Abstract
Donor specific antibodies are associated with refractory rejection episodes and poor allograft outcomes in solid organ transplantation. Our understanding of antibody mediated allograft injury is expanding beyond complement deposition. In fact, unique mechanisms of alloantibodies are advancing our knowledge about transplant vasculopathy and antibody mediated rejection. These include direct effects on the endothelium, resulting in the recruitment of leukocytes, chemokine and cytokine production, and stimulation of innate and adaptive alloresponses. These effects will be the focus of the following review.
Keywords: alloantibodies, transplant vasculopathy, antibody-mediated rejection, endothelium, HLA class I, complement, therapy
Introduction
Although short-term outcomes have improved with modern era immunosuppression, little progress has been made in long-term allograft survival in solid organ transplantation. AMR (antibody-mediated rejection) is one of the leading causes of allograft failure and contributes significantly to poor long-term outcomes, the current organ shortage and increased transplant wait times (1–3). One of the common consequences of AMR is transplant vasculopathy (TV), characterized by intimal thickening, endothelial and smooth muscle cell (EC) proliferation, survival and migration (4–7). This results in occlusion of allograft vessels leading to arteriosclerosis with subsequent deterioration of organ function.
Donor specific antibodies to HLA and non-HLA antigens are an important clinical risk factor for TV and AMR in solid organ transplantation (8–16). Traditionally the complement dependent mechanisms of AMR are emphasized in its pathological diagnosis, which focus on C4d, a degradation product of activated complement factor C4. C4d is a component of the classical complement cascade and is deposited on the capillary endothelium (17). However, C4d staining is not always a sensitive marker and its diagnostic capability is inconsistent across solid organ transplantation (18).
A growing body of literature is emerging on the importance of complement independent mechanisms as well as novel complement mechanisms of alloantibodies. An innovative paradigm for diagnosing and treating AMR is evolving. C4d negative AMR, inflammation of the microcirculation, and TV are now being recognized as important diagnostic criteria for AMR (19). The pathogenesis of alloantibodies in the context of TV and AMR is best characterized for HLA class I molecules. Therefore, new perspectives on injury mechanisms of HLA class I antibodies will be described. Additionally, the potential for existing approved therapeutic drugs to antagonize HLA antibody-activated signaling in endothelium will also be discussed.
HLA Class I Antibody Induces Actin Cytoskeleton Remodeling and Migration
The cytoskeleton consists of actin microfilaments, microtubules, and intermediate filaments that provide the necessary framework for cell motility, organelle support, and cell division (20). Dynamic remodeling of the actin cytoskeleton regulates cell proliferation and migration and is thought to contribute to TV and AMR. Crosslinking of HLA class I molecules by anti-HLA antibodies activates Rho signaling and triggers reorganization of the cytoskeleton (21). The activation of the guanosine-5′-trisphosphate (GTP)-binding protein RhoA and Rho kinase on endothelial cells is central to the formation of F-actin stress fibers and mediates phophoinositide 3-kinase (PI3K) dependent endothelial cell proliferation (22) and represents a potential therapeutic target to prevent HLA antibody-induced cell changes. Besides treatment of hypercholesterolemia, HMG- coA reductase inhibitors, or “statins” can potentially be used as adjunctive therapy to ameliorate TV and AMR in solid organ transplantation (22) (Figure 1). Simvastatin, which inhibits RhoA geranylgeranylation, significantly reduces HLA Class I induced endothelial cell proliferation in vitro (22). Other promising inhibitors of Rho and Rho kinase that are utilized in animal models of chronic rejection include fasudil and Y-27632 which reduce neointimal thickening and decrease immune cell infiltration (23–25) (Figure 1).
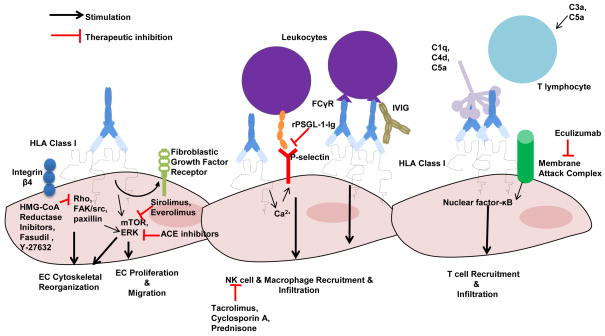
Figure 1. Potential Therapeutic Interventions for the Pleiotropic Effects of HLA Class I Antibodies on EC.
HLA Class I antibody has several mechanisms by which it can induce endothelial injury including stimulation of EC proliferation, cytoskeletal changes, and migration; the recruitment of leukocytes; chemokine and cytokine production; and stimulation of innate and adaptive alloresponses. This Figure depicts therapeutic intervention to target HLA signaling on the endothelium.
