Abstract
The hemolymph of the American horseshoe crab, Limulus polyphemus, is harvested from over 500,000 animals annually to produce Limulus Amebocyte Lysate, a medically important product used to detect pathogenic bacteria. Declining abundance of spawning Limulus females in heavily harvested regions suggests deleterious effects of this activity and, while mortality rates of the harvest process are known to be 10–30%, sub-lethal behavioral and physiological effects are not known. In this study, we determined the impact of the harvest process on locomotion and hemocyanin levels of 28 female horseshoe crabs. While mortality rates after bleeding (18%) were similar to previous studies, we found significant decreases in the linear and angular velocity of freely moving animals, as well as changes in their activity levels and expression of circatidal behavioral rhythms. Further, we found reductions in hemocyanin levels, which may alter immune function and cuticle integrity. These previously unrecognized behavioral and physiological deficits suggest that the harvest of Limulus Amebocyte Lysate may decrease female fitness, and thus may contribute to the current population decline.
Keywords: American horseshoe crabs, Limulus polyphemus, circadian, circatidal, LAL, bleeding, activity
Introduction
The American horseshoe crab, Limulus polyphemus, is valued for both its ecological and economic importance. Ecologically, L. polyphemus is a keystone species in marine ecosystems of the Atlantic and Gulf coasts of North America (Botton, 1984b), serving as a bioturbator (Krauter and Fegley, 1994; Lee, 2010), a food source for shorebirds, fish and crustaceans (Botton 1984a), and a predator of mollusks and polychaete worms (Botton, 1984b). Commercially, L. polyphemus is the preferred bait source for the whelk (Buscyon carica, Buccinum undatum, and Busycotypus canaliculatus; ASMFC, 2012) and eel (Anguilla rostra and Anguilla Anguilla: ASMFC, 1998) fisheries, and its hemolymph provides the raw material for Limulus Amebocyte Lysate (LAL), the industry standard for detection of bacterial endotoxin in pharmaceuticals, vaccines, and medical devices (Novitsky, 2009). The magnitude of the LAL harvest, principally composed of female horseshoe crabs (50–77%; Rutecki et al., 2004; ASFMC, 2012), has increased 76% since 2006, during which time New England populations of L. polyphemus have declined, despite a 45% bait harvest reduction (ASMFC, 2012). Further, population trends in heavily harvested Pleasant Bay, MA, have evoked concern over possible effects of the LAL harvest process (Malkoski, 2010; ASFMC, 2012; James-Pirri, 2012). In this region, where horseshoe crabs have been harvested for LAL production for over 30 years, but closed to the bait fishery since 2006 (Rutecki et al., 2004; Leschen and Correia, 2010), the proportion of females appearing at spawning beaches has declined from 30% (Carmichael et al., 2003) to 10% (Malkoski, 2010; James-Pirri, 2012) and egg abundances at spawning beaches have significantly decreased (James-Pirri, 2012). These trends have prompted researchers and environmental managers to suggest that the LAL harvest process, while causing moderate mortality rates of 8–15% in males (Walls and Berkson, 2003; Hurton and Berkson, 2006) and 10– 29% in females (Hurton and Berkson, 2006; Leschen and Correia, 2010), may also induce behavioral and physiological effects in L. polyphemus, which could lead to alterations in spawning activity (Malkoski, 2010; James-Pirri, 2012). Moreover, these effects could be exacerbated by the high level (50%) of the annual LAL harvest that occurs during the spawning season (Leschen and Correia, 2010).
