Abstract
Night-shift workers are prone to sleep deprivation, misalignment of circadian rhythms, and subsequent sleepiness and sleep-related performance deficits. The purpose of this narrative systematic review is to critically review and synthesize the scientific literature regarding improvements in sleepiness and sleep-related performance deficits following planned naps taken during work-shift hours by night workers and to recommend directions for future research and practice. We conducted a literature search using the Medline, PsychInfo, CINAHL, Cochrane Library, and Health and Safety Science Abstracts databases and included English-language quasi-experimental and experimental studies that evaluated the effects of a nighttime nap taken during a simulated or actual night-work shift. We identified 13 relevant studies, which consisted primarily of small samples and mixed designs. Most investigators found that, despite short periods of sleep inertia immediately following naps, night-shift napping led to decreased sleepiness and improved sleep-related performance. None of the studies examined the effects of naps on safety outcomes in the workplace. Larger-scale randomized clinical trials of night-shift napping and direct safety outcomes are needed prior to wider implementation.
Keywords: napping, sleep, sleepiness, performance, psychomotor vigilance, systematic review
Shift workers, individuals who do not work within a 09:00–17:00 time frame (National Sleep Foundation, 2009), are increasingly needed in our 24/7 society. In the United States, nearly 20% of employed adults are shift workers (Drake & Wright, 2011). Shift workers are especially prone to circadian misalignment (Monk, 2005; Rollinson et al., 2003) and sleep deprivation (Drake & Wright, 2011) that lead to sleepiness, fatigue, and sleep-related performance deficits (Belenky & Akerstedt, 2011). For most individuals, sleepiness is at its highest level during the early morning hours (Caruso & Rosa, 2007). The fatigue and other performance deficits night-shift workers experience during these hours have been implicated in major disasters such as the nuclear-plant accidents at Chernobyl (World Nuclear Association, 2011) and Three Mile Island (World Nuclear Association, 2010), and the Exxon Valdez oil spill (U.S. Environmental Protection Agency, 2011).
Scheduled naps in the workplace are recommended to combat fatigue and reduce sleepiness in health care workers (Joint Commission, 2011) and flight crews (Caldwell et al., 2009). A number of investigators have studied the effects of planned naps during the night shift on sleep-related performance deficits and sleepiness. The purpose of this systematic review is to critically analyze and synthesize this literature to recommend directions for future research and practice.
Background
Sleep is a complex physiological process comprised of two phases: non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. NREM consists of three stages of gradually deepening sleep (N1, N2, and N3, or slow wave sleep). A cyclic pattern of NREM and REM sleep (sleep cycle) occurs throughout the sleep period. Sleep cycles are approximately 60–110 min in length (Landis, 2011).
Although research has not determined the precise mechanisms that regulate sleep, the two-process model of sleep regulation (Achermann & Borbely, 2011) is frequently used to describe sleep regulation and is useful for conceptualizing sleep and fatigue in shift workers. According to this model, sleep is regulated by the interaction of two processes: the homeostatic “drive” for sleep (process S) and the circadian component of the wakefulness rhythm (process C). Process S increases as a person’s wake time increases and decreases during sleep. Process C opposes sleep propensity during the day and then dissipates during the nighttime hours to promote sleep onset and maintenance (Achermann & Borbely, 2011; Taillard, Philip, Coste, Sagaspe, & Bioulac, 2003). The nadir of the wakefulness rhythm occurs in the early morning hours and the peak occurs in the late afternoon (Akerstedt, 2003; Czeisler & Brown, 1999).
Night-shift workers accumulate a sleep “debt” over successive night shifts. Many keep a diurnal schedule on days off and start their night shifts in a sleep-deprived state. After the shift, these workers often try to sleep during the day at a time that coincides with the circadian peak in wakefulness and therefore may have poor quality or insufficient sleep. The incompatibility between circadian patterns and work demands contributes to sleep deprivation and increased homeostatic drive. Volitional (e.g., recreational or social activities) and non-volitional activities (e.g., family and household responsibilities) may also contribute to sleep deprivation (Drake & Wright, 2011; Ruggiero & Pezzino, 2006).
Fatigue (“a subjective feeling of tiredness that can vary in intensity and duration”; Piper, Lindsey, & Dodd, 1987, p. 19), sleepiness (an urge to sleep; MacFarlane & Moldofsky, 2011), and impairments in vigilance and sustained attention (Oken, Salinsky, & Elsas, 2006) are important consequences of sleep deprivation and circadian alterations that may, in turn, lead to such negative outcomes as motor vehicle accidents (Steele, Ma, Watson, Thomas, & Muelleman, 1999), drowsy driving (Scott et al., 2007), and medical errors (Balas, Scott, & Rogers, 2006). While researchers have used a variety of self-report measures to measure sleep-related performance deficits and sleepiness, self-rated estimates of fatigue are often unrelated to observed behavior. Thus, researchers often use objective measures, such as simulators and devices designed to measure vigilance (e.g., Psychomotor Vigilance Test [PVT-192], Ambulatory Monitoring, Ardsley, NY; MacFarlane & Moldofsky, 2011) to evaluate the effects of shift-work–related performance deficits.
Healthy adults need approximately 7.5–8.5 hr of sleep per day (Banks & Dinges, 2011). They often use naps, defined as sleep periods at least 50% shorter than an individual’s average nocturnal sleep length (Dinges, Orne, Whitehouse, & Orne, 1987), in addition to main sleep periods to accumulate an adequate amount of sleep. Naps are not shortened, compressed versions of nocturnal sleep (Dinges, 1989); rather stages of sleep vary with the time of day and length of the nap. For example, REM sleep may occur in a nap of 1 hr’s duration but not in a nap of 30 min. Although naps are generally restorative, sleep inertia, a brief period (20–30 min) of decreased cognitive function and performance immediately after a period of sleep, may occur and temporarily obscure the recuperative effects of sleep (Dinges et al., 1987; Kemper, 2001; Mahowald & Bornemann, 2011; Scheer, Shea, Hilton, & Shea, 2008; Tassi & Muzet, 2000). Sleep inertia has an endogenous circadian rhythm, with the most impaired cognitive performance upon awakening occurring when an individual takes a nap during the biological night (23:00–03:00; Scheer et al., 2008). The degree of sleep inertia may be related (Tassi & Muzet, 2000) or unrelated (Scheer et al., 2008) to the sleep stage upon awakening. The chronic sleep deprivation many night-shift workers experience may exacerbate the detrimental effects of sleep inertia on sleepiness and performance. These observations suggest that the practice of using scheduled naps during work hours should include time for recuperation after the nap period (Scheer et al., 2008).