Both Rho GTPase and Rho kinase are involved in class I-mediated phosphorylation of focal adhesion kinase (FAK) and paxillin (21, 26). FAK is a cytoplasmic protein kinase that localizes to regions of the cell called focal adhesions that attach to extracellular matrix. FAK is a key regulator for cell proliferation, survival, and migration and plays a critical role in wound repair, atherosclerosis and tumor angiogenesis. Ligation of HLA class I by antibody on endothelial cells stimulates phosphorylation of FAK, Src, and paxillin leading to cytoskeletal rearrangement and stabilization of focal adhesions, which is required for cell proliferation. Inhibition of FAK by small interfering RNA during HLA class I signaling reduces endothelial proliferative capacity (26).
The formation of stress fibers is an essential part of cytoskeleton remodeling. Stress fibers function in endothelial cell adhesion, migration and permeability and central for the development of TV (27, 28). HLA class I antibody can induce stress fiber formation in endothelial cells via phosphorylation of myosin light chain (MLC). This in turn activates myosin light chain kinase and Rho kinase in an ERK1/2 dependent fashion without increased intracellular calcium (29). Interestingly, angiotensin converting enzyme (ACE) inhibition with captopril has been shown to suppress TV and hypertension by reducing ERK and MLC expression (30, 31). Therefore, ACE inhibitors may be therapeutic for solid organ recipients who suffer from both and additionally may antagonize HLA I antibody-induced activation of MLC and ERK in endothelium leading to TV (Figure 1). Aside from stress fiber formation, HLA class I antibodies also stimulate the translocation of mammalian target of rapamycin complex 2 (mTORC2) and ERK1/2 from the cytoplasm to the plasma membrane which may act as a scaffold for downstream proteins (29). Furthermore, HLA class I antibody increases cell migration and wound healing through mTOR (32–34). Indeed, the mTOR inhibitors, everolimus and sirolimus, can inhibit HLA class I stimulated cell migration and wound healing (33) (Figure 1). These in vitro results are consistent with findings in animal and human studies where TV is attenuated by both everolimus and sirolimus in cardiac transplantation (35–37).
Additionally, proteomic studies have revealed novel proteins that are involved in actin remodeling induced by class I antibodies compared with other agonists including thrombin and fibroblast growth factor (38). Analysis by tandem mass spectrometry has shown unique cytoskeleton proteomes for each treatment group. Using annotation tools, a candidate list has been created that identifies 12 proteins which are unique to the HLA class I stimulated group and highlights cytoskeletal proteins such as TMP4, Nup153, and eIF4A1 (38). TMP4 may regulate HLA-class I induced cytoskeleton remodeling downstream of extracellular regulated kinases (ERK)(38). Nup153 is crucial for nucleoskeleton and cytoskeleton architecture maintenance and is necessary for cell cycle progression and migration (39). Finally the eIF4A1 protein functions downstream of mTOR complex 1 following class I ligation to promote translation and cell proliferation (29, 40). These candidate proteins may link HLA class I induced cytoskeleton changes to downstream cellular functions such as proliferation and provide novel diagnostic and therapeutic targets for TV.
HLA Class I Antibody Elicits Endothelial and Smooth Muscle Cell Proliferation
TV is a predominantly proliferative disease, in which the vessels of the allograft become occluded by severe intimal thickening, endothelial expansion and smooth muscle invasion. Many in vitro studies have suggested that HLA I antibodies can promote endothelial and smooth muscle cell changes relevant to this process. Specifically, HLA antibody stimulation of endothelial cells and smooth muscle cells increases cellular proliferation in the absence of exogenous growth factors (34, 41, 42). Studies have shown that rapidly following HLA class I ligation by antibodies, Rho-GTP, Src, and FAK become activated (22, 26). Src activation triggers the PI3K/Akt signaling axis, which is a key regulator of cell proliferation and survival. Phosphorylation of PI3K/Akt activates mTOR, a serine/threonine kinase. mTOR exists in two distinct protein complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 is composed of raptor, mTOR, and MLST8 and stimulates protein synthesis and cell proliferation via activation of S6K, S6 ribosomal protein (S6RP) and 4EBP1 (40). mTORC2, which contains mTOR, rictor, mLST8 and Sin 1, is a central regulator of cell survival, migration, and cytoskeletal rearrangement. mTORC2 also regulates ERK1/2 which to induce cell proliferation (40). Because HLA I molecules have no known signaling motif, we have postulated that HLA I associates with a coreceptor to induce its proliferative effects on the endothelium. Using immunoprecipitation, we have discovered a molecular association between HLA I and integrin β4, which is required for HLA I-mediated activation of protein phosphorylation of Src, ERK, and AKT and stimulation of cell proliferation (42).
These in vitro findings are supported by in vivo studies in animals as well as in patients with AMR (43–45). Mice treated with donor specific MHC class I antibodies show an increase in phosphorylation of proteins involved in cell proliferation on the endothelium within the cardiac allograft (46). Moreover, the phosphorylation of the mTOR effectors, S6RP and S6K, is increased in cardiac and renal transplant biopsies from patients diagnosed with allograft rejection (47–49). These markers are more sensitive than C4d in identifying patients with AMR (47). Since mTOR inhibition has been found to improve TV in patients with AMR, intragraft activation of mTOR effector proteins may be ideal to monitor TV treatment response and compare drug efficacy amongst inhibitors. Recently, we have found that everolimus more effectively inhibits HLA class I induced endothelial functional changes than sirolimus by antagonizing mTORC1 and mTORC2, as well as downstream MAPK kinase pathways (33) (Figure 1). These results suggest that everolimus may be more beneficial than sirolimus in preventing AMR and TV, which needs validation in future clinical trials.
