Abstract
Semiconducting polymer nanoparticles are utilized as a free-radical inert and light-harvesting nanoplatform for in vivo molecular imaging of reactive oxygen and nitrogen species (RONS). With its RONS-sensitive fluorescence, good biodistribution and passive targeting ability to leaky inflammatory vasculature, this nanoprobe permits detection of RONS in the microenvironment of spontaneous bacterial infection following systemic administration.
Keywords: semiconducting polymers, RONS, nanoparticles, biosensors, imaging agents
The elevated generation of reactive oxygen and nitrogen species (RONS) is a hallmark of many pathological processes ranging from acute and chronic bacterial infections to chronic diseases such as cancer, cardiovascular disease, and arthritis.[1] Therefore, the ability to detect the generation of RONS as pathological chemical messengers is critical to both understanding the etiology of these diseases and optimizing therapeutic interventions against these potentially life-threatening conditions. The main effort in RONS sensing has been devoted to the construction of fluorescence probes because of the high sensitivity and nonionizing nature of fluorescence imaging.[2] Although small-molecule fluorophores,[3] genetically encoded proteins,[4] fluorescent single-walled carbon nanotubes (SWCNs),[5] quantum dots (QDs),[6] and dye-conjugated inorganic nanoparticles[7] have been developed for this purpose, probes applicable for in vivo imaging are still very limited.[2]
In addition to near-infrared (NIR) fluorescence, one of the most important prerequisites for in vivo RONS imaging is the resistance of the reporter moiety of the probe to degradation by RONS. Failing to meet this requirement will likely result in a greatly reduced signal-to-noise ratio because persistent microenvironmental RONS in vivo will bleach the activated probe and lead to false negative signals. QDs and many small molecules dyes are not stable in the presence of highly oxidative RONS such as hypochlorite (ClO−),[6,8] and thus are not ideal to serve as the signal output units in this application of imaging probes.
Semiconducting polymer nanoparticles (SPNs) represent a new class of photostable fluorescent nanomaterials with excellent brightness that can be orders of magnitude higher than that of small-molecule fluorophores, and tens of times better than that of QDs.[9] As these nanoparticles are primarily composed of π-conjugated polymers, their utilization as fluorescent probes eliminates the possibility of heavy metal ion-induced toxicity to living organisms, potentially giving rise to good biocompatibility.[10] Despite theses advantages, exploration of SPNs in molecular imaging is still in its infancy with only a few in vivo studies reported.[11] Recently, we have developed a bioluminescence resonance energy transfer (BRET) and fluorescence resonance energy transfer (FRET) relay nanoprobe for lymph node mapping and tumor imaging by taking advantage of the efficient light-harvesting properties of SPNs.[12] In addition, SPNs are shown to maintain its fluorescence in the presence of H2O2 and ClO−.[9d] All these optical and physiological advantages of SPNs make them a promising nanoplatform for in vivo RONS imaging.
We herein report a dual-color SPN-based NIR nanoprobe for the detection of RONS (in short, NanoDRONE) in inflammatory microenvironments in living mice. NanoDRONE is designed to comprise a RONS-inert SPN core (energy donor) covered by RONS-sensitive fluorophore molecules (energy acceptors), enabling FRET from the SPN core to the fluorophore in the absence of RONS (Figure 1a). The presence of RONS can decompose the energy-accepting fluorophore and subsequently abolish the FRET within NanoDRONE, ultimately leading to enhanced emission intensity of SPN and a change in the emission spectrum that provides a RONS-dependent spectral fingerprint. By virtue of poly(ethylene glycol) (PEG) protected nanoarchitecture, NanoDRONE possesses another desirable advantage over small-molecule probes, namely passive targeting in tissues with leaky vasculature through the enhanced permeability and retention (EPR) effect. As leaky vasculature is a hallmark of inflammation,[13] NanoDRONE can effectively accumulate at inflammatory regions, permitting RONS imaging following systemic administration.
Figure 1.
