Abstract
♦ Introduction: Although previous studies have suggested associations between serum intact parathyroid hormone (iPTH), 25-hydroxyvitamin D (25(OH)D) and metabolic syndrome (MS) in the general population, these associations are still uncharacterized in peritoneal dialysis (PD) patients.
♦ Methods: In total, 837 prevalent PD patients from 5 centers in China were enrolled between April 1, 2011 and November 1, 2011. The demographic data, biochemical parameters and medical records were collected, except for serum 25(OH)D which was measured in 347 of 837 patients. The definition of MS was modified from National Cholesterol Education Program Third Adult Treatment Panel (NCEP-ATPIII).
♦ Results: 55.4% of 837 patients were found to have MS. The median concentration of iPTH, 25(OH)D and doses of oral vitamin D analogs for participants with MS was significantly lower than those without MS. The iPTH, 25(OH)D values and doses of vitamin D analogs were all associated with one or more components of MS. After multivariate adjustment, low serum iPTH values and oral vitamin D analogs, rather than serum 25(OH)D, were significantly associated with the presence of MS, abnormal fasting blood glucose (FBG) and high-density lipoprotein cholesterol (HDL-C). Compared to iPTH < 130pg/mL, iPTH 130-585 pg/mL and > 585pg/mL were associated with a lower risk of MS with adjusted odds ratio (OR) of 0.59 and 0.33, respectively. Taking vitamin D analogs was also associated with a lower risk of MS with adjusted OR of 0.55.
♦ Conclusions: Serum iPTH and the use of active vitamin D supplements rather than serum 25(OH)D were independently associated with the presence of MS in patients on PD.
Keywords: Metabolic syndrome, parathyroid hormone, peritoneal dialysis, vitamin D
Metabolic syndrome (MS) consists of a cluster of metabolic and hemodynamic abnormalities. The components of MS such as abdominal obesity, high blood pressure, insulin resistance and dyslipidemia are risk factors for chronic kidney disease (CKD) (1,2), diabetes (3), cardiovascular disease (4) and high cardiovascular mortality (5). On the other hand, the components of MS develop early in CKD and are exacerbated during the progression to end-stage renal disease (ESRD) (6,7). The prevalence of MS is remarkably high in CKD patients, being approximately 30% - 65% for non-dialysis patients (6-8) and 47% - 69% for dialysis patients (9-12). MS represents a global epidemic and it is clearly important to explore new, non-traditional risk factors.
In the general population, MS has been positively associated with serum intact parathyroid hormone (iPTH) (13-15) and negatively with vitamin D status, as measured using 25-hydroxyvitamin D (25(OH)D) concentrations (16-20). Decreased vitamin D levels and elevated iPTH levels may play critical roles in the etiology of MS, either through an association with the individual components of MS or via insulin resistance (21,22). Given that abnormalities in serum iPTH and vitamin D levels exist throughout the stages of CKD and predict the mortality of dialysis patients (23-25), determining the associations between iPTH, 25(OH)D and MS is important.
Oral vitamin D supplements are widely used in dialysis patients to treat hyperparathyroidism, and have been strongly associated with the serum iPTH and 25(OH) D levels. The benefits of oral vitamin D supplements on cardiovascular disease and death risk have been discussed broadly in recent years but results are not consistent in the general population (26-28). The impact of oral vitamin D supplements on the prevalence of MS and its components has not been well documented in dialysis patients.
We therefore aimed to determine whether serum iPTH, 25(OH)D levels and the use of oral vitamin D analogs are associated with the prevalence of MS in peritoneal dialysis (PD) patients in this large, multi-center, cross-sectional study.
Subjects and Methods
Study Design and Subjects
This is an add-on multi-center cross-sectional study of SSOP (socioeconomic status and outcome in patients on peritoneal dialysis (PD)). Five PD centers from 4 provinces (Beijing, Heilongjiang, Ningxia Hui Autonomous Region, Shanghai) located in 4 geographical regions (north, northeast, northwest, and east) in China, were selected for the study because they have professional PD clinicians providing regular clinical visits for patients every 1 to 3 months. Inclusion criteria for participants were: age ≥ 18 years; prevalent patients between April 1, 2011, and November 1, 2011; able to undergo dialysis adequacy and biochemical parameter measurements as needed during the study period. Patients were excluded if they had a history of systemic infection, cardiovascular event, active hepatitis, cancer, surgery, or trauma in the month prior to the study. All the subjects received conventional glucose-based, lactate-buffered PD solutions (Ultrabag, Baxter Healthcare, Guangzhou, China). Data from each center were collected in a strict quality control framework and further inspected and optimized to maintain integrity and accuracy of the database. All study investigators and staff members completed a training program that taught them the methods and processes of the study. A manual of detailed instructions for data collection was distributed. The Ethics Committees of Peking University First Hospital, Second Affiliated Hospital of Harbin Medical University, General Hospital of Ningxia Medical University, Peking University People’s Hospital and Huashan Hospital of Fudan University approved this study protocol and written informed consent was obtained from each participant.
MS Definition
MS was defined on the basis of the National Cholesterol Education Program Third Adult Treatment Panel (NCEP-ATPIII) (29), i.e. fulfillment of at least three of the following: abnormal waist circumference; triglyceride (TG) ≥ 1.7 mmol/L; high-density lipoprotein cholesterol (HDL-C) < 1.0 mmol/L in male or < 1.3 mmol/L in female; blood pressure (BP) ≥ 130/85 mmHg (or drug treatment), and fasting blood glucose (FBG) ≥ 6.1 mmol/L (or previously diagnosed type 2 diabetes). Due to the presumed impact of instilled PD solution, we used body mass index (BMI) ≥ 25 kg/m2 rather than waist circumference as the marker of central obesity according to Asian standards (30) and previous references (31-33).