HLA class I antibody can also induce smooth muscle cell proliferation through stress-induced activation of matrix metalloproteases/sphingolipid signaling in vitro and in vivo (34, 41, 50). TV develops in human arteries grafted into immunodeficient SCID/beige mice after stimulation with Class I antibody (41). Treatment with pharmacological inhibitors of the matrix metallopreoteases/sphingolipid pathway prevents alloantibody-provoked SMC proliferation and vascular lesion development with minimal side effects, highlighting alternative therapeutic options for TV (41).
HLA Class I Antibody Induces Cell Survival and Promotes Accommodation
Although alloantibodies have been strongly associated with graft pathogenesis, the presence of circulating donor-specific HLA antibodies may not always result in rejection or graft dysfunction. Several in vitro studies indicate possible mechanisms whereby HLA class I antibodies promote transplant accommodation and resistance to graft injury from AMR. At low concentrations, HLA class I antibodies can induce endothelial accommodation by upregulating anti-apoptotic genes B-cell lymphoma extra-large (Bcl-xL), B-cell lymphoma 2 (Bcl-2), and heme oxygenese I (HO-1) through PI3K/AKT and cAMP dependent PKA(51–54). This results in protection of endothelial cells from complement-induced cell death (53, 55). By contrast, high concentrations of HLA antibodies in combination with complement cause cell death (52–54). In vivo studies have also illustrated the importance of Bcl-2 and HO-1 in HLA Class I mediated transplant accommodation. In a murine heart transplant model, pretreatment of HLA-A2 transgenic donor hearts with low level of HLA class I antibody confers protection against AMR in highly sensitized recipients by increasing expression of Bcl-xl, Bcl-2, and HO-1 and reducing expression of adhesion molecules and inflammatory cytokines and chemokines (55). Additionally, Mannam and colleagues have found that differential renal microvascular endothelial responses to HLA class I antibody influence the fate of the allograft (56). Activation of thrombomodulin, by reducing thrombogenic activity, interleukin 11, through its cytoprotective effects, and anti-apoptotic genes promote accommodation; while upregulation of cytokine activity, chemokine and pro-apoptotic genes favor allograft rejection (56). In human studies, a subset of sensitized and non-sensitized patients has circulating donor specific HLA antibodies without evidence of diminished allograft function or rejection (57–59). Taken together, these in vitro and in vivo studies give insight into the effects of HLA Class I antibody as a dynamic spectrum with graft accommodation and/or “asymptomatic AMR” on one end and full blown rejection at the other.
HLA Class I Antibody Stimulates Production of Secondary Factors
Besides direct stimulation of intracellular signaling cascades, HLA class I antibodies augment the sensitivity to and the production of soluble mediators which stimulate autocrine proliferative signaling. Work from our group over the past years has identified a critical role for the interaction between HLA class I antibodies and fibroblast growth factors (FGFs) in the progression of TV. Ligation of HLA class I by antibodies on the endothelium stimulates proliferation and TV by up-regulating the expression of fibroblast growth factor receptors (FGFRs) on the cell surface in a dose-dependent fashion, increasing the proliferative response to basic fibroblast growth factor (60–62). HLA class I antibodies also trigger endothelial cell production of cytokines that may also enhance cell growth. For example, HLA class I antibody induces endothelial production of vascular endothelial growth factor (VEGF), which activates cells in an autocrine manner through its receptor VEGFR2 (63). Indeed, VEGF protein has been localized to the vascular endothelium in cardiac transplant biopsies, suggesting its role in TV and its utility as a biomarker (64). Additionally, HLA class I antibodies increased production of cytokines such as platelet-derived growth factor (PDGF), insulin-like growth factor 1 (IGF-1), and bFGF, which contributed to paracrine proliferation of lung airway epithelial cells in vitro and in vivo (43, 65). These findings highlight the ability of HLA class I antibodies to stimulate soluble mediators which trigger both autocrine and paracrine cell proliferative responses.