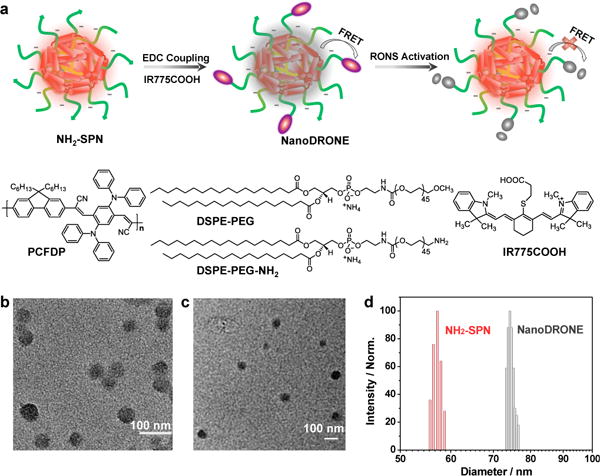
(a) Schematic of the preparation and RONS sensing of NanoDRONE, and the molecular structures of PCFDP, DSPE-PEG, DSPE-PEG-NH2 and IR775COOH. Representative TEM images of (b) NH2-SPN and (c) NanoDRONE. (d) Representative DLS profiles of NH2-SPN and NanoDRONE in 1×PBS (pH = 7.4).
The facile synthesis of NanoDRONE proceeds in two steps as illustrated in Figure 1a. We first utilized a nanoprecipitation method to prepare NH2-functionalized SPN (NH2-SPN) by choosing a NIR emissive semiconducting polymer, PCFDP, as the fluorescent polymer and DSPE-PEG and DSPE-PEG-NH2 as the matrix polymers. Then, a cyanine dye derivative (IR775COOH), which has been recently discovered to undergo oxidation-induced degradation in the presence of RONS,[14] was conjugated to the nanoparticle surface through a carbodiimide-activated coupling reaction to afford NanoDRONE. The successful conjugation of IR775COOH was confirmed by agarose gel electrophoresis (Supporting Information, Figure S1). Both NH2-SPN and NanoDRONE have spherical morphology as shown by transmission electron microscopy (TEM) images in Figure 1b and 1c, respectively. With the conjugation of the positively charged dye to the nanoparticle surface, the average diameter of the nanoparticles increased from 56 nm for NH2-SPN to 78 nm for NanoDRONE as measured by dynamic light scattering (DLS) (Figure 1d), while the zeta potential of NanoDRONE increased from −21 mV for NH2-SPN to −9 mV. Despite the relatively small absolute zeta potential, NanoDRONE has high stability in aqueous solution (Supporting Information, Figure S2), attributable to the strong steric repulsion provided by the PEG shell.
NanoDRONE has two absorption peaks at 410 and 788 nm (Figure 2a), corresponding to the SPN core and the fluorophore, respectively. Upon excitation of the SPN core, NanoDRONE exhibits an additional emission peak at 818 nm relative to NH2-SPN (678 nm) (Figure 2b), confirming FRET from the SPN core to the fluorophore on the surface. Moreover, the fluorescence of NanoDRONE at 678 nm was much weaker than that of NH2-SPN (Inset of Figure 2b): the fluorescence quantum yield of NanoDRONE decreased from the original 18% of NH2-SPN to 3%. However, simple mixing of IR775COOH with NH2-SPN at the same concentrations did not cause obvious fluorescence change to NH2-SPN (Supporting Information, Figure S3), which not only confirms the successful conjugation of the fluorophore to the nanoparticle surface but also highlights its necessity for efficient FRET.
Figure 2.
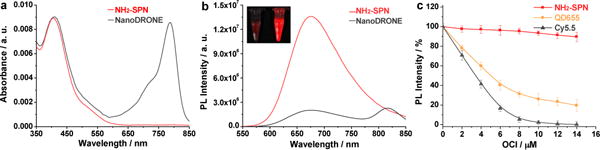
UV-Vis absorption (a) and fluorescence (b) spectra of NH2-SPN and NanoDRONE in PBS (30 mM, pH = 7.4). (c) Fluorescence changes of NH2-SPN, QD655 and Cy5.5 at 678, 655 and 693 nm, respectively, upon addition of ClO− in PBS (30 mM, pH = 7.4). [NH2-SPN] = [Cy5.5] = 1.0 μg; [QD655] = 20 nM. Excitation wavelengths for NH2-SPN, QD655 and Cy5.5 are 405, 500, 630 nm, respectively. Error bars represent the standard deviation from three measurements.
