Abstract
Theories on the functions of the hippocampal system are based largely on two fundamental discoveries: the amnestic consequences of removing the hippocampus and associated structures in the famous patient H.M. and the observation that spiking activity of hippocampal neurons is associated with the spatial position of the rat. In the footsteps of these discoveries, many attempts were made to reconcile these seemingly disparate functions. Here we propose that mechanisms of memory and planning have evolved from mechanisms of navigation in the physical world and hypothesize that the neuronal algorithms underlying navigation in real and mental space are fundamentally the same. We review experimental data in support of this hypothesis and discuss how specific firing patterns and oscillatory dynamics in the entorhinal cortex and hippocampus can support both navigation and memory.
Navigation is based on two interlinked mechanisms for representation of the spatial environment1,2, one that provides static position information in a reference frame and another that calculates coordinates based on integration of motion and knowledge of previous positions. The first is often referred to as map-based or allocentric navigation (Fig. 1), in which the spatial relationships among landmarks assist in defining the animal’s location in the environment3. The spatial metric needed for the estimation of distances between landmarks is believed to arise from a second mechanism, often referred to as path integration or egocentric navigation. Path integration requires active movement of the body and computes the distances and turns of the animal as it explores the environment; this mechanism allows the animal to return to its home base using the shortest route1,2,4,5 (Fig. 1). The essential components of the self-referenced navigation system are locomotion speed, elapsed time, head direction and the initial reference position5. Map-based and path integration-based representation always work together, but the availability of external landmarks may determine whether allocentric or egocentric strategies dominate. In cue-rich environments, representations may be updated frequently by changes in the configuration of sensory inputs. In environments with few stationary landmarks or complete darkness, path integration may be the default mode6–8.
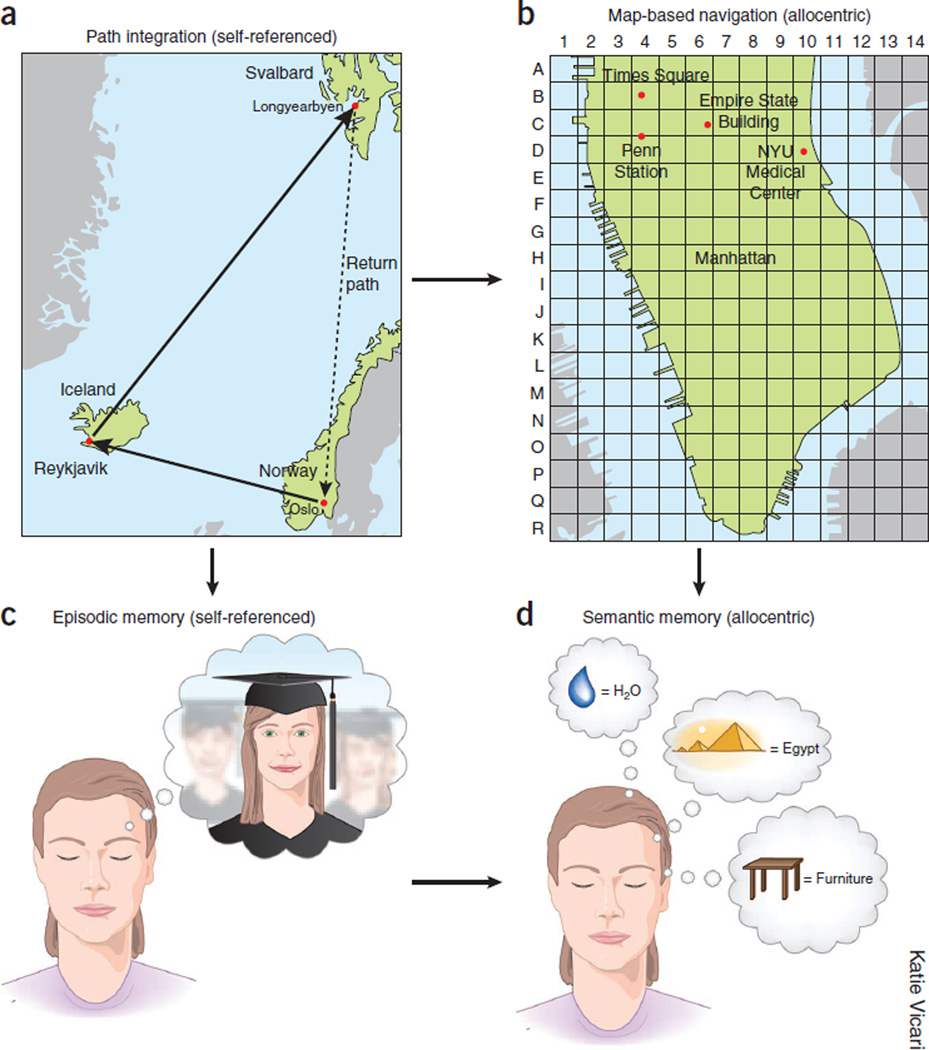
Figure 1.
Relationship between navigation and memory. (a) Path integration (also known as dead reckoning) is based on self-referenced information by keeping track of travel distances (time elapsed multiplied by speed) and direction of turns. Calculating translocation relative to the start location allows the animal to return to the start along the shortest (homing) path. (b) Map-based navigation is supported by the relationships among visible or otherwise detectable landmarks. A map is constructed by exploration (path integration). (c) Episodic memory is ‘mental travel’ in time and space referenced to self. (d) Semantic memory is explicit representation of living things, objects, places and events without temporal or contextual references. Semantic knowledge can be acquired through multiple episodes with common elements. We hypothesize that the evolutionary roots of episodic and semantic memory systems are the dead reckoning and landmark-based forms of navigation, respectively.
The brain systems involved in guiding navigation, the hippocampus and entorhinal cortex, are the same that support declarative memories9,10. How are the navigation mechanisms, then, related to the mental ‘travel’ of memory11 and planning? As in the dichotomy of the spatial representation, two forms of declarative memory can be distinguished10. Semantic memory explicitly defines living things, objects, facts and events of the surrounding world independently of temporal context10,12, much as an allocentric map defines a location largely independently of how the animal got there. Episodic memory, in contrast, endows the individual with the capacity to learn and recall first-person experiences in the context of both space and subjective time12 and to use such information for planning actions13,14, in the same way as location sequences are linked together by a neural path integrator. It has been suggested that explicit, semantic knowledge is acquired progressively as similar episodes are encoded repeatedly by the self-referenced episodic memory system, so that knowledge eventually becomes context independent10,15,16. This gradual process is reminiscent of the formation of allocentric maps based on repeated exploration of the environment17. The clear parallels between allocentric navigation and semantic memory, on one hand, and path integration and episodic memory, on the other, raise the possibility that the same networks and algorithms support both physical and mental forms of travel. However, this hypothesized evolutionary link does not imply that spatial memory ‘incorporates’ all memories or that all memories should have spatial components.
