Preface
Intermediate filaments (IFs) are cytoskeletal and nucleoskeletal structures that provide mechanical and stress-coping resilience to cells, contribute to subcellular and tissue-specific biological functions, and facilitate intracellular communication. IF proteins, including nuclear lamins and cytoplasmic keratins, vimentin, desmin, neurofilaments, and glial fibrillary acidic protein, undergo various functionally important post-translational modifications (PTMs). Proteomic advances highlight the enormous complexity of IF PTMs, which include phosphorylation, glycosylation, sumoylation, acetylation, and prenylation, as well as their ability to regulate IF proteins, and are likely to reveal novel modifications. We would like to keep the original statement, since the revised version seems to imply that proteomic advances provide insight into the ability of PTMs to regulate IF proteins, which is not the case. Future studies will need to characterize their on–off mechanisms, cross-talk, and utility as biomarkers and targets for diseases involving the IF cytoskeleton.
Introduction
Since their initial description nearly half a century ago1, research into the characteristics and functions of IFs has solved many important questions pertaining to their assembly mechanisms, structural features, mechanical and biochemical characteristics, organization within the cell, biological functions, and involvement in a large number of human diseases2–7. As our understanding of IF biology has grown, so has our appreciation for the central role of post-translational modifications (PTMs) in regulating the functional properties of IFs.
Studies on IF protein phosphorylation have provided a detailed view of the highly dynamic nature of IF structures and their cell-protective functions, owing to the fact that this PTM regulates IF assembly and disassembly8–11. Recent studies on IF protein sumoylation in different species have demonstrated that this type of modification is an evolutionarily-conserved mechanism for ensuring the structural and functional integrity of IFs via its ability to regulate IF protein solubility., which is regulated by sumoylation, is an evolutionarily-conserved mechanism for ensuring the structural and functional integrity of IFs12–14. An appreciation for the role of cellular metabolism in the regulation and function of IFs has been highlighted by studies on the glycosylation and acetylation of IF proteins 15,16. Furthermore, PTM studies have revealed important interactions between IFs and other cellular components and structures, such as the interaction of 14-3-3 proteins with multiple IFs17–19. Key fundamental and emerging concepts also indicate that modulation of PTMs is a potential mechanism to target defective IF proteins that are a direct cause of, or contribute to, the pathogenesis of neuropathies, myopathies, skin disorders, liver diseases, metabolic dysfunctions, and premature aging syndromes, among other diseases6.
A full appreciation of IF function and dysfunction will require us to understand the well-established (phosphorylation, glycosylation and prenylation), newly-characterized (sumoylation and acetylation) and putative (methylation and malonylation) IF PTMs. It will also be important to delineate the mechanisms that add and remove these PTMs from IFs and facilitate cross-talk between them. Given that ~400 different PTMs are catalogued in the Uniprot database (http://www.uniprot.org/docs/ptmlist), of which 107 have been reported in eukaryotes, the functional significance of IF PTMs will remain an important question for years to come.
In this Review we will first introduce IF proteins before describing the different PTMs that are important for their function. We focus on covalent PTMs, such as phosphorylation, sumoylation, acetylation, glycosylation, and farnesylation, rather than PTMs that involve protein processing, such as cleavage by caspases20. The complex cross-talk that occurs between different PTMs on IFs will also be considered. Finally, the role of aberrantly-modified IF proteins in disease, which underscores the importance of PTMs in regulating IF protein function, will be discussed.
IFs: the basics of form and function
Owing to their diversity in protein composition and cell-specific presence and functions, IFs are the least characterized of the three major cytoskeletal systems, the other two being microfilaments (MFs) and microtubules (MTs). Whereas actins and tubulins, which form MFs and MTs, respectively, have few major subtypes (α,β and γ), there are ~70 different human cell type-specific IF proteins21. In further contrast to MFs and MTs, which exhibit structural polarity to enable their cell motility and transport functions, IFs are apolar flexible structures that provide mechanoprotection and regulate a multitude of cellular growth- and stress-related signaling events4,5,22,23. These wide-ranging cellular roles are facilitated by the existence of six major IF protein subtypes (Table 1) and two distinct IF systems (one cytoplasmic and one nuclear), each of which are characterized by unique structural and functional features22.
Table 1.
Intermediate filaments: expression pattern and involvement in human diseases
| Cell type (IF type) | IF | Disease examples |
|---|---|---|
| All nucleated cells (V) |
Lamins Lamin A/C Lamin B |
cardiomyopathy; muscular dystrophy; lipodystrophy; leukodystrophy; progeroid syndrome (for example, Hitchinson-Gilford Progeria Syndrome) |
|
Epithelial (I and II) (Type I: K9-K29, K31-K40) (Type II: K1-K8, K71-K86) |
Keratins hair epidermal simple epithelial |
skin fragility (for example, epidermolysis bullosa simplex) and other skin disorders; hair and nail disorders; corneal dystrophy; predisposition to liver diseases |
| Mesenchymal (III) | Vimentin | cataract |
| Glia (III) | GFAP | Alexander disease |
| Neurons (III and IV) | Neurofilaments (H,M and L) Peripherin α-internexin | Predisposition to amyotrophic lateral sclerosis; Charcot-Marie Tooth disease; predisposition to Parkinson disease |
| Myocytes (III and IV) |
Desmin Synemin Syncoilin |
myopathy; muscular dystrophy |
| Developing and regenerating cells (IV) | Nestin | none reported |
| Lens (VI or orphan) |
Filensin Phakinin |
cataract |
Dynamics of IF assembly and disassembly
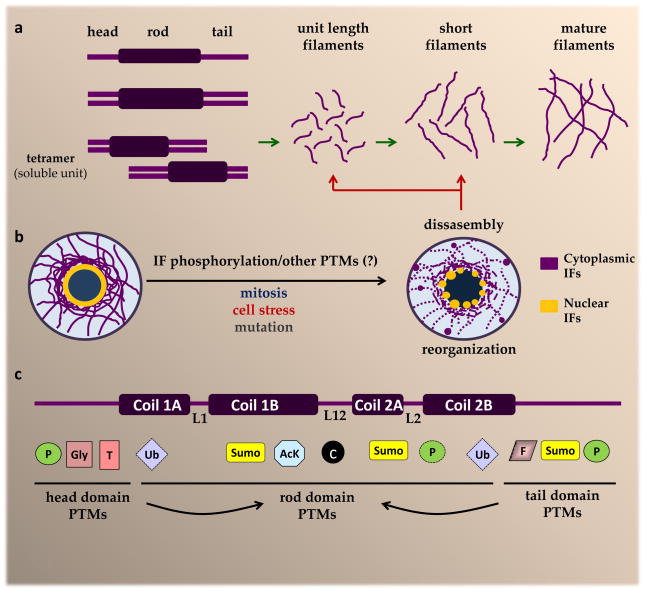
All IF proteins share a common three-domain structure that includes an N-terminal ‘head’ domain, a C-terminal ‘tail’ domain, and a central α-helical ‘rod’ domain2,4. Three linker domains (L1, L12, and L2) are interspersed between distinct rod domain sub-helices (coil-1A, coil-1B, coil-2A, and coil-2B). There is substantial variability in the amino acid sequences of the globular head and tail domains among the different IF proteins, whereas the rod domains are highly conserved. Cytoplasmic IF proteins exist as obligate homo- or heterodimers, as a result of two parallel rod domains forming a coiled-coil structure24,25 (Fig. 1a). Keratins form obligate type I–type II heterodimers, which is in contrast to many other IF proteins, such as desmin and vimentin, which homodimerize. The association between two vimentin or desmin dimers, in a half-staggered antiparallel fashion, yields soluble tetramers, which are the basic cytoplasmic IF subunits in vivo4. Further assembly involves lateral association of 8 tetramers to form a unit length filament, followed by longitudinal annealing of unit length filaments into short filaments, and then radial compaction of the annealed segments to produce the final 10nm diameter mature IFs4 (Fig. 1a). Nuclear lamins have a unique assembly pathway, which begins with rod domain-mediated homodimerization and is followed by head-to-tail dimer associations that result in polar polymers, and a final lateral joining of the anti-parallel polymers to form mature apolar lamin filaments22,26. As the only nuclear IFs, lamins form the nuclear lamina27, a complex fibrillar structure located beneath the inner nuclear membrane that also contains lamin-associated proteins. Cytoplasmic IFs, on the other hand, are flexible structures that extend from junctional complexes at the plasma membrane to anchor points at the outer nuclear membrane22 (Fig. 1b).
Figure 1. Cytoplasmic IF assembly and re-organization in response to phosphorylation and other PTMs.
a. The IF protein structure includes three distinct domains (head, rod, and tail)24. Cytoplasmic IFs undergo homo- or heterodimerization. The type III IFs vimentin and desmin undergo homodimerization, which is followed by anti-parallel association between homodimers to form soluble tetramers. The further assembly of vimentin and desmin filaments involves lateral association between 8 tetramers to form a unit length filament, followed by the longitudinal annealing of these into short filaments, and radial compaction of the annealed segments to yield mature IFs4. b. IF structures are highly dynamic and re-organize in certain physiologic situations, such as during mitosis, and under conditions of cell stress and in response to mutation. Site-specific phosphorylation of head and tail domain residues is a major facilitator of IF re-organization13–16, although other post-translational modifications (PTMs) are also likely to play a role. c. In addition to phosphorylation, IF proteins undergo several other modifications that target residues in the head, tail and rod domain, including the linker regions (L1, L12, L2). Rod domain PTMs are likely facilitated by head and tail domain PTMs, such as phosphorylation, as indicated by the arrows. The development of PTM-specific IF-transgenic mouse models, in combination with significant advances in proteomic technologies and antibodies targeted to specific modifications, have accelerated the pace of discovery for novel IF modifications, many of which are altered in the context of disease-causing IF mutants. AcK, lysine acetylation; c, caspase cleavage; F, farnesylation; Gly, O-linked glycosylation; P=phosphorylation; SUMO, sumoylation; T, transamidation; Ub, ubiquitination.
IF structures are highly dynamic, which has been most clearly illustrated in the context of keratin filament turnover cycle, which involves several distinct steps: nucleation of soluble oligomers at the cell periphery, elongation of the smaller particles into short filaments, integration of the short filaments into the peripheral keratin network and, finally, either maturation of the bundled filaments to form a perinuclear cage-like filament structure28, or disassembly into soluble oligomers used for another cycle29. Because the intracellular movement of smaller IF subunits depends on MFs, MTs, and molecular motors, drugs that target these systems induce IF re-organization30,31. In contrast to MFs and MTs, there are no known drugs that can directly and specifically bind to IFs.
Functions of IFs
IF functions are mechanical or non-mechanical in nature2,5,23,27. Mechanical functions, as the name implies, support the structural integrity of cells, and the crucial importance of these IF roles are best illustrated by the skin blistering diseases caused by keratin mutations32 (Table 1). In epidermolysis bullosa simplex (EBS), the first disease to be linked to an IF gene mutation, a disrupted K5–K14 filament network causes fragility of basal keratinocytes and epithelial rupture upon physical trauma32,33. Non-mechanical IF functions include regulation of the cellular architecture22 (for example, organelle positioning this really only applies to vimentin IFs at this point so it is best to take it out), cell growth5 (for example, promoting wound repair and tissue regeneration), cell migration34 (for example, tumor metastasis, via promoting (vimentin) or attenuating (keratins) the invasive properties of tumor cells and stress-related signaling cascades7. Mutation of LMNA, the gene encoding lamin A and lamin C, results in diseases that are known as laminopathies35. This attests to the importance of non-mechanical IF functions because laminopathies affect numerous physiological processes, including metabolism, skeletal and cardiac muscle function, and aging. As the repertoire of IF functions has grown in recent years, so too has our appreciation that many, if not most, of these functions are impacted by various PTMs (Figure 1c and Table 2).