HLA Class I Antibody Leukocyte Recruitment and Activation
Infiltrating leukocytes of the innate immune system have been implicated in the pathogenesis of TV and AMR. Ligation of HLA class I antibody to the endothelium plays an important role in leukocyte recruitment, especially of monocytes. In vitro studies have shown that HLA class I molecule ligation mobilizes endothelial vesicles called Weibel-Palade bodies (WPb) through a calcium dependent mechanism, releasing von Willebrand Factor (vWF) and cell surface P-selectin (66, 67). Recruitment and adherence of neutrophilic cells and monocytes to the endothelium occur (66, 67). Using an in vivo murine model of cardiac AMR, we have shown that blockade of P-selectin with recombinant soluble PSGL-1 Fc chimera (rPSGL-1-Ig) reduces HLA Class I antibody elicited monocyte recruitment and improves vasculopathy (66). Infiltrating macrophages have been associated with donor specific antibody and AMR in transplant recipients (68, 69). Since rPSGL-1-Ig has already been shown in clinical trials to have a beneficial effect on liver transplantation ischemia reperfusion injury, it may be a promising therapy for TV and AMR (70) (Figure 1). We have also shown that HLA Class I antibody can augment monocyte recruitment through FcγR interactions (71). Human IgG1 and human IgG3 may have a greater capacity to trigger monocyte infiltration into the graft than IgG2 or IgG4 due to enhancement by FcγR interactions (71). Delineation of human IgG subclasses may identify patients at higher risk of TV and AMR in the clinical setting. Additionally, intravenous immunoglobulin (IVIG) may play a role in reducing this Fc receptor-dependent response (72) (Figure 1).
Besides leukocyte recruitment, HLA Class I antibody can also mediate AMR through activation of natural killer (NK) cells. NK cells release IFNγ, a pro-inflammatory cytokine, as well as perforin and granzyme B, through degranulation, resulting in killing of target cells. In a murine cardiac vasculopathy model, Colvin and colleagues show that NK cells mediate TV by binding FcγRIIIa to the Fc portion of HLA Class I antibody. These effects are independent of complement. Subsequently, NK cell depletion prevents TV and chronic AMR (73). In human studies, NK-cell transcripts and intravascular accumulation of NK cells have been detected in kidney allografts with DSA and C4d negative chronic AMR (74). Shin and colleagues recently have shown that the calcineurin inhibitors, tacrolimus and cyclosporin A, in combination with prednisone synergistically suppress NK cell IFNγ production and degranulation significantly more than anti-metabolites, such as sirolimus or mycophenolate mofetil (75) (Figure 1). Given the NK cell signature of AMR in experimental models and in patients, it is possible that NK cells can be used as yet another tool to identify C4d negative rejection and may represent a therapeutic target to ameliorate AMR.
HLA Class I Antibody and T cell-mediated Alloimmunity
In transplant patients, studies have shown an association between donor specific antibodies and acute cellular rejection (76, 77). However, until recently, the mechanism by which antibodies might influence T cell mediated alloimmune responses has not been established. Microvascular endothelial cells treated with HLA class I antibody from both mice and sensitized humans produced inflammatory cytokines and chemokines such as IL-6, CXCL8, CCL5, and CXCL10 through a CREB/protein kinase-A dependent pathway (78). The corresponding receptors for these inflammatory mediators are expressed on T cells, monocytes, and neutrophils, suggesting an endothelium-leukocyte interaction and a role for alloantibody in acute cellular rejection (78).
Finally, a novel function of HLA antibody-activated complement has recently been described. Jordan Pober and colleagues have recently demonstrated that alloantibodies from highly sensitized transplant patients can deposit complement, including formation of membrane attack complexes on endothelial cells in vitro and in vivo (79). As described above, endothelial cells remain resistant to complement-mediated lysis in the presence of HLA antibodies. Rather than provoke cytolysis, membrane attack complexes upregulate inflammatory genes, increasing the capacity of endothelial cells to recruit and activate allogeneic interferon-γ-producing CD4+ T cells through noncanonical nuclear factor-κB signaling (79). Interestingly, noncanoncial NF-κB activation is also found in the peritubular capillaries of renal transplant biopsies with chronic AMR, highlighting its potential utility as a diagnostic marker and/or a therapeutic target for TV. Therefore, complement inhibition with Eculizumab may prevent antibody-activated complement T cell recruitment, alleviating TV (Figure 1). Additionally, Heeger and colleagues have shown that complement split products C3a and C5a from allogenic dendritic cells augment T cell alloresponses in vitro and in vivo and provides yet another target for organ transplant rejection (80).
Conclusion
In summary, alloantibodies are a major barrier to long-term allograft survival in solid organ transplantation. Current diagnostic markers do not always accurately detect AMR and conventional therapies have limited efficacy on TV. As delineated in Figure 1, advances in our understanding of HLA class I signaling have uncovered new diagnostic markers that can optimize the use of currently approved medications against HLA signals in the endothelium and identify novel therapeutic targets to improve transplant outcomes. New perspectives summarized in this review, regarding the pleiotropic effects of HLA class I antibody, will give us insight into the mechanisms of antibodies against other alloantigens, including HLA class II and non-HLA molecules.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases Grant R01 AI 042819, NIH U01AI077821, and the National Heart Lung and Blood Institute Grant RO1 HL 090995, Pfizer Arts Award WS2331291, Novartis Investigator Initiated Research Grant CRAD001A124T.
