Abstract
The purpose of this study is to assess whether composite or coordinate immunoexpression patterns of estrogen receptor (ER), progesterone receptor (PR) and Wilms tumor 1 (WT1) gene can significantly distinguish between endometrial serous carcinoma (ESC) and ovarian serous carcinoma (OSC). Immunohistochemical analyses were performed on whole tissue sections from 22 uterus-confined ESC and on a tissue microarray of 140 high grade, pan-stage ovarian serous carcinomas, using antibodies to ER, PR, and WT-1. ER, PR and WT1 expression were present in 37%, 49% and 81% of OSC respectively, but these markers were also present in 18%, 27% and 36% of ESC. The ER+/PR+/WT1+ coordinate profile was identified in 33.6% of OSC but in none of ESC (p=0.0006), resulting in a calculated sensitivity and specificity of this profile for OSC of 33.6% and 100% respectively. By contrast, the ER−/PR−/WT1− coordinate profile was identified in 41% of ESC but in only 6.4% of OSC (p=0.0001), resulting in a calculated sensitivity and specificity of this profile for ESC of 50% and 94%. In summary, in the differential diagnosis between OSC and ESC, positivity for all 3 markers favors an extrauterine origin whereas negativity for all 3 markers is supportive of an endometrial origin. The use of single markers for this purpose is not recommended, as each lacks optimal discriminatory power. Coordinate profiles, in general, have a high specificity but low sensitivity in this differential diagnosis.
INTRODUCTION
The histopathologic distinction of serous carcinomas of endometrial origin from those arising from extrauterine locations can pose a significant challenge, since both of these tumors have broadly similar morphologic and immunophenotypic profiles, and nearly identical patterns of metastases. This distinction is important for accurate tumor staging (and epidemiological data extracted therefrom), the reporting of probable primary sites in biopsies or cervicovaginal cytologic preparations, and occasionally, chemotherapeutic choices (1). Several biomarkers have been proposed for use in this distinction. An antibody directed against the Wilms tumor 1 (WT1) gene was originally reported by Goldstein and Uzieblo (2) to be expressed in 97% of ovarian serous carcinomas (OSC) but in none of the endometrial serous carcinomas (ESC), and several studies soon followed that to varying extents all raised the possibility of some discriminatory value for this marker (3,4). In most of these reports, WT1 expression was generally diffuse and extensive in OSC, and when present in ESC, was weak and patchy. A 2005 meta-analysis of 7 such studies by Heatley (3) found statistically significant differences in the expression rate of WT1expression in endometrial serous carcinomas (mean 29.1%) and ovarian serous carcinomas (mean 91.1%) [odds ratio of 0.03 (95% CI 0.02–0.07)]. However, with the subsequent analysis of more cases, it has become apparent that a significant proportion of ESC may exhibit WT1 expression, and that the pattern of expression may be strong and diffuse (5-8). Estrogen [ER] and progesterone [PR] receptors are 2 biomarkers that may also have discriminatory value in this context. 75.8 to 88% of OSCs are known to be ER-positive (9,10). Contemporary studies have found that up to 58.8% of ESC are indeed ER-positive (6,8), contrary to earlier reports, in which less than 30% ESC were found to be ER-positive (11,12). The purpose of this study is to assess whether coordinate immunoexpression patterns of ER, PR and WT-1 can significantly distinguish between OSC and ESC.