Night-shift workers often use naps between main sleep periods at home and at work (Ruggiero & Avi-Itzhak, 2011). A planned nap during work hours may supplement sleep duration, improve the negative health effects caused by shift-work sleep deprivation (cardiovascular disease, breast cancer, and duodenal ulcers; Drake & Wright, 2011), reduce performance deficits and sleepiness (Petrie, Powell, & Broadbent, 2004), and improve safety (Geiger-Brown & McPhaul, 2011). In this article, we critically review and synthesize the scientific literature regarding the effects of planned naps taken during the work shift by nightshift workers and make recommendations for future research and practice. Due to heterogeneity of the samples, settings, and study methodologies, a meta-analysis of these data was not feasible. Therefore, we conducted a narrative systematic review in which we addressed the following research questions:
What is the methodological quality of the studies of napping and sleepiness and sleep-related performance deficits?
What are the characteristics of the study populations, nature of the work, and workplace settings in studies of night-shift napping?
What characteristics of the naps are provided (e.g., length, time of day, location) in studies of night-shift napping?
What are the effects of night-shift napping on subsequent daytime sleep?
What are the effects of night-shift napping on sleepiness and sleep-related performance deficits?
Method
Search Strategy
We combined the key word nap* with each of the terms performance, fatigue, psychomotor vigilance, sleepiness, shift work, employment, and alert* and ran searches in the computerized databases of CINAHL (1982–November 2011), the Cochrane Library (2002–November 2011), Health and Safety Science Abstracts (1981–November 2011), Medline (1950–November 2011), and PsychInfo (1894–November 2011).
Selection Criteria
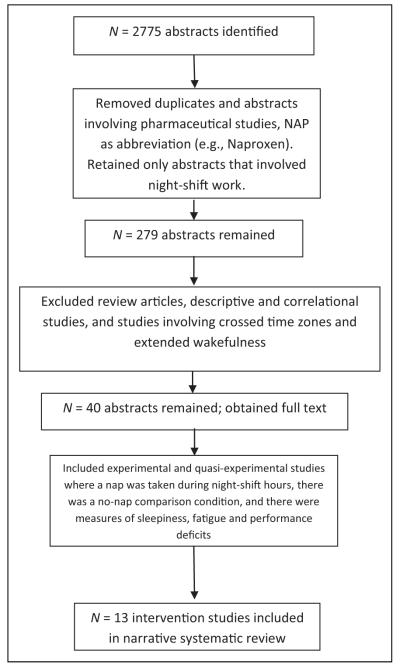
We retrieved a total of 2,775 abstracts (Figure 1) and collaborated to determine study eligibility. We removed duplicates and papers that used the word nap as an abbreviation (e.g., naproxen, nanoparticles, naphthalene). To decrease variability that could interfere with our interpretation of the findings, we eliminated nap studies that did not involve night-shift work (or simulated night-shift work) and pharmaceutical studies because their primary purpose was evaluation of drug effects. We were left with 279 abstracts. We then eliminated laboratory and field studies of situations in which the participant was expected to have extended wakefulness (e.g., long-haul truck driving) or cross time zones (airline pilots) because of the high variability in the length and timing of participants’ duty periods. We also excluded nap studies with descriptive or correlational designs because of our interest in the effects of naps and the inability to ascribe causality in studies with these designs. We retained reports of original experimental and quasi-experimental studies that included (1) a specifically assigned nap (2 hr or less) taken during a night shift (or simulated night shift) of approximately 7.5–13 hr in duration (starting at 17:00 or later and ending between 06:00 and 08:00), (2) comparison to a no-nap condition, and (3) the measurement of subjective sleepiness or fatigue or objective measures of sleep-related performance deficits including vigilance, cognitive functioning, logical reasoning performance, work tasks and driving, workload and memory recall. A hand search of the reference lists of each article did not reveal any additional relevant studies. We found 13 studies that met the inclusion criteria and included them in the analyses (Table 1).
Figure 1.
Selection criteria for systematic review.
Table 1.
Sample Characteristics, Nap and Sleep Characteristics and Measures, and Sleep-Related Performance/Sleepiness Outcomes of Reviewed Studies.