References
- 1.Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M. Diagnosis and Management of Antibody-Mediated Rejection: Current Status and Novel Approaches. Am J Transplant. 2014;14:255–71. doi: 10.1111/ajt.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–83. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 3.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–33. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson C, Southwood M, Pitman R, Phillpotts C, Wallwork J, Goddard M. Angiogenesis occurs within the intimal proliferation that characterizes transplant coronary artery vasculopathy. J Heart Lung Transplant. 2005;24:551–8. doi: 10.1016/j.healun.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Zhao DX. Allograft arteriosclerosis and immune-driven angiogenesis. Circulation. 2003;107:1237–9. doi: 10.1161/01.cir.0000059744.64373.08. [DOI] [PubMed] [Google Scholar]
- 6.Reinders ME, Rabelink TJ, Briscoe DM. Angiogenesis and endothelial cell repair in renal disease and allograft rejection. J Am Soc Nephrol. 2006;17:932–42. doi: 10.1681/ASN.2005121250. [DOI] [PubMed] [Google Scholar]
- 7.Valenzuela NM, Reed EF. The link between major histocompatibility complex antibodies and cell proliferation. Transplant Rev. 2011;25:154–66. doi: 10.1016/j.trre.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Elmagd KM, Wu G, Costa G, et al. Preformed and de novo donor specific antibodies in visceral transplantation: long-term outcome with special reference to the liver. Am J Transplant. 2012;12:3047–60. doi: 10.1111/j.1600-6143.2012.04237.x. [DOI] [PubMed] [Google Scholar]
- 9.Chih S, Chruscinski A, Ross HJ, Tinckam K, Butany J, Rao V. Antibody-mediated rejection: an evolving entity in heart transplantation. J Transplant. 2012;2012:210210. doi: 10.1155/2012/210210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hachem R. Antibody-Mediated Lung Transplant Rejection. Curr Respir Care Rep. 2012;1:157–61. doi: 10.1007/s13665-012-0019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson AM, Lucas DP, Melancon JK, Desai NM. Clinical relevance and IgG subclass determination of non-HLA antibodies identified using endothelial cell precursors isolated from donor blood. Transplantation. 2011;92:54–60. doi: 10.1097/TP.0b013e31821b60e9. [DOI] [PubMed] [Google Scholar]
- 12.Qin Z, Lavingia B, Zou Y, Stastny P. Antibodies against nucleolin in recipients of organ transplants. Transplantation. 2011;92:829–35. doi: 10.1097/TP.0b013e31822d0977. [DOI] [PubMed] [Google Scholar]
- 13.Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–99. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 14.Sigdel TK, Li L, Tran TQ, Khatri P, et al. Non-HLA antibodies to immunogenic epitopes predict the evolution of chronic renal allograft injury. J Am Soc Nephrol. 2012;23:750–63. doi: 10.1681/ASN.2011060596. [DOI] [PubMed] [Google Scholar]
- 15.Torrealba JR, Samaniego M, Pascual J, et al. C4d-positive interacinar capillaries correlates with donor-specific antibody-mediated rejection in pancreas allografts. Transplantation. 2008;86:1849–56. doi: 10.1097/TP.0b013e3181902319. [DOI] [PubMed] [Google Scholar]
- 16.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–67. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 17.Sis B, Mengel M, Haas M, et al. Banff ‘09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–71. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 18.Mengel M, Sis B, Haas M, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–70. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–83. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 20.Pollard TD. The cytoskeleton, cellular motility and the reductionist agenda. Nature. 2003;422:741–5. doi: 10.1038/nature01598. [DOI] [PubMed] [Google Scholar]
- 21.Lepin EJ, Jin YP, Barwe SP, Rozengurt E, Reed EF. HLA class I signal transduction is dependent on Rho GTPase and ROK. Biochem Biophys Res Commun. 2004;323:213–7. doi: 10.1016/j.bbrc.2004.08.082. [DOI] [PubMed] [Google Scholar]
- 22.Coupel S, Leboeuf F, Boulday G, Soulillou JP, Charreau B. RhoA activation mediates phosphatidylinositol 3-kinase-dependent proliferation of human vascular endothelial cells: an alloimmune mechanism of chronic allograft nephropathy. J Am Soc Nephrol. 2004;15:2429–39. doi: 10.1097/01.ASN.0000138237.42675.45. [DOI] [PubMed] [Google Scholar]
- 23.Furuyama T, Komori K, Shimokawa H, et al. Long-term inhibition of Rho kinase suppresses intimal thickening in autologous vein grafts in rabbits. J Vasc Surg. 2006;43:1249–56. doi: 10.1016/j.jvs.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 24.Hattori T, Shimokawa H, Higashi M, et al. Long-term treatment with a specific Rho-kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res. 2004;94:46–52. doi: 10.1161/01.RES.0000107196.21335.2B. [DOI] [PubMed] [Google Scholar]