The LAL harvest procedure incorporates multiple stressors, several of which have been shown to alter both the behavior and physiology of marine species. Briefly, hemolymph is obtained in a 24–72 h process that includes trawl or hand-harvest capture, transport to, and time spent in, containment at a biomedical facility, a 30% blood extraction, and return to the point of capture (ASMFC, 1998; Leschen and Correia, 2010). Interestingly, numerous crustaceans exhibit both transient and long-term (1–4 week) behavioral (Harris and Andrews, 2005; Parsons and Eggleston, 2005; Haupt et al., 2006) and physiological (Vermeer, 1987; Bergmann et al., 2001; Ridgway et al., 2006; Patterson et al., 2007) alterations in response to similar capture stressors, with effects including altered hemolymph biochemistry (Vermeer, 1987; Ridgway et al., 2006), reduced immune function (Ridgway et al., 2006), decreased predator avoidance behaviors (Brown and Caputi, 1983: Vermeer, 1987), altered responses to stimuli (Parsons and Eggleston, 2005), reduced locomotion (Davis et al., 1978), and diminished or altered spawning behaviors (Smith and Ritar, 2005). The LAL harvest process compounds typical capture through an extended (up to 72 h) period of aerial exposure and substantial blood loss, and thus has the potential to effect analogous behavioral and physiological changes in L. polyphemus.
Immediate and long-term behavioral responses of L. polyphemus to the biomedical bleeding process have been little studied. Both activity levels (Rudloe, 1983) and movement velocity (Kurz and James-Pirri, 2002) have been reported to be unaffected by a hemolymph extraction performed with a minimal (30 min to 3 h) amount of aerial exposure. Interestingly, a high-stress hemolymph extraction process, which includes 48 h of aerial exposure combined with thermal stress, causes the highest mortality (Hurton and Berkson, 2006), although neither activity nor velocity alterations in response to such a process have been investigated. In addition, a salient behavioral feature of L. polyphemus is the expression of tidal activity rhythms, driven by a circatidal clock (Chabot et al., 2004), which facilitates successful spawning and foraging activities in the wild (Cohen and Brockmann, 1983; Barlow et al., 1986; Watson and Chabot, 2010). In other species, disruptions of behavioral and physiological circadian rhythms occur in response to a variety of environmental stressors, including alterations in light intensities (Procambarus clarkii and Procambarus digueti; Fanjul-Moles et al., 1998), decreased water quality (Astacus astacus; Styrishave et al., 1995), and trawl capture (Nephrops norvegicus; Aguzzi et al., 2005), and disruptions in circatidal rhythms occur in response to osmotic (Ruditapes philippanarum; Kim et al., 2001) or thermal stress (Chthamalus bisinuatus; Kasten and Flores, 2013). However, to date, the ramifications of the high-stress biomedical bleeding process on behavior and physiology of horseshoe crabs have not been well characterized.
The effects of the harvest process on hemolymph properties also have not been clearly elucidated. While L. polyphemus regains its blood volume within three to 30 days after being bled (Rudloe, 1983; Novitsky, 1984), restitution of amebocytes takes up to four months (Novitsky, 1984), and the length of time required for recovery of additional hemolymph constituents is unclear. Capture stress alone decreases total hemolymph protein concentration in some crustaceans (Ridgway et al., 2006), and this decline is correlated with reduced immune system functioning and increased susceptibility to infection in lobsters, Homarus americanus (Theriault et al., 2008). The biomedical bleeding process combines capture stress with substantial hemolymph loss, and L. polyphemus exhibits significantly decreased hemolymph protein concentration for at least two weeks after extraction (James-Pirri et al., 2012). Over 90% of L. polyphemus hemolymph protein is the respiratory pigment hemocyanin (Ding et al., 2005). In addition to aiding in the circulation of oxygen, hemocyanin may also participate in the primary immune response (Coates et al., 2011), and cuticle hardening and wound repair (Adachi et al., 2005). Poor environmental conditions, including thermal and captivity stress, accelerate hemocyanin decline in L. polyphemus (Coates et al., 2012), and therefore the combination of all these stressors is likely to produce a more dramatic decline than either of them alone.