| Study (location) | Sample | Sleep and outcome measures |
Test times | Nap timing (min) |
Mean (SD) of actual sleep in min |
Significant cognitive-performance/sleepiness results postnap |
|---|---|---|---|---|---|---|
|
Howard, Radford, Jackson, Swann, & Kennedy, 2010a _ENREF_1 (Australia) |
8 sleep scientists (male and female) Age: 24–54 years Shift: 21:00–07:00 |
PVT-192, driving simulator, KSS, sleep (PSG) |
20:15, 03:45, 04:30, 06:45 | 04:00 (30) | 23.5 (5.48) | No improvement |
| Kubo et al., 2010a (Japan) | 12 male university students Age (mean ± SD): 21.6 ± 2.8 years Shift: 22:00–08:00 |
VVT, subjective sleepiness, sleep (PSG), logical reasoning performance, subjective fatigue |
Tested hourly, except during naps |
00:00 (60) 00:00 (120) 04:00 (60) 04:00 (120) |
48.4 (16.1) 111 (6.5) 54.1 (12.1) 116.2 (3.8) |
Increased RT and lapses after 1-hr nap at 04:00; RT significantly better after early naps than later naps. Reaction time and lapses effect sizes: 60-min pre/post nap condition (pç2 = 0.266 and 0.268, respectively); sleepiness effect size: 120-min pre/post nap condition (pç2 = .36) |
| Lovato, Lack, Ferguson, & Tremaine, 2009a (Australia) | 22 adults (male, female) Age: 18–35 years Shift: 17:00–07:10 |
SSS, KSS, VAS, POMS, PVT, objective sleepiness, sleep (PSG) |
Baseline and 5 times postnap |
02:30 (30) | 33.25 (5.18) | Improved SSS, KSS, VAS fatigue, POMS fatigue, sleep latency, SDST, PVT lapses |
|
Purnell, Feyer, & Herbison, 2002a (New Zealand) |
24 male aircraft maintenance engineers Age: 21–59 years Shift: 19:00–07:00 |
Sleep (actigraphy), performance test battery (including PVT), reports of sleepiness while driving to and from work |
19:00, pre-nap, immediately postnap, and end of shift |
01:00–03:00 (20) | Night 1: 19 (±11.62 SE) Night 2: 21 (±14.49 SE) |
Improved MRL vigilance (1st night only) |
|
Rogers, Spencer, Stone, & Nicholson, 1989a (United Kingdom) |
6 females Age: 20–32 years Shift: 17:00–10:30 |
Sleep (PSG), sustained attention, AV, CV, 2LC, DSS, logic, STM, VVT |
8 sessions approximately 2–3 hr apart |
02:00 (60) | 62.5 (SD not reported) | Improved AV and DSS |
| Sagaspe et al., 2007a (France) | 24 participants, Age: 20–25 years (n = 12), 40–50 years (n = 12) Shift: 20:00–03:30 |
Sleep (PSG), inappropriate line crossings while driving, KSS, fatigue VAS |
02:00 drove 125 miles on highway. Monitored while driving except for posttest KSS and fatigue VAS |
01:00 (30) | Aged 20–25: 14 ± 9 Aged 40–50: 22 ± 4 |
Reduced inappropriate line crossings while driving |
| Saito & Sasaki, 1996a (Japan) | 6 females Age: 21–23 years Shift: 00:00–10:00 |
Sleep (EEG, EOG, EMG [AKA, PSG]), Fatigue Feeling Scale, SSS |
Hourly 05:00–10:00 |
03:00 (60) 03:00 (120) |
55.6 111.8 SDs not reported) |
Lower scores in Fatigue Feelings Scale (sleepiness and dullness and difficulty in concentration subscales), improved SSS |
| Sallinen, Harma, Akerstedt, Rosa, & Lillqvist, 1998a (Finland) | 14 male process operators at oil refinery Age: 31–52 Shift: 23:00–07:10 |
Visual RT, lapses, repeated test of sustained wakefulness, KSS, sleep (PSG), numerical rating scale of sleep |
23:00, 01:50, 04:40, 06:45 | 01:00 (50) 01:00 (30) 04:00 (50) 04:00 (30) |
38.1 (12.1) 24.5 (6.7) 46.6 (2.1) 27.5 (1.9) |
RT, KSS, and lapses increased throughout night shift, but there was less of an increase for nap compared to no-nap conditions |
| Signal, Gander, Anderson, & Brash, 2009a (New Zealand) | 28 air traffic controllers (male, female) Age: 26–56 years Early shift: 22:30–06:00 Late shift: 23:30–06:30 |
Sleep (PSG), PVT, EOG for rolling eye movements |
Pre-shift, after nap opportunity and before returning to work, end of shift |
00:30 (40) 02:30 (40) |
17 (12) 19 (12) |
Decreased RT, increased alertness (decreased EEG spectral power) |
| Smith et al., 2007a (Australia) | 9 nurses and medical scientists at a public hospital Age: 45.7 ± 13.2 years |
Sleep (PSG), pictoral sleepiness scale, VAS sleepiness, NASA task load index, PVT |
Hourly from 00:00 to 06:00 |
Between 02:00 and 03:00 (30) |
16.1 (SD not reported) | Improved RT, lapses, and VAS sleepiness; pre/post nap effect sizes (pç2) for all outcome measures ranging from 0.05 to 0.97 |
|
Smith-Coggins et al., 2006b (USA) |
49 male and female MDs and RNs Age: 30 (5.5) years nap, 30 (4.3) no nap Shift start: 18:30–19:30 Shift end: 07:30–09:00 |
Sleep (PSG), PVT-192, probed memory recall task, CathSim intravenous insertion simulator, driving simulator, POMS, KSS, Owl & Lark questionnaire |
18:30–19:30, 04:00– 04:30, 07:30–09:00, driving simulator 08:00 |
03:00 (40) | 24.8 (11.1) | Fewer lapses, more vigor, and less fatigue and sleepiness |
| Takeyama, Itani, Tachi, Sakamura, & Suzumura, 2002a (Japan) | 13 male university students: 5 morning types and 8 evening types Age: 18–22 years Shift: 22:00–06:00 |
ECG, salivary cortisol, CFF, reaction time, subjective fatigue, sleep (sleep logs), typing figures, mental arithmetic |
8 times throughout shift | 02:00 (120) | Not reported | First night only: compared to no-nap condition, increased number of figures typed by morning and evening types in nap condition, fewer fatigue complaints in morning-type nap group |
| Takeyama et al., 2004a (Japan) | 6 male students Age: 19–22 years Shift: 22:00–08:00 |
CRT, VT, LRT, sleep (EEG, EOG, EMG [AKA, PSG]), continuous ECG, typing documents into computer |
Hourly | 00:00 (60) 00:00 (120) 04:00 (60) 04:00 (120) |
51 ± ppc 110 ± 6 59 ± 2 117 ± 3 |
MRT of VT and RT deteriorated over night shift but no differences among the early and no-nap conditions. Significant delays in MRT after late 60-min nap compared to no-nap condition |
Note. All studies included a no-nap control condition or group. 2LC = two-letter cancellation; AV = auditory vigilance; CFF = critical flicker fusion frequency test; CRT = choice reaction time; CV = complex vigilance; DSS = digit symbol substitution, ECG = electrocardiogram; EEG = electroencephalogram; EMG = electromyogram; EOG = electrooculogram; KSS = Karolinska Sleepiness Scale; LRT = logical reasoning test; MRL = mean response latency; MRT = mean reaction time; NASA = National Aeronautics & Space Administration; POMS = Profile of Mood States; PSG = polysomnography; PVT = psychomotor vigilance task; RT = reaction time; SDST = symbol digit substitution task; SSS = Stanford Sleepiness Scale; STM = short-term memory; VAS = visual analog scale; VT = vigilance task; VVT = visual vigilance task.
quasi-experimental.
experimental.