To support memory effectively, a neural system evolved for navigation must meet two more requirements. It must have the capacity to store large quantities of seemingly unrelated, or orthogonal, representations, and it must be able to self-generate temporally evolving cell assembly sequences. We suggest that the hippocampus–entorhinal cortex system has the anatomical and physiological properties that make it especially suitable for meeting these requirements and present data that support the hypothesis of phylogenetic continuity of navigation and memory. Most experiments that we discuss were carried out on rodents, but we believe that the conclusions and interpretations based on these ‘simpler’ animals bear validity for the mechanisms in the human brain as well.
Allocentric maps and semantic memory
There is a general agreement that explicit memories depend on the entorhinal cortex–hippocampal system10,18, although debate persists whether consolidated semantic information becomes hippocampus independent or continues to depend on the hippocampus forever19–21. In the following section, we shall discuss the neuronal processes that support allocentric, map-based navigation22 and illustrate how these algorithms might at the same time support semantic memory. Our central claim is that the neuronal mechanisms that evolved to define the spatial relationship among landmarks can also serve to embody associations among objects, events and other types of factual information.
An animal’s spatial coordinates are encoded by a range of interacting cell types with defined activity profiles. The two most striking firing patterns are perhaps those of the ‘place cells’, discovered in the hippocampus1, and the ‘grid cells’, discovered in the medial entorhinal cortex (Fig. 2)23. Grid cells have multiple firing fields that span the entire available space in a periodic hexagonal pattern, which provides a metric to the neural representation of space23. Grid cells are present throughout the medial entorhinal cortex and in the pre- and parasubiculum but are most abundant in layer 2 of medial entorhinal cortex24,25. Deeper layers often contain cells with stronger representation of some grid axes than others26, and grid cells are intermingled with head direction cells25. In addition, the medial entorhinal cortex contains a smaller number of ‘border cells’, which line up in specific orientations along specific geometric boundaries of the environment27. These cell types were all discovered in rats, but place cells, grid cells and head direction cells have since been found in other species28–30.
Figure 2.
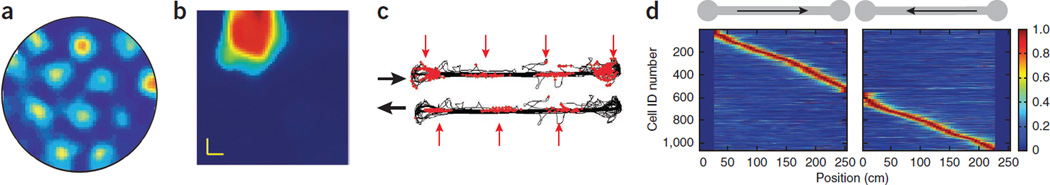
Grid cells and place cells. (a–d) Firing patterns of entorhinal (a,c) and hippocampal (b,d) principal cells in a two-dimensional open field (a,b) and one-dimensional track (c,d). (a) Grid cell from layer 2 of the entorhinal cortex recorded from a rat exploring a 2-m cylinder. Note increased firing rates (warmer colors) throughout the environment at the apexes of tiling triangles. (b) Singular place field of a CA1 pyramidal neuron recorded from a rodent exploring a square 1 × 1 m. Firing rate of the neuron is color-coded. (c) On a 2-m-long linear track, entorhinal neurons fire at regular intervals (red arrows) but at different positions during left and right journeys. (d) Firing rates of pyramidal cells on a track. CA1 pyramidal cells have typically a single field, present mainly in one direction of travel. Each row represents a neuron. Firing rates are peak-normalized, color-coded and ordered by their peak rates during right or left direction (arrow) of travel. Panels a,c reproduced with permission from ref. 23, b from ref. 74 and d from ref. 35.
The functions of each cell type are yet to be determined, but the periodic fields of the grid cells provide a metric to the neural representation of space, in the same way that head direction cells provide a directional reference frame. Border cells may assist in the assessment of the allocentric distances by triangulation and perhaps in scaling the grid size to accommodate to the size of the discoverable environment. The availability of direction, position, distance and boundary information in the entorhinal cortex makes this brain structure an ideal candidate for computing the spatial metric of the surrounding environment22,25. Unlike hippocampal place cells, which fire differentially in different environments31, grid cells, border cells and head direction cells are active in all environments and often behave in a coherent manner27. The preservation of spatial and directional firing relationships in local ensembles of such cells implies that the entorhinal cortex generates a metric that can be applied universally across environments5,22,23.
How could the spatial maps of the medial entorhinal cortex sub-serve memory formation? The organization of the grid-cell network provides some clues. Grid cells are arranged as semi-independent modules or clusters along the dorsal-to-ventral axis of the medial entorhinal cortex, with small grid scales dominating in dorsal parts and large grid scales being more abundant in ventral parts. Steps in grid scale go hand in hand with quantal shifts of orientation and asymmetry in the grid pattern32. Different modules may respond independently to changes in the geometry of the environment. The functional autonomy of the grid modules has important consequences for representation of space in downstream hippocampal cell populations (Fig. 3a). Their ability to respond differentially to reconfiguration of the environment supplies a rich combination of efferent patterns to the hippocampus33, which may be sufficient to generate large numbers of discrete maps individualized to the vast number of environments the animal visits in its lifetime.
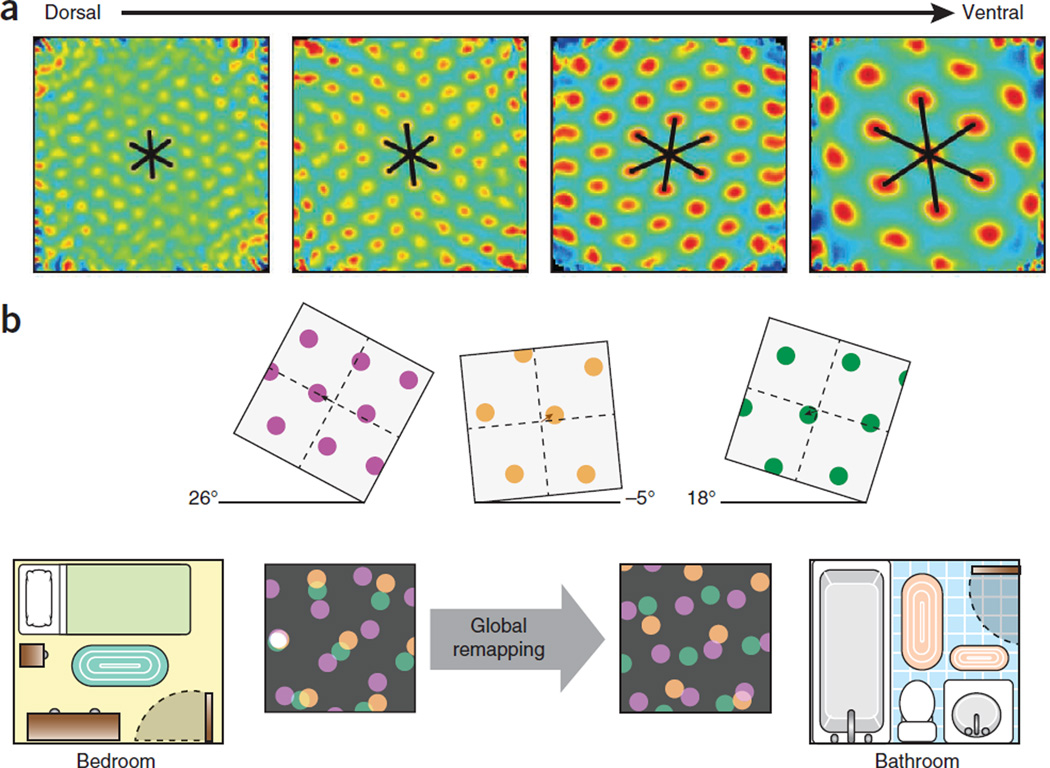
Figure 3.