Table 2.
IF protein PTMs, modifying enzymes, and methods of analysis
| PTM | IFs | PTM enzyme (on) | PTM enzyme (off) | Tools for PTM analysis |
|---|---|---|---|---|
| Extensively studied | ||||
| Phosphorylation | most IFs* | SAPKs, AKT1, PKC, Cdk1, Cdk5, other | PP1, PP2A | LC-MS; antibodies; phosphomutant transgenic mice; enzyme inhibitors |
| Farnesylation | lamins | farnesyl transferase | Zmpste24 (via proteolysis) | Zmpste−/− mice, FTIs |
| Ubiquitination | most IFs** | CHIP/STUB, Ubc3, UbcH5, Siah1, Trim32 | not known | LC-MS, in vitro ubiquitination; stress models; proteasome inhibitors |
| Accumulating data | ||||
| Sumoylation | lamins keratins vimentin |
Ubc9 | not known | LC-MS; in vitro sumoylation, SUMO antibodies |
| Glycosylation | keratins neurofilaments vimentin |
OGT | O-GlcNAcase | LC-MS, transgenic mice (for example, K18-Gly-), antibodies (for example, NL6) |
| Acetylation | keratins | not known | SIRT2 | LC-MS, pan-acetyllysine antibodies, acid-urea gels, deacetylase inhibitors |
| Limited data | ||||
| Transamidation | keratins | transglutaminases | none | in vitro and in vivo cross-linking analysis |
| ADP ribosylation | desmin vimentin |
ADP-ribosyl transferase SpyA | not known | LC-MS, in vitro ribosylation |
phosphorylation of filensin, phakinin, and syncoilin is based on proteomic evidence
putative ubiquitination sites for most IFs have been identified by large scale proteomic studies
IF phosphorylation: multifunctional PTM
IFs must reorganize and, in some cases, disassemble during physiological and pathophysiological events that change the physical and functional properties of a cell36,37 (Fig. 1b). A general role for IF phosphorylation, which is modulated by numerous kinases and phosphatases (see Table 2)10,11, is to facilitate IF re-organization by targeting one or more Ser/Thr residues, typically in the head and tail domains. Phosphorylation may also regulate ‘dynamic subunit exchange’, whereby new IF subunits can become incorporated anywhere along the length of IF structures8,10,38,39. In general, Ser/Thr phosphorylation promotes IF protein solubility, which is a critical factor in maintaining filament structural dynamics. Appreciation of IF protein Tyrosine phosphorylation is growing as phosphoproteomic tools expand40–42. Known IF targets for Tyrosine phosphorylation include K843, K1942,44 and vimentin45. K8 phosphorylation at a highly conserved residue in the rod domain (Tyr-267) promotes keratin insolubility and proper filament formation46, whereas the effects of Tyr phosphorylation on other IF proteins is poorly understood. The subsections below incorporate established and new evidence implicating IF phosphorylation in various physiologic and disease mechanisms (Fig. 2).
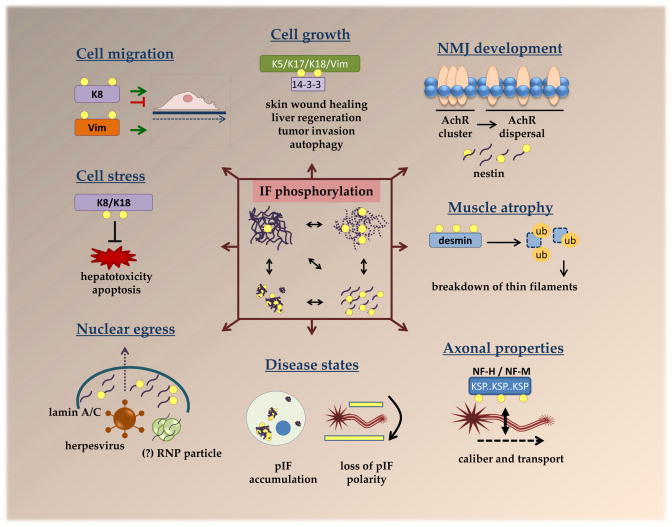
Figure 2. Multifunctional roles for IF protein phosphorylation.
Numerous cellular properties are affected by IF phosphorylation, which, as depicted in the central box, leads to filament reorganization, disassembly and aggregation. Phosphorylation of K5, K17, K18 and vimentin facilitates their association with 14-3-3 to promote cellular growth via several mechanisms, including regulation of 14-3-3 localization and its association with other proteins, such as the autophagy regulator Beclin 117–19,77. Keratin phosphorylation has been shown to both inhibit and promote cell migration, depending on the type of cell and stimulus, whereas vimentin phosphorylation is strongly associated with promoting cell migration34. phosphorylation by host and viral kinases facilitates nuclear egress of herpesviruses64. A similar nuclear egress mechanism may be utilized by RNPs68, although this has not been fully confirmed with respect to the requirement for lamin phosphorylation Phosphorylation-mediated dissasembly of myoblast nestin IFs appears to be important for neuromuscular junction development by facilitating acetylcholine receptor dispersal69. Fasting-induced desmin phosphorylation leads to its subsequent ubiquitination and proteasomal degradation, which, in turn, results in the breakdown of thin filaments to promote muscle atrophy73. While phosphorylation of most IFs is low basally, but triggered by stress or physiological stimuli, neurofilaments (NF-M and NF-H) are heavily phosphorylated under basal conditions within tail-domain K-S-P repeats10. This is thought to regulate axonal stability, caliber, and transport51,52. Hyperphosphorylated IF aggregates are present in various diseases and, in neurodegenerative diseases, hyperphosphorylated NF aggregates appear in neuronal cell bodies, leading to loss in the polarity of phosphorylated NFs50.
NF phosphorylation and regulation of axon properties
Neurofilaments, which include light polypeptides (NF-L), as well as medium (NF-M) and heavy polypeptides (NF-H), were among the first identified IF phosphorylation targets11,47. While the basal stoichiometry of phosphorylation for most IF proteins is low, NF-M and NF-H are highly phosphorylated basally by Proline-directed kinases on multiple Serines located within tail-domain Lys-Ser-Pro (KSP) motifs,10,48 with likely consequences to axonal caliber, stability, and transport48,49. All NF head domains also are phosphorylated, but in a transient manner10. Furthermore, NF head domain and tail-domain regulation is highly compartmentalized, such that tail-domain phosphorylation via several kinases (Table 2) predominates in axons, whereas head-domain phosphorylation occurs within neuronal cell bodies50. The compartmentalized NF phosphorylation is a tightly regulated process, and deregulation of NF phosphorylation topography has been implicated in several neurodegenerative diseases50.
NF tail-domain phosphorylation is thought to increase axonal caliber and stability by promoting cross-bridging between the lateral projections (side arms) of interacting neurofilament subunits10. There is controversy with respect to the effect of neurofilament phosphorylation on axonal transport, as different methodologies have led to opposing conclusions51. For example, the slowest rate of transport was associated with axons that were highly reactive to antibody RT9752, which recognizes phosphorylated mouse and human NF-H at KSPXK and KSPXXXK motifs in the tail-domain53. However, ablation of the NF-H C-terminal tail in mice did not alter axonal transport speed54, although this might be explained by a compensatory increase in NF-M phosphorylation51. Nevertheless, the current model suggests that NF phosphorylation slows the speed of axonal transport10,51,55, possibly by promoting NF bundling, which, in turn, competes with kinesin-mediated NF-MT association55–57. Determining which kinases phosphorylate which sites in vivo may provide further insight into the significance of NF tail phosphorylation.
IF phosphorylation in stress responses
Re-organization of the IF network is a universal response to stress and this has been most extensively studied in the context of keratins11,29. Several transgenic mouse models demonstrate that keratin phosphorylation may serve as a phosphate “sponge” to protect hepatocytes during liver injury. Protection occurs, in part, by shunting phosphorylation away from pro-apoptotic proteins that are themselves activated by phosphorylation11. Mutation of the major phosphorylation site in the head domain of K8 (Ser74) or K18 (Ser54) increases apoptosis and liver injury in mice58,59. Importantly, the effects observed in mice in which K8 Ser74 had been substituted with Ala (S74A) are similar to those seen in mice over-expressing a K8 variant (G62C) that predisposes to liver disease58. This suggests that the absence of K8 phosphorylation at Ser74 may predispose humans harboring the G62C variant to liver disease progression. The phosphorylation motif in K8 that includes Ser74 (LLSPL) is conserved in several type II IFs, including K4, K5 and K6, where the phosphorylated residue is a Threonine (LLTPL). Similar to K8, K4–K6 undergo phosphorylation at the paralogous sites in response to stress (for example, UV radiation and apoptosis) or disease (for example, psoriasis and squamous cell carcinoma)60. Phosphorylation of K8 Ser74 is mediated by p38 and Jun kinases, which are rapidly activated and recruited to keratin IFs upon stress38,61,62. Following phosphorylation by p38, keratin IFs are disassembled into granules and this reversible event appears necessary for the short-term regulation of epithelial cell plasticity38.
IF phosphorylation in viral egress
Mitotic lamin A/C disassembly, which contributes to the breakdown of the nuclear membrane, is facilitated by phosphorylation of its head domain by Cdk163. Interestingly, this pathway is copied by herpesviruses to facilitate their egress out of the nucleus64. Herpesvirus progeny are assembled inside nuclei of infected cells and require an envelopment–deenvelopment process to traverse the inner and outer nuclear membranes before the herpesvirus nucleocapsids can be re-enveloped in the cytoplasm. In the absence of lamin phosphorylation, viral access to the nuclear membrane is precluded by an intact nuclear lamina65. However, just as Cdk1 phosphorylates lamin A at Ser22 to promote its disassembly during mitosis, the viral kinase UL97 phosphorylates this site to promote nuclear lamina disassembly during human cytomegalovirus (HCMV) egress64. Phase III clinical trials testing the effectiveness of the UL97-selective inhibitor maribavir in preventing HCMV infection in liver and hematopoetic stem cell transplant patients were unsuccessful66, suggesting that other mediators of lamin disassembly could be involved. For example, the combined kinase activities of viral UL97 and host protein kinase C (PKC) are needed to generate a binding motif (TPLpSPT) for the peptidyl/prolyl cis/trans isomerase Pin1 on lamin A/C67. The subsequent Pin1-induced conformational changes in lamin A/C might mediate HCMV egress. Therefore, changes in IF site-specific phosphorylation might regulate the tissue-specific cytopathic effects of various pathogens, in addition to being a general marker of cell stress7,11.
IF phosphorylation in neuromuscular function
Like viral egress, a novel nucleo-cytoplasmic transport mechanism for endogenous ribonucleoprotein (RNP) particles appears dependent on lamin A/C phosphorylation68. During Drosophila melanogaster synaptic development, RNP particles containing synaptic protein transcripts are targeted to the D. melanogaster A-type lamin, LamC. Their nuclear exit follows a mechanism akin to herpesviruses, requiring the activity of atypical PKC and, likely, lamin phosphorylation. RNP export was compromised in D. melanogaster lacking LamC globally or in muscle, resulting in neuromuscular junction (NMJ) defects. Future studies on this pathway in mammalian systems should reveal whether it is regulated by lamin phosphorylation and compromised in laminopathies characterized by neuromuscular deficits.