According to the sensing mechanism in Figure 1a, the fluorescence response of the probe toward RONS is not only determined by the efficacy of the decomposition of energy acceptor upon reaction with RONS, but also dependent on the stability of energy donor in the presence of RONS. To confirm that SPN is a suitable donor in FRET-based RONS sensing, the fluorescence stability of NH2-SPN was studied and compared with QD655 and Cy5.5 (Figure 2c). With increasing concentrations of ClO−, one of the most oxidative RONS, both QD655 and Cy5.5 showed significantly decreased fluorescence, while NH2-SPN was significantly more resistant, showing only slight decreases in its fluorescence. At [ClO−] = 14 μM, the fluorescence of NH2-SPN only decreases by 9%; in contrast, the fluorescence of Cy5.5 nearly vanished, and QD655 showed a decrease of 86%. In addition to ClO−, NH2-SPN was tolerant to other RONS, such as ONOO−, •OH, O2•−, 1O2 and H2O2 (Supporting Information, Figure S4), indicating its superiority over QD655 and Cy5.5 as a reliable and inert energy donor for RONS sensing.
The fluorescence responses of NanoDRONE toward RONS were evaluated in solution under physiological conditions. Figure 3a shows the representative fluorescence spectral changes of NanoDRONE upon addition of RONS such as ONOO−. With increasing concentrations of ONOO−, the emission peak at 678 nm correspondingly increased with the concurrent loss of emission at 818 nm. This is due to the rapid oxidative cleavage of the oligomethine moiety of the FRET acceptor (IR775COOH),[14] which abolished FRET and recovered the donor fluorescence (SPN) at 678 nm. Thereby, NanoDRONE has a spectral fingerprint that can change from a dual-peak profile (678/818 nm) to a single-peak profile (678 nm) upon RONS activation. Figure 3b summarizes the discriminatory fluorescence responses of NanoDRONE at 678 nm to a number of RONS in N2-purged PBS. The probe emission can be significantly enhanced by ONOO− (4.0 times), ClO− (3.6 times) and •OH (2.5 times), and slightly enhanced by 1O2 (1.4 times), O2•− (1.3 times) and NO (1.2 times), but not by H2O2. In PBS without N2 purging, the intensity incensements are nearly the same for RONS expect NO (2.9 times), which should be due to generation of other stronger RONS from the autooxidation between NO and O2 (Figure 3b). Noteworthy is that the fluorescence responses toward RONS are nearly instant, and the response time is mainly constrained by the reaction methods used to generate RONS (Supporting Information, Figure S5). In addition, the fluorescence enhancement of NanoDRONE shows an excellent linear correlation with the concentration of the RONS such as ONOO−, ClO− and •OH (R2 > 0.99) (Figure 3c). The limit of detection for those RONS is determined to be ~10 nM in solution, which is comparable to or even lower than previously reported probes.[2–7] The combination of the RONS sensitivity of NanoDRONE with its nearly unchanged fluorescence after RONS activation under physiological conditions (Supporting Information, Figure S6) makes it feasible for in vivo RONS imaging.
Figure 3.
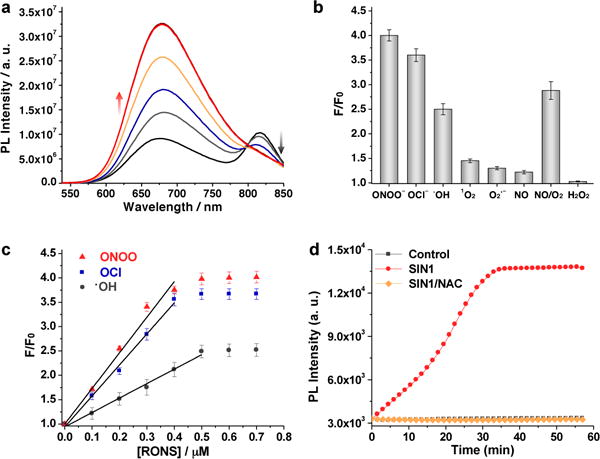
(a) Fluorescence spectra of NanoDRONE in PBS (30 mM, pH = 7.4) in the absence or presence of ONOO− with concentrations ranging from 0 to 0.5 μM at intervals of 0.1 μM. (b) Fluorescence responses of NanoDRONE toward RONS in nitrogen-purged PBS (pH = 7.4). F and F0 stand for the fluorescence intensities at λem = 678 nm in the presence and absence of RONS, respectively. [NanoDRONE] = 0.1 μg/mL; [RONS] = 1 μM. Excitation at 405 nm. (c) F/F0 as a function of RONS concentration. (d) Time course of changes in the fluorescence of NanoDRONE at 678 nm in the absence or presence of SIN1 or SIN1/NAC in PBS (30 mM, pH = 7.4). [NanoDRONE] = 0.1 μg/mL; [SIN1] = 5 μM; [NAC] = 50 μM. Error bars represent the standard deviation from three measurements.