Modular organization of the grid-cell network. (a) Stepwise increase of grid spacing at successive dorsoventral levels of medial entorhinal cortex. Spatial autocorrelograms for four example cells (one per dorsoventral module). (b) Remapping of hippocampal place cells in two environments. Top panels show responses of three grid cells to a change in the environment. Independent responses are illustrated by different degrees of rotation and translation. Bottom panels show inputs from the grid cells, each from a different module (purple, green and orange), at different locations in the environments. The three grid cells provide input to a particular CA3 cell (white spot at left) sufficient to cause it to fire when, and only when, the nodes of the three grids overlap. This occurs only at one location in this example. In the second environment, the altered coactivity of the grid cells activates a different subset of place cells at each location, and global remapping is observed in hippocampal place ensembles. Panel a reproduced with permission after ref. 32, b after ref. 33.
The formation of numerous independent maps in the hippocampus has obvious advantages for memory formation, as it allows the network to store new representations in a manner that minimizes interference with previously stored memories. The orthogonal nature of hippocampal representations is expressed in its ability to ‘remap’ between experiences and environments31 (Fig. 3). Even minor changes in the configuration of landmarks or the motivational context can completely alter the firing pattern of the hippocampal place cell population. As a result, each environment can be represented by a unique combination of active place cells and place fields. The apparent orthogonalization is strongest in the CA3 subfield34,35, where firing locations of any two cells across environments are no more correlated than expected by chance34. By virtue of the vast and densely connected random graph of recurrent collaterals between neurons in this area36, the CA3 region has the ability to read and register the rich combinatorial output of the entorhinal cortex grid modules. The multiplicity of representations may be generated by independent changes of grid maps in the medial entorhinal cortex, but the orthogonalization may also benefit from intrinsic architectural properties of the hippocampus. The latter may be accomplished in two steps. First, the entorhinal cortex–mediated pattern is separated into subpatterns by the distinctive and low-divergence connectivity of granule cells to CA3 targets37,38. Second, the CA3 region can compute the distance relations among the discretized subpatterns and represent those relationships by the synaptic weights between the CA3 place cells and CA3-CA1 projections39. Taken together, the combined entorhinal cortex and hippocampal mechanisms may enable the storage of very large numbers of arbitrary associations without interfering with one another.
If the hippocampal–entorhinal cortex system were developed only for navigation, the large combinatorial space and the large numbers of potential cell assembly combinations would be surprising. First, insects can navigate effectively with much simpler circuits and many fewer neurons40. Second, in navigating rats, the local environment can be mapped at centimeter precision by just a dozen or so grid cells33 or place cells41. We suggest that the rich variety of orthogonal representations generated by the modular representation of space during mammalian evolution laid the ground for storing independent representations for the wide variety of environments and experiences encountered by the animal every day. This enlarged representational capacity of environmental details could be what distinguishes the mammalian brain from the brains of species in which navigation is based on much smaller circuits.
The growth of networks that enable the storage of millions of situations in the mammalian brain and the evaluation of the relationships among them may also form the basis for representing and categorizing explicit knowledge. The same mechanisms that define unique positions and their relationships in a map can be used to define or symbolize events, objects and living things10,19. Many experiments demonstrate that recognition and recall of objects or events are associated with unique constellations of firing patterns in the entorhinal cortex–hippocampal system in a variety of species21,41–46. Examples of semantic ‘encoding’ are also available from human epilepsy patients, in which selective firing of hippocampal and entorhinal cortex neurons has been evoked by specific words, objects or individuals, largely independently of their physical characteristics47,48. The classification of items, events and situations on the basis of semantic proximity shares many features with the distance relationship among landmarks49. Similarly to the embodiment of the spatial relations among objects in the cognitive map1, models of semantic relatedness use a metric based on topological similarity and a neuronal network equivalent of vector distance for defining relationships among words49,50, which can be ably assisted by the pattern completion and separation mechanisms of the entorhinal cortex–hippocampus system38,51 in partnership with the neocortex19–21.
Self-referenced navigation and episodic memory
Beyond phenomenological similarities between episodic memory and egocentric travel, recent work in humans indicates an extensive overlap in the brain networks supporting navigation, remembering the past and thinking about the future52,53. The key properties of episodic memory involve binding disparate and often arbitrary details together into a coherent event and the recollection of self-centered past experiences in the context of time and space in which the events occurred1,14. Characteristically, snippets of cues can trigger a long process of recollection, which is often grouped or chunked into shorter sub-episodes. Although re-experiencing the past appears as a continuous process, we are consciously aware of only short segments of the episodes at any one time, pointing to an important cooperation between structures and mechanisms responsible for long-term storage of declarative knowledge and working memory. Many excellent reviews have summarized the overlapping nature of brain systems involved in navigation, episodic memory, imagination and planning of actions1,10,18,52,53. Below we will discuss the physiological mechanisms that may support these seemingly disparate functions, focusing on self-organized cell assembly sequences, multiple-timescale representation and theta oscillations.
Episodic recall by internally generated cell assembly sequences
How does the brain generate and store sequences? We hypothesize that the mechanisms for representing a path through an environment are similar to those used to represent sequences in memory (Fig. 4a). Like the position-dependent sequential firing of neurons along a linear path, sequences of arbitrary items in episodic memory tasks are essentially unidimensional. Therefore, the same encoding mechanisms can be used for temporal integration of positions and episodic items54. The dominantly unidirectional linking of items in episodic memory, analogous to linking of place cells55, can explain two important principles of free recall: asymmetry, or the fact that forward associations are stronger than backward associations56, and temporal contiguity, or the fact that recollection of an item is facilitated by the presentation or spontaneous recall of another item that occurred close in time to the item just recalled56. There are also key differences between mechanisms of navigation in the physical world and mental recall. A fundamental difference is that while the navigation system can rely on environmental or body-derived cues, as it does in invertebrates, ‘simulated’ or ‘internalized’ travel12,14,57 requires internally generated cell assembly sequences (Fig. 4).