MATERIALS AND METHODS
After approval from our institutional review board, we selected from our files 162 serous carcinomas in which the primary sites were not in question. This included 22 ESC cases, International Federation of Gynecology and Obstetrics stage I or II (uterus confined as determined by surgical staging, and exclusive of any cases with involvement of the outer 10% of the myometrium), and 140 cases of pan stage, high grade ovarian serous carcinomas, the latter in a previously constructed tissue microarray. This microarray was constructed as previously described (13) from 209 patient tumor blocks, 140 of which were serous. Each patient sample had at least 2 representative 2 mm tissue cores. The arrays were constructed using a manual arrayer (Beecher Instruments, Sun Prairie, WI, USA). Immunohistochemical analyses using antibodies raised against ER, PR and WT1 were performed on all cases. All studies were performed on formalin-fixed, paraffin-embedded tissue sections. Paraffin slides were cut at 4 microns and baked for 15 minutes at 60°C. Slides were stained on the Leica Bondmax platform (Leica Microsystems, Buffalo Grove, IL) or the Ventana Benchmark Ultra or XT platform (Ventana Medical Systems, Tucson, AZ). Deparaffinization and antigen retrieval was performed on the instrument. The 3 primary antibodies included ER (clone SP-1, prediluted, antigen retrieval using the Ventana CC1 [Cell Conditioning Solution 1] for 30 minutes, Detection with the Ventana Ultraview 3, 3′-diaminobenzidine [DAB] system), PR (clone 1E2, prediluted, antigen retrieval using the Ventana CC1 for 30 minutes, Detection with the Ventana Ultraview DAB), and WT-1 (clone WT49 from Leica Microsystems [Buffalo Grove, IL], prediluted, antigen retrieval using the Leica Epitope Retrieval Solution 2 for 30 minutes, Detection with the Leica DAB). Following the application of primary antibody a secondary antibody and then a tertiary or polymer was applied. Endogenous peroxidase was blocked using 3% hydrogen peroxide. Slides were then stained with DAB chromogen and counterstained in hematoxylin for visualization. Positive and negative controls were run in parallel as appropriate. For each case and each marker, “composite scores” (0 to 9+) were generated by multiplying the extent of staining score (0-3+) by the intensity of staining score (0-3+), figure 1. The extent of staining was semi-quantitatively assessed as follows: 0 (0-9%), 1 (10-25%), 2 (26-50%), 3 (51-100%). Any composite score above 0 was considered to be positive. When the 2 cores in any cases did not display identical composite scores, the core displaying the highest score was used. The sensitivity, specificity, positive predictive value (PPV) and negative predictive values (NPV) for each singular and coordinate immunoprofile using ESC and OSC as endpoints, were calculated. Staining patterns on the tissue microarray were confirmed on 7 randomly selected cases from the array, wherein whole sections were also evaluated. For ER, the average difference in composite scores between the TMA and the whole section was 0.7 (range 1-3); For PR, this difference was 0 (range 0-1); For WT1, the average difference was 1.75 (range 1-5). However, in no case was a stain positive in one modality and negative in the other (and vice versa). The differences between the OSC and the ESC groups regarding the frequency of each singular and coordinate profile were assessed using the Fisher Exact test, with a 2-tailed p value of <0.05 considered as significant.
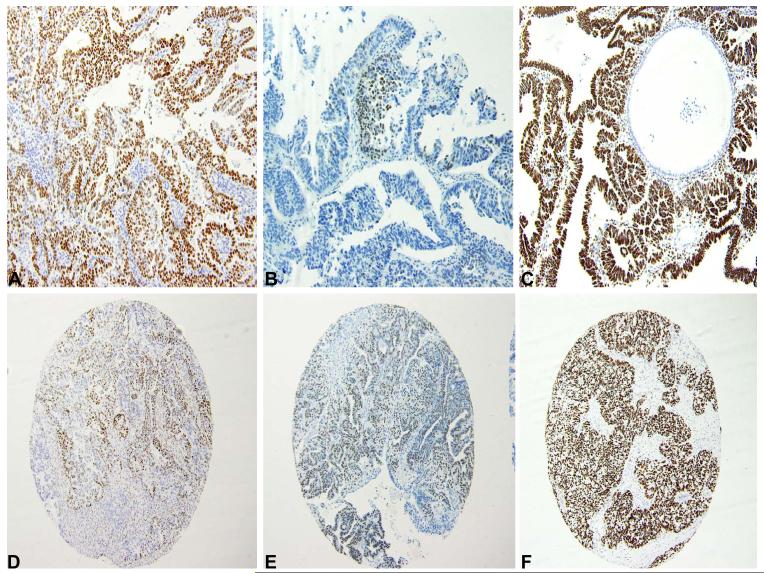
Figure 1.