- 25.Ohki S, Iizuka K, Ishikawa S, et al. A highly selective inhibitor of Rho-associated coiled-coil forming protein kinase, Y-27632, prolongs cardiac allograft survival of the BALB/c-to-C3H/He mouse model. J Heart Lung Transplant. 2001;20:956–63. doi: 10.1016/s1053-2498(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 26.Jin YP, Korin Y, Zhang X, Jindra PT, Rozengurt E, Reed EF. RNA interference elucidates the role of focal adhesion kinase in HLA class I-mediated focal adhesion complex formation and proliferation in human endothelial cells. J Immunol. 2007;178:7911–22. doi: 10.4049/jimmunol.178.12.7911. [DOI] [PubMed] [Google Scholar]
- 27.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–94. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shasby DM, Shasby SS, Sullivan JM, Peach MJ. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982;51:657–61. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler ME, Jin YP, Young SH, Rozengurt E, Reed EF. HLA class I-mediated stress fiber formation requires ERK1/2 activation in the absence of an increase in intracellular Ca2+ in human aortic endothelial cells. Am J Physiol Cell Physiol. 2012;303:C872–82. doi: 10.1152/ajpcell.00199.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford SE, Mavroudis C, Backer CL, et al. Captopril suppresses post-transplantation angiogenic activity in rat allograft coronary vessels. J Heart Lung Transplant. 2004;23:666–73. doi: 10.1016/j.healun.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Hu WY, Han YJ, Gu LZ, Piano M, de Lanerolle P. Involvement of Ras-regulated myosin light chain phosphorylation in the captopril effects in spontaneously hypertensive rats. Am J Hypertens. 2007;20:53–61. doi: 10.1016/j.amjhyper.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Jin YP, Singh RP, Du ZY, Rajasekaran AK, Rozengurt E, Reed EF. Ligation of HLA class I molecules on endothelial cells induces phosphorylation of Src, paxillin, and focal adhesion kinase in an actin-dependent manner. J Immunol. 2002;168:5415–23. doi: 10.4049/jimmunol.168.11.5415. [DOI] [PubMed] [Google Scholar]
- 33.Jin YP, Valenzuela NM, Ziegler ME, Rozengurt E, Reed EF. Everolimus Inhibits Anti-HLA I Antibody-Mediated Endothelial Cell Signaling, Migration and Proliferation More Potently Than Sirolimus. Am J Transplant. 2014 Mar 1; doi: 10.1111/ajt.12669. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li F, Zhang X, Jin YP, Mulder A, Reed EF. Antibody ligation of human leukocyte antigen class I molecules stimulates migration and proliferation of smooth muscle cells in a focal adhesion kinase-dependent manner. Hum Immunol. 2011;72:1150–9. doi: 10.1016/j.humimm.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dambrin C, Klupp J, Birsan T, et al. Sirolimus (rapamycin) monotherapy prevents graft vascular disease in nonhuman primate recipients of orthotopic aortic allografts. Circulation. 2003;107:2369–74. doi: 10.1161/01.CIR.0000065576.80196.A4. [DOI] [PubMed] [Google Scholar]
- 36.Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847–58. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 37.Vigano M, Tuzcu M, Benza R, et al. Prevention of acute rejection and allograft vasculopathy by everolimus in cardiac transplants recipients: a 24-month analysis. J Heart Lung Transplant. 2007;26:584–92. doi: 10.1016/j.healun.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Ziegler ME, Souda P, Jin YP, Whitelegge JP, Reed EF. Characterization of the endothelial cell cytoskeleton following HLA class I ligation. PloS One. 2012;7:e29472. doi: 10.1371/journal.pone.0029472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou L, Pante N. The nucleoporin Nup153 maintains nuclear envelope architecture and is required for cell migration in tumor cells. FEBS Lett. 2010;584:3013–20. doi: 10.1016/j.febslet.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 40.Jindra PT, Jin YP, Rozengurt E, Reed EF. HLA class I antibody-mediated endothelial cell proliferation via the mTOR pathway. J Immunol. 2008;180:2357–66. doi: 10.4049/jimmunol.180.4.2357. [DOI] [PubMed] [Google Scholar]
- 41.Galvani S, Auge N, Calise D, et al. HLA class I antibodies provoke graft arteriosclerosis in human arteries transplanted into SCID/beige mice. Am J Transplant. 2009;9:2607–14. doi: 10.1111/j.1600-6143.2009.02804.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Rozengurt E, Reed EF. HLA class I molecules partner with integrin beta4 to stimulate endothelial cell proliferation and migration. Sci Signal. 2010;3:ra85. doi: 10.1126/scisignal.2001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maruyama T, Jaramillo A, Narayanan K, Higuchi T, Mohanakumar T. Induction of obliterative airway disease by anti-HLA class I antibodies. Am J Transplant. 2005;5:2126–34. doi: 10.1111/j.1600-6143.2005.00999.x. [DOI] [PubMed] [Google Scholar]
