Abstract
Background
Working memory and underlying functional brain deficits have been observed in euthymic bipolar disorder (BD) patients, though there is heterogeneity in the degree of deficits. Sleep/circadian rhythm abnormalities are thought to be a core component of BD and may explain some of the heterogeneity in functional abnormalities. This preliminary study examined associations between sleep/circadian rhythm abnormalities and functional magnetic resonance imaging (fMRI) brain response on a working memory task among BD patients.
Methods
Fourteen euthymic medicated BD patients wore an actigraph for seven days before undergoing fMRI with a working memory task. Two matched healthy comparison (HC) groups were used (14 in each sample). One group completed the actigraphy portion and one completed the fMRI portion of the study. Circadian activity rhythm and sleep variables were calculated and compared between BD and HC participants. Variables that significantly differed were used to examine the association between activity rhythms/sleep abnormalities and fMRI working memory brain response in anatomically defined regions.
Results
Sleep efficiency and the rhythm robustness, mesor, and amplitude-to-width ratio were significantly abnormal in BD patients. Individual variability in all the sleep/circadian variables was significantly associated with the degree of abnormality of brain response in the dorsolateral prefrontal cortex and supramarginal gyri.
Limitations
Small sample size and multiple comparison groups limit the interpretability of these findings.
Conclusions
BD patients have abnormal activity rhythms and sleep efficiency, which are associated with abnormal working memory brain response. These preliminary findings support the notion that the sleep/circadian system is important in the functional brain deficits among BD patients.
Keywords: Bipolar Disorder, Euthymic, Sleep, Circadian Rhythms, Working Memory, Functional Magnetic Resonance Imaging
Introduction
Bipolar Disorder (BD) is characterized by deficits in many cognitive domains and associated changes to the underlying neural circuitry, with working memory (WM) comprising one prominent domain (see Bearden et al., 2010 for a review). While cognitive deficits and neuronal changes are most severe during a mood episode, there is a growing body of evidence suggesting cognitive impairment persists during periods of remission or euthymia (Bearden et al., 2010; Chen et al., 2011). However, there is heterogeneity in findings where some patients do not demonstrate the same degree of cognitive impairment between mood episodes as others. The variable findings suggest other factors, such as age, medication, and sleep/circadian abnormalities could influence the degree of cognitive deficits. Abnormalities in sleep timing and architecture are recognized as common co-morbidities in a number of psychiatric disorders, including BD. Several lines of research suggest sleep/circadian changes are core features of BD (see Murray and Harvey, 2010 for a review). For example, rates of insomnia and hypersomnia during bipolar depression are usually very high (Harvey, 2008) and mania is defined, in part, by a lack of need for sleep (American Psychiatric Association, 2000). Interestingly, during periods of reported clinical remission, sleep disturbance remains prominent in BD patients with sleep patterns and frequencies resembling those seen in insomnia groups as measured by actigraphy and polysomnography (Harvey, 2008). Studies utilizing actigraphy to measure sleep-wake patterns and circadian activity rhythms find euthymic BD patients have fragmented sleep, changes to the amplitude and period of circadian activity rhythms, and overall less physical activity (Jones et al., 2005; Murray and Harvey, 2010). Other associational studies utilizing actigraphy have demonstrated altered sleep duration is associated with more severe BD symptoms and worse functioning and quality of life (Harvey, 2008; Murray and Harvey, 2010). These studies suggest actigraphic assessment is a useful tool in characterizing abnormalities of sleep and circadian activity rhythms in BD patients. Actigraphic assessment offers an objective measure of circadian activity rhythms, sleep quality, and light exposure with minimal restriction on normal routines. Circadian measurements derived from wrist actigraphy have shown good sensitivity/specificity when compared to measures derived from the gold standard of polysomnography (Ancoli-Israel et al., 2003; Pollak et al., 2001).