The potential significance of IF phosphorylation to NMJ function also extends to nestin, a cytoplasmic IF expressed during early CNS and muscle development69. During myoblast differentiation, nestin is phosphorylated on Thr316 by Cdk5, which ultimately leads to nestin dissasembly70. However, nestin also forms a scaffold for both Cdk5 and the Cdk5 co-activator p35 at NMJ synapses, thus providing a mechanism for continuous regulation of Cdk5/p35 activity69. Prolonged Cdk5 activation, which is enabled after its dissociation from dissasembled phospho-nestin, is required for acetylcholine (Ach)-induced dispersal of Ach receptor (AchR) clusters71. Similar to Cdk5-null mice, nestin-null mice exhibit impaired motor coordination and an increased number of AchR clusters in NMJ areas72. These studies demonstrate the importance of IF phosphorylation during neuromuscular development.
In mature myofibers, nestin is downregulated and its expression is replaced by another IF protein, desmin, which also undergoes phosphorylation-mediated depolymerization with in vivo significance. During fasting-induced muscle atrophy, desmin is phosphorylated in the head domain at Ser28, Ser32 and Ser68. This results in its association with the RING finger ubiquitin ligase Trim32 and, ultimately, its disassembly and degradation73. This appears to be a critical event leading to the breakdown of thin filaments and Z-band components, highlighting an important role for desmin phosphorylation in myofibril destruction during muscle atrophy.
IF phosphorylation in the regulation of cell growth
The phosphorylation of IFs has long been known to modulate their interactions with IF-associated proteins74. The adaptor protein 14-3-3 binds phosphorylated Ser/Thr residues on various protein substrates that, in many cases, contain the motif RSXpS/TXP75. The association of several IF proteins, including K5, K17, K18 and vimentin with 14-3-3 influences cell growth and tumorigenesis (Fig. 2) via a number of mechanisms17–19.
K18 was identified as the first 14-3-3 IF-binding partner, their association being shown to occur during cell cycle progression76. K18 phosphorylation at Ser34 in the head domain promotes its solubility and binding to 14-3-3. In mice that over-express K18-S34A, K8 and K18 filaments in pancreatic acinar cells retract from the basal nuclear region and become apically concentrated, while nuclear retention of 14-3-3 leads to partial mitotic arrest in the livers18. Additionally, interactions between 14-3-3 and Thr9 and Ser44 of K17 stimulate mammalian target of rapamycin (mTOR) activation and cell growth during wound healing in skin epithelia17. Elimination of the 14-3-3 binding sites in K17 and K18 increases 14-3-3 nuclear retention, which is associated with decreased mammalian target of rapamycin activation, and is a likely mechanism for the observed effects on cell growth17,18. The role of keratin binding to 14-3-3 proteins in modulating mitosis progression likely involves other pathways, including keratin phosphorylation-mediated regulation of Raf-1 binding to 14-3-3 (Ku et al. J Cell Biol 2004), cdc25 binding to 14-3-3 (Margolis et al., Cell 2006) and the nuclear distribution of the 14-3-3zeta protein. Additionally, 14-3-3 promotes mammary tumorigenesis by stabilizing a soluble K5–K17–actin complex; this function of 14-3-3 is dependent on PKCζ phosphorylation 77. Maintenance of this complex is thought to facilitate polarized cytoskeletal filament assembly during cell migration.
The binding of 14-3-3 to vimentin inhibits autophagy and promotes Akt-mediated tumorigenesis19. Vimentin is an established marker for epithelial-to-mesenchymal transition (EMT)78 and it is phosphorylated by Akt1 at Ser3979; this phosphorylation event promotes in vivo tumor metastasis79. Notably, 14-3-3 serves as a linker between phosphorylated Beclin1 (a protein required for the initiation of autophagy) and phosphorylated vimentin, and this association promotes autophagy inhibition and tumorigenesis19.
IF phosphorylation in cell migration
The role of IFs, in particular keratins and vimentin, in cell migration is becoming increasingly appreciated34 and phosphorylation serves a critical function in this regard. In general, vimentin expression is associated with increased migration of immune80 and cancer cells81,82, while keratin expression is associated with decreased migration of epithelial83,84 and cancer cells85,86. Numerous vimentin phosphorylation sites have been identified in vivo8 and they appear to promote its pro-migratory function, in part by enhancing recycling87 and activation88 of integrins. K8 phosphorylation at Ser432 has been linked to increased migration of pancreatic and gastric cells89 but decreased migration of oral squamous carcinoma cells90, highlighting a context-specific function of this phospho-residue. Increased cellular elasticity caused by the bioactive lipid sphingosylphosphorylcholine,91 which was used in the study of pancreatic and gastric cells, likely orchestrates a unique migratory program that is dependent on pSer432-mediated perinuclear K8 and K18 re-reorganization.
In light of these broad functions of phosphorylation in IF biology, it is important to highlight that significant changes in IF protein phosphorylation are observed in various human diseases (see below).
Sumoylation: a conserved PTM for IFs
IFs are modified by small ubiquitin-like modifier (SUMO)92 proteins in a covalent and reversible manner. Conjugation of SUMO (SUMO-1, -2 and -3) to mammalian proteins involves a single E2 ubiquitin ligase (Ubc9) that commonly targets lysine residues within the classical consensus linear motif ΨKX(D/E) [where Ψ=hydrophobic residue, K=target lysine, X=any residue, D=aspartic acid and E=glutamic acid], although many proteins are modified on lysines outside of this motif92,93. SUMO-1 is mostly nuclear and conjugated as a monomer, while SUMO-2 and -3, which are nearly identical in sequence and, most probably, function, can be conjugated as polymers to cytoplasmic and nuclear proteins. As discussed below, sumoylation of cytoplasmic and nuclear IFs regulates filament formation and solubility (Fig. 3), two properties that are significantly altered by disease-causing mutations in IFs, in part due to sumoylation defects.
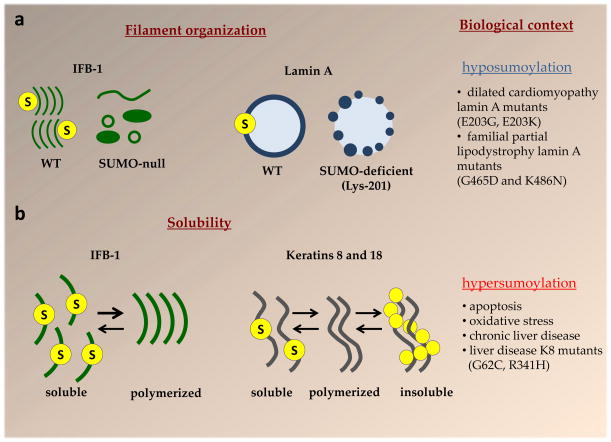
Figure 3. IF protein sumoylation regulates filament organization and solubility properties.
a. Sumoylation regulates the organization of IFs. Sumoylation is important for the proper filament organization of IFB-1, a C. elegans cytoplasmic IF that forms epidermal attachment structures12. In SUMO-null worms IFB-1 filaments appear as a heterogeneous population of different size aggregates, long filaments and ring-like structures, and the occurrence of large aggregates increases with age. 12 Similarly, sumoylation of human lamin A in the highly-conserved rod domain at Lys-201 is important for its proper nuclear localization14. The sumoylation deficient lamin A K201R forms abnormal puncta inside the nuclei or at the nuclear periphery14. Laminopathy-associated lamin A mutants, as exemplified by those listed in the figure, are hyposumoylated and exhibit abnormal organization in comparison to normal lamin A14,96. b. Sumoylation regulates the solubility of IFs. Sumoylation sequesters a fraction of IFB-1 into a soluble cytoplasmic pool and facilitates the exchange between the soluble and polymerized IFB-1 pool12. Sumoylation of wild-type polymerized human K8 and K18 (depicted by the yellow filaments in the middle) is not readily detectable under basal but is highly upregulated upon the re-organization of these IFs in response to apoptosis, stress, disease, or in the context of K8 variants that predispose to liver disease13. Monosumoylation promotes K8 solubility in vitro, similar to IFB-1, whereas pathologic hypersumoylation is associated with diminished K8 solubility and formation of high molecular mass complexes13.
Sumoylation of nuclear lamins
The initial evidence that IF proteins were sumoylated came from a yeast two-hybrid screen identifying Ubc9 as an interacting partner of progerin,94 a truncated version of lamin A that leads to Hutchinson-Gilford Progeria Syndrome (HGPS). It was found later that nuclear levels of Ubc9 in HGPS fibroblasts were reduced markedly, which resulted in impaired nucleo-cytoplasmic protein transport owing to mislocalization of Ran and disruption of the Ran GTPase gradient95. While sumoylation processes are adversely affected by HGPS pathology, it is unknown if sumoylation of lamin A/C and progerin results in different functional outcomes. The probability that some laminopathy-causing mutants have defective sumoylation was raised by evidence that lamin A sumoylation is important for its proper localization14. However, the relationship between sumoylation and the various laminopathies remains correlative at this point, since nearly every known lamin mutation results in disease.
Lamin A is monosumoylated by SUMO-2 at the highly-conserved rod-domain residue Lys201, which is part of a SUMO consensus motif (MKEE)14. Mutations within this motif (K201R, E203G, E203K) eliminate lamin A sumoylation and result in large lamin A puncta inside the nucleus or at the nuclear periphery (Fig. 3a). These findings suggest that defective lamin A sumoylation could possibly contribute to the pathogenesis of familial dilated cardiomyopathy (DCM) in patients carrying lamin A E203G and E203K mutations96. Similarly, several DCM-causing lamin A mutants (D192G, Q353K, R386K) formed SUMO-1-containing aggregates96. As these mutants also increased overall nuclear protein sumoylation, it is possible that the sequestered SUMO-1 may have been conjugated to other proteins, to which access by desumoylases may have been prevented by the aggregation.and/or prevented the access of desumoylases to these targets.
Lys420 and Lys486 in the tail-domain of lamin A are direct SUMO-1 targets97 and the lamin A mutants G465D and K486N, which cause familial partial lipodystrophy, are deficient in SUMO-1 conjugation97. While lamin A Lys420 is nested within a linear SUMO consensus motif; Lys486, located within the Ig-fold, is not. It was proposed that Lys486 sumoylation becomes enabled by a consensus “patch” of several acidic residues, which formed a three-dimensional Ubc9-recognition site97. Loss of this putative Ubc-9 recognition site may be the reason for the lack of sumoylation in the G465 mutant, it localizes within the Ig fold. Interestingly, sumoylation of the Ig-fold in lamin A may represent an evolutionarily-conserved process for modulating the properties of this domain, since sumoylation of the Caenorhabditis elegans IF protein IFB-1 occurs at Lys460 within the Ig-fold domain12.
Sumoylation of cytoplasmic IFs
C. elegans IFB-1 is functionally similar to epidermal keratins12,98 although it contains an Ig-fold similar to vertebrate nuclear lamins. During embryonic development, IFB-1 is required for elongation. During post-embryonic development IFB-1 provides a hemidesmosome-like mechanical linkage between muscle and the cuticle, an exoskeleton composed of a collagenous extracellular matrix. The normal organization of IFB-1 is a striped pattern of filaments along the basal and apical membrane of the epidermis. In contrast, SUMO-null worms exhibit ectopic circular and long filaments and cytoplasmic inclusions of various size and morphology that increase with age12 (Fig. 3a). These abnormalities were attributed to a decrease in IFB-1 solubility in the absence of sumoylation at Lys46012 (Fig. 3b). A reduction in the levels of soluble IFB-1 available for exchange with the polymerized IFB-1 pool caused embryonic elongation defects, thus providing evidence that IF solubility is of physioloigcal importance and prompting investigation into the effects of sumoylation on mammalian cytoplasmic IFs.