The ability of NanoDRONE to detect RONS was further examined in solution under bio-inspired conditions of RONS generation. 3-Morpholinosydnonimine (SIN1), which spontaneously releases NO and O2•− to form ONOO− in aqueous solution, was used to mimic the generation of ONOO− by macrophages. With increasing incubation time with SIN1, the fluorescence of NanoDRONE at 678 nm gradually increased to a maximum at 35 min (Figure 3d). As compared to its initial state, the intensity at 678 nm was increased by 4 times, which is consistent with the fluorescence enhancement observed by the direct addition of ONOO− into the probe solution. This activation of NanoDRONE was prevented in the presence of N-acetylcysteine (NAC), a general antioxidant.[15] The fluorescence of the nanoprobe remained unchanged after incubation with either SIN1 (Figure 3d) or ClO− (Supporting Information, Figure S7), revealing the successful scavenging of both ONOO− and ClO−, respectively, by NAC.
NanoDRONE was then applied to detect endogenously generated RONS in cultured cell types relevant to inflammation. RAW264.7, a murine macrophage cell line, showed very weak fluorescence after incubation with NanoDRONE in its resting state (Figure 4a). To mimic the inflammatory condition that activates resting tissue macrophages, RAW264.7 cells were successively pretreated with bacterial cell wall lipopolysaccharide (LPS) and phorbol 12-myristate 13-acetate (PMA) to elicit the elevated production of RONS such as ONOO− and ClO−.[16] With LPS/PMA stimulation, strong fluorescence was observed from the cells (Figure 4b), indicating the activation of the nanoprobe by RONS under conditions relevant to inflammation. When NAC, a free-radical scavenger with high membrane permeability, was used to treat the cells along with LPS/PMA stimulation, no obvious fluorescence was observed after incubation with NanoDRONE (Figure 4c). This indicates that NAC scavenges endogenously generated RONS from macrophage cells and effectively inhibits the activation of NanoDRONE, similarly to that observed in solution. Additionally, NanoDRONE was found to be non-toxic to tested cell lines, including RAW264.7, RWPE-1 and HeLa cells (Supporting Information, Figure S8). These in vitro data clearly demonstrate that NanoDRONE can efficiently detect RONS produced by stressed cells.
Figure 4.
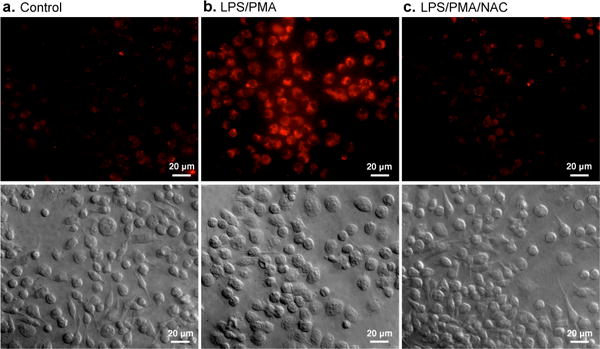
Fluorescence and DIC images of live murine macrophages (RAW 264.7) incubated with NanoDRONE for 3 h before imaging: (a) non-treated cells, (b) cells successively treated with LPS (2 h) and PMA (0.5 h), and (c) cells pretreated with NAC 2 h before treated with LPS (2 h) and PMA (0.5 h), followed with NAC for 1 h. [NanoDRONE] = 1.5 μg/mL; [LPS] = 1 μg/mL; [PMA] = 5 μg/mL; [NAC] = 1 mM. Scale bar: 30 μm.