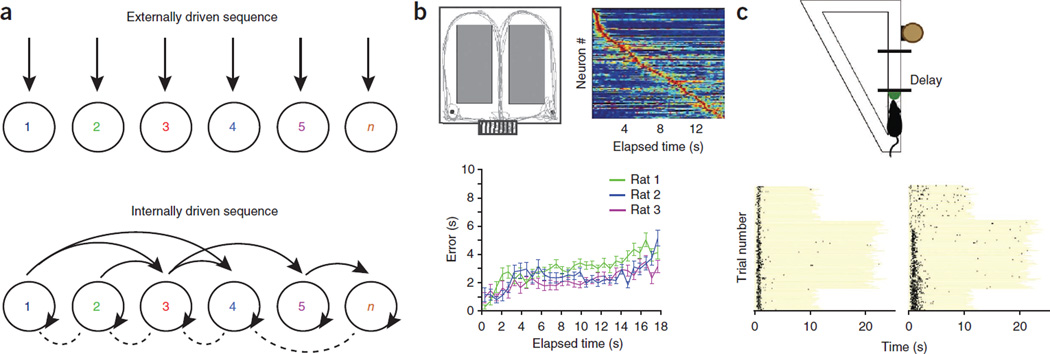
Figure 4.
Cell assembly sequences, space and time tracking. (a) During physical travel, successive assemblies of neurons (1 to n) respond sequentially owing to the changing constellation of environmental landmarks and/or proprioceptive information from the body (top). During mental travel, sequential activation is supported by self-organized patterning59. Not only first order (neighbor) but also higher order (non-neighbor) connections can be represented in strongly connected recurrent networks. (b) Inferring elapsed time from self-organized cell assembly sequences during wheel running in a spontaneous alternation task. Top left, the rat’s travel path superimposed on the maze. The start area is the wheel (bottom). Top right, normalized firing rate sequence of neurons during wheel running, ordered by the latency of their peak firing rates (each line represents a cell). Bottom, accumulating errors of time prediction (in seconds) calculated from a probabilistic model for inferring elapsed time from the phases of spikes with respect to theta oscillation. Note high precision of time prediction during the entire episode of wheel running. (c) Time tracking by neuron sequences during the delay part of a task of a working memory go/no-go task in a linear maze (top). The delay varied between 10 and 20 s in consecutive blocks, as indicated by the length of the yellow lines (bottom). Some neurons maintain their spike timing (left), whereas others ‘re-time’ (right) when the length is abruptly changed. Panel b reprinted with permission from ref. 65, c from ref. 93.
In addition to reporting the instantaneous position of the animal or the explicit identity of objects and events, neurons in the hippocampus can also predict where the animal is coming from or where it is going45,58. When environmental or body-derived signals are kept constant—for example, during running in a wheel in a memory task—perpetually changing neuronal assembly sequences are present in the hippocampus59 (Fig. 4b). The unique patterns of the cell assembly sequences can reliably predict an animal’s correct or even erroneous choice in a maze many seconds before the actual motor turn. It has been hypothesized that such self-organized cell assembly sequences or neural ‘trajectories’ underlie the numerous episodes recorded in one’s lifetime59. Because many more sequences can be generated than the numbers of the member neurons, the syntactical rules underlying self-evolving neuronal trajectories can enormously expand the memory capacity of the hippocampal system60. In short, assembly sequences in the hippocampus can be generated by two different but possibly interacting mechanisms, driven, respectively, by external inputs (environmental or body-derived cues) and internal self-organization (Fig. 4a). Self-organized cell assembly sequences disengaged from the environmental or body-derived inputs, in turn, may support mental travel.
Chunking of paths and memories
How long is a sequence? Neuronal representation of travel paths does not consist of long uninterrupted neuronal chains but are often broken up into repeating chunks by prominent landmarks, state changes or reinforcers. Furthermore, the firing characteristics of grid cells and place cells are context dependent. In corridors or on elevated tracks, the firing fields of entorhinal cortex grid cells in one direction are generally independent of the positions in the other direction61. Similarly, the omnidirectional place fields of hippocampal neurons62 become unidirectional in linear environments, such that different sets of neurons are active at each location in the two directions of travel63. The independent representations of travel paths in opposite directions (Fig. 2) may reflect mechanisms similar to those underlying remapping between different environments, such as independent resetting among modules of the entorhinal cortex grid map. Chunking is even more prominent in complex environments. When different segments of a maze are geometrically similar, such as for the corridors of a hairpin labyrinth, hippocampal and entorhinal cortex neurons fire the same sequences in each corridor, although distinct sets of neurons fire on opposite journeys7,35,64. The chunking of the neuronal representation at each entry point can be assisted by resetting of the path integrator7 or of the self-organized cell assembly trajectories by a new initial condition65. Chunking is an efficient way to limit the accumulation of error inherent in long sequences and is a frequent strategy for encoding episodic information66. We suggest that chunking is a common property of path integration–based navigation and episodic memory systems.
Temporal organization of exploration and episodic memory
The hallmark of episodic recall is that fragments of information in short time windows predict the spatio-temporal evolution of episodes at longer timescales12,14. A narrative can be initiated by a single cue and can move forward more effectively than it can move in the reverse direction56. We suggest that both of these features can be achieved by a multiple-timescale organization of neuronal assemblies that hippocampal theta oscillations enable.
The multiple-timescale organization of neuronal assemblies
A postulated mechanism of internally generated neuronal sequences is the perpetual interaction among the multitude of brain rhythms maintained by cross-frequency coupling67. A prominent example of cross-frequency coupling in the hippocampus and entorhinal cortex is the theta phase-modulation of gamma power68,69, which has been shown to correlate with memory performance in both rats70 and humans71–73. This multiple-timescale organization is also evident in the spiking activity of hippocampal neurons (Fig. 5). Place cells representing the same spatial position or item form assemblies in the time window of gamma cycles54,74,75. Which neurons are considered members of an assembly is determined by their downstream ‘reader’ neurons. If the collective spiking of an upstream population occurs within the membrane time constant76 (10–30 ms) of the reader neurons, it will be classified as a single event by the readers. Spikes of other upstream neurons outside this integration window are relegated to other assemblies, thus representing separate events and resulting in the discharge of other reader neurons60. The ‘assembly window’ coincides with the time window of spike timing–dependent plasticity and the time constant of GABAA and AMPA receptors, whose interactions give rise to the gamma rhythm77,78. Given the 10- to 30-ms lifetime of hippocampal cell assemblies74, five to nine assemblies, each residing in a gamma cycle, can be active in a theta cycle68.