Clinical Characteristics
General information including age, gender, body weight, height, primary renal diseases, the presence of diabetes, duration of dialysis, and history of hypertension was collected. Body weight and height were measured with the subject barefoot, without PD solution in the abdomen and wearing light clothing, and was used to calculate the BMI using the formula, body weight/(body height)2 (kg/m2). Systolic and diastolic blood pressure (SBP and DBP) were measured according to the guidelines presented in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood pressure (34). Patients took usual antihypertensive medications the morning of each clinic visit. A trained nurse measured patients’ brachial blood pressures with a mercury sphygmomanometer in a sitting position after resting for at least 5 minutes in a quiet room. In addition, antihypertensive and diabetic medication regimens and oral vitamin D supplements as well as PD prescription for each patient were recorded. Whether glucose-containing dialysate dwell was used during the night prior to blood sampling was also recorded. Alfacacidol doses were converted to the calcitriol equivalent by multiplying by 0.75 (35).
Laboratory Methods
After overnight fasting while continuing PD therapy, venous blood was sampled for routine and biochemical measurements for each subject. All laboratory samples except for serum 25(OH)D were analyzed by standard laboratory techniques in the local hospitals including hemoglobin, serum albumin, fasting blood glucose, calcium, phosphorus, TG, HDL-C, LDL-C, total cholesterol (TCHO), iPTH and C-reactive protein (CRP). Dialysis adequacy and residual renal function (RRF) were also measured. RRF was defined as the mean of residual creatinine and urea clearance. Dialysis adequacy was presented as total Kt/V and total creatinine clearance. Serum iPTH was measured by the chemiluminescence assay in each local hospital (reference range: 15∼65pg/mL). The measurement of serum 25(OH)D was not the primary goal of study and was performed in a subset of volunteers who were prepared to have extra blood samples and signed an appropriate consent form and subject to separate ethics review. Only 347 patients provided extra serum samples for vitamin D measurement. They all were from the cities of Harbin or Peking, in the northern region of China. Blood samples for 25(OH)D were collected during April to June and centrifuged at 3000 rpm for 10 minutes before storing at -80°C until analysis. All samples for 25(OH)D were measured by enzyme-linked immunosorbent assay (Immunodiagnostic Systems Ltd., Bolden, UK) in the laboratory of Peking University First Hospital.
Statistical Analysis
Continuous data are presented as mean ± standard deviation for parametric data or median values with their lower and upper quartiles for nonparametric data. Categorical variables were expressed as a percentage or ratio. Patients’ data were compared by using the t-test or ANOVA F-test for normally distributed continuous variables, chi-square test for categorical variables, and Mann-Whitney U test for skewed continuous variables.
Spearman correlation analysis was used to examine the coefficient correlations between serum iPTH, 25(OH) D, doses of oral vitamin D analogs and components of MS, respectively. In order to determine risk factors of MS, binary logistic multivariable regression models were constructed to explore the relationship between serum iPTH, 25(OH)D, doses of oral vitamin D analogs, and the prevalence of MS adjusted for age and gender. At the next step, dialysis duration, serum calcium, phosphorus, albumin, CRP, hemoglogin, total Kt/V and RRF were also included. Finally, the confounding effect of oral vitamin D supplements on associations of serum iPTH, 25(OH)D and MS risk was explored. Associations between serum iPTH, 25(OH)D and oral vitamin D and the presence of MS were examined as continuous variables as well as categorized variables according to recognized cut-off points or tertiles. Next, we examined the association between iPTH, 25(OH)D and oral vitamin D supplements and the individual components of MS including BMI, FBG, BP, TG and HDL-C respectively after adjustment for age, gender, dialysis duration, serum calcium, phosphorus, albumin, CRP, hemoglobin, total Kt/V and RRF. All models determining the correlations between components of MS and iPTH/25(OH)D were additionally adjusted for the doses of oral vitamin D analogs. Models determining the correlations of FBG and iPTH/25(OH) D/doses of oral vitamin D were additionally adjusted for the presence or absence of an overnight dwell of glucose-containing dialysate.
We report the multivariable adjusted odds ratios (ORs) with 95% confidence intervals (CIs). All probabilities were two-tailed, and the level of significance was set at 0.05. Statistical analysis was performed by SPSS for Windows software version 13.0 (SPSS Inc., Chicago, IL).
Results
Demographic Data and Clinical Characteristics
We enrolled 837 prevalent PD patients (407 men and 430 women; mean age, 59.47 ± 14.21 years). Data on serum 25(OH)D levels was only available in 347 of the 837 patients. MS was identified in 464 of the 837 (55.4%) patients. The median (lower and upper quartiles) of serum concentrations of iPTH and 25(OH)D, and the dose of oral vitamin analogs were 148.6 pg/mL (56.7 - 307.1 pg/mL), 16.1 nmol/l (12.1 - 21.6 nmol/l) and 0 μg/week (0 - 0.5 μg/week), respectively. Comparison of demographic data and clinical characteristics between patients with and without 25(OH)D measurements did not reveal any significant differences in age, gender, BMI or MS prevalence. However, patients with 25(OH)D measurements had been on dialysis for a longer duration than patients without these measurements; median of 28 months (13 - 52months) vs 23 months (12 - 43 months) (p = 0.048).