A: Diffuse expression of ER in ESC (composite score 9)
B: Focal expression of PR in ESC (composite score 3)
C: Diffuse expression of WT1 in ESC (composite score 9)
D: Sporadic but strong expression of ER in OSC (composite score 6)
E: Diffuse expression of PR of intermediate intensity in OSC (composite score 6)
F: Diffuse expression of WT1 in OSC (composite score 9)
RESULTS
The distribution of composite scores, and the coordinate patterns of expression are outlined in tables 1 and 2. WT1 expression was more commonly identified in OSC (81%) than ESC (36%), p=0.0001. However, the expression of WT1 in 36% of ESC indicated that it has limited value as a specific marker of OSC when used in isolation. Both ER and PR also approached, but did not attain statistical significance as more prevalent antigens in OSC (p=0.0954 and 0.0668 respectively). As with WT1, their utility was limited when used as a single marker, since 18% and 27% of ESC expressed ER and PR respectively. The sensitivity, specificity, PPV and NPV for ER expression for OSC were 37%, 78%, 93% and 14% respectively. Parallel values for PR were 49%, 73%, 92% and 18% respectively. For WT1, the respective values were 81%, 67%, 93% and 37%. There was generally uniform correlation in staining patterns between the 2 cores on each array. However, in 7 of 140 cases, one core showed composite WT1 scores between 1+ to 3+ while the other showed scores between 7+ and 9+. For ER, this level of discrepancy was present in only 2 cases. For PR, no pair of cores showed a greater than 2+ difference in composite scores. Wherever there was a discrepancy between cores, the core showing the highest composite score was used for final analyses.
Table 1.
Distribution of composite scores
| Composite scores, Number of cases | ||||
|---|---|---|---|---|
| 0 | 1+ to 3+ | 4+ to 6+ | 7+ to 9+ | |
| Endometrial Serous carcinomas, n=22 | ||||
| ER | 18 | 1 | 2 | 1 |
| PR | 16 | 4 | 2 | 0 |
| WT1 | 14 | 2 | 1 | 5 |
| Ovarian Serous Carcinomas, n=140 | ||||
| ER | 88 | 4 | 40 | 8 |
| PR | 71 | 11 | 49 | 9 |
| WT1 | 27 | 7 | 22 | 84 |
Table 2.
Coordinate and Singular Immunophenotypic Patterns
| Coordinate and Singular Immunophenotypes |
Endometrial Serous Carcinomas (n=22) |
Ovarian Serous Carcinoma (n=140) |
P value* |
|---|---|---|---|
| Number positive (percentage) |
Number positive (percentage) |
||
| ER+/PR+/WT1+ | 0 (0) | 47 (33.6) | 0.0006 |
| ER+/PR+/WT1− | 4 (18) | 3 (2) | 0.0070 |
| ER+/PR−/WT1− | 0 (0) | 0 (0) | 1.0 |
| ER+/PR−/WT1+ | 0 (0) | 2 (1.4) | 1.0 |
| ER−/PR−/WT1− | 9 (41) | 9 (6.4) | 0.0001 |
| ER−/PR−/WT1+ | 7 (32) | 62 (44) | 0.3553 |
| ER−/PR+/WT1+ | 1 (4.5) | 15 (11) | 0.6994 |
| ER−/PR+/WT1− | 1 (4.5) | 2 (1.4) | 0.3565 |
| ER+/PR+ | 4 (18) | 50 (36) | 0.1444 |
| ER+/PR− | 0 (0) | 2 (1.4) | 1.0 |
| ER−/PR− | 16 (73) | 71(51) | 0.0668 |
| ER−/PR+ | 2 (9) | 17 (12) | 1.000 |
| ER+ | 4 (18) | 52 (37) | 0.0954 |
| PR+ | 6 (27) | 69 (49) | 0.0668 |
| WT1+ | 8 (36) | 113 (81) | 0.0001 |
Fisher Exact Test
As is outlined in table 2, the ER+/PR+/WT1+ coordinate profile was identified in 33.6% of OSC but in none of ESC (p=0.0006), resulting in a calculated sensitivity of this profile for OSC of 33.6%. Specificity, PPV and NPV were 100%, 100% and 19%. By contrast, the ER−/PR−/WT1− coordinate profile was identified in 41% of ESC but only 6.4% of OSC (p=0.0001). The calculated sensitivity, specificity, PPV and NPV of the ER−/PR−/WT1− coordinate profile for ESC were 50%, 94%, 50% and 94% respectively. In either analysis, the removal of ER or PR from the panel (i.e. the use of a ER−/WT1−, PR−/WT1−, ER+/WT1+, or PR+/WT1+ coordinate profiles in the aforementioned calculations), only slightly decreased sensitivity without significantly affecting specificity.