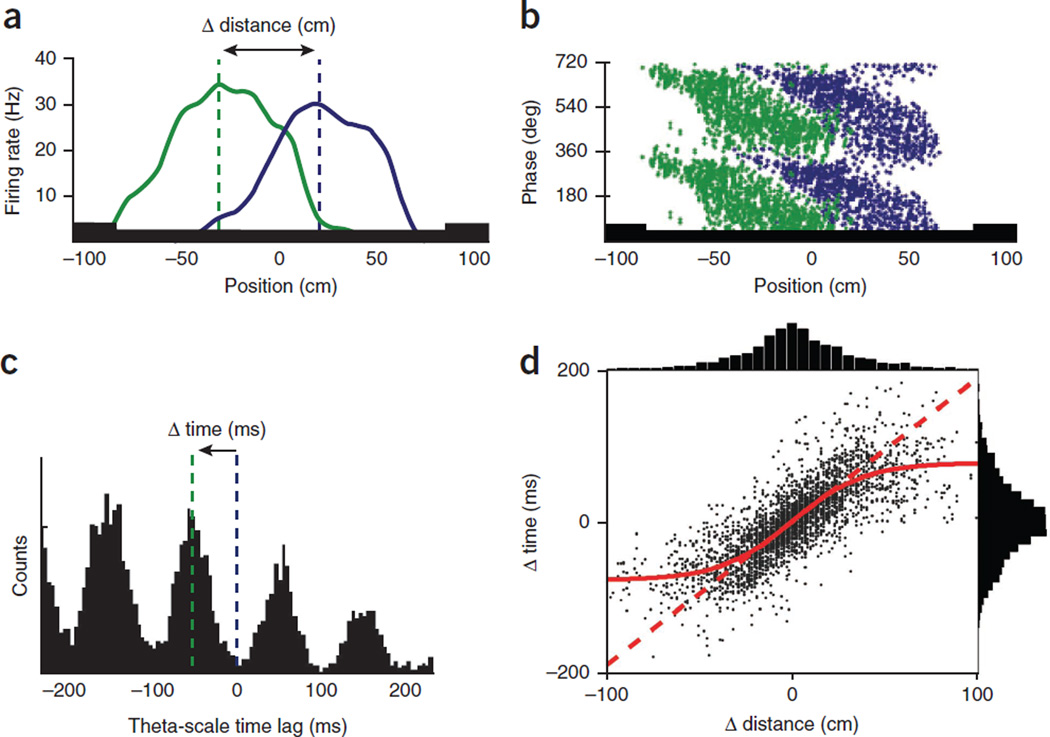
Figure 5.
Theta oscillations link assembly sequences. (a) Overlapping place fields of two hippocampal neurons (green and blue) on a track. (b) Theta phase of each spike as a function of position in the place fields of the two neurons. Note precession of spikes from late to early phases as the rat crosses the place fields83. Two theta cycles are shown for clarity. (c) Cross-correlation between the reference (blue) and overlapping (green) place cells. Δ time is the time lag between the spikes of two neurons within the theta cycle (‘theta time’). (d) Correlation between the distances of place field peaks and theta-scale time lags for >3,000 pairs of CA1 place cells. Note that the duration of the theta cycle limits distance resolution (red sigmoid curve). Panels a–c reproduced with permission after ref. 86, d after ref. 84.
Thus, the dual functions of the theta oscillation mechanism are to bring together and link cell assemblies into the temporal range where they can be modulated by synaptic plasticity, and at the same time segregate them within the available phase space54,60. The phase segregation of adjacent place cells may be brought about by perisomatic interneuron-mediated inhibition79–81 (Fig. 6). There are three interrelated consequences of the theta phase segregation of cell assemblies. First, the firing sequences of neurons on the descending phase, trough and ascending phase of the theta waves represent the sequences of the past, current and future positions of the animal’s journey75,82. Second, the time delays between overlapping place cells at the sub-theta-cycle scale are proportional to the time differences between their peak firing activities in physical space; that is, the time it takes for the animal to travel between the peaks of their place fields (Fig. 5a–d). As a result of this relationship, the travel distances between landmarks during navigation are expressed in the temporal domain by a time-compressed format in the theta waves75,82. Third, the oscillation frequency of the waxing-waning spike activity of place cells is faster than the frequency of the ‘background’ population, which is also reflected by the local field potential theta rhythm. This brings about a frequency interference pattern, known as phase precession83 (Fig. 5b), which is due, at least partially, to the millisecond time delays between adjacent place cells82,84. These temporal mechanisms determine the size of the place field (that is, its ‘lifetime’) and the slope of phase precession82,84. Similar temporal rules may govern the phase precession and the size of the grid in the entorhinal cortex85.
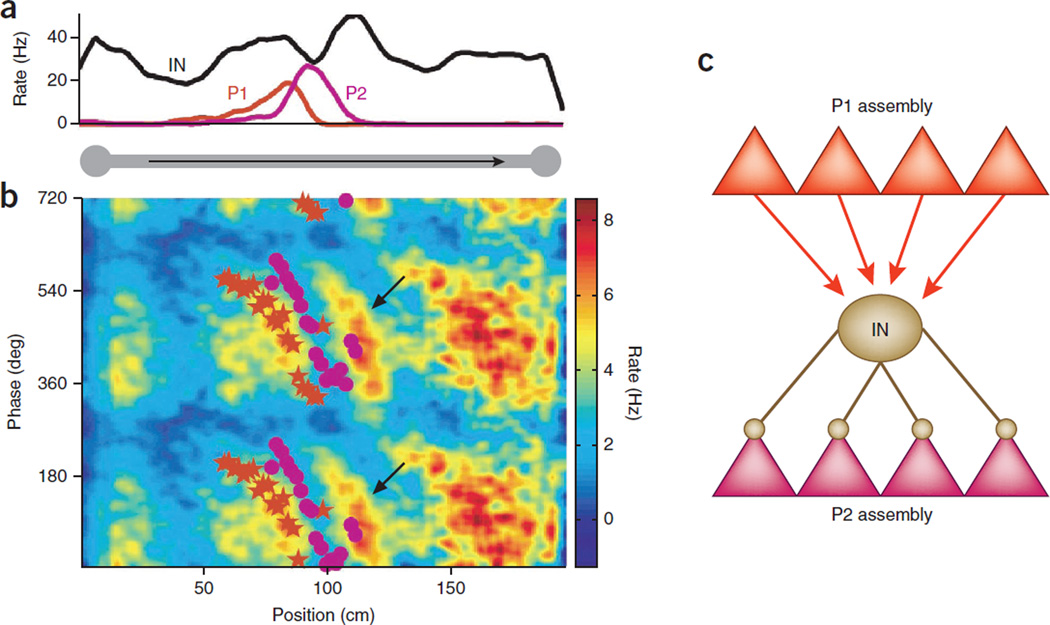
Figure 6.
Cell assembly segregation role of theta oscillation. (a) Place fields of two pyramidal cells (P1 and P2) and a putative basket cell interneuron (IN) on a linear track. (b) Mean phase precession of P1 (red stars) and P2 (magenta dots), superimposed on the interneuron’s color-coded and smoothed density of firing in theta phase space. Note the similar phase slopes of P1 and the interneuron and firing minima of the interneuron in the phase space of P2. (c) Model of theta phase selection by means of inhibition between competing assemblies. The interneuron is driven by the assembly of which P1 is a member and prevents discharge activity of a competing assembly of which P2 is a member. (d) Cartoon of phase segregation of seven cell assemblies in the entire phase space of the theta cycle (top). Perturbation of perisomatic inhibition reduces assembly segregation81 so that ‘information’ from the scrambled assembly sequence will become difficult to read out by downstream observer mechanisms (bottom). Panel a reproduced with permission after ref. 80.