Human keratins (K5, K8, K14, K18 and K19) and vimentin are extensively and preferentially modified by SUMO-2/3 over SUMO-1 in vitro13. The modification of K8, K18, and K19 by SUMO-2/3 chains takes place on multiple conserved rod domain sites within consensus motifs, including at Lys207 in K18 and at Lys208 in K19-, which are paralogs of the SUMO-2 target, Lys201, on lamin A. In contrast to lamin A, significant keratin sumoylation was not detected under basal conditions. However, the induction of filament re-organization by oxidative and apoptotic stress, or hyperphosphorylation, dramatically upregulated keratin sumoylation13. Findings in mouse and human chronic liver injury supported the stress-induced mode of keratin sumoylation, while in vitro evidence suggested that keratin mono-sumoylation increases, while hyper-sumoylation decreases, keratin solubility13 (Fig. 3b). Together with the findings on IFB-1, these data suggest that the role of SUMO in regulating the solubility of cytoplasmic IFs is evolutionarily conserved.
From a human disease perspective, the most common known K8 variants that predispose individuals to acute and chronic liver disease progression (G62C and R341H)99,100 have decreased solubility and are extensively sumoylated under basal conditions13. Mutation-induced conformational changes may facilitate access of SUMO and its ligases to the keratin rod domains under basal conditions. The decreased solubility likely compromises the dynamics of mutant keratin filaments by limiting the availability of substrate for the nucleation step of the keratin cycle29.
In addition to the liver, K8 is also critical for the integrity of the intestinal epithelium, since K8-null mice develop colitis, hyperplasia, diarrhea, and show aberrant targeting of apical and basolateral proteins101. Interestingly, inducible Ubc9-null mice also display a profound loss of baso-apical enterocyte polarity and enterocyte detachment from the basal lamina102, a phenotype reminiscent of tissue fragility disorders due to keratin loss or dysfunction32. Loss of K8 sumoylation was proposed as one potential mechanism for the compromised enterocyte integrity in adult mice lacking Ubc9102. Therefore, regulated sumoylation appears to be essential for keratins in digestive epithelia, and this likely extends to other IFs.
IF lysine acetylation: metabolic sensor
Conventionally regarded as a modification for histones and microtubules, lysine acetylation is now known to regulate many other proteins. IF proteins are among ~1000–1700 acetylation targets, based on identification of their putative acetylation sites through large-scale studies103,104. Validation of these sites and delineation of their context-specific functions will take time as specific tools (for example, site-specific acetyl-lysine antibodies105) for studying this modification are developed and refined. Based on the evidence to date, lysine acetylation appears to modulate IF properties in response to metabolic changes16.
A conserved lysine in the rod-domain of K8, Lys207, was identified as a putative acetylation target by mass spectrometry103,104 and confirmed as a functionally-important K8 acetylation site16. Basal acetylation at Lys207 reduces K8 solubility and promotes formation of a dense perinuclear K8 filament network. Increased K8 acetylation is observed during hyperglycemia and upon inhibition of the NAD-dependent deacetylase SIRT2, with accompanying changes in K8 biochemical and filament properties16, including K8 oligomerization and formation of perinuclear aggregates in a subset of cells. Therefore, SIRT2 is likely to be a broad regulator of the cytoskeleton since it is also an α-tubulin deacetylase106.
A survey of published data103 and datasets curated by the bioinformatics resource ‘Phosphosite’107 reveals that sites mutated in IF diseases, including Lys97 of lamin A (K97E in DCM108) and Lys86 of glial fibrillary acidic protein (GFAP; K86E in Alexander disease; AxD109), might be acetylated. The change from a positively-charged Lys to a negatively-charged Glu in each case suggests that the charge at this site, which would be neutralized by addition of an acetyl group to the lysine, is likely to be an important determinant of filament properties. Furthermore, the end of coil 1A in K5, which is a mutation ‘hot-spot’ area for EBS32, contains two putative acetylation sites (Lys178 and Lys185). It remains to be determined whether these are bona fide acetylation sites and if defective acetylation is involved in the pathogenesis of these K5 variants.
O-linked glycosylation of IFs
O-linked glycosylation is the enzymatic addition of β-N-acetylglucosamine (GlcNAc) by a single enzyme, O-GlcNAc transferase (OGT), to protein Ser/Thr residues110. The importance of this modification in mammals is evidenced by the fact that knockout of OGT111 or O-GlcNAcase112 (the enzyme that deglycosylates O-GlcNAc-modified proteins) in mice is embryonic lethal. As O-linked glycosylation is labile under most types of mass spectrometry, our understanding of this modification has been slower110. O-linked glycosylation regulates signaling events related to nutrient sensing and stress responses, among other functions. Known IF glycosylation targets are K8, K13, K18, vimentin, and neurofilaments.113–116 Here we focus specifically on the glycosylation of K18 and neurofilaments, the best characterized IFs in that context.
Glycosylation of K18 was reported years113 before its biological role became known15, demonstrating the difficulties in studying this modification. Nevertheless, it is clear that K18 glycosylation at three residues in the head domain (Ser30, Ser31 and Ser49) helps protect epithelial cells from injury15. Mice that over-express glycosylation-deficient human K18 show elevated susceptibility to liver and pancreatic injury induced by streptozotocin, which inhibits O-GlcNAcase. The underlying mechanism involves decreased phosphorylation of Akt1 and PKCθ (at sites that are critical for their kinase activities) when K18 glycosylation is disabled15. Akt1 phosphorylation at Thr308, which is critical for its cell survival function, is dependent both on its direct association with the K18 heterodimeric partner, K8, as well as K18 glycosylation15. Therefore, it appears that a major role for keratin glycosylation in simple-type epithelia is to facilitate the phosphorylation and activation of cell survival kinases during stress and injury.
The functional role of neurofilament glycosylation is less clear, although it is known to be dynamically regulated by metabolic changes. NF-L glycosylation occurs on Thr21 and Ser27 in the head domain 115, and NF-M glycosylation occurs on Thr48 in the head domain and Thr431 in the tail domain 117. Additional NF-M glycosylation sites are present based on the fact that the tail domain of NF-M that cannot be glycosylated on Thr431 still reacts to an antibody (NL6) that detects glycosylation118. The amount of glycosylated NF-M, which is highest in axons of human neurons, is significantly decreased in brains from patients with Alzheimer disease (AD)119. Animal models further support a metabolically-linked and pathologic decrease in NF-M glycosylation, as NF-M glycosylation was decreased in fasted rats119 and in a rat model of amyotrophic lateral sclerosis (ALS)118. In contrast to NF-M, NF-H glycosylation is increased in response to glucose deprivation, owing to an increase in OGT activity, which is dependent on AMP-activated protein kinase120. It remains to be determined whether the reported differences in NF-H and NF-M glycosylation are nonenzymatic or technical in nature (glycosylation is altered in post-mortem tissue118) or are rooted in different biological mechanisms. The functional significance of NF and other IF glycosylation awaits further investigation and development of better tools to study this significant modification.
Farnesylation of nuclear lamins
Nuclear A and B-type lamins are modified by farnesylation, which is the modification (prenylation) of proteins with lipid intermediates from the cholesterol biosynthesis pathway121. Farnesylation of normal lamin A by farnesyltransferase (FTase) is transient and thought to facilitate its initial association with the inner nuclear membrane via hydrophobic interactions27. Full maturation of lamin A requires proteolytic removal of 15 tail domain residues, including the farnesylated cysteine, by the zinc metalloendoprotease Zmpste2427. Progerin does not undergo this last step because it lacks the Zmpste24 internal cleavage site and thus becomes permanently associated with the inner nuclear membrane. This is considered the major underlying mechanism in the pathology of HGPS, which has been targeted by FTase inhibitors (FTIs) in recent clinical trials (Box 1).
Box. 1. Targeting defective lamin A prenylation in the clinic.
Therapeutic strategies have been developed to target the defective prenylation of lamin A seen in Hutchinson-Gilford Progeria Syndrome (HGPS). The main distinguishing feature between lamin A and progerin, which is the HGPS truncated mutant (C1824T) form of lamin A, is the permanent farnesylation of the latter that prevents its release from the inner nuclear membrane, resulting in severe abnormalities in nuclear morphology and function. Farnesylation is the modification of proteins with lipid intermediates from the cholesterol biosynthesis pathway121. Farnesylation of normal lamin A by farnesyltransferase (FTase) is transient and thought to facilitate its initial association with the inner nuclear membrane via hydrophobic interactions27 (see the figure; left). Full maturation of lamin A requires proteolytic removal of 15 tail-domain residues, including the farnesylated cysteine, by the zinc metalloendoprotease Zmpste2427. Progerin does not undergo this last step because it lacks the Zmpste24 internal cleavage site and thus becomes permanently associated with the inner nuclear membrane (see the figure; right). Dashed lines indicate an indirect proces. For a detailed overview of lamin processing, please see Ref.
Farnesyltransferase inhibition in HGPS. The establishment of persistent farnesylation as being, at least partly, responsible for the HGPS phenotype formed the basis for use of farnesyltransferase inhibitors (FTIs) to potentially treat HGPS165. FTIs alleviated the characteristic nuclear morphology defects in progerin-expressing cells, and extended lifespan, while improving weight gain and cardiovascular function in HGPS mouse models166. Given that HGPS patients die (at an average age of 13) of a heart attack or stroke, and FTIs have a favorable safety profile in humans, 25 HGPS patients were enrolled in a multi-year prospective single-arm phase-II clinical trial of the FTI lonafarnib162. The results of this initial trial revealed varied success with respect to the primary outcome (weight gain) but significant improvements in secondary outcomes (vascular stiffness, bone structure and hearing). New insights from basic research should help inform potential future studies in patients using combination therapy. For example, lamin A mutants can undergo another type of prenylation, geranylgeranylation (see the figure; right), which may surmount the effect of an FTI167. Consistent with this idea, upstream targeting of farnesyl pyrophosphate, a shared precursor of farnesylation and geranylgeranylation reactions, with bisphopshonates and statins, extends lifespan in the Zmpste−/− mouse model of HGPS167. The rapid developments that have taken place in the HGPS field over the past 10 years stress the value of understanding how post-translational modifications affect IF proteins at a fundamental level.

B-type lamins (encoded by two different genes, LMNB1 and LMNB2) are processed similarly to lamin-A/C, except they do not undergo the last cleavage step and therefore they permanently retain their farnesyl lipid anchor. Knock-in mice expressing non-farnesylatable lamin B2 appear to develop normally, whereas lamin B1 that cannot be farnesylated results in severe impairment in neuronal development and perinatal death122. At the cellular level, non-farnesylated lamin B1 displays an abnormal “honeycomb” pattern of nuclear distribution in mouse embryonic fibroblasts and leads to nuclear blebbing and chromatin detachment from the nuclear lamina in neurons122. Of note, treatment of normal human fibroblasts with FTIs disrupts lamin B processing by decreasing the levels of mature B1 and causing accumulation of unprocessed lamin B2 in the nucleoplasm123. The numerous studies on lamin farnesylation have provided strong evidence for the importance of this IF modification in cellular function and organismal development.
Modification of IFs by other PTMs
IF proteins can be modified by PTMs in addition to those already discussed, although the mechanics of these modifications with respect to IFs, and their functions, are just beginning to emerge.
Ubiquitination
The obligatory nature of keratin heterodimers suggests that either keratin might be degraded by the ubiquitin-proteasome system in the absence of its partner, which usually provides a stabilizing function124–126. Various E2 ubiquitin ligases, including Ubc3, Ubc5, UbcH5, and Ubc6, are required for keratin degradation in response to mechanical stress and hypoxia, presumably as a stress-adaptation mechanism125. The ubiquitin ligase CHIP/STUB1, on the other hand, appears to target mutant keratins for degradation127. Ubiquitination sites on IF proteins are not known, aside from putative lysines identified via mass spectrometry128.