In terms of cognition, it is well established that sleep and circadian disruption can interfere with almost all cognitive processes studied to date in humans, ranging from the most basic (e.g., alertness and vigilance) to the most advanced cognitive abilities (e.g., decision making and problem solving; Killgore, 2010; Lim and Dinges, 2011; McKenna et al., 2007). Those cognitive functions subserved by the prefrontal cortex, such as WM, are often most impacted (McKenna and Eyler, 2012). Findings in healthy individuals shed light into the neurobiological mechanisms by which individuals may be susceptible or resilient to abnormal changes in the sleep/circadian system. Disruptions in cognitive processing due to abnormal sleep patterns observed in normal populations are similar to those typically observed among BD individuals, suggesting a relationship between circadian rhythms and cognitive changes in BD (McKenna and Eyler, 2012). For example, one study demonstrated self-reported rhythms in euthymic BD patients predicted daily functioning. Interestingly, a subsample received a measure of executive functioning, which was found to moderate this association (Giglio et al., 2010). However, no studies to date have examined the associations of circadian activity rhythm abnormalities in BD patients with the underlying neuronal activity of cognitive functions.
The aim of the present preliminary study is to replicate findings of circadian activity rhythm abnormalities in a euthymic BD patient sample and, more importantly, to examine the association of sleep and circadian activity rhythm abnormalities with brain functioning on a WM task as measured with functional magnetic resonance imaging (fMRI). The task paradigm employs a slow event-related design allowing the examination of WM encoding and maintenance processes separately (McKenna et al., 2013a). We predict that euthymic BD patients will have significantly lower sleep efficiency and abnormal circadian activity rhythms characterized by changes to the amplitude, period, mesor (i.e., mean activity), and robustness (i.e., variability) of the rhythm. We also predict that among euthymic BD patients, the degree of abnormal activity rhythms and sleep will be associated with the degree of abnormal blood oxygen-dependent level (BOLD) response in regions underlying WM encoding and maintenance including the dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus, supramarginal gyrus, and visual cortex (Champod and Petrides, 2010; Cowan, 1999; Ravizza et al., 2004).
Methods
Participants
Following procedures approved by the University of California, San Diego (UCSD) and Veterans Affairs San Diego Healthcare System, written informed consent was obtained from 14 BD patients and two groups of age-, education-, and gender-comparable healthy participants (14 in each group). All participants were deemed eligible if they were between the ages of 30 and 79, right handed, free of diagnoses of substance abuse for 6 months and dependence for 12 months, free of any serious neurological or medical condition, suitable for MRI, native English speakers, and not currently under conservatorship. Patients met Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV; American Psychiatric Association, 2000) criteria for the diagnosis of bipolar I disorder with first mood episode occurring between ages 13 and 30 as determined by an expanded version of the Structured Clinical Interview for DSM-IV (SCID-IV; Spitzer et al., 1995), and on stable doses of psychotropic medication for at least 6 weeks. Potential BD patients were excluded from the study if they were currently experiencing a mood episode as determined by the SCID-IV, or significant residual symptoms as determined by assessment of depressive (Hamilton Rating Scale for Depression; Trajković et al., 2011), manic (Young Mania Rating Scale; YMRS; Young et al., 1978), or psychotic (Positive and Negative Syndrome Scale; PANSS; Kay et al., 1987) symptoms, or had a history of any other Axis I DSM-IV diagnosis. Two samples of healthy comparison (HC) participants were used. One sample completed a separate protocol with identical actigraphic assessment (actigraphy HC) and one sample completed identical MRI procedures (MRI HC). All HC participants were eligible for analyses if they had no Axis I DSM-IV diagnosis, were not taking medication known to interfere with cognitive functioning, and had no first-degree relatives with a diagnosis of major depressive disorder, bipolar disorder, or schizophrenia. One BD patient did not complete the MRI protocol due to not being able to tolerate the MRI. Demographic information and clinical rating scores are presented in Table 1.
Table 1.