The overall goal of this investigation was to determine whether the biomedical bleeding process induces sub-lethal physiological and behavioral effects in female horseshoe crabs during their spawning season. Our specific aims were to evaluate: (1) overall activity, linear and angular velocities of their movements, and expression of tidal rhythms for two weeks before and four weeks after a 52 h bleeding process, and (2) hemocyanin concentrations immediately prior to, and six weeks after, a 52 h bleeding process. We evaluated these parameters in L. polyphemus from Great Bay, NH. While this population is genetically distinct from the harvested mid-Atlantic populations, the genetic distance is low (King et al., 2005); further, this population has not historically been harvested for biomedical bleeding (ASFMC, 2012), and so animals were presumably naïve to the process. We performed both laboratory studies, which allowed continuous monitoring of animal activities, and an outdoor study, which enabled evaluation of bleeding impacts in a quasi-natural environment. Our results suggest that L. polyphemus experiences sub-lethal behavioral (reduced activity, velocity of movements, and expression of circatidal rhythms) and physiological (chronic hemocyanin loss) alterations in response to the bleeding process, and these should be considered when assessing the impact of this procedure on horseshoe crab populations.
Materials and Methods
2.1 Animals- Treatment Groups and Conditions
Fifty-six female horseshoe crabs were collected during high tide at spawning beaches on Adams Point, Durham, New Hampshire, from May 15–23, 2012, and their prosomal width was measured. Animals were distributed by size into four experimental groups; this distribution by size was necessary because of constraints of experimental equipment. The largest 14 animals (prosomal width: 18–23 cm) were assigned to the outdoor unrestrained (OU) group, and they were placed in tanks outside of the University of New Hampshire Jackson Estuarine Laboratory (JEL). These outdoor tanks could accommodate larger animals than could our indoor laboratory tanks. The remaining 42 animals were transported by van to Plymouth State University (PSU; trip duration was 2 h) in polyurethane coolers. The smallest 14 animals (prosomal width: 16–18 cm) were assigned to the laboratory running wheel (LRW) group; the smallest animals were selected for this group because pre-constructed running wheels could only fit animals of prosomal width less than 18.5 cm. The remaining 28 animals were equally divided between the laboratory unrestrained (LU) and laboratory communal tank (LCT) groups. Because smaller animals were selected for the LRW group, prosomal width varied significantly (F(3,52) = 6.05, P = 0.002; Table 1) across the four groups, with prosomal width in the OU and LU groups greater than that of the LRW group (P < 0.05), while prosomal width in the LCT group was not significantly different from any of the other groups.
Table 1.
Size, amount of hemolymph extracted, and mortality in the four female bled groups.
| Treatment | Prosomal Width (cm) | Extracted Hemolymph Volume (mL) | Estimated Percent Extraction | Total Mortality | Percent Mortality |
|---|---|---|---|---|---|
| Lab Running Wheel | 17.5 ± 0.8 | 28.9 ± 4.7 | 15.5 ± 2.5 | 1 | 14 |
| Lab Unrestrained | 18.9 ± 0.3 | 47.6 ± 7.0 | 21.0 ± 3.0 | 1 | 14 |
| Outdoor Unrestrained | 19.1 ± 0.4 | 64.4 ± 2.8 | 28.1 ± 1.1 | 0 | 0 |
| Lab Communal Tank | 18.4 ± 0.4 | 29.0 ± 4.9 | 14.2 ± 2.4 | 3 | 42 |
Values are mean ± SEM; n = 7 per group. Estimated percent extraction determined by comparing actual amount extracted to expected hemolymph volume, calculated using prosomal width.