NanoDRONE were applied to the imaging of RONS in living mice in an animal model of peritonitis induced by intraperitoneal (i.p.) injection of LPS. NanoDRONE was administered via i.p. 4 h after the injection of saline or LPS (Figure 5a), and fluorescence images were acquired 30 min after particle administration. The fluorescence intensities of the LPS-treated mice were 2.7-times higher than those of the saline-treated mice (Figure 5b). In addition, exposure of the peritoneal cavity during necropsy confirmed the isolation of the observed fluorescence to the peritoneum and not the overlying skin (Supporting Information, Figure S9).
Figure 5.
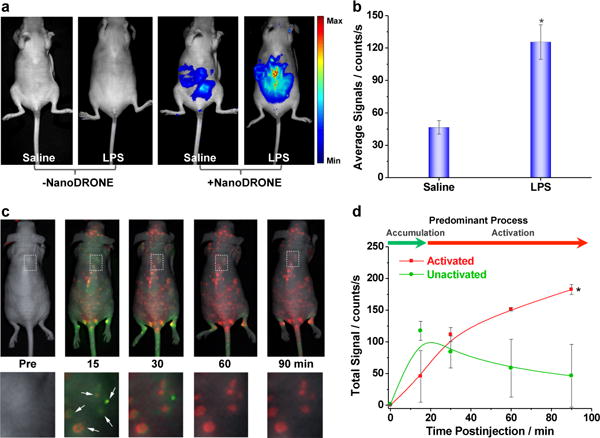
(a) In vivo imaging of RONS with NanoDRONE in a LPS-induced acute peritonitis mouse model. Saline (n=4) and LPS (n=4) were administered i.p., followed 4 h later by i.p. administration of NanoDRONE. Images were acquired before and 30 min after particle administration. (b) The quantified average fluorescence intensities of the peritoneal cavity for each treatment group. * indicates statistically significant increase of fluorescence intensity relative to the control groups (p<0.05). (c) Imaging RONS with NanoDRONE in mice with spontaneous systemic C. bovis bacterial infection. Overlaid images of activated (red) and unactivated (green) NanoDRONE following i.v. administration to mice with spontaneous infections (n=4). Enlargements of the regions indicated by dashed white boxes are given below each corresponding image. White arrows indicate localized regions of bacterial infection. (d) Quantification of activated (red) and non-activated (green) NanoDRONE fluorescence over time. † Significantly different change in fluorescence between unactivated and activated nanoprobe (p<0.05).
The in vivo application of existing RONS probes in living mouse models are mainly limited to local administration via i. p. injection, possibly owing to the unfavorable pharmacokinetics and biodistribution associated with these probes.[2–7] NanoDRONE was injected intravenously (i.v.) to test its suitability for systemic administration, which resulted in dynamic accumulation in the liver, kidney, gastrointestinal tract, skin, heart and brain, all of which decreased with time (Supporting Information, Figure S10a). Notably, organs of the reticuloendothelial system (RES) such as the liver and spleen did not display higher nanoparticle uptake than non-reticuloendothelial tissues, suggesting the ability of NanoDRONE to evade RES uptake. The blood circulation half-life was measured to be approximately 6 h through repeated saphenous vein sampling (Supporting Information, Figure S10b). The relatively long circulation time and favorable biodistribution of NanoDRONE, in conjunction with the lack of any overt systemic toxicity of the particle ingredients reveal that NanoDRONEs are amenable to i. v. administration for in vivo imaging of RONS in whole animals.
Since inflammation can be induced by pathogen-associated molecular patterns (PAMPs) following bacterial infection, NanoDRONE was evaluated for the systemic imaging of the RONS pool following i.v. administration in mouse models of spontaneous Corynebacterium bovis (C. bovis) bacterial infection. Nude mice are susceptible to skin infection by C. bovis,[17] which results in inflammation localized to regions of infection. As NanoDRONE has a spectral fingerprint dependent on their state of activation (Figure 2a), hyperspectral in vivo fluorescence imaging can be employed to deconvolve unactivated from activated probe states in live animals (Supporting Information, Figure S11). Following the intravenous injection of NanoDRONE to mice spontaneously infected by C. bovis, fluorescence images were taken, and signals from unactivated (illustrated in pseudo green color) and activated (illustrated in pseudo red color) probes were deconvolved (Supporting Information, Figure S12a), overlaid (Figure 5c) and quantified (Figure 5d). It was found that NanoDRONE can first specifically accumulate in the infected foci within 15 min through the EPR effect, and then progressively be induced to change from unactivated (green) to activated (red) states by microenvironmental RONS in the bacterial infection regions, with complete probe activation by 60 min. Histological analysis also showed specific accumulation of NanoDRONE to infected regions (Figure S12b in Supporting Information), which further proves its propensity for passive targeting to inflammatory microenvironments via the EPR effect following systemic administration.