Demographic and clinical characteristics of samples
| Group | Age (Years) | Education (Years) | Duration of Illness (Years) | Number of Depressive Episodes | Number of Manic Episodes | Number of Mixed Episodes | YMRS | HAMD-17 | PANSS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | General | |||||||||
| Bipolar I patients (n = 14; 3 males) | |||||||||||
| Mean | 49.07 | 15.36 | 30.25 | 14.71 | 3.86 | 4.86 | 1.21 | 3.57 | 9.79 | 9.86 | 23.00 |
| Standard deviation | 11.34 | 2.31 | 9.72 | 16.41 | 2.25 | 3.67 | 1.35 | 2.38 | 1.76 | 2.21 | 3.26 |
| Actigraphy HC (n = 14; 4 males) | |||||||||||
| Mean | 46.36 | 15.86 | |||||||||
| Standard deviation | 15.04 | 1.79 | |||||||||
| MRI HC (n = 14; 4 males) | |||||||||||
| Mean | 49.20 | 14.71 | |||||||||
| Standard deviation | 12.12 | 2.40 | |||||||||
n: number of participants in each group; HC: healthy comparison; YMRS: Young Mania Rating Scale; HAMD-17: Hamilton Rating Scale for Depression; PANSS: Positive and Negative Syndrome Scale
Activity Rhythm and Sleep-Wake Assessment
Participants wore Respironics™ wrist actiwatches on their left wrist for 7 days prior to their MRI assessment. Actigraphic data was collected in 60 second epochs over the recording period. To complement the actigraphic data, participants completed subjective diaries of their sleep each morning.
Delayed Match-to-Sample Task
Participants were administered a delayed match-to-sample WM task while undergoing fMRI. The task has been previously validated and used with HC and BD patients to examine WM encoding and maintenance processes (McKenna et al., 2013a; McKenna et al., 2013b). Stimuli consisted of multi-syllabic pronounceable pseudowords, with each constituent syllable consisting of a consonant-vowel-consonant letter string (e.g., MUV). The number of syllabi was parametrically manipulated such that participants were presented with 2-, 3-, and 4-syllable pseudowords with seven stimuli presented in each condition. Pseudowords were visually presented followed by a maintenance interval and then a forced choice recognition trial. The forced choice involved two choices where the target pseudoword differed from the foil by one letter. Participants used their dominant right-hand to respond by using a response box (Current Designs). Each trial condition (e.g., 2-, 3-, or 4-syllable pseudoword) was pseudo-randomly presented with an inter-trial interval (ITI) between trials. The ITI, composed to two phases, served as the baseline condition, and was designed to keep mental set fixed on anticipating the next trial, similar to visual detection paradigms used to study attention. The first involved the fading out of an orientation cross, where the cross becomes progressively less visible over a four-second interval. The second involved a blank screen where the next trial could start at any moment over the next 20 seconds. The median length was 6 seconds as governed by the hazard function of a percentile distribution derived from a geometric distribution. The total time for each task depended on which maintenance interval and ITI was chosen for each task trial, with an expected average time of 9 minutes 52 seconds. The instructions were to memorize the visually-presented pseudowords, mentally rehearse pseudowords by repeating them continuously (akin to subvocalization), and choose the correct pseudoword on a recognition test. Once tested, participants were further instructed to no longer rehearse pseudowords.
Data Acquisition
Participants were scanned at the UCSD Keck Center for fMRI using a GE Signa EXCITE 3.0 Tesla whole-body imaging system. Anatomical scans utilized a T1-weighted fast spoiled gradient echo pulse sequence (TE=4ms, flip angle= 90°, 1mm3 resolution). The functional scans were sensitive to the T2*-weighted BOLD signal. 32 echoplanar 4mm axial slices covering the whole brain (TR=2000 ms, TE=30 ms, image matrix=64×64, 4mm × 4mm resolution) were acquired parallel to the intercommissural plane in an interleaved manner using a gradient echo pulse sequence. The number of repetitions acquired varied over the task, as described above. To correct for warping in the echo-planar images due to inhomogeneties in the magnetic field, scans with opposite phase encoding polarities resulting in opposite spatial distortion patterns were acquired. The resulting images were aligned using a fast nonlinear registration procedure (Holland et al., 2010).