2.1.1 Outdoor Unrestrained Group
The purpose of the OU group (n = 14; prosomal width: 19.1 ± 0.4 cm, mean ± SEM) was to monitor activity in animals exposed to a natural photoperiod and constantly replenished estuarine water. Accelerometers (Onset Computer, Pocasset, MA), set to measure acceleration (g) in the three orthogonal axes, were affixed to the prosoma of each animal using cable ties, duct tape, and cyanoacrylate (Schaller et al., 2010). Animals were placed in separate cylindrical wire enclosures (70 cm diameter × 48 cm height) within seven 850-L tanks (183 cm × 92 cm × 50 cm) containing approximately 15 cm of sand so animals had the opportunity to bury. Water from the estuary continuously flowed (~4 L/min) through the tanks, keeping salinity and temperature consistent with that of Great Bay, NH, and the tanks remained uncovered and exposed to the natural light/dark cycle (approximate sunrise: 5:01–5:15 am; sunset: 8:00–8:28 pm). The animals were allowed access to 2% of their body weight in diced quahogs three times a week by placing the food in the bottom of the tanks. Activity was logged via the accelerometers for two weeks prior to the biomedical bleeding process.
2.1.2 Indoor Groups at Plymouth State University
To simulate the summer photoperiod, all indoor groups were maintained under a 14:10 light/dark (LD) cycle with instantaneous photic transitions, salinity between 25 and 30 psu, and temperature between 18–21°C. Temperature and lighting conditions were continuously recorded using Vernier Labquest handheld data collection units connected to a light sensor and temperature probe (Vernier Software and Technology LCC, Beaverton, OR). Activity was measured in two separate laboratory groups via two different monitoring systems, enabling comparison between the two techniques for validation of results.
Laboratory Running Wheel Group
The activity of this group of 14 animals (prosomal width: 17.5 ± 0.8 cm) was monitored using “running wheels,” constructed as described in Chabot et al. (2004, 2007). The animal was secured within the wheel with its telson sticking out through a slit. Then a polypropylene plastic golf ball was placed on the telson to prevent it from being drawn into the wheel. Cable ties were used to attach the front of the carapace to the frame. After all animals were prepared, the running wheels were distributed among four custom-made acrylic recirculating open top tanks (80 cm L × 65 cm W × 32 cm D). Wheel rotations were recorded with ClockLab Data Collection Software (Actimetrics, Wilmette, IL).
Laboratory Unrestrained Group
The activity of this group of 14 freely moving animals (prosomal width: 18.9 ± 0.3 cm) was monitored using video recording. The animals were distributed between two large (1.7 m L × .9 m W × .75 m D) tanks, each with a separate filter system. The tanks were subdivided by plastic egg grating (1 cm × 1 cm) into a total of eight arenas per tank (each 21 cm × 45 cm), and a 4 m length of waterproof red LED Ribbon Flex strip lighting (λ = 630–660 nm; LED Liquidators, Inc., Westlake Village, CA) was threaded through the egg grating in each tank to provide continuous illumination for video recording. One animal was placed into each of seven arenas per tank (one arena in each tank was excluded to house the filtration system). An infrared video camera was suspended 1 m above the tanks, and digital video recordings were obtained at a rate of one frame per 20 s using Gawker software (Piwonka, Seattle, WA). The videos were then analyzed for total distance moved, linear velocity, and angular velocity using Ethovision XT software (Noldus Information Technology Inc., Wageningen, Netherlands).
Laboratory Communal Tank Group
The 14 animals in this group were kept in one acrylic recirculating open top tank (80 cm L × 65 cm W × 32 cm D). Activity of this group was not measured; hemocyanin concentrations in control and bled horseshoe crabs of the LCT group (n = 14; prosomal width: 18.4 ± 0.4 cm) were measured from 1–2 mL blood samples taken each week, using the “Hemolymph Sampling” process detailed below.
2.2 Biomedical Bleeding Procedure
After collecting behavioral data for two weeks, 28 animals were bled using a process that approximated the standard biomedical bleeding procedure (high stress: Hurton and Berkson, 2006).
2.2.1 Laboratory Groups
The bleeding process for these groups was completed from June 1–3, 2012. Half of the horseshoe crabs from each of the LRW, LCT, and LU groups (n = 7 each) were randomly selected to undergo the bleeding process and distributed among three 50-gallon plastic barrels; the remaining 21 remained in their treatment conditions as controls. The temperature within the barrels was monitored using a Vernier Labquest with thermometer attachment during the 52 h process.