In summary, we have developed a SPN-based NIR nanoprobe by taking advantage of the RONS-inert property of SPN in conjunction with the RONS sensitivity of a cyanine derivative for in vitro and in vivo imaging of RONS. With high physiological stability, good biodistribution and long circulation half-life, and passive targeting to inflammatory regions provided by its unique nanoarchitecture, this nanoprobe allows for detection of RONS in the microenvironment of inflammation via systemic administration. Additionally, the efficient RONS-mediated FRET of the nanoprobe results in a RONS-dependent fluorescence spectral fingerprint in the near infrared region, ultimately permitting real-time probe tracking and easy differentiation of probe activation from accumulation in living mice. Our probe design thus potentially provides more accurate spatial resolution for in vivo RONS imaging as compared with ‘off-on’ imaging probes that can only be seen once activated. The current probe does not display specificity over ONOO−, ClO−, and •OH (Figure 3b), and thus is more suitable for detection of the RONS pool at inflammation sites in living animals. However, the modular core-shell probe design allows the use of other RONS-selective NIR fluorophores as the energy acceptor to generate nanoprobes with specificity for individual RONS.[18] Thus, in addition to monitoring of bacterial infection as demonstrated herein, this SPN nanoplatform has the potential to provide real-time, in situ information regarding the RONS status of many other diseases such as cancer, cardiomyopathy, diabetes, stroke, and neurodegeneration.
Supplementary Material
Acknowledgments
This work was supported by the NIH National Cancer Institute (NCI) grants R01CA135294, R21CA138353A2, and the Stanford University NCI CCNE-T grant (U54CA119367). We acknowledge the use of the SCi3 Core Facility at Stanford, and the expertise of Pauline Chu in preparing histology samples. AJS also thanks the Susan G. Komen For The Cure for fellowship support.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a) Carden DL, Granger DN. J Pathol. 2000;190:255. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]; b) Medzhitov R. Nature. 2008;454:428. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]; c) Querfurth HW, LaFerla FM. N Engl J Med. 2010;362:329. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Chan J, Dodani SC, Chang CJ. Nat Chem. 2012;4:973. doi: 10.1038/nchem.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Lim MH, Xu D, Lippard SJ. Nat Chem Biol. 2006;2:375. doi: 10.1038/nchembio794. [DOI] [PubMed] [Google Scholar]; b) Koide Y, Urano Y, Kenmoku S, Kojima H, Nagano T. J Am Chem Soc. 2007;129:10324. doi: 10.1021/ja073220m. [DOI] [PubMed] [Google Scholar]; c) Kundu K, Knight SF, Willett N, Lee S, Taylor WR, Murthy N. Angew Chem Int Ed. 2009;48:299. doi: 10.1002/anie.200804851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Nat Methods. 2006;3:281. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 5.Jin H, Heller DA, Kalbacova M, Kim JH, Zhang J, Boghossian AA, Maheshri N, Strano MS. Nat Nanotechnol. 2010;5:302. doi: 10.1038/nnano.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancini MC, Kairdolf BA, Smith AM, Nie S. J Am Chem Soc. 2008;130:10836. doi: 10.1021/ja8040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panizzi P, Nahrendorf M, Wildgruber M, Waterman P, Figueiredo JL, Aikawa E, McCarthy J, Weissleder R, Hilderbrand SA. J Am Chem Soc. 131;43:15739. doi: 10.1021/ja903922u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oakes J, Gratton P. J Chem Soc, Perkin Trans. 