Image Processing
We used local software, FSL (Smith et al., 2004), and the Analysis of Functional NeuroImages (AFNI; Cox, 1996) for analyses. To improve structural to functional image correspondence we used a cost function optimized for T2*-to T1-weighted image alignment (Saad et al., 2009). Echo planar slices were aligned to have the same temporal origin and then corrected for motion artifact by co-registering to a base image. A general linear model was applied to each participant’s time series yielding brain response estimates during encode, maintenance, and recognition intervals using the ITI as the common baseline. For this study, we focused only on encode and maintenance intervals as these were the component processes of interest and only examined the mean BOLD response averaging across 2, 3, and 4 syllable conditions. Raw BOLD signal data was transformed into a percent signal change from baseline, spatially smoothed using a Gaussian filter (FWHM=6 mm), and normalized into standard Talairach atlas space (Talairach and Tournoux, 1988).
Regions of Interest
We defined anatomical regions of interest (ROIs) implicated in verbal WM and used in our previous work with this task paradigm (McKenna et al., 2013a) based on the AFNI daemon (Cox, 1996). These included the DLPFC, inferior frontal gyrus, supramarginal gyrus, and visual cortex. For a more detailed description on the creation of these regions please refer to McKenna, et al., 2013a. Voxel-based correlation analyses were conducted within the ROIs as described below.
Actigraphy Data Analyses
An extension to a traditional cosine model was used to map a 24-hour circadian activity rhythm to the actigraphy data (Marler et al., 2006) and the following rhythmicity measures were calculated: amplitude, phase, amplitude-to-width ratio (a marker of amplitude relative to period), mesor (mean level of activity), and an overall measure of the robustness of individual’s circadian activity rhythm. We also calculated total sleep time and sleep efficiency from actigraphic and sleep diary data. These variables were compared between BD patients and the actigraphy HC participants using independent-samples t-tests assuming unequal variances between groups. Bonferroni corrections were used to control for multiple comparisons for each family of comparisons (α = 0.01 level for circadian variables and α = 0.025 for sleep variables). We also examined the association of the clinical data with circadian/sleep variables and BOLD signal among BD patients using the Spearman’s correlation coefficient.
MRI Data Analyses
Standardized Z-scores were created for BD patients’ raw circadian/sleep variables relative to the actigraphy HC group and BD patients’ mean BOLD response relative to the MRI HC group. For those circadian/sleep variables that were significantly abnormal between BD and actigraphy HC participants, we examined the association between circadian/sleep and BOLD Z-scores using linear regression within the anatomical WM ROIs. We controlled Type I error using a cluster threshold method (Forman et al., 1995). This method utilizes Monte Carlo simulations to determine the probability that a single significantly activated voxel is also part of a contiguous cluster of N voxels that are all individually significantly activated at p ≤ .05 in each ROI. That is, we only considered an area of activation as reliable if it contained a threshold of 18 contiguous voxels (1152 μL) in the DLPFC/inferior frontal gyrus, 12 voxels (768 μL) in the supramarginal gyrus, or 8 voxels (512 μL) in the visual cortex, each of which were individually activated at the p ≤ .05 level, providing a corrected p-value of .05.
Results
Activity Rhythms and Sleep
Table 2 presents the circadian and sleep variables for BD and actigraphy HC participants. We found medium-to-large effects such that the BD sample demonstrated less efficient sleep, a lower mesor (i.e., less mean activity), a less robust rhythm (i.e., increased variability), and a smaller amplitude-to-width ratio, which reflects a longer period given no differences in amplitude. Spearman correlational analyses among the BD patients revealed significant associations between the amplitude-to-width ratio and age of illness onset and number of major depressive episodes (ρ = 0.595, p = 0.025; ρ = −0.751, p = 0.002, respectively). Specifically, those patients with a longer period relative to amplitude had an earlier age of onset and an increased number of lifetime depressive episodes. Circadian phase was also associated with age of onset (ρ = −0.633, p = 0.015) such that those patients with a later phase had an earlier age of onset. Lastly, the mesor was associated with patients’ YMRS and PANSS total score (ρ = 0.709, p = 0.004; ρ = 0.637, p = 0.014, respectively) such that those patients with increased mean activity (similar to levels of the actigraphy HC group) had higher scores on the YMRS and PANSS total score.
Table 2.