Many IF-associated diseases, including myopathies, neuropathies and liver diseases are characterized by IF-containing aggregates that contain ubiquitin99,129,130. In AxD mouse models, dissolution of GFAP aggregates into smaller oligomers and monomers, via the actions of the small heat shock protein αβ-crystallin, results in improved proteasomal clearance of the mutant GFAP with reduced brain defects and mortality131,132. Furthermore, a mouse progeroid phenotype involves the selective ubiquitination and proteasomal degradation of mature lamin A, facilitated by its interaction with the E3 ubiquitin ligase Siah1, which does not bind lamin C or progerin133. Therefore, ubiquitination of IF proteins is a normal physiologic process that is involved in IF turnover, while accumulation of ubiquitinated IF proteins occurs in the context of cellular dysfunction that is accompanied by proteasome inhibition.
Transamidation
The activity of transglutaminase-2 (TG2), an inducible acyltransferase that catalyzes the formation of amide bonds (transamidation) between the ε-amino group of lysine and the γ-carboxyl group of glutamine, regulates IF function under physiologic and pathologic contexts. Transamidation seems to be essential for the attachment of several epidermal type II keratins to the cornified envelope of the skin, which provides a critical barrier function134. In the setting of certain chronic liver disease, most commonly alcoholic and nonalcoholic steatohepatitis, K8 in hepatocytes becomes crosslinked by TG2135, resulting in the formation of K8–K18 aggregates termed Mallory-Denk bodies (MDBs); these also contain ubiquitin and the ubiquitin-binding protein p62 136. The first, of likely multiple, transamidation sites on K8 was recently identified as Gln70 in the head-domain 137.
A major challenge is to identify other relevant IF protein transamidation targets and sites in vivo, which will undoubtedly require the development of new methods to study this modification, as it is not routinely evaluated by mass spectrometry. Examining multiple potential TG mechanisms will also be essential. For example, neuronal tubulin undergoes TG-mediated polyamination, a covalent conjugation to polyamines such as putrescine, spermidine, and spermine, which decreases tubulin solubility and it is likely to aid the formation of stable axonal microtubules138. Such future studies will likely yield important mechanistic insights into protein aggregation mechanisms in the liver and other tissues (for example, brain and muscle) that are known to harbor pathologic IF inclusions.
ADP-ribosylation
The type III IFs desmin and vimentin undergo covalent modification with ADP-ribose catalyzed by different enzymes, but with similar functional outcomes. Vimentin is ADP-ribosylated in vitro by bacterial SpyA139, and the same reaction on desmin is facilitated by endogenous muscle ADP-ribosyltransferase140,141. In each case, head-domain arginines are modified, inhibiting filament formation. In the case of desmin, the presence of phosphates is critical for the assembly defect because ribosylated desmin retained filament-forming capabilities. The physiological importance of this IF modification remains to be defined.
Putative IF modifications
Lysine methylation142,143, lysine malonylation144, and arginine mono- and di-methylation145 have been identified as putative PTMs of IF proteins via large-scale mass spectrometry studies, but await experimental validation and functional assessment.
PTM crosstalk and IF proteins
Various PTMs participate in complex cross-talk to regulate the properties and cellular function of proteins at a global level110,146,147. As new modifications of IFs become identified and characterized, the next challenge will be to consolidate the evidence and construct a cohesive portrait of their combined effects. Figure 4 summarizes the known mechanisms of IF regulation by the individual PTMs and their cross-talk.
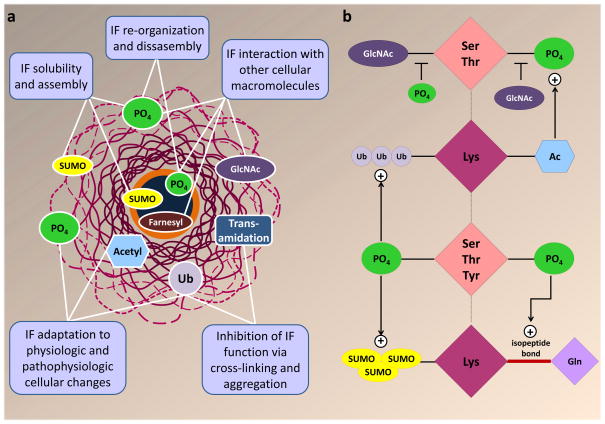
Figure 4. Mechanisms of IF PTM functions and cross-talk.
a. Summary of IF PTM functions based on current evidence. Phosphorylation and sumoylation regulate the solubility and filament formation of both nuclear and cytoplasmic IFs. Phosphorylation also regulates IF re-organization and disassembly. Associations between cytoplasmic IFs and other proteins are regulated by O-linked glycosylation (GlcNAc) and phosphorylation, whereas lamin interactions with the nuclear membrane are regulated by farnesylation. Phosphorylation also regulates the interaction of lamins with lamin associated proteins, including transcription factors and other lamin-binding partners168,169. Lysine acetylation, phosphorylation and ubiquitination regulate IF filament formation, disassembly, and turnover in response to physiological and pathophysiologic cellular changes. Functional inhibition of IFs under severe cellular stress is mediated by IF cross-linking and aggregation via transamidation and poly-ubiquitination. b. Cross-talk between IF PTMs (the plus sign indicates where one PTM promotes another). There is a reciprocal regulation between phosphorylation and glycosylation of neurofilaments and keratins. Hyperphosphorylation and hypoglycosylation of neurofilaments are observed in neurodegeneration and in aging, whereas K18 hypoglycosylation leads to hyperphosphorylation of its partner, K8 during epithelial cell injury. Stress-induced keratin phosphorylation is limited in the absence of basal acetylation16. Ubiquitination is frequently observed on hyperphosphorylated IFs124, and is associated with proteasomal inhibition131. Transamidation, which involves isopeptide bond formation between lysine and glutamine, is facilitated by phosphorylation of keratin IFs137. Sumoylation of keratin IFs is strongly enhanced in the presence of phosphorylation13, which may facilitate IF rod domain access to SUMO proteins and ligases via its ability to induce filament reorganization. While there is ample evidence for IF PTM cross-regulation, the exact mechanisms and regulators remain to be determined. Ac, acetylation; GlcNAc, O-linked glycosylation; PO4, phosphorylation; SUMO, sumoylation; Ub, ubiquitination.
Phosphorylation and sumoylation
Sumoylation of keratin IFs is strongly enhanced by phosphorylation. Treatment with the phosphatase inhibitor okadaic acid induces keratin hyperphosphorylation with a concomitant increase in sumoylation13. In the case of K8, this is partially dependent on phosphorylation at Ser74 (a stress-sensitive site), which, by promoting filament reorganization, presumably facilitates access of the sumoylation machinery to Lys285 and Lys364 of the K8 coil-2 domain. It is also plausible that putative phosphorylation sites in coil-2 and the preceding L12 domain of K8, which have been identified by large scale studies148,149 but not confirmed experimentally, affect K8 sumoylation more directly. Since the stress-protective functions of keratins are intimately tied to their ability to undergo site-specific phosphorylation58, exactly how phosphorylation modulates the sumoylation of keratins and other IFs remains an important avenue for future studies.
Phosphorylation and glycosylation
Similar to what has been reported for other proteins110, there appears to be an inverse correlation between phosphorylation and glycosylation on neurofilaments and keratins. Aging-related decline in the expression and activity of protein phosphatase 2A (PP2A) is related to NF-H hyperphosphorylation at tail-domain KSP repeats150. Furthermore, a similar increase in NF-M phosphorylation is observed in brains from patients with Alzheimer disease, which is paralleled by a decrease in NF-M glycosylation119. Since this effect was also observed in the brains of fasting rats, it was postulated that Alzheimer disease-associated impairments of glucose uptake and metabolism lead to NF-M hypoglycosylation119. A similar inverse relationship between phosphorylation and glycosylation is observed for keratins in the context of mouse liver injury151. Mechanistic studies examining the effects of IF phosphorylation on glycosylation, and vice versa, are needed to reveal whether the two pathways regulate each other. K18 glycosylation occurs independently of phosphorylation in the head domain of K1815. However, blockade of K18 glycosylation leads to an increase in the phosphorylation of its partner, K8, at Ser7415. Since Akt1 binds directly to K8 and Akt1 phosphorylation is critically modulated by the glycosylation status of K1815, this may represent a signaling hub for complex PTM cross-talk between modifications on three closely-interacting proteins.
Phosphorylation and acetylation
K8 lysine acetylation may function as a sensor to link metabolic status to K8 phosphorylation and filament re-organization. Keratin filaments significantly re-organize in response to metabolic stress, such as autophagy inhibition, where K8 undergoes aggregation in a Ser74-phosphorylation-mediated manner152. As discussed above, this residue is an important stress-induced K8 phosphorylation site that is phosphorylated by p38 and Jun kinases to shape the keratin filament cytoskeleton by increasing K8 solubility38,61,62,153. In contrast, acetylation of Lys207 on K8 under basal conditions promotes insolubility, and blocking Lys207 acetylation significantly reduces phosphorylation of K8 at Ser74 16. The mechanism for this remains to be addressed, including the possibility that acetylation may facilitate the recruitment of p38 or other kinases to K8.
Phosphorylation, ubiquitination and transamidation
Ubiquitination is frequently observed in the context of hyperphosphorylated IFs.124 The mechanistic connection between phosphorylation and ubiquitination requires more work, but it is possible that site-specific IF phosphorylation participates in the recruitment of ubiquitin ligases and it has already been shown to promote the recruitment of CHIP/STUB1127 to mutant keratins. IF protein ubiquitination is also associated with proteasomal inhibition131 and the formation of intracellular IF protein inclusions, such as hepatocyte MDBs136, the formation of which also requires TG2-mediated K8 transamidation. Stress-induced phosphorylation of K8 at Ser74 renders K8 a significantly better substrate for TG2 and, consistent with this, mice over-expressing human K8-S74A form significantly fewer MDBs than mice expressing human wild type K8137. These studies show that pathologic IF protein aggregation involves complex cross-talk between multiple IF PTMs.
IF PTMs: disease biomarkers and targets
Given the numerous functions ascribed to IF phosphorylation, it is not surprising that significant changes in IF phosphorylation are observed in many human diseases, including myopathies154, neurodegeneration155, and liver diseases99,156. Pathologic IF hyperphosphorylation is frequently accompanied by IF reorganization and aggregation, as demonstrated for NFs,48,157 lamins154, and keratins99,156,158. Furthermore, aggregates of hyperphosphorylated NFs are found in the soma of neurons, in stark contrast to their exclusive presence in axons under normal conditions50. It was demonstrated that Pin1 isomerase blocks dephosphorylation of NFs and that Pin1 inhibition may be a potential strategy to prevent aberrant NF hyperphosphorylation in neurodegenerative diseases159. Future translational studies may be aimed at modulating NF and other IF domain-specific phosphorylation changes.
IF Tyr phosphorylation also may mediate some disease mechanisms. Mutation of the highly conserved phosphotyrosine, Tyr267, in the K8 rod domain and the paralogous residue on GFAP (Tyr242) leads to defective filament formation in each case46. Of note, GFAP Tyr242 is mutated in AxD (Y242D) and its proximity to Arg239, the most common AxD GFAP mutation site130, raises the possibility that disease-causing mutations in GFAP by lead to aberrant phosphorylation.
