice opportunities for money, the break point value during the fourth opportunity was significantly higher than during the other choice opportunities under the placebo and 8 mg BUP/NX condition, and they approached statistical significance under the 2 mg BUP/NX condition. These data raise the possibility that responding for money may have continued to increase (and conversely, responding for drug may have decreased) if participants were given more opportunities to self-administer placebo or BUP/NX.
In sum, results from the present study are consistent with previous studies conducted in non-human primates demonstrating that buprenorphine serves as a reinforcer, and they provide additional evidence that the buprenorphine/naloxone combination also serves as a reinforcer. It is important to emphasize, however, that the present study was conducted in recently detoxified individuals. The buprenorphine/naloxone combination was thought to have limited parenteral abuse because of two mechanisms: 1) naloxone would antagonize the initial effects produced by buprenorphine, which are important in a drug’s reinforcing effects, and 2) naloxone would precipitate withdrawal in opioid-dependent individuals. Although the present study showed no differences in the reinforcing effects of buprenorphine compared to the buprenorphine/naloxone combination in non-dependent individuals, it is likely that different results would have been obtained in opioid-dependent individuals. The buprenorphine/naloxone combination has mostly antagonist-like effects in individuals maintained on morphine (Fudala et al., 1998; Mendelson et al., 1999), hydromorphone (Preston et al., 1988; Stoller et al., 2001), or methadone (Bigelow et al., 1987; Mendelson et al., 1997). What is not clear, however, is whether buprenorphine or the combination has abuse liability in individuals maintained on buprenorphine. The study by Amass et al. (2000) suggests that buprenorphine alone (4, 8 mg) and the buprenorphine/naloxone combination (4:1, 8:2 mg) would have no abuse liability in buprenorphine-maintained individuals. However, a study by Strain et al. (1997) demonstrated that when higher doses of intramuscular buprenorphine (4, 8, 16 mg) were administered to individuals maintained on 8 mg sublingual buprenorphine, opioid-like effects were produced, suggesting that buprenorphine alone may have abuse liability in buprenorphine-maintained individuals. Furthermore, Harris et al. (2000) showed that intravenous administration of the buprenorphine/naloxone combination (4 mg of each drug) did not precipitate withdrawal in individuals maintained on 8 mg sublingual buprenorphine (but see Eissenberg et al., 1996). Whether high doses of the buprenorphine/naloxone combination would have abuse liability in buprenorphine-maintained individuals is currently unknown. Therefore, further studies evaluating the reinforcing effects of buprenorphine and the buprenorphine/naloxone combination in buprenorphine-maintained individuals are warranted.
Buprenorphine produced few effects on performance, which is consistent with other studies showing either mild (Comer et al., 2002; Pickworth et al., 1993) or no performance-impairing effects of buprenorphine (Walsh et al., 1994). Buprenorphine did decrease pupil diameter, an effect consistent with other mu opioid agonists. Similar to results reported by Strain et al. (2000), naloxone had few statistically significant effects on buprenorphine-induced miosis in the present study. However, there were trends in the current data that were similar to those reported by Weinhold et al. (1992), who showed that naloxone antagonized the miotic effects of buprenorphine. The buprenorphine to naloxone dose ratio (1:1, 1:2, and 2:1) was lower in the study reported by Weinhold et al. (1992), compared to the present study (4:1) and the study conducted by Strain et al. (2000), which may account for the differences between the studies. In contrast to naloxone’s limited effects on pupil diameter, naloxone did antagonize the subjective effects of buprenorphine in the present study. Although Weinhold et al. (1992) and Mendelson et al. (1996) reported that naloxone tended to delay the onset of buprenorphine’s agonist effects, such that subjective effects were significantly elevated beyond the first hour following drug administration, a similar pattern of results was not obtained in the present study. Our results were more consistent with those reported by Stoller et al. (2001), who showed that while the buprenorphine/naloxone combination increased symptoms of withdrawal during the first hour after drug administration in individuals maintained on hydromorphone, positive opioid subjective effects did not emerge after the withdrawal effects dissipated. In the present study, naloxone diminished the effects of buprenorphine at all assessment points.
In conclusion, the present results confirmed our hypothesis that buprenorphine would serve as a reinforcer under these laboratory conditions. The fact that the maximum break points did not differ between buprenorphine alone and the combination was surprising, however, given the clear antagonism of buprenorphine’s subjective effects by naloxone. It is possible that the experimental conditions under which buprenorphine and the combination were self-administered in the present study contributed to the lack of difference in break point values between buprenorphine alone and the combination. The increases in money self-administration during the fourth choice session when BUP/NX was available are provocative, and suggest that BUP/NX self-administration eventually may have extinguished if participants were given more opportunities to self-administer the combination. Nevertheless, these results clearly demonstrate that both buprenorphine and the combination may be self-administered under some conditions. In order to obtain a clear assessment of the abuse liability of a drug (i.e., the risk of harmful use and/or dependence), a variety of measures should be assessed including both positive and negative subjective responses, actual drug-taking behavior, and the context under which drug is self-administered. Other important factors to consider include the presence/absence of tolerance and dependence, and participant characteristics (drug abusers versus non-drug users). The results of the present study should therefore be considered with these variables in mind.