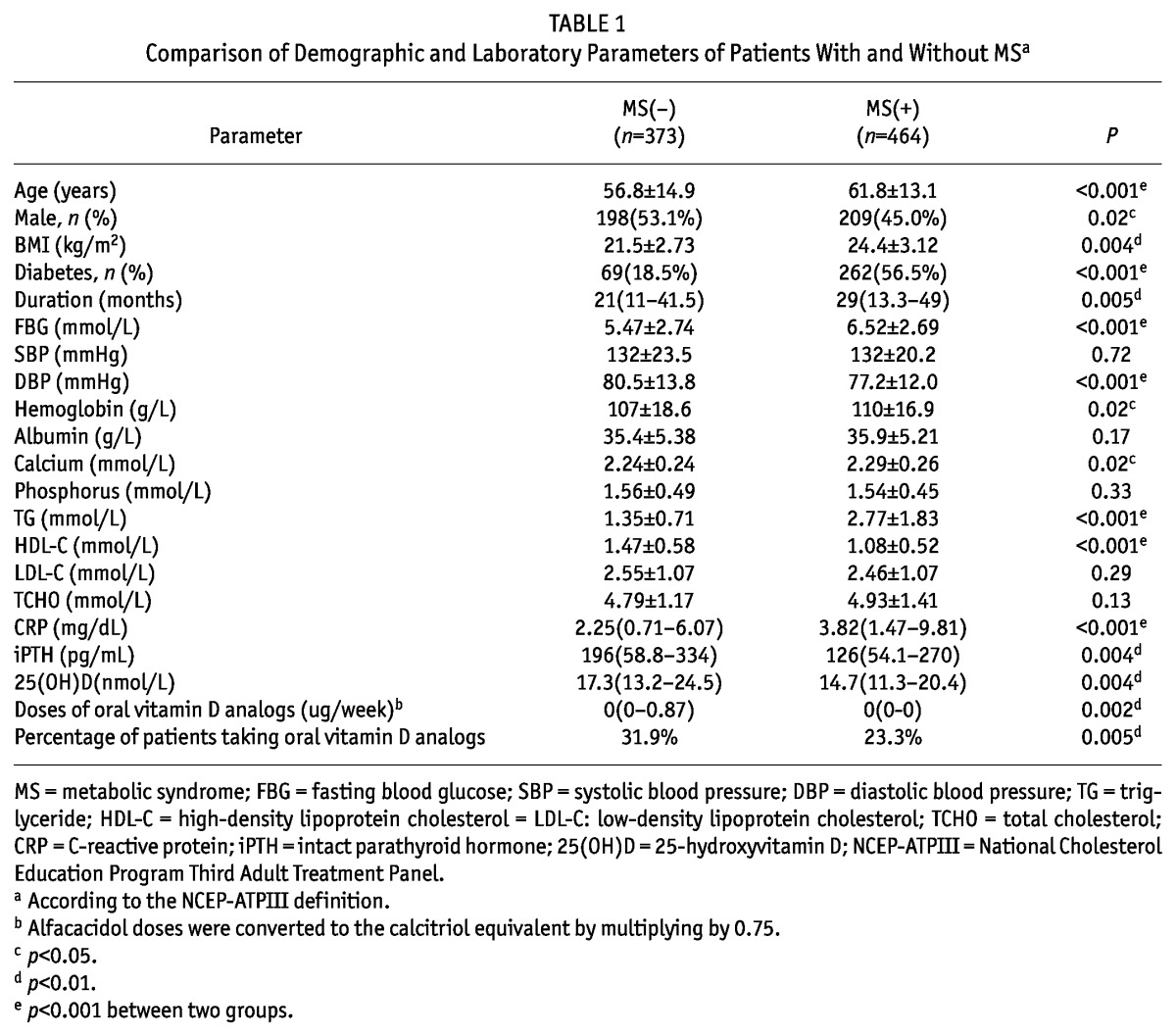
Compared to patients without MS, those with MS tended to be older, female, diabetic and on dialysis for a longer duration. As expected, patients with MS had significantly higher BMI, FBG, DBP, TG and lower HDL-C levels, which are typical features of MS. In addition, the values of CRP were significantly higher in the MS group. By contrast, the serum iPTH and 25(OH)D concentrations were significantly lower in the MS group. The dose of oral vitamin D analogs was significantly lower in patients with MS than in those without MS. In addition, the percentage of patients taking oral vitamin supplements was significantly lower in the MS group. These data are shown in Table 1.
TABLE 1.