Differences in circadian and sleep variables among bipolar I patients
| Variable | BD Mean Z-score | t-value | Df | p-value |
|---|---|---|---|---|
| Amplitude | −0.326 | 0.694 | 23.15 | 0.494 |
| Amplitude-Width Ratio | −1.787 | 2.912 | 18.77 | 0.009 |
| Mesor | −1.402 | 3.214 | 24.48 | 0.004 |
| Phase | 0.586 | 0.857 | 17.53 | 0.403 |
| Rhythm Robustness | −1.524 | 3.471 | 24.37 | 0.002 |
|
| ||||
| Total Sleep Time | 1.493 | 1.964 | 16.87 | 0.066 |
| Sleep Efficiency | −2.516 | 4.280 | 19.76 | < 0.001 |
BD: bipolar disorder group; Df: degrees of freedom. The top panel presents circadian variables while the bottom presents sleep variables. Z-scores were created by standardizing patients’ raw variables to the healthy comparison group. Statistical results are presented from an independent-samples t-test assuming unequal variance, thus there are fractional degrees of freedom. Bold values reflect significant differences after family-wise bonferroni corrections.
Associations with Working Memory Brain Functions
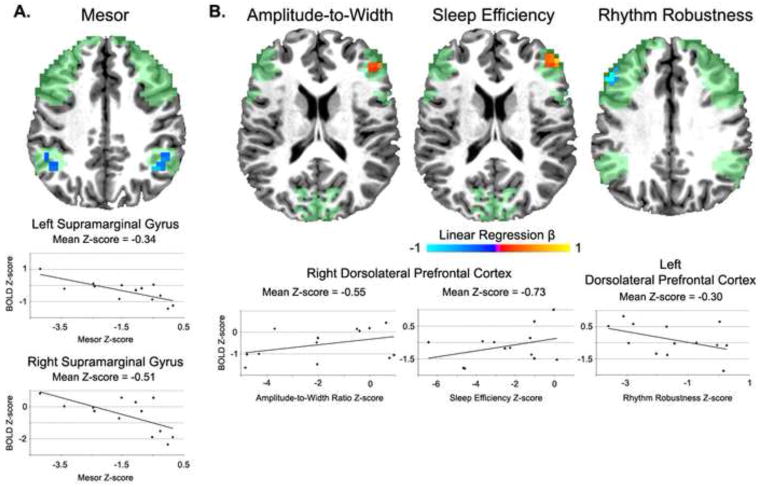
Within the WM task encode interval only the mesor was significantly associated with the mean BOLD response in bilateral supramarginal gyri among BD patients. Specifically, a negative association was found in both hemispheres using linear regression such that a decreased mesor was associated with a greater mean BOLD response (see Figure 1A). Within the maintenance interval, significant associations were found with sleep efficiency, rhythm robustness, and amplitude-to-width ratio. Specifically, within the right DLPFC, positive associations were found between the mean BOLD response and sleep efficiency and the amplitude-to-width ratio such that greater sleep efficiency and a larger amplitude-to-width ratio (i.e., shorter period relative to amplitude) was associated with an increased mean BOLD response. Contrarily, within the left DLPFC a less robust rhythm among BD patients was associated with greater mean BOLD response (see Figure 1B). Spearman correlational analyses did not find any significant associations between clinical data and the BOLD signal among BD patients.
Figure 1.
Associations between circadian/sleep variables and brain functioning among euthymic bipolar I patients. The green regions represent the anatomical regions of interest where linear regression analyses were conducted. Panel A: Findings during the task encode interval. Panel B: Findings during the task maintenance interval. All clusters are significant at a corrected p < .05
Discussion
These data provide preliminary evidence that 1) specific aspects of BD patients’ activity rhythms are abnormal and 2) there is an association between activity rhythms and brain activation on a WM task. Consistent with the literature, in medication-stable euthymic BD patients we found lower sleep efficiency and an abnormal activity rhythm characterized by a decreased mesor (i.e., mean level of activity), a less robust rhythm (i.e., increased rhythm variability), and a smaller amplitude-to-width ratio, which reflects a longer period given no differences in amplitude. Further, several circadian variables, including the mesor, amplitude-to-width ratio, and phase, were associated with the course and symptoms of euthymic BD patients such that the more abnormal the circadian measure the longer the duration of illness or the greater the severity of clinical symptoms. These findings reinforce that circadian and sleep disruption are core components of BD (Harvey, 2008; Murray and Harvey, 2010).