Vast advances in proteomic capabilities may facilitate ‘finger-printing’ of IF PTMs as potential disease biomarkers 160. One advantage that IFs offer over many other proteins in this regard is their cellular abundance. In addition, targeting IF PTMs represents a potential avenue for devising therapeutic strategies to overcome the detrimental effects of IF mutations. The first bench-to-bedside example of this is the recently-concluded phase-II clinical trial targeting the aberrant farnesylation of progerin in HGPS with the farnesyltransferase inhibitor lonafarnib161,162 (Box 1). The study provided a clinical example that pharmacological targeting of an aberrantly-modified IF protein can be used as a potential treatment of human disease.
Conclusion and perspective
The importance of PTMs in the function of the IF cytoskeleton is evident from several perspectives. With respect to normal cellular physiology, PTMs regulate IF organization and the binding of IFs to IF-associated proteins, which consequently regulates numerous cellular processes and cell-specific functions. Many human diseases are marked by significant changes in IF PTMs. These changes may be caused or aggravated by a mutation in an IF gene, or may be simply related to the stress or pathogenesis of the disease and the pathological condition resulting from it,] independent of IF mutation. In principle, it is possible that alterations in disease-associated IF PTMs might protect from the disease pathology or perpetuate the disease and its manifestations, thus providing a potential treatment target.
There are several critical areas that will warrant extensive future studies with regard to IF PTMs. The first is to experimentally validate large-scale proteomic data and ascribe functions to the different IF modifications. The second is to define the physical and temporal relationship and crosstalk between the various types of PTMs. The third is to characterize the on–off enzymatic machinery behind the various IF modifications. The fourth is to evaluate the PTM signature of disease-causing IF mutants, compared to normal IFs, as potential biomarkers. The fifth is to assess the suitability of IF PTMs as potential therapeutic targets. From a human health perspective, the latter two areas may offer exciting opportunities. For example, antibodies to specific aberrant PTMs could be used as effective diagnostic and prognostic tools. Furthermore, results of the use of kinase inhibitors such as selumetinib in cardiomyopathy animal models163 and muscular dystrophy164 caused by lamin A/C mutation provide an promising proof-of-principle for the utility of PTM targeting in disease.
Acknowledgments
Our work is supported by National Institutes of Health grants DK47918 and DK52951 (MBO), DK093776 (NTS) and Department of Veterans Affairs Merit Review Award (MBO)
References
- 1.Ishikawa H, Bischoff R, Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968;38:538–55. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–82. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- 3.Goldman RD, Cleland MM, Murthy SN, Mahammad S, Kuczmarski ER. Inroads into the structure and function of intermediate filament networks. J Struct Biol. 2012;177:14–23. doi: 10.1016/j.jsb.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrmann H, Strelkov SV, Burkhard P, Aebi U. Intermediate filaments: primary determinants of cell architecture and plasticity. J Clin Invest. 2009;119:1772–83. doi: 10.1172/JCI38214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Coulombe PA. Emerging role for the cytoskeleton as an organizer and regulator of translation. Nat Rev Mol Cell Biol. 2010;11:75–81. doi: 10.1038/nrm2818. [DOI] [PubMed] [Google Scholar]
- 6.Omary MB. “IF-pathies”: a broad spectrum of intermediate filament-associated diseases. J Clin Invest. 2009;119:1756–62. doi: 10.1172/JCI39894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toivola DM, Strnad P, Habtezion A, Omary MB. Intermediate filaments take the heat as stress proteins. Trends Cell Biol. 2010;20:79–91. doi: 10.1016/j.tcb.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyder CL, Pallari HM, Kochin V, Eriksson JE. Providing cellular signposts--post-translational modifications of intermediate filaments. FEBS Lett. 2008;582:2140–8. doi: 10.1016/j.febslet.2008.04.064. [DOI] [PubMed] [Google Scholar]
- 9.Izawa I, Inagaki M. Regulatory mechanisms and functions of intermediate filaments: a study using site- and phosphorylation state-specific antibodies. Cancer Sci. 2006;97:167–74. doi: 10.1111/j.1349-7006.2006.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res. 2007;313:2098–109. doi: 10.1016/j.yexcr.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omary MB, Ku NO, Tao GZ, Toivola DM, Liao J. “Heads and tails” of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem Sci. 2006;31:383–94. doi: 10.1016/j.tibs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Kaminsky R, et al. SUMO regulates the assembly and function of a cytoplasmic intermediate filament protein in C. elegans. Dev Cell. 2009;17:724–35. doi: 10.1016/j.devcel.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snider NT, Weerasinghe SV, Iniguez-Lluhi JA, Herrmann H, Omary MB. Keratin hypersumoylation alters filament dynamics and is a marker for human liver disease and keratin mutation. J Biol Chem. 2011;286:2273–84. doi: 10.1074/jbc.M110.171314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YQ, Sarge KD. Sumoylation regulates lamin A function and is lost in lamin A mutants associated with familial cardiomyopathies. J Cell Biol. 2008;182:35–9. doi: 10.1083/jcb.200712124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ku NO, Toivola DM, Strnad P, Omary MB. Cytoskeletal keratin glycosylation protects epithelial tissue from injury. Nat Cell Biol. 2010;12:876–85. doi: 10.1038/ncb2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snider NT, et al. Glucose and SIRT2 reciprocally mediate the regulation of keratin 8 by lysine acetylation. J Cell Biol. 2013;200:241–7. doi: 10.1083/jcb.201209028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362–5. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- 18.Ku NO, Michie S, Resurreccion EZ, Broome RL, Omary MB. Keratin binding to 14-3-3 proteins modulates keratin filaments and hepatocyte mitotic progression. Proc Natl Acad Sci U S A. 2002;99:4373–8. doi: 10.1073/pnas.072624299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang RC, et al. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science. 2012;338:956–9. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ku NO, Omary MB. Effect of mutation and phosphorylation of type I keratins on their caspase-mediated degradation. J Biol Chem. 2001;276:26792–8. doi: 10.1074/jbc.M103315200. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson JE, et al. Introducing intermediate filaments: from discovery to disease. J Clin Invest. 2009;119:1763–71. doi: 10.1172/JCI38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol. 2007;8:562–73. doi: 10.1038/nrm2197. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Coulombe PA. Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 2007;21:1581–97. doi: 10.1101/gad.1552107. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu Rev Biochem. 2004;73:749–89. doi: 10.1146/annurev.biochem.73.011303.073823. [DOI] [PubMed] [Google Scholar]
- 25.Parry DA, Strelkov SV, Burkhard P, Aebi U, Herrmann H. Towards a molecular description of intermediate filament structure and assembly. Exp Cell Res. 2007;313:2204–16. doi: 10.1016/j.yexcr.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Ho CY, Lammerding J. Lamins at a glance. J Cell Sci. 2012;125:2087–93. doi: 10.1242/jcs.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol. 2013;14:13–24. doi: 10.1038/nrm3488. [DOI] [PubMed] [Google Scholar]
- 28.Lee CH, Kim MS, Chung BM, Leahy DJ, Coulombe PA. Structural basis for heteromeric assembly and perinuclear organization of keratin filaments. Nat Struct Mol Biol. 2012;19:707–15. doi: 10.1038/nsmb.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Windoffer R, Beil M, Magin TM, Leube RE. Cytoskeleton in motion: the dynamics of keratin intermediate filaments in epithelia. J Cell Biol. 2011;194:669–78. doi: 10.1083/jcb.201008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prahlad V, Yoon M, Moir RD, Vale RD, Goldman RD. Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J Cell Biol. 1998;143:159–70. doi: 10.1083/jcb.143.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolsch A, Windoffer R, Leube RE. Actin-dependent dynamics of keratin filament precursors. Cell Motil Cytoskeleton. 2009;66:976–85. doi: 10.1002/cm.20395. [DOI] [PubMed] [Google Scholar]
- 32.Coulombe PA, Kerns ML, Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J Clin Invest. 2009;119:1784–93. doi: 10.1172/JCI38177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coulombe PA, et al. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991;66:1301–11. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- 34.Chung BM, Rotty JD, Coulombe PA. Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol. 2013 doi: 10.1016/j.ceb.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 2009;119:1825–36. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leube RE, Moch M, Kolsch A, Windoffer R. “Panta rhei”: Perpetual cycling of the keratin cytoskeleton. Bioarchitecture. 2011;1:39–44. doi: 10.4161/bioa.1.1.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fois G, et al. Effects of keratin phosphorylation on the mechanical properties of keratin filaments in living cells. FASEB J. 2013;27:1322–9. doi: 10.1096/fj.12-215632. [DOI] [PubMed] [Google Scholar]
- 38.Woll S, Windoffer R, Leube RE. p38 MAPK-dependent shaping of the keratin cytoskeleton in cultured cells. J Cell Biol. 2007;177:795–807. doi: 10.1083/jcb.200703174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivaramakrishnan S, Schneider JL, Sitikov A, Goldman RD, Ridge KM. Shear stress induced reorganization of the keratin intermediate filament network requires phosphorylation by protein kinase C zeta. Mol Biol Cell. 2009;20:2755–65. doi: 10.1091/mbc.E08-10-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rikova K, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 41.Moritz A, et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal. 2010;3:ra64. doi: 10.1126/scisignal.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo A, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci U S A. 2008;105:692–7. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng L, Zhou X, Liao J, Omary MB. Pervanadate-mediated tyrosine phosphorylation of keratins 8 and 19 via a p38 mitogen-activated protein kinase-dependent pathway. J Cell Sci. 1999;112 (Pt 13):2081–90. doi: 10.1242/jcs.112.13.2081. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Q, et al. Characterization of in vivo keratin 19 phosphorylation on tyrosine-391. PLoS One. 2010;5:e13538. doi: 10.1371/journal.pone.0013538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valgeirsdottir S, et al. PDGF induces reorganization of vimentin filaments. J Cell Sci. 1998;111 (Pt 14):1973–80. doi: 10.1242/jcs.111.14.1973. [DOI] [PubMed] [Google Scholar]
- 46.Snider NT, Park H, Omary MB. A Conserved Rod Domain Phosphotyrosine That Is Targeted by the Phosphatase PTP1B Promotes Keratin 8 Protein Insolubility and Filament Organization. J Biol Chem. 2013;288:31329–37. doi: 10.1074/jbc.M113.502724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pant HC, Shecket G, Gainer H, Lasek RJ. Neurofilament protein is phosphorylated in the squid giant axon. J Cell Biol. 1978;78:R23–7. doi: 10.1083/jcb.78.2.r23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan A, Rao MV, Veeranna &, Nixon RA. Neurofilaments at a glance. J Cell Sci. 2012;125:3257–63. doi: 10.1242/jcs.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shea TB, Jung C, Pant HC. Does neurofilament phosphorylation regulate axonal transport? Trends Neurosci. 2003;26:397–400. doi: 10.1016/S0166-2236(03)00199-1. [DOI] [PubMed] [Google Scholar]
- 50.Binukumar BK, et al. Topographic regulation of neuronal intermediate filaments by phosphorylation, role of peptidyl-prolyl isomerase 1: significance in neurodegeneration. Histochem Cell Biol. 2013;140:23–32. doi: 10.1007/s00418-013-1108-7. [DOI] [PubMed] [Google Scholar]
- 51.Shea TB, Chan WK. Regulation of neurofilament dynamics by phosphorylation. Eur J Neurosci. 2008;27:1893–901. doi: 10.1111/j.1460-9568.2008.06165.x. [DOI] [PubMed] [Google Scholar]