The theta time compression mechanisms constrain how space and memory are represented, because the delays between the place cells limit the number of cell assemblies in the theta cycle54,86. In turn, the duration of the theta cycle limits the distances that can be linearly resolved by the sequential activity of neurons in a given environment, resulting in a sigmoid relationship between theta-scale time lags of spikes and distance representations by place cell pairs75,79,86 (Fig. 5d). As a result, upcoming locations that are more proximal to the animal are given stronger representation within a given theta cycle, with poorer resolution of locations in the distant future86. The size of place fields and grids of individual neurons and the distance representations of neuron pairs scale with the size of the environment3,31,86. In a way, the theta-nested assembly organization can be conceived as a ‘zooming’ mechanism, which provides a relatively coarse resolution of a large environment but an increasingly finer spatial resolution in smaller environments, with more neurons devoted to a given area of space, increased co-firing among cells in each theta cycle and increased overall firing in the population86. An attractive property of this arrangement is that sub-theta cycle time lags also allow place and grid cells to continue to represent the same positions and distances at different speeds of locomotion80,83,86 because the oscillation frequency of place and grid cells increases in proportion with the animal’s velocity80,85 and the time lags within the theta cycle remain largely independent of firing rate changes86.
The mechanisms of theta compression and expansion can effectively serve memory mechanisms. If locations are regarded as analogous to individual items in a memory buffer15,54,87, the theta-nested assembly organization limits the number of items that can be stored within a single theta cycle54. Accordingly, only a limited amount of information can be recalled from memory stores and consciously experienced at any given time. Analogously to zoom representation of distances, an episodic recall can have a fine spatiotemporal resolution for the conditions and context that surround a recalled event, whereas the relationships among items with longer temporal separation are progressively less resolved56. Moreover, temporally closer events are recalled with more sensory and contextual details than temporally distant events49. As the content of recall moves forward in time, the upcoming events can gain high contextual resolution75, as predicted by the physiological organization of theta-nested assemblies.
Linking past and present experiences by theta oscillations
In addition to creating chains of events surrounding the seed situation, theta oscillation mechanisms may help link events that are separated at longer timescales. For example, during navigation in the rat, the interleaving assemblies representing the current location are occasionally replaced by an entirely different assembly in a single theta cycle74, as if the rat ‘mentally jumped’ transiently to another location. Such jumps from one representation to another are frequent when the identity of the environment is made ambiguous by training rats in two visually distinct environments and ‘teleporting’ them from the one to another by changing the light patterns. In this case as well, the cell assembly contents, representing either one or the other environment, can switch back and forth in a few or even single theta cycles88. Extended, ‘look-ahead’ sequences representing large distances, from the start to the goal, can also occur, especially at critical junctures of choice behavior in memory tasks89,90. Place cells also often intermingle with internally generated ‘retrospective’ and ‘prospective’ neurons45,58,59.
The above examples demonstrate that theta cycles do not simply compress first-order sequences of past and upcoming positions of travel82 but can chunk large parts of the environment, without replaying all actually visited locations. Such nonsequential, higher-order connections are possible because place fields have long ‘tails’ that provide opportunities for spikes of neuron pairs with distant place fields to fire together, at least occasionally, in the same theta cycle. The large place fields of neurons in more ventral parts of the hippocampus and the large grid size in the more ventral parts of the medial entorhinal cortex may be especially important in creating higher-order links and may provide the flexibility needed for efficient navigation64,91. These same neuronal mechanisms can enhance the flexibility of episodic memory as well. In the same way that an infinite number of paths can connect the origin and end point of a journey, a recalled story can be told in many ways, connecting the beginning and end through innumerable variations. The neuronal mechanisms that allow the selection of the optimal path in navigation in the physical world can also support the optimal selection of the sequence parts of the experienced memory. Episodic recall is rarely a true sequence of the original experience, and an episode experienced over hours, days or at longer scales can be summarized in a compressed sequence that includes many shortcuts. Theta timescale compression is also beneficial for the continuity and temporal asymmetry requirements of free recall56, for solving transitive inference problems92, for supporting higher-order associations and for solving shortcut and detour problems in both spatial and mental navigation39. Overall, the ability of the theta mechanism to flicker between current, past and future situations provides physiological support for the mental travel model and may also be the mechanism for imagining past and future situations13,53.
Tracking subjective time by cell assembly sequences
Temporal ordering of events is fundamental to both episodic experience and action planning14. However, no dedicated mechanisms may be needed for sensing time. The same cell assembly sequences that keep information about past memories and planned goals of the animal can also faithfully track the passage of time and bridge noncontiguous events59,65,93,94 (Fig. 4b). Notably, time estimation error from the theta-bound evolving cell assembly sequences does not increase proportionally to the duration of elapsed time but stays asymptotically low even after tens of seconds of delays65. The within-cycle sequences may serve as an error-correcting mechanism of time tracking65. As is the case of place cells in a maze, the compressed temporal sequences within theta cycles predict the evolution of the real time sequences of neuronal activity during the delay portion of a memory task59. This multiple-timescale mechanism may explain why each snippet of a recalled episode is experienced as if it was occurring in real time, because the temporal dynamic during recall is supported by the same mechanism as during the encoding process.
The hypothesis that a single neuronal algorithm serves both to order events and to provide temporal context is also supported by experiments showing that, when the imposed delay between the cue and response is altered, the sequential structure of cell assemblies changes in accordance with the response patterns of place cells during navigation. Although some cells remain anchored to the beginning of the delay, as place cells are anchored to the start box8,86, most neurons form a completely new trajectory, in a manner resembling the remapping of place cells in response to context change93 (Fig. 4c). Keeping track of time by evolving cell assembly sequences at the seconds to minutes timescale may be a general rule in the cerebral cortex, including in the prefrontal95 and retrosplenial96 areas. To bridge longer intervals, further mechanisms may be required97,98.
Overall, the available experimental evidence indicates that mechanisms of entorhinal cortex-hippocampus-dependent memories evolved from mechanisms introduced to compute relationships of landmarks and to track the movements of the body in the environment. As active exploration is a prerequisite for the computation of distances and calibration of landmark relationships, we submit that movement is the primary source of the brain’s ability to remember past experiences and plan future actions.
Challenges and questions
Recent progress on the relationship among navigation, memory and oscillations raises as many questions as it has answered. We list here a few pertinent issues.
Perhaps the most challenging question is the meaning of the various firing patterns, including place cells, grid cells and irregularly spaced neurons, to the target networks of the hippocampus-entorhinal cortex system. Does it matter for the downstream actuator networks whether space and memory items are represented by geometrically spaced or irregular patterns? Do regularly firing neurons carry more weight than neurons with less rigorous firing patterns? Presumably, the code has time frames with clear demarcation of the beginning and end of messages, which should be coherent across neuronal populations. Is theta oscillation a critical part of this mechanism, or do other forms of coordinating mechanisms exist?