Comparison of Demographic and Laboratory Parameters of Patients With and Without MSa
The Correlations of Serum iPTH/25(OH)D, Doses of Oral Vitamin D Analogs and MS Components
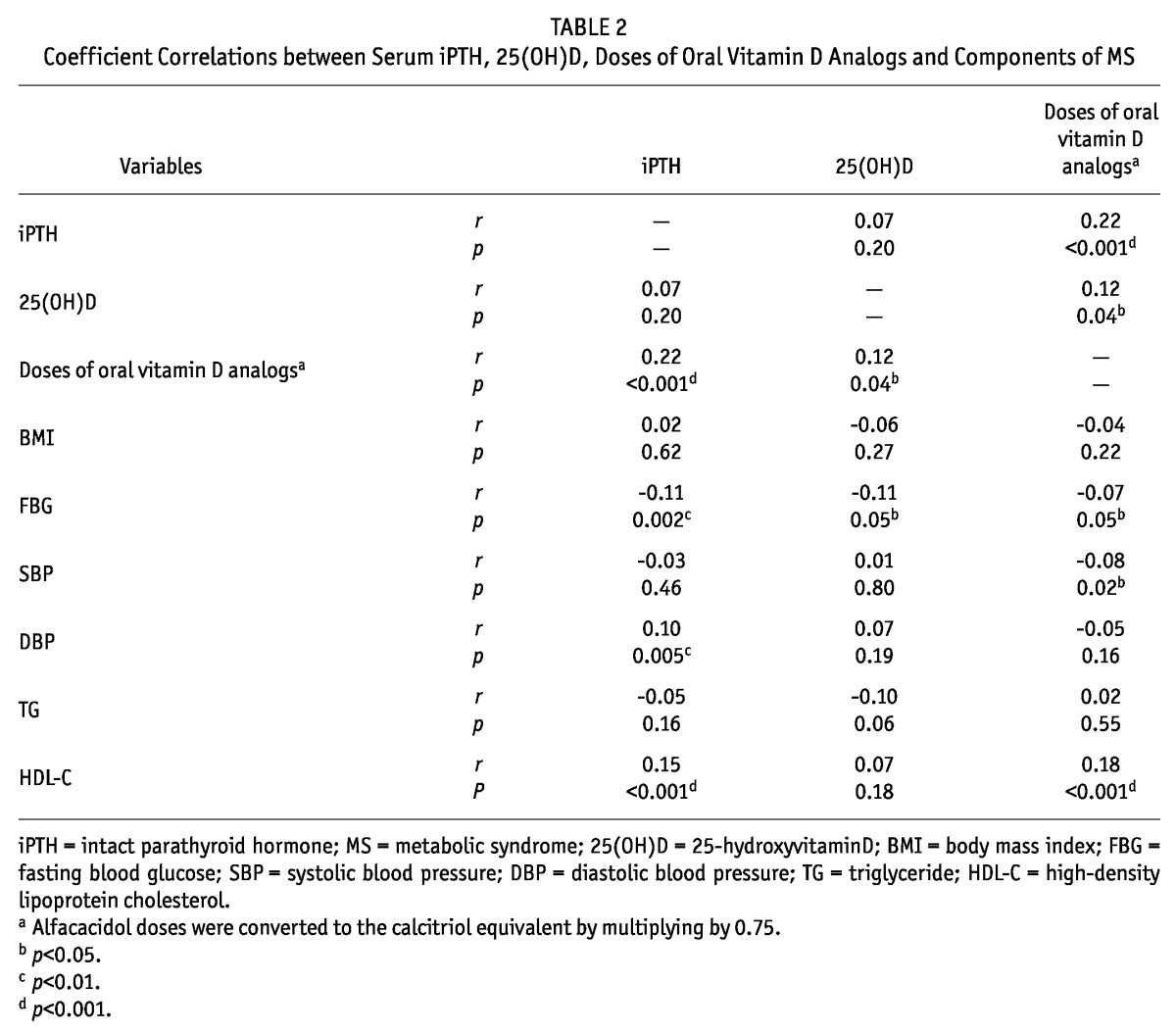
Doses of oral vitamin D analogs were significantly and positively associated with values of serum 25(OH)D and iPTH. The fasting blood glucose was weakly inversely associated with serum iPTH/25(OH)D/doses of oral vitamin D analogs (r: -0.07 to -0.11, p < 0.05). The SBP was negatively correlated with the doses of oral vitamin D analogs, while the DBP was positively associated with the serum iPTH level. The serum iPTH and doses of oral vitamin D analogs were positively associated with HDL-C (r: 0.15 to 0.18, p < 0.001). These data are shown in Table 2.
TABLE 2.
Coefficient Correlations between Serum iPTH, 25(OH)D, Doses of Oral Vitamin D Analogs and Components of MS
The Correlations of Serum iPTH, 25(OH)D, Doses of Oral Vitamin D Analogs and Risk for MS or Components of MS
By binary logistic multivariate analysis, serum iPTH, as a continuous variable, was found to be an independent risk factor for MS prevalence after adjusting for age and gender or additionally for dialysis duration, serum calcium, phosphorus, albumin, CRP and hemoglobin levels, total Kt/V urea and RRF. Further adjustment for oral vitamin D supplements weakened this correlation. For each 100 pg/mL increase in serum iPTH, MS risk decreased by 8% (Table 3). We therefore categorized patients into three groups according to iPTH in line with the KDIGO (Kidney Disease: Improving Global Outcomes) guideline (36): < 130 pg/mL, 130 - 585 pg/mL and > 585 pg/mL. Compared to serum iPTH < 130 pg/mL, 130 - 585 pg/mL or > 585 pg/mL had a lower risk of MS, with ratios ORs of 0.53 (0.34 - 0.82) and 0.35 (0.15 - 0.79), respectively. There was no difference in MS risk between patients with 130 - 585 pg/mL and > 585 pg/mL of iPTH levels (Figure 1).
TABLE 3.