Acknowledgments
We would like to thank Dr. Marian Fischman for her invaluable guidance and support during the planning of these studies. In addition, the assistance of Christy Aberg, Berena Cabarcas, Mabel Torres, Michael R. Donovan, Ronnie M. Shapiro, and Drs. Anthony Tranguch, Vladimir Ginsburg, Adam Bisaga, Maria Sullivan, and Margaret Haney is also gratefully acknowledged.
This research was supported by grant DA10909.
References
- Amass L, Kamien JB, Reiber C, Branstetter SA. Abuse liability of IV buprenorphine-naloxone, buprenorphine, and hydromorphone in buprenorphine-naloxone maintained volunteers. Drug Alcohol Depend. 2000;60(Suppl. 1):S6–S7. [Google Scholar]
- Baumevieille M, Haramburu F, Begaud B. Abuse of prescription medicines in southwestern France. Ann Pharmacother. 1997;31:847–850. doi: 10.1177/106002809703100706. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L. Buprenorphine treatment of opioid dependence: A review. Exp Clin Psychopharmacol. 1995;3(4):477–489. [Google Scholar]
- Bigelow GE, Preston KL, Liebson IA. Abuse liability assessment of buprenorphine-naloxone combinations. In: Harris LS, editor. Problems of Drug Dependence 1986 Proceeding of the 48th Annual Scientific Meeting (National Institute on Drug Abuse Research Monograph, No. 76. Washington D.C.: U.S. Government Printing Office; 1987. pp. 145–149. [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol. 1997;8:677–690. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Intravenous buprenorphine self-administration by detoxified heroin abusers. J Pharmacol Exp Ther. 2002;301:266–276. doi: 10.1124/jpet.301.1.266. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, Fischman MW. Comparison of intravenous and intranasal heroin self-administration by morphine-maintained humans. Psychopharmacology (Berl) 1999;143:327–338. doi: 10.1007/s002130050956. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Wilson ST, Donovan MR, Foltin RW, Fischman MW. Effects of an alternative reinforcer on i.v. heroin self-administration by humans. Eur J Pharmacol. 1998;345:13–26. doi: 10.1016/s0014-2999(97)01572-0. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Greenwald MK, Johnson RE, Liebson IA, Bigelow GE, Stitzer ML. Buprenorphine’s physical dependence potential: Antagonist-precipitated withdrawal in humans. J Pharmacol Exp Ther. 1996;276:449–459. [PubMed] [Google Scholar]
- Fudala PJ, Yu E, Macfadden W, Boardman C, Chiang CN. Effects of buprenorphine and naloxone in morphine-stabilized opioid addicts. Drug Alcohol Depend. 1998;50:1–8. doi: 10.1016/s0376-8716(98)00008-8. [DOI] [PubMed] [Google Scholar]
- Harris DS, Jones RT, Welm S, Upton RA, Lin E, Mendelson J. Buprenorphine and naloxone co-administration in opiate-dependent patients stabilized on sublingual buprenorphine. Drug Alcohol Depend. 2000;61:85–94. doi: 10.1016/s0376-8716(00)00126-5. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Schuh KJ, Schuster CR, Henningfield JE, Goldberg SR. Reinforcing and subjective effects of morphine in human opioid abusers: Effect of dose and alternative reinforcer. Psychopharmacology. 2000;148:272–280. doi: 10.1007/s002130050051. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR. Influence of an alternative reinforcer on human cocaine self-administration. Life Sci. 1994;55:179–187. doi: 10.1016/0024-3205(94)00878-7. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. New Engl J Med. 2000;343(18):1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Kishioka S, Paronis CA, Lewis JW, Woods JH. Buprenorphine and methoclocinnamox: Agonist and antagonist effects on respiratory function in rhesus monkeys. Eur J Pharmacol. 2000;391:289–297. doi: 10.1016/s0014-2999(00)00039-x. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, Henningfield JE, Goldberg SR. The reinforcing and subjective effects of morphine in post-addicts: A dose-response study. J Pharmacol Exp Ther. 1991;259:1165–1173. [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Bree MP, Mendelson JH. Progressive ratio performance maintained by buprenorphine, heroin and methadone in Macaque monkeys. Drug Alcohol Depend. 1988;21:81–97. doi: 10.1016/0376-8716(88)90053-1. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Fernandez I, Welm S, Melby AK, Baggot MJ. Buprenorphine and naloxone interactions in opiate-dependent volunteers. Clin Pharmacol Ther. 1996;60:105–114. doi: 10.1016/S0009-9236(96)90173-3. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Welm S, Baggot M, Fernandez I, Melby AK, Nath RP. Buprenorphine and naloxone combinations: The effects of three dose ratios in morphine-stabilized, opiate-dependent volunteers. Psychopharmacology. 1999;141:37–46. doi: 10.1007/s002130050804. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Welm S, Brown J, Batki SL. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095–1101. doi: 10.1016/S0006-3223(96)00266-1. [DOI] [PubMed] [Google Scholar]