Term not defined in source.
Data Extraction
For each study, we extracted the following information using an investigator-developed form: the purpose of the study or research question(s), sample characteristics and sampling procedure, setting, random/nonrandom assignment and selection, blinding/double blinding, data collection methods, intervention characteristics, outcome variables and measures, internal and external validity, statistical methods, results, and conclusions.
Results
Methodological Quality
Based on published criteria for findings sufficient to support evidenced-based practice (Newhouse, Dearhold, Poe, Pugh, & White, 2007), 1 of the reviewed studies is a Level 1 (experimental study/randomized control trial; Smith-Coggins et al., 2006), while the remaining 12 are Level II (quasi-experimental; Table 1). Overall, the studies have reasonably consistent results, control conditions, and recommendations. Only one (Howard et al., 2010) included a determination of statistical power and rationale for sample size. The studies were likely underpowered, which may account for the lack of statistically significant effects in some studies. Because only two groups of investigators reported effect sizes (Kubo et al., 2010; Smith, Kilby, Jorgensen, & Douglas, 2007), it was hard to determine the clinical significance of the findings. We attempted to contact the corresponding authors for each study, but only one responded with effect sizes (Lovato et al., 2009). Investigators from two of the studies compared the effects of caffeine or naps with no-nap conditions (Rogers et al., 1989; Sagaspe et al., 2007). All studies had convenience samples (N = 6–49), and investigators recruited participants from a wide variety of international locations, work settings, and college student populations (Table 1). This variation in sample characteristics makes the studies difficult to compare.
Investigators used polysomnography (PSG) to measure nap duration, sleep architecture, and post-shift daytime sleep in 11 studies (Table 1), but only two groups reported the interrater reliability of the PSG scoring (Rogers et al., 1989; Signal et al., 2009). The studies included nearly 40 different tests of sleepiness and sleep-related performance deficits (Table 1). With the exception of Smith-Coggins et al. (2006), investigators did not report the reliability or validity of these measurements. Statistical methods were appropriate in all studies.
Participants in the quasi-experimental studies (Table 1) served as their own controls. Some of the investigators in these studies scheduled the nap and non-nap conditions 5–27 days apart in order to “wash out” the effects of the intervention (Howard et al., 2010; Kubo et al., 2010; Lovato et al., 2009; Saito & Sasaki, 1996; Sallinen et al., 1998; Signal et al., 2009; Smith et al., 2007; Takeyama et al., 2002; Takeyama et al., 2004). Some investigators used random assignment (Lovato et al., 2009; Purnell et al., 2002; Rogers et al., 1989; Sagaspe et al., 2007; Saito & Sasaki, 1996; Sallinen et al., 1998; Smith et al., 2007; Smith-Coggins et al., 2006; Takeyama et al., 2002; Takeyama et al., 2004) and blinded the order of testing (Howard et al., 2010; Rogers et al., 1989; Sagaspe et al., 2007; Smith et al., 2007; Smith-Coggins et al., 2006). Only four reports included explanations of control-condition activities, but the investigators did not report the environmental and temporal characteristics of the control conditions. In these four studies, instead of napping, participants either worked as usual (Smith-Coggins et al., 2006) or engaged in activities such as reading, watching television, or conversing with others (Lovato et al., 2009; Purnell et al., 2002; Takeyama et al., 2002).
Sample Characteristics
The samples included men and women with a variety of ages and occupations (Table 1). Some, but not all, investigators reported that participants were “healthy” (Howard et al., 2010; Kubo et al., 2010; Rogers et al., 1989; Saito & Sasaki, 1996; Takeyama et al., 2002; Takeyama et al., 2004), but the methods they used to determine health status were inconsistent. Some, but not all, investigators screened participants for primary sleep disorders (Howard et al., 2010; Kubo et al., 2010; Lovato et al., 2009; Sagaspe et al., 2007; Saito & Sasaki, 1996; Sallinen et al., 1998; Signal et al., 2009), while one group screened participants for psychological distress (Smith et al., 2007). In one study, participants had a legal responsibility to report “fit for work” (Signal et al., 2009).
There was thus a great deal of heterogeneity among the study samples, inconsistency in the methods investigators used to evaluate health and sleep disorders, and wide variability in employment settings, job characteristics and possible job stress.
Sleep Before, During, and After Nap Opportunities
Preexperimental Sleep
Mean diurnal sleep duration for the night prior to the night-shift nap study ranged from 7.1 to 8.5 hr in the studies in which investigators measured this variable (Kubo et al., 2010; Sagaspe et al., 2007; Sallinen et al., 1998; Signal et al., 2009; Smith-Coggins et al., 2006). Two studies involved pre-shift naps in which participants slept 105.66–106.91 min between the hours of 15:00 and 17:00 (Lovato et al., 2009) or 4.88 min between 20:15 and 20:45 hr (Howard et al., 2010).
Nap Opportunities and Settings
Planned nap start times occurred between 00:00 and 04:00 hours. Nap opportunities were between 20 min and 2 hr in duration (Table 1). Some investigators explained that naps greater than 30 min were impractical(Howard et al., 2010; Lovato et al., 2009) and could lead to sleep inertia (Lovato et al., 2009). Also, naps of 20 (Purnell et al., 2002) to 30 (Smith et al., 2007) min were consistent with employee break durations (Sagaspe et al., 2007; Smith et al., 2007). Purnell, Feyer, and Herbison (2002) further explained that scheduling planned naps for a time between 01:00 and 03:00 hr may reduce the risk of sleep inertia upon awakening. Only 3 of the studies included 2 (Purnell et al., 2002) or 3 nights of consecutive naps (Takeyama et al., 2002; Takeyama et al., 2004), while the remaining 10 studies used a single night of nap testing during a night shift or laboratory-based simulated night shift “awake.”