- 52.Yabe JT, et al. Neurofilaments consist of distinct populations that can be distinguished by C-terminal phosphorylation, bundling, and axonal transport rate in growing axonal neurites. J Neurosci. 2001;21:2195–205. doi: 10.1523/JNEUROSCI.21-07-02195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veeranna, et al. Neurofilament tail phosphorylation: identity of the RT-97 phosphoepitope and regulation in neurons by cross-talk among proline-directed kinases. J Neurochem. 2008;107:35–49. doi: 10.1111/j.1471-4159.2008.05547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao MV, et al. Gene replacement in mice reveals that the heavily phosphorylated tail of neurofilament heavy subunit does not affect axonal caliber or the transit of cargoes in slow axonal transport. J Cell Biol. 2002;158:681–93. doi: 10.1083/jcb.200202037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holmgren A, Bouhy D, Timmerman V. Neurofilament phosphorylation and their proline-directed kinases in health and disease. J Peripher Nerv Syst. 2012;17:365–76. doi: 10.1111/j.1529-8027.2012.00434.x. [DOI] [PubMed] [Google Scholar]
- 56.Kushkuley J, et al. Neurofilament cross-bridging competes with kinesin-dependent association of neurofilaments with microtubules. J Cell Sci. 2009;122:3579–86. doi: 10.1242/jcs.051318. [DOI] [PubMed] [Google Scholar]
- 57.Lee S, Sunil N, Tejada JM, Shea TB. Differential roles of kinesin and dynein in translocation of neurofilaments into axonal neurites. J Cell Sci. 2011;124:1022–31. doi: 10.1242/jcs.079046. [DOI] [PubMed] [Google Scholar]
- 58.Ku NO, Omary MB. A disease- and phosphorylation-related nonmechanical function for keratin 8. J Cell Biol. 2006;174:115–25. doi: 10.1083/jcb.200602146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ku NO, et al. Mutation of a major keratin phosphorylation site predisposes to hepatotoxic injury in transgenic mice. J Cell Biol. 1998;143:2023–32. doi: 10.1083/jcb.143.7.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toivola DM, Zhou Q, English LS, Omary MB. Type II keratins are phosphorylated on a unique motif during stress and mitosis in tissues and cultured cells. Mol Biol Cell. 2002;13:1857–70. doi: 10.1091/mbc.01-12-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ku NO, Azhar S, Omary MB. Keratin 8 phosphorylation by p38 kinase regulates cellular keratin filament reorganization: modulation by a keratin 1-like disease causing mutation. J Biol Chem. 2002;277:10775–82. doi: 10.1074/jbc.M107623200. [DOI] [PubMed] [Google Scholar]
- 62.He T, Stepulak A, Holmstrom TH, Omary MB, Eriksson JE. The intermediate filament protein keratin 8 is a novel cytoplasmic substrate for c-Jun N-terminal kinase. J Biol Chem. 2002;277:10767–74. doi: 10.1074/jbc.M111436200. [DOI] [PubMed] [Google Scholar]
- 63.Peter M, Nakagawa J, Doree M, Labbe JC, Nigg EA. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990;61:591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- 64.Hamirally S, et al. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 2009;5:e1000275. doi: 10.1371/journal.ppat.1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morrison LA, DeLassus GS. Breach of the nuclear lamina during assembly of herpes simplex viruses. Nucleus. 2011;2:271–6. doi: 10.4161/nucl.2.4.16334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marty FM, Boeckh M. Maribavir and human cytomegalovirus-what happened in the clinical trials and why might the drug have failed? Curr Opin Virol. 2011;1:555–62. doi: 10.1016/j.coviro.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 67.Milbradt J, Webel R, Auerochs S, Sticht H, Marschall M. Novel mode of phosphorylation-triggered reorganization of the nuclear lamina during nuclear egress of human cytomegalovirus. J Biol Chem. 2010;285:13979–89. doi: 10.1074/jbc.M109.063628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Speese SD, et al. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell. 2012;149:832–46. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J, et al. Nestin negatively regulates postsynaptic differentiation of the neuromuscular synapse. Nat Neurosci. 2011;14:324–30. doi: 10.1038/nn.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sahlgren CM, et al. Cdk5 regulates the organization of Nestin and its association with p35. Mol Cell Biol. 2003;23:5090–106. doi: 10.1128/MCB.23.14.5090-5106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin W, et al. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46:569–79. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Mohseni P, et al. Nestin is not essential for development of the CNS but required for dispersion of acetylcholine receptor clusters at the area of neuromuscular junctions. J Neurosci. 2011;31:11547–52. doi: 10.1523/JNEUROSCI.4396-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen S, Zhai B, Gygi SP, Goldberg AL. Ubiquitylation by Trim32 causes coupled loss of desmin, Z-bands, and thin filaments in muscle atrophy. J Cell Biol. 2012;198:575–89. doi: 10.1083/jcb.201110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green KJ, Bohringer M, Gocken T, Jones JC. Intermediate filament associated proteins. Adv Protein Chem. 2005;70:143–202. doi: 10.1016/S0065-3233(05)70006-1. [DOI] [PubMed] [Google Scholar]
- 75.Freeman AK, Morrison DK. 14-3-3 Proteins: diverse functions in cell proliferation and cancer progression. Semin Cell Dev Biol. 2011;22:681–7. doi: 10.1016/j.semcdb.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liao J, Omary MB. 14-3-3 proteins associate with phosphorylated simple epithelial keratins during cell cycle progression and act as a solubility cofactor. J Cell Biol. 1996;133:345–57. doi: 10.1083/jcb.133.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boudreau A, et al. 14-3-3sigma stabilizes a complex of soluble actin and intermediate filament to enable breast tumor invasion. Proc Natl Acad Sci U S A. 2013;110:E3937–44. doi: 10.1073/pnas.1315022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24:1838–51. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu QS, et al. Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene. 2011;30:457–70. doi: 10.1038/onc.2010.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nieminen M, et al. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–62. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 81.Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007;313:2050–62. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 82.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–56. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seltmann K, et al. Keratins mediate localization of hemidesmosomes and repress cell motility. J Invest Dermatol. 2013;133:181–90. doi: 10.1038/jid.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rotty JD, Coulombe PA. A wound-induced keratin inhibits Src activity during keratinocyte migration and tissue repair. J Cell Biol. 2012;197:381–9. doi: 10.1083/jcb.201107078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iyer SV, et al. Understanding the role of keratins 8 and 18 in neoplastic potential of breast cancer derived cell lines. PLoS One. 2013;8:e53532. doi: 10.1371/journal.pone.0053532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fortier AM, Asselin E, Cadrin M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin1 up-regulation. J Biol Chem. 2013;288:11555–71. doi: 10.1074/jbc.M112.428920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ivaska J, et al. PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 2005;24:3834–45. doi: 10.1038/sj.emboj.7600847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang IA, et al. Vimentin phosphorylation by Cdc2 in Schwann cell controls axon growth via beta1-integrin activation. FASEB J. 2012;26:2401–13. doi: 10.1096/fj.11-199018. [DOI] [PubMed] [Google Scholar]
- 89.Busch T, et al. Keratin 8 phosphorylation regulates keratin reorganization and migration of epithelial tumor cells. J Cell Sci. 2012;125:2148–59. doi: 10.1242/jcs.080127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alam H, et al. Loss of keratin 8 phosphorylation leads to increased tumor progression and correlates with clinico-pathological parameters of OSCC patients. PLoS One. 2011;6:e27767. doi: 10.1371/journal.pone.0027767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beil M, et al. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nat Cell Biol. 2003;5:803–11. doi: 10.1038/ncb1037. [DOI] [PubMed] [Google Scholar]
- 92.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–56. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 93.Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 2013;82:357–85. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- 94.Zhong N, Radu G, Ju W, Brown WT. Novel progerin-interactive partner proteins hnRNP E1, EGF, Mel 18, and UBC9 interact with lamin A/C. Biochem Biophys Res Commun. 2005;338:855–61. doi: 10.1016/j.bbrc.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 95.Kelley JB, et al. The defective nuclear lamina in Hutchinson-gilford progeria syndrome disrupts the nucleocytoplasmic Ran gradient and inhibits nuclear localization of Ubc9. Mol Cell Biol. 2011;31:3378–95. doi: 10.1128/MCB.05087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boudreau E, et al. Lamin A/C mutants disturb sumo1 localization and sumoylation in vitro and in vivo. PLoS One. 2012;7:e45918. doi: 10.1371/journal.pone.0045918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simon DN, Domaradzki T, Hofmann WA, Wilson KL. Lamin A tail modification by SUMO1 is disrupted by familial partial lipodystrophy-causing mutations. Mol Biol Cell. 2013;24:342–50. doi: 10.1091/mbc.E12-07-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carberry K, Wiesenfahrt T, Windoffer R, Bossinger O, Leube RE. Intermediate filaments in Caenorhabditis elegans. Cell Motil Cytoskeleton. 2009;66:852–64. doi: 10.1002/cm.20372. [DOI] [PubMed] [Google Scholar]
- 99.Omary MB, Ku NO, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest. 2009;119:1794–805. doi: 10.1172/JCI37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strnad P, et al. Keratin variants predispose to acute liver failure and adverse outcome: race and ethnic associations. Gastroenterology. 2010;139:828–35. 835 e1–3. doi: 10.1053/j.gastro.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toivola DM, Krishnan S, Binder HJ, Singh SK, Omary MB. Keratins modulate colonocyte electrolyte transport via protein mistargeting. J Cell Biol. 2004;164:911–21. doi: 10.1083/jcb.200308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Demarque MD, et al. Sumoylation by Ubc9 regulates the stem cell compartment and structure and function of the intestinal epithelium in mice. Gastroenterology. 2011;140:286–96. doi: 10.1053/j.gastro.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 103.Choudhary C, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–40. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 104.Zhao S, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–4. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guan KL, Yu W, Lin Y, Xiong Y, Zhao S. Generation of acetyllysine antibodies and affinity enrichment of acetylated peptides. Nat Protoc. 2010;5:1583–95. doi: 10.1038/nprot.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–44. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 107.Hornbeck PV, Chabra I, Kornhauser JM, Skrzypek E, Zhang B. PhosphoSite: A bioinformatics resource dedicated to physiological protein phosphorylation. Proteomics. 2004;4:1551–61. doi: 10.1002/pmic.200300772. [DOI] [PubMed] [Google Scholar]
- 108.Arbustini E, et al. Autosomal dominant dilated cardiomyopathy with atrioventricular block: a lamin A/C defect-related disease. J Am Coll Cardiol. 2002;39:981–90. doi: 10.1016/s0735-1097(02)01724-2. [DOI] [PubMed] [Google Scholar]
- 109.Prust M, et al. GFAP mutations, age at onset, and clinical subtypes in Alexander disease. Neurology. 2011;77:1287–94. doi: 10.1212/WNL.0b013e3182309f72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–58. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shafi R, et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci U S A. 2000;97:5735–9. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang YR, et al. O-GlcNAcase is essential for embryonic development and maintenance of genomic stability. Aging Cell. 2012;11:439–48. doi: 10.1111/j.1474-9726.2012.00801.x. [DOI] [PubMed] [Google Scholar]