1998;2:2201. [Google Scholar]; de Urzedo1 APFM, Nascentes CC, Diniz1 MER, Catharino RR, Eberlin MN, Augusti R. Rapid Commun Mass Spectrom. 2007;21:1893. [Google Scholar]; Toutchkine A, Nguyen DV, Hahn KM. Org Lett. 2007;9:2775. doi: 10.1021/ol070780h. [DOI] [PubMed] [Google Scholar]; Yan Y, Wang S, Liu Z, Wang H, Huang D. Anal Chem. 2010;82:9775. doi: 10.1021/ac101929q. [DOI] [PubMed] [Google Scholar]
- 9.a) Wu C, Szymanski C, Cain Z, McNeill J. J Am Chem Soc. 2007;129:12904. doi: 10.1021/ja074590d. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Moon JH, McDaniel W, MacLean P, Hancock LE. Angew Chem Int Ed. 2007;46:8223. doi: 10.1002/anie.200701991. [DOI] [PubMed] [Google Scholar]; c) Pecher J, Mecking S. Chem Rev. 2010;110:6260. doi: 10.1021/cr100132y. [DOI] [PubMed] [Google Scholar]; d) Zhu C, Liu L, Yang Q, Lv F, Wang S. Chem Rev. 2012;112:4687. doi: 10.1021/cr200263w. [DOI] [PubMed] [Google Scholar]; e) Li K, Liu B. J Mater Chem. 2012;22:1257. [Google Scholar]; f) Wu C, Chiu DT. Angew Chem Int Ed. 2013;52:3086–3109. doi: 10.1002/anie.201205133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernando LP, Kandel PK, Yu JB, McNeill J, Ackroyd PC, Christensen KA. Biomacromolecules. 2010;11:2675. doi: 10.1021/bm1007103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Kim S, Lim CK, Na J, Lee YD, Kim K, Choi K, Leary JF, Kwon IC. Chem Commun. 2010;46:1617. doi: 10.1039/b923309a. [DOI] [PubMed] [Google Scholar]; b) Wu C, Hansen S, Hou Q, Yu J, Zeigler M, Jin Y, Burnham D, McNeill J, Olson J, Chiu DT. Angew Chem. 2011;123:3492. doi: 10.1002/anie.201007461. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:3430. [Google Scholar]; c) Li K, Ding D, Huo D, Pu KY, Thao NNP, Hu Y, Li Z, Liu B. Adv Funct Mater. 2012;22:3107. [Google Scholar]
- 12.Xiong L, Shuhendler AJ, Rao J. Nat Commun. 2012;3:1193. doi: 10.1038/ncomms2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Sci Transl Med. 2011;3:88ps25. doi: 10.1126/scitranslmed.3002011. [DOI] [PubMed] [Google Scholar]
- 14.Oushiki D, Kojima H, Terai T, Arita M, Hanaoka K, Urano Y, Nagano T. J Am Chem Soc. 2010;132:2795. doi: 10.1021/ja910090v. [DOI] [PubMed] [Google Scholar]
- 15.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Cell Mol Life Sci. 2003;60:6. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sheng KC, Pietersz GA, Tang CK, Ramsland PA, Apostolopoulos V. J Immunol. 2010;184:2863. doi: 10.4049/jimmunol.0903458. [DOI] [PubMed] [Google Scholar]; Victor VM, Rocha M, De la Fuente M. Free Radic Res. 2003;37:919. doi: 10.1080/1071576031000148727. [DOI] [PubMed] [Google Scholar]
- 16.Hashioka S, Han YH, Fujii S, Kato T, Monji A, Utsumi H, Sawada M, Nakanishi H, Kanba S. Neurochem Int. 2007;50:499. doi: 10.1016/j.neuint.2006.10.006. [DOI] [PubMed] [Google Scholar]; Bergt C, Marsche G, Panzenboeck U, Heinecke JW, Malle E, Sattler W. Eur J Biochem. 2001;268:3523. doi: 10.1046/j.1432-1327.2001.02253.x. [DOI] [PubMed] [Google Scholar]
- 17.Burr HN, Lipman NS, White JR, Zheng J, Wolf FR. J Am Assoc Lab Anim Sci. 2011;50:378. [PMC free article] [PubMed] [Google Scholar]
- 18.Tian J, Chen H, Zhuo L, Xie Y, Li N, Tang B. Chem Eur J. 2011;17:6626. doi: 10.1002/chem.201100148. [DOI] [PubMed] [Google Scholar]; Yuan L, Lin W, Yang Y, Chen H. J Am Chem Soc. 2012;134:1200. doi: 10.1021/ja209292b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