Settings for the naps included sleep laboratories (Howard et al., 2010; Kubo et al., 2010; Lovato et al., 2009; Rogers et al., 1989; Sallinen et al., 1998; Takeyama et al., 2002; Takeyama et al., 2004), an airplane hangar (Purnell et al., 2002), participants’ homes (Saito & Sasaki, 1996), cars (Sagaspe et al., 2007), a room away from the clinical work site (Smith-Coggins et al., 2006), and darkened, quiet rooms (Lovato et al., 2009; Rogers et al., 1989; Signal et al., 2009; Smith et al., 2007; Smith-Coggins et al., 2006). Most investigators reported the mean duration of sleep during the nap opportunities, and participants slept for the majority of the allotted time (Table 1). However, 50% of the sample on the first night and 42% on the second night in one study were not able to sleep during their nap opportunity (taken between 01:00 and 03:00 hr) because of “too much noise” (Purnell et al., 2002).
Effects of Night-Shift Naps on Daytime Sleep
Some investigators recorded daytime sleep after the night shift/simulated night shift. There was wide variation in postshift daytime sleep duration (190 min to almost 8 hr; Kubo et al., 2007; Purnell et al., 2002; Rogers et al., 1989; Saito & Sasaki, 1996; Signal et al., 2009; Smith et al., 2007). Most participants had similar postshift daytime sleep durations in the nap and no-nap conditions (Kubo et al., 2007; Purnell et al., 2002; Sallinen et al., 1998; Signal et al., 2009; Smith et al., 2007; Takeyama et al., 2002), with the exception of participants in Saito and Sasaki’s study, who slept longer in the no-nap condition.
Summary
Diurnal schedules with nighttime sleep during the 24 hr before the night shift led to prolonged wakefulness that may have increased homeostatic sleep pressure and the likelihood of sleep during the planned nap interval. Most participants slept during their nap opportunities, but the wide variety of nap opportunity times, lengths, and settings makes it difficult to ascertain the best nap intervention. Most participants, regardless of nap condition and characteristics, did not have difficulty sleeping during the daytime after their shifts, although, with the exception of participants in Smith, Kilby, Jorgensen, and Douglas’s (2007) study, sleep times were short. Because most of the investigators studied only 1 night of planned napping, little is known about the effects of naps over consecutive nights.
Sleepiness and Sleep-Related Performance Deficits
The investigators evaluated subjective and objective sleepiness and numerous sleep-related performance deficits, including reaction time and vigilance, cognitive functioning, subjective fatigue, logical reasoning performance, work tasks and driving, workload and memory recall. Most studies measured these phenomena at the start and end of the shifts and before and after nap opportunities. Table 1 lists the specific variables, measures and timing for each study, and Table 2 categorizes the instruments and methods investigators used by performance outcome.
Table 2.
Instruments and Tests Used in Reviewed Studies Categorized by Performance Outcome.
| Performance outcome | Instruments/tests used |
|---|---|
| Subjective (self-reported) sleepiness | Karolinska sleepiness scale, Stanford Sleepiness Scale, VAS for sleepiness, pictorial sleepiness scale |
| Objective sleepiness | Repeated test of sustained wakefulness, EOG for rolling eye movements, videotapes of behavioral alertness, sleep latency test |
| Reaction time and vigilance, cognitive functioning |
PVT-192 metrics: fastest 10% of responses, slowest 10% of responses, lapses, mean and median reaction times; visual vigilance task; critical flicker fusion frequency; 3-choice reaction time test; 2-choice visual reaction time test; Mackworth clock vigilance task; simple reaction time task; choice reaction time test; symbol-digit substitution task; letter cancellation task |
| Subjective fatigue | VAS; Profile of Mood States, Fatigue and Vigor subscales; Fatigue Feeling scale |
| Logical reasoning performance | Logical reasoning performance test, logical reasoning test |
| Work task and driving | Typing words into documents, mental arithmetic, driving simulator, catheter insertion simulation |
| Workload | NASA Task Load Index (mental, physical, temporal demands, performance effort, frustration) |
| Memory recall | Probed memory recall test |
Note. EOG = electrooculogram; PVT = psychomotor vigilance task; VAS = visual analog scale.
Effects of Naps Taken at 00:00 or 01:00 hr
When compared to a no-nap condition, 30-, 40-, and 50-min naps taken between 00:00 and 01:00 hr led to faster reaction time, fewer lapses, less subjective sleepiness, and improved driving performance at the end of a shift (Sagaspe et al., 2007; Sallinen et al., 1998; Signal et al., 2009). A nap of 60 or 120 min taken at 00:00 did not improve reaction time (Takeyama et al., 2004) compared to a no-nap condition.
Effects of Naps Taken at 02:00, 02:30, 03:00 Hr
When compared to no-nap conditions, 20-, 30-, 40-, 60- and 120-min naps taken between 02:00 and 03:00 hr resulted in improved outcomes, including mean response latency, reaction time, subjective sleepiness, sleep latency, fatigue, lapses, and probed memory recall testing through the end of the shift (Lovato et al., 2009; Purnell et al., 2002; Saito & Sasaki, 1996; Signal et al., 2009; Smith-Coggins et al., 2006; Smith et al., 2007; Takeyama et al., 2002). Effect sizes (pη2) ranging from 0.31 to 0.64 indicate that the naps and their timing had a robust influence on sleepiness, task load and psychomotor vigilance task (PVT) performance (Smith et al., 2007). Naps had moderate-to-large effects (Cohen’s d) on sleepiness and mean reaction time 15–225 min post nap (Lovato et al., 2009).