- 113.Chou CF, Smith AJ, Omary MB. Characterization and dynamics of O-linked glycosylation of human cytokeratin 8 and 18. J Biol Chem. 1992;267:3901–6. [PubMed] [Google Scholar]
- 114.Slawson C, Lakshmanan T, Knapp S, Hart GW. A mitotic GlcNAcylation/phosphorylation signaling complex alters the posttranslational state of the cytoskeletal protein vimentin. Mol Biol Cell. 2008;19:4130–40. doi: 10.1091/mbc.E07-11-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dong DL, Xu ZS, Hart GW, Cleveland DW. Cytoplasmic O-GlcNAc modification of the head domain and the KSP repeat motif of the neurofilament protein neurofilament-H. J Biol Chem. 1996;271:20845–52. doi: 10.1074/jbc.271.34.20845. [DOI] [PubMed] [Google Scholar]
- 116.King IA, Hounsell EF. Cytokeratin 13 contains O-glycosidically linked N-acetylglucosamine residues. J Biol Chem. 1989;264:14022–8. [PubMed] [Google Scholar]
- 117.Dong DL, et al. Glycosylation of mammalian neurofilaments. Localization of multiple O-linked N-acetylglucosamine moieties on neurofilament polypeptides L and M. J Biol Chem. 1993;268:16679–87. [PubMed] [Google Scholar]
- 118.Ludemann N, et al. O-glycosylation of the tail domain of neurofilament protein M in human neurons and in spinal cord tissue of a rat model of amyotrophic lateral sclerosis (ALS) J Biol Chem. 2005;280:31648–58. doi: 10.1074/jbc.M504395200. [DOI] [PubMed] [Google Scholar]
- 119.Deng Y, et al. Regulation between O-GlcNAcylation and phosphorylation of neurofilament-M and their dysregulation in Alzheimer disease. FASEB J. 2008;22:138–45. doi: 10.1096/fj.07-8309com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheung WD, Hart GW. AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J Biol Chem. 2008;283:13009–20. doi: 10.1074/jbc.M801222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–69. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 122.Jung HJ, et al. Farnesylation of lamin B1 is important for retention of nuclear chromatin during neuronal migration. Proc Natl Acad Sci U S A. 2013;110:E1923–32. doi: 10.1073/pnas.1303916110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Adam SA, Butin-Israeli V, Cleland MM, Shimi T, Goldman RD. Disruption of lamin B1 and lamin B2 processing and localization by farnesyltransferase inhibitors. Nucleus. 2013;4:142–50. doi: 10.4161/nucl.24089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ku NO, Omary MB. Keratins turn over by ubiquitination in a phosphorylation-modulated fashion. J Cell Biol. 2000;149:547–52. doi: 10.1083/jcb.149.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rogel MR, Jaitovich A, Ridge KM. The role of the ubiquitin proteasome pathway in keratin intermediate filament protein degradation. Proc Am Thorac Soc. 2010;7:71–6. doi: 10.1513/pats.200908-089JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jaitovich A, et al. Ubiquitin-proteasome-mediated degradation of keratin intermediate filaments in mechanically stimulated A549 cells. J Biol Chem. 2008;283:25348–55. doi: 10.1074/jbc.M801635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loffek S, et al. The ubiquitin ligase CHIP/STUB1 targets mutant keratins for degradation. Hum Mutat. 2010;31:466–76. doi: 10.1002/humu.21222. [DOI] [PubMed] [Google Scholar]
- 128.Kim W, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell. 2011;44:325–40. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goldfarb LG, Dalakas MC. Tragedy in a heartbeat: malfunctioning desmin causes skeletal and cardiac muscle disease. J Clin Invest. 2009;119:1806–13. doi: 10.1172/JCI38027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liem RK, Messing A. Dysfunctions of neuronal and glial intermediate filaments in disease. J Clin Invest. 2009;119:1814–24. doi: 10.1172/JCI38003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tang G, Perng MD, Wilk S, Quinlan R, Goldman JE. Oligomers of mutant glial fibrillary acidic protein (GFAP) Inhibit the proteasome system in Alexander disease astrocytes, and the small heat shock protein alphaB-crystallin reverses the inhibition. J Biol Chem. 2010;285:10527–37. doi: 10.1074/jbc.M109.067975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hagemann TL, Boelens WC, Wawrousek EF, Messing A. Suppression of GFAP toxicity by alphaB-crystallin in mouse models of Alexander disease. Hum Mol Genet. 2009;18:1190–9. doi: 10.1093/hmg/ddp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oh YS, et al. Downregulation of lamin A by tumor suppressor AIMP3/p18 leads to a progeroid phenotype in mice. Aging Cell. 2010;9:810–22. doi: 10.1111/j.1474-9726.2010.00614.x. [DOI] [PubMed] [Google Scholar]
- 134.Candi E, et al. A highly conserved lysine residue on the head domain of type II keratins is essential for the attachment of keratin intermediate filaments to the cornified cell envelope through isopeptide crosslinking by transglutaminases. Proc Natl Acad Sci U S A. 1998;95:2067–72. doi: 10.1073/pnas.95.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Strnad P, et al. Transglutaminase 2 regulates mallory body inclusion formation and injury-associated liver enlargement. Gastroenterology. 2007;132:1515–26. doi: 10.1053/j.gastro.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 136.Ku NO, Strnad P, Zhong BH, Tao GZ, Omary MB. Keratins let liver live: Mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies. Hepatology. 2007;46:1639–49. doi: 10.1002/hep.21976. [DOI] [PubMed] [Google Scholar]
- 137.Kwan R, et al. Keratin 8 phosphorylation regulates its transamidation and hepatocyte Mallory-Denk body formation. FASEB J. 2012;26:2318–26. doi: 10.1096/fj.11-198580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Song Y, et al. Transglutaminase and polyamination of tubulin: posttranslational modification for stabilizing axonal microtubules. Neuron. 2013;78:109–23. doi: 10.1016/j.neuron.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Icenogle LM, et al. Molecular and biological characterization of Streptococcal SpyA-mediated ADP-ribosylation of intermediate filament protein vimentin. J Biol Chem. 2012;287:21481–91. doi: 10.1074/jbc.M112.370791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yuan J, Huiatt TW, Liao CX, Robson RM, Graves DJ. The effects of mono-ADP-ribosylation on desmin assembly-disassembly. Arch Biochem Biophys. 1999;363:314–22. doi: 10.1006/abbi.1998.1096. [DOI] [PubMed] [Google Scholar]
- 141.Winter DL, Paulin D, Mericskay M, Li Z. Posttranslational modifications of desmin and their implication in biological processes and pathologies. Histochem Cell Biol. 2013 doi: 10.1007/s00418-013-1148-z. [DOI] [PubMed] [Google Scholar]
- 142.Liu H, et al. A Method for Systematic Mapping of Protein Lysine Methylation Identifies Functions for HP1beta in DNA Damage Response. Mol Cell. 2013;50:723–35. doi: 10.1016/j.molcel.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moore KE, et al. A general molecular affinity strategy for global detection and proteomic analysis of lysine methylation. Mol Cell. 2013;50:444–56. doi: 10.1016/j.molcel.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peng C, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10:M111 012658. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bremang M, et al. Mass spectrometry-based identification and characterisation of lysine and arginine methylation in the human proteome. Mol Biosyst. 2013 doi: 10.1039/c3mb00009e. [DOI] [PubMed] [Google Scholar]
- 146.Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–61. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hunter T, Sun H. Crosstalk between the SUMO and ubiquitin pathways. Ernst Schering Found Symp Proc. 2008:1–16. doi: 10.1007/2789_2008_098. [DOI] [PubMed] [Google Scholar]
- 148.Daub H, et al. Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol Cell. 2008;31:438–48. doi: 10.1016/j.molcel.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 149.Dephoure N, et al. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci U S A. 2008;105:10762–7. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Veeranna, et al. Declining phosphatases underlie aging-related hyperphosphorylation of neurofilaments. Neurobiol Aging. 2011;32:2016–29. doi: 10.1016/j.neurobiolaging.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ku NO, et al. Susceptibility to hepatotoxicity in transgenic mice that express a dominant-negative human keratin 18 mutant. J Clin Invest. 1996;98:1034–46. doi: 10.1172/JCI118864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kongara S, et al. Autophagy regulates keratin 8 homeostasis in mammary epithelial cells and in breast tumors. Mol Cancer Res. 2010;8:873–84. doi: 10.1158/1541-7786.MCR-09-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liao J, Ku NO, Omary MB. Stress, apoptosis, and mitosis induce phosphorylation of human keratin 8 at Ser-73 in tissues and cultured cells. J Biol Chem. 1997;272:17565–73. doi: 10.1074/jbc.272.28.17565. [DOI] [PubMed] [Google Scholar]
- 154.Mitsuhashi H, et al. Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients. J Cell Sci. 2010;123:3893–900. doi: 10.1242/jcs.072157. [DOI] [PubMed] [Google Scholar]
- 155.Rudrabhatla P, Grant P, Jaffe H, Strong MJ, Pant HC. Quantitative phosphoproteomic analysis of neuronal intermediate filament proteins (NF-M/H) in Alzheimer’s disease by iTRAQ. FASEB J. 2010;24:4396–407. doi: 10.1096/fj.10-157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Toivola DM, et al. Keratin 8 and 18 hyperphosphorylation is a marker of progression of human liver disease. Hepatology. 2004;40:459–66. doi: 10.1002/hep.20277. [DOI] [PubMed] [Google Scholar]
- 157.Dale JM, Garcia ML. Neurofilament Phosphorylation during Development and Disease: Which Came First, the Phosphorylation or the Accumulation? J Amino Acids. 2012;2012:382107. doi: 10.1155/2012/382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Strnad P, Paschke S, Jang KH, Ku NO. Keratins: markers and modulators of liver disease. Curr Opin Gastroenterol. 2012;28:209–16. doi: 10.1097/MOG.0b013e3283525cb8. [DOI] [PubMed] [Google Scholar]
- 159.Rudrabhatla P, Albers W, Pant HC. Peptidyl-prolyl isomerase 1 regulates protein phosphatase 2A-mediated topographic phosphorylation of neurofilament proteins. J Neurosci. 2009;29:14869–80. doi: 10.1523/JNEUROSCI.4469-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Altelaar AF, Munoz J, Heck AJ. Next-generation proteomics: towards an integrative view of proteome dynamics. Nat Rev Genet. 2013;14:35–48. doi: 10.1038/nrg3356. [DOI] [PubMed] [Google Scholar]
- 161.Gordon LB, Cao K, Collins FS. Progeria: translational insights from cell biology. J Cell Biol. 2012;199:9–13. doi: 10.1083/jcb.201207072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gordon LB, et al. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2012;109:16666–71. doi: 10.1073/pnas.1202529109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Muchir A, et al. Treatment with selumetinib preserves cardiac function and improves survival in cardiomyopathy caused by mutation in the lamin A/C gene. Cardiovasc Res. 2012;93:311–9. doi: 10.1093/cvr/cvr301. [DOI] [PubMed] [Google Scholar]
- 164.Muchir A, et al. Inhibition of extracellular signal-regulated kinase 1/2 signaling has beneficial effects on skeletal muscle in a mouse model of Emery-Dreifuss muscular dystrophy caused by lamin A/C gene mutation. Skelet Muscle. 2013;3:17. doi: 10.1186/2044-5040-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Young SG, Yang SH, Davies BS, Jung HJ, Fong LG. Targeting protein prenylation in progeria. Sci Transl Med. 2013;5:171ps3. doi: 10.1126/scitranslmed.3005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schreiber KH, Kennedy BK. When lamins go bad: nuclear structure and disease. Cell. 2013;152:1365–75. doi: 10.1016/j.cell.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Varela I, et al. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat Med. 2008;14:767–72. doi: 10.1038/nm1786. [DOI] [PubMed] [Google Scholar]
- 168.Harper M, Tillit J, Kress M, Ernoult-Lange M. Phosphorylation-dependent binding of human transcription factor MOK2 to lamin A/C. FEBS J. 2009;276:3137–47. doi: 10.1111/j.1742-4658.2009.07032.x. [DOI] [PubMed] [Google Scholar]
- 169.Bengtsson L, Wilson KL. Barrier-to-autointegration factor phosphorylation on Ser-4 regulates emerin binding to lamin A in vitro and emerin localization in vivo. Mol Biol Cell. 2006;17:1154–63. doi: 10.1091/mbc.E05-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]