Adjusted OR of Serum iPTH, 25(OH)D, Oral Vitamin D Analogs for Prevalence of MS in PD Patients
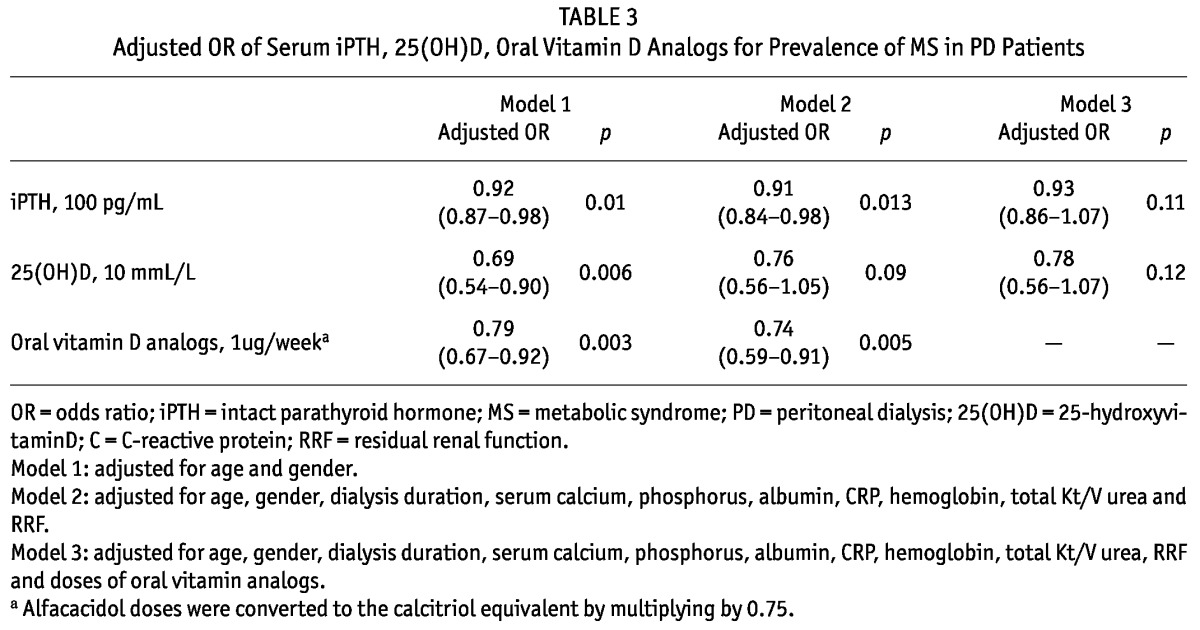
Figure 1 —
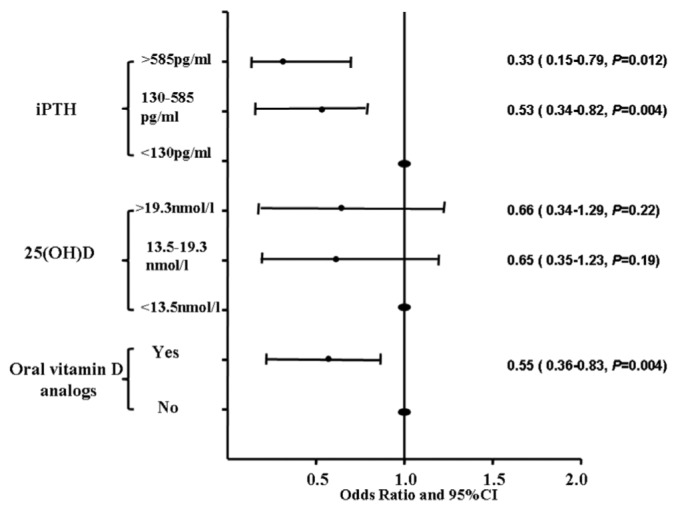
Adjusted ratio of varied levels of serum iPTH, 25(OH)D, oral vitamin D analogs for risk of MS. All models are adjusted for age, gender, body mass index, dialysis duration, serum calcium, phosphorus, albumin and C-reactive protein, hemoglobin, total Kt/V urea and residual renal function. iPTH = intact parathyroid hormone; 25(OH)D = 25-hydroxyvitaminD; CI = confidence interval.
Although serum 25(OH)D was also associated with MS risk adjusted for age and gender, this relationship weakened after multivariate adjustments (Table 3). There were no differences in the risk for MS across tertiles of serum 25(OH)D (Figure 1).
Patients taking oral vitamin D analogs had a significantly lower risk of MS, with an adjusted OR of 0.55 (0.36 - 0.83) (Figure 1). For a 1-μg/week increase in the doses of oral vitamin D analogs, the adjusted risk of MS decreased by 26% (Table 3).
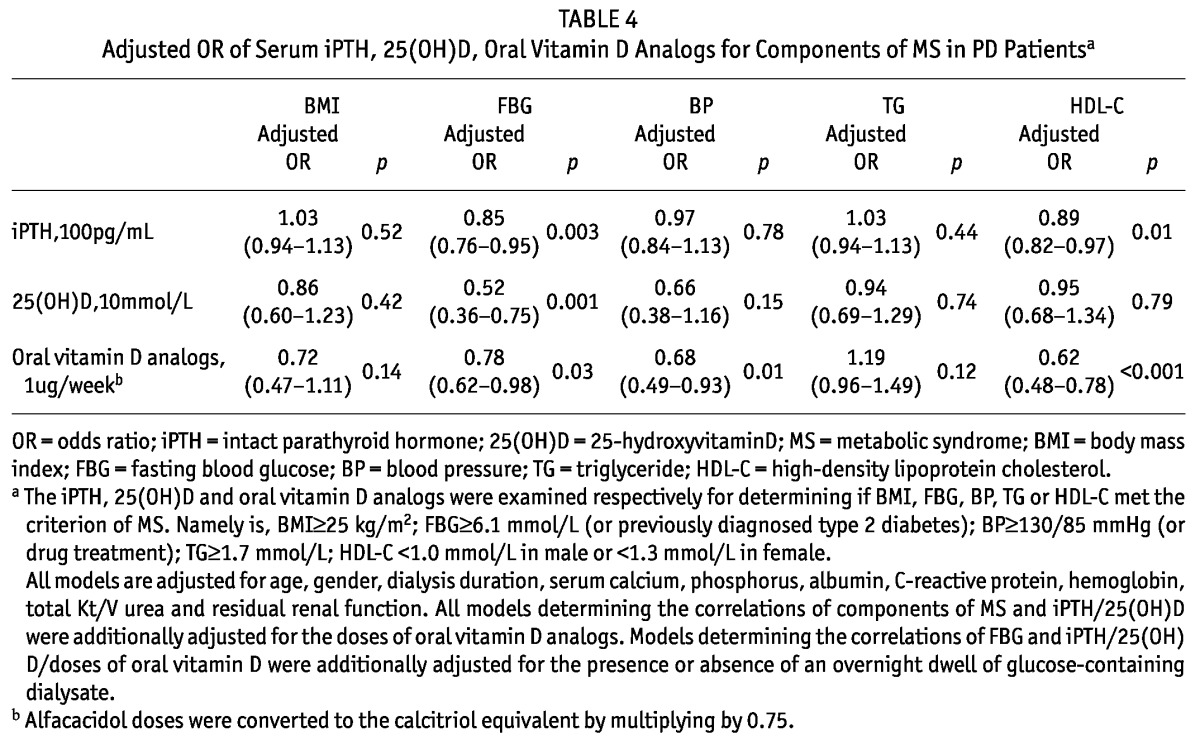
Next, we examined the association between values of iPTH, 25(OH)D and doses of oral vitamin D supplements and the individual components of MS, i.e. BMI, FBG, BP, TG and HDL-C (Table 4). After adjustment for age, gender, dialysis duration, serum calcium, phosphorus, albumin, CRP, hemoglobin, total Kt/V, RRF and whether a glucose-containing dialysate dwell was used during the night prior to the blood draw, serum iPTH/25(OH) D and doses of oral vitamin D analogs independently predicted FBG meeting the criteria of MS. Serum iPTH/doses of oral vitamin D analogs also independently predicted abnormal HDL-C. Only oral vitamin D analogs was an independent risk factor for BP ≥ 130/85 mmHg. Neither serum iPHT/25(OH)D nor oral vitamin D could predict BMI and TG meeting criteria for MS.
TABLE 4.
Adjusted OR of Serum iPTH, 25(OH)D, Oral Vitamin D Analogs for Components of MS in PD Patientsa
Discussion
The prevalence of MS in PD patients in this large, multi-center, cross-sectional study was 55.4%, which is in agreement with previous studies (9-12). The key finding of our study is that serum iPTH and oral vitamin D supplementation rather than serum 25(OH)D levels were inversely associated with MS prevalence and some MS components after adjustment for multivariates.
In the general population, serum iPTH has been positively correlated with the risk of MS, with ORs ranging from 1.9 to 3.7 (13-15). By contrast, our study is the first to show the opposite trend among PD patients. We hypothesized that the relationship between iPTH and MS risk is described by a non-linear curve. The iPTH at a relatively low range is directly associated with MS risk in the general population. However, when iPTH increases to a higher level in dialysis populations, it might be inversely associated with MS risk. Our data did not confirm this since serum iPTH higher than 585 pg/mL was not associated with a lower risk for MS than in the 130 - 585 pg/mL group. However, only relatively few patients (55 of 837) had a serum iPTH level > 585 pg/mL, and this might have reduced the power to demonstrate an effect. Therefore, our observational data do not clarify whether the target of iPTH levels recommended by KDIGO (36) favors MS prevalence.