Hippocampal neurons in primates rarely fire in theta-rhythmic manner, and continuous local field potential theta rhythm is observed only infrequently30. Place and grid cells have been described in bats without apparent theta modulation28,29. Can the temporal coordination role of internal rhythms be substituted by other means, such as echolocation emission in bats, as occurs with saccadic eye movements in the visual system in primates? Neurons in the hippocampus and entorhinal cortex can be ‘driven’ by external cues and may persist in showing position-dependent firing even after theta oscillations cease24,99. Grid cells with either strong or no theta modulation often coexist, even at the same location. Yet in the presence of theta oscillations, place and grid cells are more pronounced, have higher peak rates and are more stable. Is theta coordination more important for memory than navigation, as memory depends primarily on internally generated neuronal sequences?
Are independent changes in grid maps of different modules instrumental in generating new representations in the hippocampus, and if so, by what mechanisms? Are there intrahippocampal mechanisms for further segregation of representations? How many representations can be generated, and what mechanisms determine their independence? Is the hippocampal output important for generating and maintaining grid formation in the entorhinal cortex and subicular complex, and if so, how do hippocampal activity patterns contribute to properties of grid cells and grid maps?
The hippocampal-entorhinal cortex system has a bidirectional, topographically arranged communication with the cortical mantle. Do the different septotemporal segments of this system map onto the neocortex similarly in animals with more complex brains and with a growing share of higher-order cortical areas? Does the region corresponding to the ventral quadrant of the rodent hippocampus grow disproportionally relative to the dorsal part in primates, and what are the consequences of any such shifts in cortical-hippocampal communication?
An interesting problem is the dominant direction of flow of neuronal activity in the entorhinal cortex–subiculum–hippocampus system. During memory encoding, the entorhinal cortex is believed to drive the hippocampal circuits, as it does in navigation, in a manner that, at least partly, must rely on external cues32. During spontaneous recall, the direction of flow is from the hippocampus to the entorhinal cortex43,100 but it is not known how this reversed flow is used during navigation. Yet on account of the postulated similar algorithms governing navigation and memory, one may speculate that once a path in the maze is learned, the sequential activation of hippocampal neurons can be temporally disengaged from external landmarks, as when driving a car to work on a long-practiced route. This raises the question of whether the direction of neuronal activity in the entorhinal cortex–hippocampus flips back and forth during navigation.
We become aware of our recollections only after segments of the neuronal sequences enter the working memory system. Does this conscious operation require only the prefrontal cortex, or are interactions with the rest of the cerebral cortex also needed?
ACKNOWLEDGMENTS
We thank G. Fishell for comments, Heather McKellar for assistance, and the US National Institutes of Health (NS34994; MH54671; NS 074015), International Human Frontiers Science Program Organization (RGP0032/2011), James S. McDonnell Foundation, Global Institute for Scientific Thinking, European Research Council (‘CIRCUIT’ Advanced Investigator Grant, Grant Agreement 232608), Louis-Jeantet Prize for Medicine, Kavli Foundation and Centre of Excellence scheme of the Research Council of Norway for support.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. New York: Oxford Univ. Press; 1978. [Google Scholar]
- 2.McNaughton BL, et al. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J. Exp. Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- 3.O’Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- 4.Thompson E, Varela FJ. Radical embodiment: neural dynamics and consciousness. Trends Cogn. Sci. 2001;5:418–425. doi: 10.1016/s1364-6613(00)01750-2. [DOI] [PubMed] [Google Scholar]
- 5.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat. Rev. Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 6.Knierim JJ, Kudrimoti HS, McNaughton BL. Interactions between idiothetic cues and external landmarks in the control of place cells and head direction cells. J. Neurophysiol. 1998;80:425–446. doi: 10.1152/jn.1998.80.1.425. [DOI] [PubMed] [Google Scholar]
- 7.Derdikman D, et al. Fragmentation of grid cell maps in a multicompartment environment. Nat. Neurosci. 2009;12:1325–1332. doi: 10.1038/nn.2396. [DOI] [PubMed] [Google Scholar]
- 8.Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J. Neurosci. 1996;16:823–835. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 11.Suddendorf T, Corballis MC. The evolution of foresight: what is mental time travel, and is it unique to humans? Behav. Brain Sci. 2007;30:299–313. doi: 10.1017/S0140525X07001975. discussion 313-251. [DOI] [PubMed] [Google Scholar]
- 12.Tulving E, Donaldson W, Bower GH United States Office of Naval Research. Organization of Memory. New York: Academic; 1972. [Google Scholar]
- 13.Buckner RL. The role of the hippocampus in prediction and imagination. Annu. Rev. Psychol. 2010;61:27–48. C21–C28. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- 14.Tulving E. Chronesthesia: conscious awareness of subjective time. in. In: Stuss DT, Knight RC, editors. Principles of Frontal Lobe Function. New York: Oxford Univ. Press; 2002. pp. 311–325. [Google Scholar]
- 15.Buzsáki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- 16.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 17.Lever C, Wills T, Cacucci F, Burgess N, O’Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature. 2002;416:90–94. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- 18.Hasselmo ME. How We Remember: Brain Mechanisms of Episodic Memory. Cambridge, Massachusetts, USA: MIT Press; 2012. [Google Scholar]
- 19.McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 20.Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 21.Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- 22.Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Annu. Rev. Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- 23.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 24.Boccara CN, et al. Grid cells in pre- and parasubiculum. Nat. Neurosci. 2010;13:987–994. doi: 10.1038/nn.2602. [DOI] [PubMed] [Google Scholar]
- 25.Sargolini F, et al. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- 26.Krupic J, Burgess N, O’Keefe J. Neural representations of location composed of spatially periodic bands. Science. 2012;337:853–857. doi: 10.1126/science.1222403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- 28.Yartsev MM, Witter MP, Ulanovsky N. Grid cells without theta oscillations in the entorhinal cortex of bats. Nature. 2011;479:103–107. doi: 10.1038/nature10583. [DOI] [PubMed] [Google Scholar]
- 29.Ulanovsky N, Moss CF. Hippocampal cellular and network activity in freely moving echolocating bats. Nat. Neurosci. 2007;10:224–233. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
- 30.Ekstrom AD, et al. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- 31.Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J. Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stensola H, et al. The entorhinal grid map is discretized. Nature. 2012;492:72–78. doi: 10.1038/nature11649. [DOI] [PubMed] [Google Scholar]
- 33.Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- 34.Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 35.Mizuseki K, Royer S, Diba K, Buzsáki G. Activity dynamics and behavioral correlates of CA3 and CA1 hippocampal pyramidal neurons. Hippocampus. 2012;22:1659–1680. doi: 10.1002/hipo.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li XG, Somogyi P, Ylinen A, Buzsáki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J. Comp. Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- 37.Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 38.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 39.Muller RU, Stead M, Pach J. The hippocampus as a cognitive graph. J. Gen. Physiol. 1996;107:663–694. doi: 10.1085/jgp.107.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menzel R, Geiger K, Joerges J, Muller U, Chittka L. Bees travel novel homeward routes by integrating separately acquired vector memories. Anim. Behav. 1998;55:139–152. doi: 10.1006/anbe.1997.0574. [DOI] [PubMed] [Google Scholar]