Outcomes of Naps Taken at 04:00
Naps of 30 and 50 min taken at 04:00 resulted in improved subjective sleepiness, driving performance, lapses, and reaction time post nap and through the end of the shift when compared to no-nap conditions (Howard et al., 2010; Sallinen et al., 1998). However, there were significant delays in reaction time after a 60-min nap, a finding that the researchers attributed to sleep inertia (Kubo et al., 2010; Takeyama et al., 2004). Effect sizes for reaction time and lapses in the 60-min nap condition (pη2 = 0.266 and 0.268, respectively) and sleepiness in the 120-min nap condition (pη2=.36) indicate a moderate influence of naps and their timing on these variables (Kubo et al., 2010).
Summary
Most investigators tested nap opportunities of 20–40 min with start times ranging from 0200 to 0300 hr. In most cases, the rationale was feasibility related to scheduling of work activities and break guidelines. In several studies (Kubo et al., 2010; Smith-Coggins et al., 2006; Takeyama et al., 2004), investigators attributed lack of improvement in some outcomes to sleep inertia despite improvements in other outcomes.
Discussion
The findings of this review indicate that planned naps during night shifts or simulated night shifts reduced nocturnal sleepiness and improved sleep-related performance deficits in a number of populations and settings. Although the wide variability in study designs, populations, settings, and measures make the studies difficult to compare, the findings do provide important information that may be useful in supporting future systematic research and prescriptions for napping in shift-work settings.
Although length and timing of naps varied widely among the reviewed studies, both 30- to 40-min nap opportunities between the hours of 02:00 and 03:00 (Lovato et al., 2009; Smith et al., 2007) and naps as short as 20 min during this time frame had beneficial effects (Purnell et al., 2002). While later naps (04:00) had small-to-moderate effects on improving reaction time and sleepiness (Table 1), one group of investigators suggested that sleep inertia may have an impact on the outcomes of naps taken during this time frame (Kubo et al., 2010). There is no agreement in the literature as to the time frame for the maximal impact of sleep inertia, with Kubo et al. (2010) suggesting 05:00 and Scheer, Shea, Hilton, and Shea (2008) suggesting 23:00–03:00. Given the potential deleterious effects on work performance of sleep inertia, a phenomenon that may last as long as 30 min (Dinges et al., 1987), further studies are needed to determine the optimal timing of naps in order to minimize the possible hazards associated with sleep inertia.
Although naps quite consistently improved sleepiness and sleep-related performance deficits in the reviewed studies, they seldom affected daytime sleep. The brief duration of daytime sleep in most studies suggests that participants remained sleep deprived. A single nap is not sufficient to make up for the sleep debt incurred by working all night. Individuals designing scheduled-nap interventions or studies should consider naps in the context of 24-hr sleep patterns and ensure that they are not used as a substitute for obtaining adequate sleep during the day following the shift work.
Investigators conducted the reviewed nap studies in a variety of workplace and laboratory settings. While laboratory studies afford the ability to control environmental and behavioral conditions, they lack ecological validity and may not adequately address the nature of the work (e.g., intensity, complexity), work context (stress, noise, inability to nap at specific times), shift-work patterns (e.g., days on/off, shift length), or the characteristics of the worker (e.g., age, gender, non-work role responsibilities). Future studies should systematically address these factors.
The feasibility of napping is an important consideration in planning shift-work nap studies or interventions. For example, those who work in health care settings or other highly stressful, demanding, and time-pressured environments may lack the time or be unable to sleep due to stress during the scheduled nap period. In other settings, quiet space appropriate for napping may be unavailable (Purnell et al., 2002).
Many characteristics of the workers and the contexts of their lives may influence napping, as well as performance, during shift work. For example, aging, gender, and years of experience on the night shift may contribute. Aging is associated with increased tendency to nap (Dinges, 1989), and women have more self-reported sleep complaints than men, which are often associated with hormonal factors (Redeker, 2011). The “healthy worker effect” (Oginska, Pokorski, & Oginski, 1993) associated with good accommodation to night shift work may explain the abilities of some workers to nap at night, sleep during the daytime, and also perform effectively. Nonwork-related role demands, such as caring for dependents, may also contribute to sleep deprivation and the ability to nap as well as to sleepiness and performance outcomes for shift workers.
Choice and timing of performance measures varied greatly across the reviewed studies, although all explored some aspect of sleepiness or psychomotor performance. Advances in the field are greatly dependent on in-depth understanding of the conceptual underpinnings and methodological issues associated with choice of outcomes. Most of the measures the investigators used are surrogates for actual work performance; therefore, an investigator must consider the attributes that are most important to the work being performed (e.g., driving, decision making, motor coordination) when selecting measures. In addition, each of the objective and subjective measures may assess different aspects of sleepiness or performance (Oken et al., 2006). It is also important to consider the feasibility of measurements and factors that might influence the results, including sensitivity to sleep loss and sleep inertia, practice effects, attention effects, testing conditions, and reliability.
Limitations
In this review, we addressed evidence available in the published literature. It is possible that investigators have conducted other studies but have not published them due to negative findings. In addition, few investigators reported effect sizes or were able to provide these data to the authors. Therefore, we were unable to conduct a meta-analysis, which would have been a more objective review approach.
Conclusion
The findings of this review suggest that planned naps hold promise as the means to improve sleepiness and sleep-related performance deficits among shift workers. Although there is some evidence of possible sleep inertia, these data are not consistent, and naps appear to be safe. Therefore, it may be feasible to implement nap programs in current workplace studies. Nevertheless, studies to date have been small and heterogeneous and have not documented the effects of napping on work-specific outcomes, such as decision making, errors, and safety in actual work-related tasks. Future studies should include larger, randomized controlled trials to establish the efficacy of napping in improving performance and safety. These studies need to confirm the appropriate dose and timing for a nap and subsequent recovery from sleep inertia and the interactions among nap behavior, job and shift-worker characteristics, and the nature of the workplace environment prior to wider-spread implementation of nap programs.