Another novel finding in this study is that doses of oral vitamin D analogs were significantly inversely associated with the prevalence of MS. Indeed adjusting for oral vitamin D supplementation weakened the association of iPTH with MS prevalence, providing further evidence of the relationship between oral vitamin D and MS. Since vitamin D supplementation has been reported to improve glycemic control (37), reduce proteinuria (38) and inflammation, and improve cardiac dysfunction (39), nephrologists would be very interested to know if oral vitamin D supplementation is an easy and relatively inexpensive way to mitigate MS risk.
Although some studies have indicated that serum 25(OH)D levels independently predict MS (16-20), this association was not proved in our study. Our finding supports previous studies performed in severely obese subjects (25,40,41) and older adults (42), which showed that the significance of serum 25 (OH)D in MS prevalence disappeared after adjustments for age, gender, BMI and season (43). Of note, both ours and previous studies indicating no association of 25(OH) D and MS included subjects with obvious vitamin D deficiency (14,40,41), whereas other studies reporting an inverse correlation of 25(OH)D with MS prevalence enrolled subjects with > 50 nmol/L of serum 25(oH) D levels (16-20). This suggests that the relationship between 25 (OH)D and MS prevalence might also be non-linear.
From out data, serum iPTH levels were associated with the risk for some but not all of the individual components of MS, i.e. FBG and HDL-C, and oral vitamin D supplements with FBG, HDL-C and BP. The underlying mechanisms for these findings are not clear. Some studies have shown that insulin resistance is closely associated with vitamin D deficiency in dialysis patients and active vitamin D supplements improved insulin resistance (44-46). This data may explain the correlation of iPTH/oral vitamin D and FBG in this study. However, the relationship between iPTH, vitamin D status and BP or HDL has not been indicated.
The major strength of our study is that this is the first study to explore the relationships between serum iPTH, 25(OH)D, oral vitamin D supplements and MS in a PD cohort. A prospective study design within a strict, quality-control framework is also a merit of this report. Our analysis took into account a number of potential covariates that might confound the observed associations, such as age, gender, BMI, dialysis duration and biochemical parameters. All measurements of serum 25(OH)D concentrations were made in the same season, thus avoiding the seasonal variations in this hormone.
Nevertheless, our study had several limitations. First, the cross-sectional design makes it difficult to establish a cause-effect relationship. Second, the enrolled patients for the measurement of 25(OH)D concentrations might not be representative of the characteristics of all PD patients. Finally, the definition of MS still has certain limitations in terms of the measurement of plasma glucose levels. The inherent continuous absorption of glucose from dialysate is a risk factor for hyperglycemia and hyperinsulinemia. The timing of blood collection for the measurement of fasting glucose (PD fluids drained out or dwelled) and the dwell times were inconsistent across the participants. Unfortunately, this is a common limitation of studies on MS in PD patients (47).