- 41.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 42.Hampson RE, Byrd DR, Konstantopoulos JK, Bunn T, Deadwyler SA. Hippocampal place fields: relationship between degree of field overlap and crosscorrelations within ensembles of hippocampal neurons. Hippocampus. 1996;6:281–293. doi: 10.1002/(SICI)1098-1063(1996)6:3<281::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 43.Miyashita Y. Cognitive memory: cellular and network machineries and their topdown control. Science. 2004;306:435–440. doi: 10.1126/science.1101864. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J. Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- 45.Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 46.Kumaran D, McClelland JL. Generalization through the recurrent interaction of episodic memories: a model of the hippocampal system. Psychol. Rev. 2012;119:573–616. doi: 10.1037/a0028681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heit G, Smith ME, Halgren E. Neural encoding of individual words and faces by the human hippocampus and amygdala. Nature. 1988;333:773–775. doi: 10.1038/333773a0. [DOI] [PubMed] [Google Scholar]
- 48.Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- 49.Trope Y, Liberman N. Construal-level theory of psychological distance. Psychol. Rev. 2010;117:440–463. doi: 10.1037/a0018963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navigli R, Lapata M. An experimental study of graph connectivity for unsupervised word sense disambiguation. IEEE Trans. Pattern Anal. Mach. Intell. 2010;32:678–692. doi: 10.1109/TPAMI.2009.36. [DOI] [PubMed] [Google Scholar]
- 51.Nakashiba T, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn. Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 54.Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- 56.Howard MW, Kahana MJ. Contextual variability and serial position effects in free recall. J. Exp. Psychol. Learn. Mem. Cogn. 1999;25:923–941. doi: 10.1037//0278-7393.25.4.923. [DOI] [PubMed] [Google Scholar]
- 57.Shrager Y, Kirwan CB, Squire LR. Neural basis of the cognitive map: path integration does not require hippocampus or entorhinal cortex. Proc. Natl. Acad. Sci. USA. 2008;105:12034–12038. doi: 10.1073/pnas.0805414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 59.Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buzsáki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizuseki K, Sirota A, Pastalkova E, Buzsáki G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron. 2009;64:267–280. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller RU, Bostock E, Taube JS, Kubie JL. On the directional firing properties of hippocampal place cells. J. Neurosci. 1994;14:7235–7251. doi: 10.1523/JNEUROSCI.14-12-07235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McNaughton BL, Barnes CA, O’Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp. Brain Res. 1983;52:41–49. doi: 10.1007/BF00237147. [DOI] [PubMed] [Google Scholar]
- 64.Royer S, Sirota A, Patel J, Buzsáki G. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J. Neurosci. 2010;30:1777–1787. doi: 10.1523/JNEUROSCI.4681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Itskov V, Pastalkova E, Mizuseki K, Buzsáki G, Harris KD. Theta-mediated dynamics of spatial information in hippocampus. J. Neurosci. 2008;28:5959–5964. doi: 10.1523/JNEUROSCI.5262-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wickelgren WA. Webs, cell assemblies, and chunking in neural nets: introduction. Can. J. Exp. Psychol. 1999;53:118–131. doi: 10.1037/h0087304. [DOI] [PubMed] [Google Scholar]
- 67.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 68.Bragin A, et al. Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. J. Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colgin LL, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 70.Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Thetagamma coupling increases during the learning of item-context associations. Proc. Natl. Acad. Sci. USA. 2009;106:20942–20947. doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Axmacher N, et al. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl. Acad. Sci. USA. 2010;107:3228–3233. doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Canolty RT, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griesmayr B, Gruber WR, Klimesch W, Sauseng P. Human frontal midline theta and its synchronization to gamma during a verbal delayed match to sample task. Neurobiol. Learn. Mem. 2010;93:208–215. doi: 10.1016/j.nlm.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 74.Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsáki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- 75.Dragoi G, Buzsáki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 76.Koch C, Rapp M, Segev I. A brief history of time (constants) Cereb. Cortex. 1996;6:93–101. doi: 10.1093/cercor/6.2.93. [DOI] [PubMed] [Google Scholar]
- 77.Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibitionbased rhythms: experimental and mathematical observations on network dynamics. Int. J. Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 78.Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL. Phase precession in hippocampal interneurons showing strong functional coupling to individual pyramidal cells. J. Neurosci. 2006;26:13485–13492. doi: 10.1523/JNEUROSCI.2882-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geisler C, Robbe D, Zugaro M, Sirota A, Buzsáki G. Hippocampal place cell assemblies are speed-controlled oscillators. Proc. Natl. Acad. Sci. USA. 2007;104:8149–8154. doi: 10.1073/pnas.0610121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Royer S, et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci. 2012;15:769–775. doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 83.O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 84.Geisler C, et al. Temporal delays among place cells determine the frequency of population theta oscillations in the hippocampus. Proc. Natl. Acad. Sci. USA. 2010;107:7957–7962. doi: 10.1073/pnas.0912478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burgess N, O’Keefe J. Models of place and grid cell firing and theta rhythmicity. Curr. Opin. Neurobiol. 2011;21:734–744. doi: 10.1016/j.conb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diba K, Buzsáki G. Hippocampal network dynamics constrain the time lag between pyramidal cells across modified environments. J. Neurosci. 2008;28:13448–13456. doi: 10.1523/JNEUROSCI.3824-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 88.Jezek K, Henriksen EJ, Treves A, Moser EI, Moser MB. Theta-paced flickering between place-cell maps in the hippocampus. Nature. 2011;478:246–249. doi: 10.1038/nature10439. [DOI] [PubMed] [Google Scholar]
- 89.Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J. Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Segmentation of spatial experience by hippocampal theta sequences. Nat. Neurosci. 2012;15:1032–1039. doi: 10.1038/nn.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kjelstrup KB, et al. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- 92.Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc. Natl. Acad. Sci. USA. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hasselmo ME. Arc length coding by interference of theta frequency oscillations may underlie context-dependent hippocampal unit data and episodic memory function. Learn. Mem. 2007;14:782–794. doi: 10.1101/lm.686607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fujisawa S, Amarasingham A, Harrison MT, Buzsáki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat. Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature. 2012;484:62–68. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mankin EA, et al. Neuronal code for extended time in the hippocampus. Proc. Natl. Acad. Sci. USA. 2012;109:19462–19467. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333:773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- 99.Koenig J, Linder AN, Leutgeb JK, Leutgeb S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science. 2011;332:592–595. doi: 10.1126/science.1201685. [DOI] [PubMed] [Google Scholar]
- 100.Fell J, Klaver P, Elger CE, Fernandez G. The interaction of rhinal cortex and hippocampus in human declarative memory formation. Rev. Neurosci. 2002;13:299–312. doi: 10.1515/revneuro.2002.13.4.299. [DOI] [PubMed] [Google Scholar]