The pattern of associations among sleep/circadian variables and brain function presents a process by which circadian and sleep abnormalities may impact the neural functioning underlying cognitive performance in BD. During WM encoding processing only the mesor was associated with the mean BOLD response within the supramarginal gryi where a lower mesor (i.e., less mean activity) among BD patients was associated with similar to greater activation relative to HC participants. It may be that euthymic BD patients require lower than normal levels of activity to illicit normal brain function. Alternatively, those patients who have a higher mesor relative to their peers may have higher symptom severity between mood episodes and thus abnormal brain functioning. This is consistent with the significant associations with our clinical measures where a higher mesor was associated with higher scores on the YMRS and PANSS.
Within the task maintenance interval, the predicted associations among sleep efficiency and amplitude-to-width ratio were found where greater abnormalities on these measures were associated with greater deficits in brain functioning within the right DLPFC. These findings suggest that one possible explanation for the variability in the literature on WM functions among euthymic BD patients lies in aspects of the sleep and circadian system. This is consistent with findings in healthy young adults showing lower sleep efficiency predicts lower activation during a verbal learning task (Jonelis et al., 2012). Here, our preliminary findings suggest those patients with longer period lengths relative to amplitude in their activity rhythms and worse sleep efficiency are the patients with the greatest deficits in the DLPFC, a core WM neural structure required for maintaining verbal information within WM (Champod and Petrides, 2010). However, contrary to our predictions, a less robust circadian rhythm among euthymic BD patients was associated with greater activation relative to HC participants in the left DLPFC. Further research is required in parsing out the complex interaction between sleep and circadian disruption with brain function within BD where there are likely both deficits and compensatory neuronal processes interacting.
Interestingly, the abnormal sleep and circadian variables were associated with brain activation primarily during WM maintenance, as opposed to encoding processing. Our previous research with this task paradigm found our sample of euthymic BD patients had less overall accuracy and lower mean BOLD response primarily in the encode interval supporting the notion that attentional changes underlie WM impairment at the group level (McKenna et al., 2013b). However, individual differences among BD patients may be more detectable in brain regions or cognitive operations that do not show group differences, as there is likely increased variability among patients in these regions. The sleep literature also supports the notion of individual differences in response to sleep loss. For example, our findings are consistent with a study utilizing a similar task as ours that showed, at the group-level, WM maintenance was most impacted by sleep loss while WM encoding was only mildly impacted. However, at the individual-level effects to sleep loss varied (Turner et al., 2007). Due to limitations in sample sizes and multiple comparison groups we chose to focus our analyses on predefined anatomical ROIs implicated within our WM paradigm and only examine the mean BOLD response on the task. As such, this preliminary study cannot provide mechanistic role of sleep circadian rhythms with the brain function of BD patients. Further research is needed examining the links between sleep/circadian rhythms, mood, and cognition in BD. Future studies designed and powered to fully examine the role of sleep and circadian rhythms in brain functions would prove beneficial, such as examining increasing cognitive demands, changes over time, and changes with mood states.
In summary, this preliminary study supports the notion the sleep and circadian system is a core component of BD with abnormalities that persistent between mood episodes and likely play a role in the neural processes underlying differences in cognitive function among patients. A better understanding of the role of the circadian system in the neuropsychology of BD would aid in understanding the variability within the disorder with implications for improving the everyday functioning of patients.
Acknowledgments
We would like to thank the Desert Pacific Mental Illness Research Education and Clinical Center for infrastructure support and Ashley Sutherland for assistance. We also would like to thank the San Diego Depression and Bipolar Support Alliance, International Bipolar Foundation, San Diego Chapter of the National Alliance for Mental Illness, and the dedicated volunteer participants.
Role of Funding Source
This work was supported by the VA Desert-Pacific Mental Illness Research, Education, and Clinical Center and the VA San Diego Healthcare System. This study was also funded, in part, by National Institute of Health grants R01-MH083968, P30-MH080002, and R01-AG024506.
Footnotes
Conflict of Interest
All authors declare they have no conflict of interest related to this study.