- 44.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. II. Importance of humoral immunity. J Immunol. 1994;152:5135–41. [PubMed] [Google Scholar]
- 45.Uehara S, Chase CM, Cornell LD, Madsen JC, Russell PS, Colvin RB. Chronic cardiac transplant arteriopathy in mice: relationship of alloantibody, C4d deposition and neointimal fibrosis. Am J Transplant. 2007;7:57–65. doi: 10.1111/j.1600-6143.2006.01599.x. [DOI] [PubMed] [Google Scholar]
- 46.Jindra PT, Hsueh A, Hong L, et al. Anti-MHC class I antibody activation of proliferation and survival signaling in murine cardiac allografts. J Immunol. 2008;180:2214–24. doi: 10.4049/jimmunol.180.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lepin EJ, Zhang Q, Zhang X, et al. Phosphorylated S6 ribosomal protein: a novel biomarker of antibody-mediated rejection in heart allografts. Am J Transplant. 2006;6:1560–71. doi: 10.1111/j.1600-6143.2006.01355.x. [DOI] [PubMed] [Google Scholar]
- 48.Tible M, Loupy A, Vernerey D, et al. Pathologic classification of antibody-mediated rejection correlates with donor-specific antibodies and endothelial cell activation. J Heart Lung Transplant. 2013;32:769–76. doi: 10.1016/j.healun.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Tsai EW, Wallace WD, Gjertson DW, Reed EF, Ettenger RB. Significance of intragraft CD138+ lymphocytes and p-S6RP in pediatric kidney transplant biopsies. Transplantation. 2010;90:875–81. doi: 10.1097/TP.0b013e3181f24e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galvani S, Trayssac M, Auge N, et al. A key role for matrix metalloproteinases and neutral sphingomyelinase-2 in transplant vasculopathy triggered by anti-HLA antibody. Circulation. 2011;124:2725–34. doi: 10.1161/CIRCULATIONAHA.111.021790. [DOI] [PubMed] [Google Scholar]
- 51.Iwasaki K, Miwa Y, Haneda M, Uchida K, Nakao A, Kobayashi T. Significance of HLA class I antibody-induced antioxidant gene expression for endothelial cell protection against complement attack. Biochem Biophys Res Commun. 2010;391:1210–5. doi: 10.1016/j.bbrc.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 52.Jin YP, Fishbein MC, Said JW, et al. Anti-HLA class I antibody-mediated activation of the PI3K/Akt signaling pathway and induction of Bcl-2 and Bcl-xL expression in endothelial cells. Hum Immunol. 2004;65:291–302. doi: 10.1016/j.humimm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Narayanan K, Jaramillo A, Phelan DL, Mohanakumar T. Pre-exposure to sub-saturating concentrations of HLA class I antibodies confers resistance to endothelial cells against antibody complement-mediated lysis by regulating Bad through the phosphatidylinositol 3-kinase/Akt pathway. Eur J Immunol. 2004;34:2303–12. doi: 10.1002/eji.200324843. [DOI] [PubMed] [Google Scholar]
- 54.Narayanan K, Jendrisak MD, Phelan DL, Mohanakumar T. HLA class I antibody mediated accommodation of endothelial cells via the activation of PI3K/cAMP dependent PKA pathway. Transplant Immunol. 2006;15:187–97. doi: 10.1016/j.trim.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Iwasaki K, Miwa Y, Ogawa H, et al. Comparative study on signal transduction in endothelial cells after anti-a/b and human leukocyte antigen antibody reaction: implication of accommodation. Transplantation. 2012;93:390–7. doi: 10.1097/TP.0b013e3182424df3. [DOI] [PubMed] [Google Scholar]
- 56.Mannam VK, Lewis RE, Cruse JM. The fate of renal allografts hinges on responses of the microvascular endothelium. Exp Mol Pathol. 2013;94:398–411. doi: 10.1016/j.yexmp.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Lachmann N, Terasaki PI, Budde K, Liefeldt L, Kahl A, Reinke P, et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation. 2009;87:1505–13. doi: 10.1097/TP.0b013e3181a44206. [DOI] [PubMed] [Google Scholar]
- 58.Aubert V, Venetz JP, Pantaleo G, Pascual M. Low levels of human leukocyte antigen donor-specific antibodies detected by solid phase assay before transplantation are frequently clinically irrelevant. Hum Immunol. 2009;70:580–3. doi: 10.1016/j.humimm.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 59.Kraus ES, Parekh RS, Oberai P, et al. Subclinical rejection in stable positive crossmatch kidney transplant patients: incidence and correlations. Am J Transplant. 2009;9:1826–34. doi: 10.1111/j.1600-6143.2009.02701.x. [DOI] [PubMed] [Google Scholar]
- 60.Bian H, Harris PE, Mulder A, Reed EF. Anti-HLA antibody ligation to HLA class I molecules expressed by endothelial cells stimulates tyrosine phosphorylation, inositol phosphate generation, and proliferation. Hum Immunol. 1997;53:90–7. doi: 10.1016/S0198-8859(96)00272-8. [DOI] [PubMed] [Google Scholar]
- 61.Bian H, Harris PE, Reed EF. Ligation of HLA class I molecules on smooth muscle cells with anti-HLA antibodies induces tyrosine phosphorylation, fibroblast growth factor receptor expression and cell proliferation. Int Immunol. 1998;10:1315–23. doi: 10.1093/intimm/10.9.1315. [DOI] [PubMed] [Google Scholar]