In conclusion, this study indicated that elevated iPTH levels were associated with a lower risk of MS and some of MS components in dialysis patients. Further exploration is required to determine whether the current iPTH target recommended by KDIGO favors MS and cardiovascular disease risk. Since oral vitamin D supplementation was an important protective factor against MS, interventional trials are necessary to explore this effect.
Disclosures
No conflict of interest exists.
Acknowledgments
The authors express their appreciation to the patients, doctors, and nursing staff of the peritoneal dialysis center of Peking University First Hospital, General Hospital of Ningxia Medical University, the people’s Hospital of Peking University, Huashan Hospital and the Second Affiliated Hospital of Harbin Medical University for their continuing contribution to this study. The authors also thank Dr. Martin Wilkie and Dr. Tim Ellam, Sheffield Kidney Institute, Sheffield. UK, for their valuable revision of the draft. This work is supported in part by New Century Excellent Talents from Education Department of China, Clinic Research Award from ISN GO R&P Committee, and Renal Research Grant (Baxter, China).
References
- 1. Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol 2005; 16:2134–40 [DOI] [PubMed] [Google Scholar]
- 2. Tanaka H, Shiohira Y, Uezu Y, Higa A, Iseki K. Metabolic syndrome and chronic kidney disease in Okinawa, Japan. Kidney Int 2006; 69:369–74 [DOI] [PubMed] [Google Scholar]
- 3. Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol 2002; 156:1070–7 [DOI] [PubMed] [Google Scholar]
- 4. Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation 2004; 109:42–6 [DOI] [PubMed] [Google Scholar]
- 5. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002; 288:2709–16 [DOI] [PubMed] [Google Scholar]
- 6. Townsend RR, Anderson AH, Chen J, Gadebegku CA, Feldman HI, Fink JC, et al. Metabolic syndrome, components, and cardiovascular disease prevalence in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Nephrol 2011; 33:477–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thorn LM, Forsblom C, Fagerudd J, Thomas MC, Pettersson-Fernholm K, Saraheimo M, et al. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care 2005; 28:2019–24 [DOI] [PubMed] [Google Scholar]
- 8. Johnson DW, Armstrong K, Campbell SB, Mudge DW, Hawley CM, Coombes JS, et al. Metabolic syndrome in severe chronic kidney disease: Prevalence, predictors, prognostic significance and effects of risk factor modification. Nephrology (Carlton) 2007; 12:391–8 [DOI] [PubMed] [Google Scholar]
- 9. Lee CJ, Subeq YM, Wang CH, Lee RP, Fang TC, Hsu BG. Fasting serum ghrelin level is associated with metabolic syndrome in peritoneal dialysis patients. Perit Dial Int 2008; 28(Suppl 3):S196–200 [PubMed] [Google Scholar]
- 10. Park JT, Chang TI, Kim DK, Choi HY, Lee JE, Kim HW, et al. Association of white blood cell count with metabolic syndrome in patients undergoing peritoneal dialysis. Metabolism 2009; 58:1379–85 [DOI] [PubMed] [Google Scholar]
- 11. Park JT, Chang TI, Kim DK, Lee JE, Choi HY, Kim HW, et al. Metabolic syndrome predicts mortality in non-diabetic patients on continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 2010; 25:599–604 [DOI] [PubMed] [Google Scholar]
- 12. Young DO, Lund RJ, Haynatzki G, Dunlay RW. Prevalence of the metabolic syndrome in an incident dialysis population. Hemodial Int 2007; 11:86–95 [DOI] [PubMed] [Google Scholar]
- 13. Ahlström T, Hagström E, Larsson A, Rudberg C, Lind L, Hellman P. Correlation between plasma calcium, parathyroid hormone (PTH) and the metabolic syndrome (MetS) in a community-based cohort of men and women. Clin Endocrinol (Oxf) 2009; 71:673–8 [DOI] [PubMed] [Google Scholar]
- 14. Hjelmesæth J, Hofsø D, Aasheim ET, Jenssen T, Moan J, Hager H, et al. Parathyroid hormone, but not vitamin D, is associated with the metabolic syndrome in morbidly obese women and men: a cross-sectional study. Cardiovasc Diabetol 2009; 8:7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saab G, Whaley-Connell A, Bombeck A, Kurella Tamura M, Li S, Chen S-C, et al. The association between parathyroid hormone levels and the cardiorenal metabolic syndrome in non-diabetic chronic kidney disease. Cardiorenal Medicine 2011; 1:123–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ford ES, Zhao G, Li C, Pearson WS. Serum concentrations of vitamin D and parathyroid hormone and prevalent metabolic syndrome among adults in the United States. J Diabetes 2009; 1:296–303 [DOI] [PubMed] [Google Scholar]
- 17. Kayaniyil S, Vieth R, Harris SB, Retnakaran R, Knight JA, Gerstein HC, et al. Association of 25(OH)D and PTH with metabolic syndrome and its traditional and nontraditional components. J Clin Endocrinol & Metabol 2010; 96:168–175 [DOI] [PubMed] [Google Scholar]
- 18. Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: The Medical Research Council Ely Prospective Study 1990-2000. Diabetes 2008; 57:2619–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reis JP, von Muhlen D, Miller ER. Relation of 25-hydroxyvitamin D and parathyroid hormone levels with metabolic syndrome among US adults. Eur J Endocrinol 2008; 159:41–8 [DOI] [PubMed] [Google Scholar]