Acknowledgments
This research was supported by a Rutgers, the State University of New Jersey, College of Nursing Faculty Research Award and the Hurdis M. Griffith Faculty Research Award.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Achermann P, Borbely AA. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed Elsevier; St. Louis, MO: 2011. pp. 431–444. [Google Scholar]
- Akerstedt T. Shift work and disturbed sleep/wakefulness. Occupational Medicine. 2003;53:89–94. doi: 10.1093/occmed/kqg046. [DOI] [PubMed] [Google Scholar]
- Balas MC, Scott LD, Rogers AE. Frequency and type of errors and near errors reported by critical care nurses. Canadian Journal of Nursing Research. 2006;38:24–41. [PubMed] [Google Scholar]
- Banks S, Dinges DF. Chronic sleep deprivation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed Elsevier; St. Louis, MO: 2011. pp. 67–75. [Google Scholar]
- Belenky G, Akerstedt T. Occupational sleep medicine: Introduction. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed Elsevier; St. Louis, MO: 2011. pp. 734–737. [Google Scholar]
- Caldwell JA, Mallis MM, Caldwell JL, Paul MA, Miller JC, Neri DF. Fatigue countermeasures in aviation. Aviation, Space, and Environmental Medicine. 2009;80:29–59. doi: 10.3357/asem.2435.2009. [DOI] [PubMed] [Google Scholar]
- Caruso CC, Rosa R. Shift work and long work hours. In: Rom WM, editor. Environmental and occupational medicine. 4th ed Lippincott, Williams & Wilkins; Philadelphia, PA: 2007. pp. 1359–1363. [Google Scholar]
- Czeisler CA, Brown EN. Commentary: Models of the effect of light on the human circadian system: Current state of the art. Journal of Biological Rhythms. 1999;14:538–543. doi: 10.1177/074873099129000876. [DOI] [PubMed] [Google Scholar]
- Dinges DF. Napping patterns and effects in human adults. In: Dinges DF, Broughton RJ, editors. Sleep and alertness: Chronobiological, behavioural, and Medical Aspects of Napping. Raven Press; New York, NY: 1989. pp. 171–204. [Google Scholar]
- Dinges DF, Orne MT, Whitehouse WG, Orne EC. Temporal placement of a nap for alertness: Contributions of circa-dian phase and prior wakefulness. Sleep. 1987;10:313–329. [PubMed] [Google Scholar]
- Drake CL, Wright KP. Shift work, shift work disorder, and jet lag. In: Krieger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed Elsevier; St. Louis, MO: 2011. pp. 784–798. [Google Scholar]
- Geiger-Brown J, McPhaul KM. Sleep promotion in occupational health settings. In: Redeker NS, McEnany GP, editors. Sleep disorders and sleep promotion in nursing practice. Springer; New York, NY: 2011. pp. 355–369. [Google Scholar]
- Howard ME, Radford L, Jackson ML, Swann P, Kennedy GA. The effects of a 30-minute napping opportunity during an actual night shift on performance and sleepiness in shift workers. Biological Rhythm Research. 2010;41:137–148. doi:10.1080/09291010903030946. [Google Scholar]
- Joint Commission Health care worker fatigue and patient safety. 2011 Retrieved from http://www.pwrnewmedia.com/2011/joint_commission/fatigue/downloads/printfriendly.pdf. [PubMed]
- Kemper M. The role and effectiveness of napping on the work performances of shift workers. Work. 2001;16:153. [PubMed] [Google Scholar]
- Kubo T, Takahashi M, Takeyama H, Matsumoto S, Ebara T, Murata K, Itani T. How do the timing and length of a night-shift nap affect sleep inertia? Chronobiology International. 2010;27:1031–1044. doi: 10.3109/07420528.2010.489502. doi:10.3109/07420528.2010.489502. [DOI] [PubMed] [Google Scholar]
- Kubo T, Takeyama H, Matsumoto S, Ebara T, Murata K, Tachi N, Itani T. Impact of nap length, nap timing and sleep quality on sustaining early morning performance. Industrial Health. 2007;45:552–563. doi: 10.2486/indhealth.45.552. [DOI] [PubMed] [Google Scholar]
- Landis CA. Physiological and behavioral aspects of sleep. In: Redeker NS, McEnany G, editors. Sleep disorders and sleep promotion in nursing practice. Springer; New York, NY: 2011. pp. 1–18. [Google Scholar]
- Lovato N, Lack L, Ferguson SJ, Tremaine R. The effects of a 30-min nap during night shift following a prophylactic sleep in the afternoon. Sleep & Biological Rhythms. 2009;7:34–42. doi: 10.1111/j.1479-8425.2009.00382.x. [Google Scholar]
- MacFarlane JG, Moldofsky H. Fibromyalgia and chronic fatigue syndromes. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed Elsevier; St. Louis, MO: 2011. pp. 1422–1434. [Google Scholar]
- Mahowald MW, Bornemann MAC. Parasomnias. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed Elsevier; St. Louis, MO: 2011. pp. 1075–1082. [Google Scholar]
- Monk TH. Shift work: Basic principles. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed Elsevier-Saunders; Philadelphia, PA: 2005. pp. 673–679. [Google Scholar]
- National Sleep Foundation National Sleep Foundation 2009 Sleep in America Poll. 2009 Retrieved from http://healthyliving.free-domblogging.com/files/2009/03/2009sleeppoll.pdf.