Contributors
Author BM aided in design and implementation of the study, in addition to performing the statistical analyses, literature search, and preparation of the first draft of the manuscript. Author SD conducted a portion of the study. Author LT aided in the designed and implementation of the study. All authors contributed to preparation of the manuscript and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. APA; Washington, DC: 2000. 4th Text Revision. [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Woogen M, Glahn DC. Neurocognitive and neuroimaging predictors of clinical outcome in bipolar disorder. Curr Psychiatry Rep. 2010;12:499–504. doi: 10.1007/s11920-010-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociation within the Frontoparietal Network in Verbal Working Memory: A Parametric Functional Magnetic Resonance Imagining Study. J Neurosci. 2010;30:3849–3856. doi: 10.1523/JNEUROSCI.0097-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox B, Ooi C, Bullmore E. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- Cowan N. An embedded-process model of working memory. In: Miyake A, Shah P, editors. Models of working memory: mechanisms of active maintenance and executive control. Cambridge University Press; Cambridge, UK: 1999. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Giglio L, Magalhães P, Kapczinski N, Walz J, Kapczinski F. Functional impact of biological rhythm disturbance in bipolar disorder. J Psychiatr Res. 2010;44:220–223. doi: 10.1016/j.jpsychires.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Harvey A. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- Holland D, Kuperman J, Dale A. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. Neuroimage. 2010;50:175–183. doi: 10.1016/j.neuroimage.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonelis M, Drummond S, Salamat J, McKenna B, Ancoli-Israel S, Bondi M. Age-related influences of prior sleep on brain activation during verbal encoding. Front Neurol. 2012;3:1–8. doi: 10.3389/fneur.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Hare D, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005;7:176–186. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Kay S, Fiszbein A, Opler L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Killgore W. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges D. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2011;136:375–389. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler M, Gehrman P, Martin J, Ancoli-Israel S. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25:3893–3904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- McKenna B, Brown G, Drummond S, Turner T, Mano Q. Linking mathematical modeling with human neuroimaging to segregate verbal working memory maintenance processes from stimulus encoding. Neuropsychology. 2013a;27:243–255. doi: 10.1037/a0031515. [DOI] [PubMed] [Google Scholar]
- McKenna B, Eyler L. Overlapping prefrontal systems involved in cognitive and emotional processing in euthymic bipolar disorder and following sleep deprivation: A review of functional neuroimaging studies. Clin Psychol Rev. 2012;32:650–663. doi: 10.1016/j.cpr.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna B, Sutherland A, Legenkaya A, Eyler L. Verbal working memory encoding deficits in euthymic bipolar patients. Bipolar disord. 2013b doi: 10.1111/bdi.12126. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna BS, Dickinson DL, Orff HJ, Drummond SP. The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. J Sleep Res. 2007;16:245–252. doi: 10.1111/j.1365-2869.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- Murray G, Harvey A. Circadian rhythms and sleep in bipolar disorder. Bipolar Disord. 2010;12:459–472. doi: 10.1111/j.1399-5618.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- Pollak C, Tryon W, Nagaraja H, Dzwonczyk R. How accurately does wrist actigraphy identify the states of sleep and wakefulness? Sleep. 2001;24:957–965. doi: 10.1093/sleep/24.8.957. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22:562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M. Structured Clinical Interview for DSM-IV Patient Version (SCIDI/P, Version 2.0) New York State Psychiatric Institute; 1995. [Google Scholar]
- Talairach J, Tournoux P. Co-Planar stereotaxic atlas of the human brain. Thieme Medical; New York: 1988. [Google Scholar]
- Trajković G, Starčević V, Latas M, Leštarević M, Ille T, Bukumirić Z, Marinković J. Reliability of the Hamilton Rating Scale for Depression: a meta-analysis over a period of 49 years. Psychol Res. 2011;189:1–9. doi: 10.1016/j.psychres.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Turner TH, Brown GG, Drummond SPA. Effects of 42 Hr of Total Sleep Deprivation on Component Processes of Verbal Working Memory. Neuropsychology. 2007;21:787–795. doi: 10.1037/0894-4105.21.6.787. [DOI] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]