DISCUSSION
Serous carcinomas of the endometrium and ovary have traditionally been conceptualized as histotypic analogues due to their myriad of shared phenotypic and genotypic attributes (14). However, these apparent similarities may belie important differences. Gene expression profiling studies have clearly highlighted some of these differences (15). Furthermore, at the morphologic level, although ESC and OSC share core morphologic features, the morphologic spectrum of OSC is recognized to be substantially wider (16,17). Accordingly, differences between OSC and ESC in their rates of expression of selected antigens could potentially be exploited for diagnostic purposes.
In this study, we demonstrated that each of the 3 markers that we evaluated (ER, PR and WT1) is more frequently expressed in OSC than ESC, and we affirm the differential localization of these antigens in these morphologically similar tumors. The sensitivity of each of these markers, when used in isolation for identifying OSC, was 37%, 49% and 81% respectively. Specificity values were 78%, 73% and 67% respectively. Therefore, for diagnostic purposes, none of these markers are optimally discriminatory, and a reliance on single markers has a substantial probability of generating erroneous results regarding the site of origin. In our investigation of coordinate profiles for this purpose, we found that the ER+/PR+/WT1+ coordinate profile is highly specific for OSC. This profile was only identified in OSC in this study and as such had a high positive predictive value for OSC. However, it lacked sensitivity since it was only identified in 33.6% of OSC. The ER−/PR−/WT1− coordinate profiles had a similarly high specificity for ESC of 94%, however, this profile was also seen in 6.4% of OSC, and its positive predictive value for ESC was only 50%. The fact that none of the ESC cases had an ER+/PR+/WT1+ profile is probably an artifact of the study, and an analysis of a larger dataset of ESC may reveal At least a few cases showing at least focal co-expression of all of these markers. At minimum, our findings suggest that the ER+/PR+/WT1+ profile is decidedly uncommon in ESC. The ER−/PR−/WT1− coordinate profile is consistent with ESC, since it was identified in 41% of cases but in only 6.4% of OSC. The drawback to this profile in identifying ESC, similar to the ER+/PR+/WT1+ profile in identifying OSC, is its low sensitivity.
In summary, in the differential diagnosis between OSC and ESC, positivity for all 3 markers favors an extrauterine origin whereas negativity for all 3 markers is supportive of an endometrial origin. The use of single markers for this purpose is not recommended, as each lacks optimal discriminatory power. Coordinate profiles, in general, substantially increase the specificity of this determination but are hampered by their low sensitivity.
ACKNOWLEDGEMENT
This study was presented in part at the United States & Canadian Academy of Pathology’s 102nd Annual Meeting, Baltimore, MD, March 2-8, 2013.