- Newhouse R, Dearhold S, Poe S, Pugh LC, White K. The Johns Hopkins nursing evidence-based practice rating scale. Johns Hopkins Hospital, Johns Hopkins University School of Nursing; Baltimore, MD: 2007. [Google Scholar]
- Oginska H, Pokorski J, Oginski A. Gender, aging, and shiftwork intolerance. Ergonomics. 1993;36:161–168. doi: 10.1080/00140139308967868. [DOI] [PubMed] [Google Scholar]
- Oken BS, Salinsky MC, Elsas SM. Vigilance, alertness, or sustained attention: Physiological basis and measurement. Clinical Neurophysiology. 2006;117:1885–1901. doi: 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie KD, Powell D, Broadbent E. Fatigue self-management strategies and reported fatigue in international pilots. Ergonomics. 2004;47:461–468. doi: 10.1080/0014013031000085653. [DOI] [PubMed] [Google Scholar]
- Piper BF, Lindsey AM, Dodd MJ. Fatigue mechanisms in cancer patients: Developing nursing theory. Oncology Nursing Forum. 1987;14:17–23. [PubMed] [Google Scholar]
- Purnell MT, Feyer AM, Herbison GP. The impact of a nap opportunity during the night shift on the performance and alertness of 12-hr shift workers. Journal of Sleep Research. 2002;11:219–227. doi: 10.1046/j.1365-2869.2002.00309.x. [DOI] [PubMed] [Google Scholar]
- Redeker NS. Developmental aspects of normal sleep. In: Redeker NS, McEnany G, editors. Sleep disorders and sleep promotion in nursing practice. Springer; New York, NY: 2011. pp. 19–32. [Google Scholar]
- Rogers AS, Spencer MB, Stone BM, Nicholson AN. The influence of a 1 h nap on performance overnight. Ergo-nomics. 1989;32:1193–1205. doi: 10.1080/00140138908966890. [DOI] [PubMed] [Google Scholar]
- Rollinson DC, Rathlev NK, Moss M, Killiany R, Sassower KC, Auerbach S, Fish SS. The effects of consecutive night shifts on the neuropsychological performance of interns in the emergency department: A pilot study. Annals of Emergency Medicine. 2003;41:400–406. doi: 10.1067/mem.2003.77. [DOI] [PubMed] [Google Scholar]
- Ruggiero JS, Avi-Itzhak T. [Schedules and shifts of emergency nurses] Unpublished raw data. 2011.
- Ruggiero JS, Pezzino JM. Nurses’ perceptions of the advantages and disadvantages of their shift and work schedules. Journal of Nursing Administration. 2006;36:450–453. doi: 10.1097/00005110-200610000-00004. doi:00005110-200610000-00004 [pii] [DOI] [PubMed] [Google Scholar]
- Sagaspe P, Taillard J, Chaumet G, Moore N, Bioulac B, Philip P. Aging and nocturnal driving: Better with coffee or a nap? A randomized study. Sleep. 2007;30:1808–1813. doi: 10.1093/sleep/30.12.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Sasaki T. The effect of length of a nocturnal nap on fatigue feelings during subsequent early morning hours. Journal of Science of Labour. 1996;72:15–23. [Google Scholar]
- Sallinen M, Harma M, Akerstedt T, Rosa R, Lillqvist O. Promoting alertness with a short nap during a night shift. Journal of Sleep Research. 1998;7:240–247. doi: 10.1046/j.1365-2869.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. Journal of Biological Rhythms. 2008;23:353–361. doi: 10.1177/0748730408318081. doi:10.1177/0748730408318081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LD, Hwang WT, Rogers AE, Nysse T, Dean GE, Dinges DF. The relationship between nurse work schedules, sleep duration, and drowsy driving. Sleep. 2007;30:1801–1807. doi: 10.1093/sleep/30.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signal TL, Gander PH, Anderson H, Brash S. Scheduled napping as a countermeasure to sleepiness in air traffic controllers. Journal of Sleep Research. 2009;18:11–19. doi: 10.1111/j.1365-2869.2008.00702.x. doi:JSR702 [pii] 10.1111/j.1365-2869.2008.00702.x. [DOI] [PubMed] [Google Scholar]
- Smith-Coggins R, Howard SK, Mac DT, Wang C, Kwan S, Rosekind MR, Gaba DM. Improving alertness and performance in emergency department physicians and nurses: The use of planned naps. Annals of Emergency Medicine. 2006;48:596–604. e3. doi: 10.1016/j.annemergmed.2006.02.005. doi:10.1016/j.annemergmed.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Smith SS, Kilby S, Jorgensen G, Douglas JA. Napping and nightshift work: Effects of a short nap on psychomotor vigilance and subjective sleepiness in health workers. Sleep & Biological Rhythms. 2007;5:117–125. doi:10.1111/j.1479-8425.2007.00261.x. [Google Scholar]
- Steele MT, Ma OJ, Watson WA, Thomas HA, Jr., Muelleman RL. The occupational risk of motor vehicle collisions for emergency medicine residents. Academic Emergency Medicine. 1999;6:1050–1053. doi: 10.1111/j.1553-2712.1999.tb01191.x. [DOI] [PubMed] [Google Scholar]
- Taillard J, Philip P, Coste O, Sagaspe P, Bioulac B. The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. Journal of Sleep Research. 2003;12:275–282. doi: 10.1046/j.0962-1105.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- Takeyama H, Itani T, Tachi N, Sakamura O, Suzumura H. Psycho-physiological effects of naps during night shifts on morning types and evening types. Journal of Occupational Health. 2002;44:89–98. [Google Scholar]
- Takeyama H, Matsumoto S, Murata K, Ebara T, Kubo T, Tachi N, Itani T. Effects of the length and timing of nighttime naps on task performance and physiological function. Revista de Saude Publica. 2004;38:32–37. doi: 10.1590/s0034-89102004000700006. doi:/S0034-89102004000700006. [DOI] [PubMed] [Google Scholar]
- Tassi P, Muzet A. Sleep inertia. Sleep Medicine Reviews. 2000;4:341–353. doi: 10.1053/smrv.2000.0098. doi:10.1053/smrv.2000.0098. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency Emergency management: Exxon Valdez. 2011 Retrieved from http://www.epa.gov/oem/content/learning/exxon.htm.
- World Nuclear Association Three Mile Island accident. 2010 Retrieved from http://www.world-nuclear.org/info/inf36.html.
- World Nuclear Association Chernobyl accident 1986. 2011 Retrieved from http://www.world-nuclear.org/info/chernobyl/inf07.html.