- Negus SS, Woods JH. Reinforcing effects, discriminative stimulus effects, and physical dependence liability of buprenorphine. In: Cowan A, Lewis JW, editors. Buprenorphine: Combatting Drug Abuse with a Unique Opioid. New York: Wiley-Liss, Inc; 1995. pp. 71–101. [Google Scholar]
- O’Connor JJ, Moloney E, Travers R, Campbell A. Buprenorphine abuse among opiate addicts. Brit J Addiction. 1988;83:1085–1087. doi: 10.1111/j.1360-0443.1988.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK. Buprenorphine dose self-selection: Effects of an alternative reinforcer, behavioral economic analysis of demand, and examination of subjective drug effects. Exp Clin Psychopharmacol. 1999;7:38–48. doi: 10.1037//1064-1297.7.1.38. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Johnson RE, Holicky BA, Cone EJ. Subjective and physiologic effects of intravenous buprenorphine in humans. Clin Pharmacol Ther. 1993;53(5):570–576. doi: 10.1038/clpt.1993.72. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Liebson IA. Buprenorphine and naloxone alone and in combination in opioid-dependent humans. Psychopharmacology. 1988;94:484–490. doi: 10.1007/BF00212842. [DOI] [PubMed] [Google Scholar]
- Robinson GM, Dukes PD, Robinson BJ, Cooke RR, Mahoney GN. The misuse of buprenorphine and a buprenorphine-naloxone combination in Wellington, New Zealand. Drug Alcohol Depend. 1993;33:81–86. doi: 10.1016/0376-8716(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Sakol MS, Stark C, Sykes R. Buprenorphine and temazepam abuse by drug takers in Glasgow – an increase. Br J Addiction. 1989;84:439–441. doi: 10.1111/j.1360-0443.1989.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Walsh SL, Stitzer ML. Onset, magnitude and duration of opioid blockade produced by buprenorphine and naltrexone in humans. Psychopharmacology. 1999;145:162–174. doi: 10.1007/s002130051045. [DOI] [PubMed] [Google Scholar]
- Singh RA, Mattoo SK, Malhotra A, Varma VK. Cases of buprenorphine abuse in India. Acta Psychiatr Scand. 1992;86:46–48. doi: 10.1111/j.1600-0447.1992.tb03224.x. [DOI] [PubMed] [Google Scholar]
- Stoller KB, Bigelow GE, Walsh SL, Strain EC. Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology. 2001;154:230–242. doi: 10.1007/s002130000637. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stoller KB, Walsh SL, Bigelow GE. Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers. Psychopharmacology. 2000;148:374–383. doi: 10.1007/s002130050066. [DOI] [PubMed] [Google Scholar]
- Strain EC, Walsh SL, Preston KL, Liebson IA, Bigelow GE. The effects of buprenorphine in buprenorphine-maintained volunteers. Psychopharmacology (Berl) 1997;129:329–338. doi: 10.1007/s002130050199. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, McCaul ME, Bigelow GE, Liebson IA. Oral methadone self-administration: Effects of dose and alternative reinforcers. Clin Pharmacol Ther. 1983;34:29–35. doi: 10.1038/clpt.1983.124. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Tucker JA. Behavioral theories of choice as a framework for studying drinking behavior. J Abn Psychol. 1983;92:408–416. doi: 10.1037//0021-843x.92.4.408. [DOI] [PubMed] [Google Scholar]
- Walker EA, Zernig G, Woods JH. Buprenorphine antagonism of mu opioids in the rhesus monkey tail-withdrawal procedure. J Pharmacol Exp Ther. 1995;273:1345–1352. [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Weinhold LL, Preston KL, Farre M, Liebson IA, Bigelow GE. Buprenorphine alone and in combination with naloxone in non-dependent humans. Drug Alcohol Depend. 1992;30:263–274. doi: 10.1016/0376-8716(92)90061-g. [DOI] [PubMed] [Google Scholar]
- Winger G, Woods JH. The effects of chronic morphine on behavior reinforced by several opioids or by cocaine in rhesus monkeys. Drug Alcohol Depend. 2001;62:181–189. doi: 10.1016/s0376-8716(00)00166-6. [DOI] [PubMed] [Google Scholar]