REFERENCES
- 1.Resnik E, Taxy JB. Neoadjuvant chemotherapy in uterine papillary serous carcinoma. Gynecol Oncol. 1996;62:123–127. doi: 10.1006/gyno.1996.0201. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein NS, Uzieblo A. WT-1 immunoreactivity in uterine papillary serous carcinomas is different from ovarian serous carcinomas. Am J Clin Pathol. 2002;117:541–5. doi: 10.1309/K84K-005F-TCB8-FV4B. [DOI] [PubMed] [Google Scholar]
- 3.Heatley MK. WT-1 in ovarian and endometrioid serous carcinoma: a meta-analysis. Histopathology. 2005;46:468. doi: 10.1111/j.1365-2559.2004.02005.x. [DOI] [PubMed] [Google Scholar]
- 4.McCluggage WG. WT1 is of value in ascertaining the site of origin of serous carcinomas within the female genital tract. Int J Gynecol Pathol. 2004;23:97–9. doi: 10.1097/00004347-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Dupont J, Wang X, Marshall DS, Leitao M, Hedvat CV, Hummer A, Thaler H, O’Reilly RJ, Soslow RA. Wilms Tumor Gene (WT1) and p53 expression in endometrial carcinomas: a study of 130 cases using a tissue microarray. Gynecol Oncol. 2004;94:449–55. doi: 10.1016/j.ygyno.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Hirschowitz L, Ganesan R, McCluggage WG. WT1, p53 and hormone receptor expression in uterine serous carcinoma. Histopathology. 2009;55:478–82. doi: 10.1111/j.1365-2559.2009.03390.x. [DOI] [PubMed] [Google Scholar]
- 7.Acs G, Pasha T, Zhang PJ. WT1 is differentially expressed in serous, endometrioid, clear cell, and mucinous carcinomas of the peritoneum, fallopian tube, ovary, and endometrium. Int J Gynecol Pathol. 2004;23:110–118. doi: 10.1097/00004347-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Nofech-Mozes S, Khalifa MA, Ismiil N, et al. Immunophenotyping of serous carcinoma of the female genital tract. Mod Pathol. 2008;21:1147–1155. doi: 10.1038/modpathol.2008.108. [DOI] [PubMed] [Google Scholar]
- 9.Köbel M, Kalloger SE, Carrick J, Huntsman D, Asad H, Oliva E, Ewanowich CA, Soslow RA, Gilks CB. A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. Am J Surg Pathol. 2009;33:14–21. doi: 10.1097/PAS.0b013e3181788546. [DOI] [PubMed] [Google Scholar]
- 10.Ordóñez NG. Value of estrogen and progesterone receptor immunostaining in distinguishing between peritoneal mesotheliomas and serous carcinomas. Hum Pathol. 2005;36:1163–7. doi: 10.1016/j.humpath.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Kounelis S, Kapranos N, Kouri E, Coppola D, Papadaki H, Jones MW. Immunohistochemical profile of endometrial adenocarcinoma: a study of 61 cases and review of the literature. Mod Pathol. 2000;13:379–88. doi: 10.1038/modpathol.3880062. [DOI] [PubMed] [Google Scholar]
- 12.Carcangiu ML, Chambers JT, Voynick IM, Pirro M, Schwartz PE. Immunohistochemical evaluation of estrogen and progesterone receptor content in 183 patients with endometrial carcinoma. Part I: Clinical and histologic correlations. Am J Clin Pathol. 1990;94:247–54. doi: 10.1093/ajcp/94.3.247. [DOI] [PubMed] [Google Scholar]
- 13.Rimm DL, Camp RL, Charette LA, Costa J, Olsen DA, Reiss M. Tissue microarray: a new technology for amplification of tissue resources. Cancer J. 2001 Jan-Feb;7(1):24–31. [PubMed] [Google Scholar]
- 14.Caduff RF, Svoboda-Newman SM, Bartos RE, Ferguson AW, Frank TS. Comparative analysis of histologic homologues of endometrial and ovarian carcinoma. Am J Surg Pathol. 1998;22:319–26. doi: 10.1097/00000478-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Zorn KK, Bonome T, Gangi L, Chandramouli GV, Awtrey CS, Gardner GJ, Barrett JC, Boyd J, Birrer MJ. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res. 2005;11:6422–30. doi: 10.1158/1078-0432.CCR-05-0508. [DOI] [PubMed] [Google Scholar]
- 16.Soslow RA. Histologic subtypes of ovarian carcinoma: an overview. Int J Gynecol Pathol. 2008;27:161–74. doi: 10.1097/PGP.0b013e31815ea812. [DOI] [PubMed] [Google Scholar]
- 17.Clement PB, Young RH. Non-endometrioid carcinomas of the uterine corpus: a review of their pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2004;11:117–42. doi: 10.1097/00125480-200405000-00001. [DOI] [PubMed] [Google Scholar]