- 20. Lee DM, Rutter MK, O’Neill TW, Boonen S, Vanderschueren D, Bouillon R, et al. Vitamin D, parathyroid hormone and the metabolic syndrome in middle-aged and older European men. Eur J Endocrinol 2009; 161:947–54 [DOI] [PubMed] [Google Scholar]
- 21. Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 2004; 79:820–25 [DOI] [PubMed] [Google Scholar]
- 22. Chiu KC, Chuang LM, Lee NP, Ryu JM, McGullam JL, Tsai GP, et al. Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism 2000; 49:1501–5 [DOI] [PubMed] [Google Scholar]
- 23. Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71:31–8 [DOI] [PubMed] [Google Scholar]
- 24. Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Jain A, Schreiber MJ, Jr., et al. Low 25-hydroxyvitamin D levels and mortality in non-dialysis-dependent CKD. Am J Kidney Dis 2011; 58:536–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 2006; 70:771–80 [DOI] [PubMed] [Google Scholar]
- 26. Vacek JL, Vanga SR, Good M, Lai SM, Lakkireddy D, Howard PA. Vitamin D deficiency and supplementation and relation to cardiovascular health. Am J Cardiol 2012; 109:359–63 [DOI] [PubMed] [Google Scholar]
- 27. Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am J Clin Nutr 2011; 94:1144–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salum E, Kampus P, Zilmer M, Eha J, Butlin M, Avolio AP, et al. Effect of vitamin D on aortic remodeling in streptozotocin-induced diabetes. Cardiovasc Diabetol 2012; 11:58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–97 [DOI] [PubMed] [Google Scholar]
- 30. WHO/IASO/IOTF. The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications Australia: Melbourne 2000. [Google Scholar]
- 31. Park JT, Chang TI, Kim DK, Choi HY, Lee JE, Kim HW, et al. Association of white blood cell count with metabolic syndrome in patients undergoing peritoneal dialysis. Metabolism 2009; 58:1379–85 [DOI] [PubMed] [Google Scholar]
- 32. Liao CT, Kao TW, Chou YH, Wu MS, Chen YM, Chuang HF, et al. Associations of metabolic syndrome and its components with cardiovascular outcomes among non-diabetic patients undergoing maintenance peritoneal dialysis. Nephrol Dial Transplant 2011; 26:4047–54 [DOI] [PubMed] [Google Scholar]
- 33. Li PK, Kwan BC, Ko GT, Chow KM, Leung CB, Szeto CC. Treatment of metabolic syndrome in peritoneal dialysis patients. Perit Dial Int 2009; 29 Suppl 2:S149–52 [PubMed] [Google Scholar]
- 34. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–72 [DOI] [PubMed] [Google Scholar]
- 35. Matsumoto T, Ito M, Hayashi Y, Hirota T, Tanigawara Y, Sone T, et al. A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures — A randomized, active comparator, double-blind study. Bone 2011; 49:605–12 [DOI] [PubMed] [Google Scholar]
- 36. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009; S1–130 [DOI] [PubMed] [Google Scholar]
- 37. Blair D, Byham-Gray L, Lewis E, McCaffrey S. Prevalence of vitamin D [25(OH)D] deficiency and effects of supplementation with ergocalciferol (vitamin D2) in stage 5 chronic kidney disease patients. J Ren Nutr 2008; 18:375–82 [DOI] [PubMed] [Google Scholar]
- 38. Liu L-J, Lv J-C, Shi S-F, Chen Y-Q, Zhang H, Wang H-Y. Oral Calcitriol for reduction of proteinuria in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis 2012; 59:67–74 [DOI] [PubMed] [Google Scholar]
- 39. Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, et al. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol 2010; 5:905–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rueda S, Fernández-Fernández C, Romero F, Martínez de Osaba MJ, Vidal J. Vitamin D, PTH, and the metabolic syndrome in severely obese subjects. Obesity Surgery 2008; 18:151–4 [DOI] [PubMed] [Google Scholar]
- 41. Neyestani T, Salekzamani Alavi Majd H, Houshiarrad Kalayi, Shariatzadeh Gharavi. Is vitamin D status a determining factor for metabolic syndrome? A case-control study. Diabetes Metab Syndr Obes 2011; 205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, Parathyroid Hormone Levels, and the Prevalence of Metabolic Syndrome in Community-Dwelling Older Adults. Diabetes Care 2007; 30:1549–55 [DOI] [PubMed] [Google Scholar]
- 43. Røislien J, Van Calster B, Hjelmesæth J. Parathyroid hormone is a plausible mediator for the metabolic syndrome in the morbidly obese: a cross-sectional study. Cardiovascular Diabetology 2011; 10:17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hung AM, Ikizler TA. Factors determining insulin resistance in chronic hemodialysis patients. Contrib Nephrol 2011; 171:127–34 [DOI] [PubMed] [Google Scholar]
- 45. Bindal ME, Taskapan H. Hypovitaminosis D and insulin resistance in peritoneal dialysis patients. Int Urol Nephrol 2011; 43:527–34 [DOI] [PubMed] [Google Scholar]
- 46. Ulutas O, Taskapan H, Taskapan MC, Temel I. Vitamin D deficiency, insulin resistance, serum adipokine, and leptin levels in peritoneal dialysis patients. Int Urol Nephrol 2012; 45(3):879–84 [DOI] [PubMed] [Google Scholar]
- 47. He Q, Zhang W, Chen J. A meta-analysis of icodextrin versus glucose containing peritoneal dialysis in metabolic management of peritoneal dialysis patients. Ren Fail 2011; 33:943–8 [DOI] [PubMed] [Google Scholar]