- 62.Bian H, Reed EF. Alloantibody-mediated class I signal transduction in endothelial cells and smooth muscle cells: enhancement by IFN-gamma and TNF-alpha. J Immunol. 1999;163:1010–8. [PubMed] [Google Scholar]
- 63.Bieri M, Oroszlan M, Farkas A, Ligeti N, Bieri J, Mohacsi P. Anti-HLA I antibodies induce VEGF production by endothelial cells, which increases proliferation and paracellular permeability. Int J Biochem Cell Biol. 2009;41:2422–30. doi: 10.1016/j.biocel.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Bayliss J, Bailey M, Leet A, Stein AN, Thomson NM, McLean CA. Late onset antibody-mediated rejection and endothelial localization of vascular endothelial growth factor are associated with development of cardiac allograft vasculopathy. Transplantation. 2008;86:991–7. doi: 10.1097/TP.0b013e318186d734. [DOI] [PubMed] [Google Scholar]
- 65.Jaramillo A, Zhang L, Mohanakumar T. Binding of anti-HLA class I antibodies to airway epithelial cells induces activation and growth factor production and indirectly upregulates lung fibroblast proliferation. J Heart Lung Transplant. 2001;20:166. doi: 10.1016/s1053-2498(00)00304-1. [DOI] [PubMed] [Google Scholar]
- 66.Valenzuela NM, Hong L, Shen XD, et al. Blockade of p-selectin is sufficient to reduce MHC I antibody-elicited monocyte recruitment in vitro and in vivo. Am J Transplant. 2013;13:299–311. doi: 10.1111/ajt.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamakuchi M, Kirkiles-Smith NC, Ferlito M, et al. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proc Natl Acad Sci U S A. 2007;104:1301–6. doi: 10.1073/pnas.0602035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ashokkumar C, Ningappa M, Ranganathan S, et al. Increased expression of peripheral blood leukocyte genes implicate CD14+ tissue macrophages in cellular intestine allograft rejection. Am J Pathol. 2011;179:1929–38. doi: 10.1016/j.ajpath.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tinckam KJ, Djurdjev O, Magil AB. Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney Int. 2005;68:1866–74. doi: 10.1111/j.1523-1755.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- 70.Busuttil RW, Lipshutz GS, Kupiec-Weglinski JW, et al. rPSGL-Ig for improvement of early liver allograft function: a double-blind, placebo-controlled, single-center phase II study. Am J Transplant. 2011;11:786–97. doi: 10.1111/j.1600-6143.2011.03441.x. [DOI] [PubMed] [Google Scholar]
- 71.Valenzuela NM, Mulder A, Reed EF. HLA class I antibodies trigger increased adherence of monocytes to endothelial cells by eliciting an increase in endothelial P-selectin and, depending on subclass, by engaging FcgammaRs. J Immunol. 2013;190:6635–50. doi: 10.4049/jimmunol.1201434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013;13:176–89. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 73.Hirohashi T, Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2012;12:313–21. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hidalgo LG, Sis B, Sellares J, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant. 2010;10:1812–22. doi: 10.1111/j.1600-6143.2010.03201.x. [DOI] [PubMed] [Google Scholar]
- 75.Shin BH, Ge S, Mirocha J, et al. Regulation of Anti-HLA Antibody-Dependent Natural Killer Cell Activation by Immunosuppressive Agents. Transplantation. 2014;97:294–300. doi: 10.1097/01.TP.0000438636.52085.50. [DOI] [PubMed] [Google Scholar]
- 76.Lobo LJ, Aris RM, Schmitz J, Neuringer IP. Donor-specific antibodies are associated with antibody-mediated rejection, acute cellular rejection, bronchiolitis obliterans syndrome, and cystic fibrosis after lung transplantation. J Heart Lung Transplant. 2013;32:70–7. doi: 10.1016/j.healun.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Willicombe M, Roufosse C, Brookes P, et al. Acute cellular rejection: impact of donor-specific antibodies and c4d. Transplantation. 2014;97:433–9. doi: 10.1097/01.TP.0000437431.97108.8f. [DOI] [PubMed] [Google Scholar]
- 78.Naemi FM, Carter V, Kirby JA, Ali S. Anti-donor HLA class I antibodies: pathways to endothelial cell activation and cell-mediated allograft rejection. Transplantation. 2013;96:258–66. doi: 10.1097/TP.0b013e3182985504. [DOI] [PubMed] [Google Scholar]
- 79.Jane-Wit D, Manes TD, Yi T, et al. Alloantibody and complement promote T cell-mediated cardiac allograft vasculopathy through noncanonical nuclear factor-kappaB signaling in endothelial cells. Circulation. 2013;128:2504–16. doi: 10.1161/CIRCULATIONAHA.113.002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cravedi P, Leventhal J, Lakhani P, Ward SC, Donovan MJ, Heeger PS. Immune cell-derived C3a and C5a costimulate human T cell alloimmunity. Am J Transplant. 2013;13:2530–9. doi: 10.1111/ajt.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]