Pre-Bleeding Procedure
To replicate the capture and transportation during a typical biomedical bleeding process, the barrels were placed on the roof of Boyd Hall at PSU for 8 h. For the first 4 h, the barrels were kept in direct sunlight to simulate time spent on the deck of a boat; during this time, temperatures reached 37°C (mean ± SD: 32.0 ± 2.9°C). For the next 4 h, the barrels were covered with cardboard and moved into shade to simulate time spent in a truck en route to a bleeding facility (26.1 ± 1.1°C). Then the covered barrels were moved indoors (20.8 ± 0.9°C) for 16 h to simulate time spent overnight at the bleeding facility and then hemolymph was withdrawn.
Bleeding Procedure
Hemolymph was extracted using the procedure of Armstrong and Conrad (2008). One person held the animal in the abdominal flexure position, exposing the arthrodial membrane of the medial dorsal surface at the joint between the prosoma and the opisthosoma, while a second person withdrew the hemolymph. The arthrodial membrane was sterilized with 70% ethanol and punctured with a 14 gauge needle. Hemolymph was collected in 50 mL conical tubes, pre-chilled on ice, until the flow stopped or the estimated 30% volume had been reached. The equation of Hurton et al. (2005) was used to estimate total hemolymph volume for each horseshoe crab:
Extraction volumes ranged from 30 to 75 mL (mean ± SE: 35.8 ± 4.6 mL) and were generally less than the calculated 30% volume (17.3 ± 2.0%; Table 1). The hemolymph was kept on ice until processed further.
Post Bleeding Procedure
Animals were returned to their barrels and held indoors for 24 h (19.8 ± 0.9°C) to simulate a second overnight at a bleeding facility. The barrels were placed next to a heater (24.0 ± 1.3°C) and shaken periodically for 4 h to simulate transportation by truck back to the ocean. Finally, they were returned to their treatment conditions after a total of 52 h out of water. Activity in the LU and LRW groups was recorded for the next six weeks, and weekly hemolymph samples were taken from the LCT group. Tanks were checked daily for mortalities.
2.2.2 Outdoor group
The bleeding process for the OU group took place from June 6–8 and used a treatment paradigm similar to that used on the laboratory groups, though with minor modifications to adjust for poor weather conditions. Seven horseshoe crabs (one per tank) were selected to undergo the bleeding process; their accelerometers were detached, and they were distributed between two 50-gallon plastic barrels. The barrels were first kept under a heat lamp for 4 h to simulate time spent on the deck of a boat; during this time, temperature reached 28°C (26.1 ± 2.1°C). Then the barrels were transported by van to PSU (2 h; 21.3 ± 2.7°C) and placed indoors (18.2 ± 1.7°C) overnight (16 h). Then hemolymph was extracted as described for the indoor groups and the animals were returned to their barrels and kept indoors, uncovered, for 24 h (18.0 ± 0.5°C). The barrels were transported by van back to JEL (2 h; 25.6 ± 0.3°C) and placed outdoors, covered, for the final 4 h to complete the simulation of transport back to the ocean (24.2 ± 1.1°C). Finally, the accelerometers were reattached and the horseshoe crabs were returned to their original tanks, after being out of water for 52 h, and their activity was recorded for the next six weeks.
The percent of hemolymph extracted varied significantly (F(3,24) = 7.2, P = 0.001; Table 1) across the four groups. Percent extracted in the OU group significantly exceeded that of the LRW and LCT groups (P < 0.05), while percent extracted in the laboratory unrestrained group was not significantly different from the other groups.
2.3 Hemolymph Sampling
Weekly 1–2 mL hemolymph samples were taken from all animals in the LCT group. Samples were taken from all control animals at the time of the bleeding process for the bled groups, and from all animals six weeks after the bleeding process. Hemolymph was extracted following the procedure used for the bled group, except it was collected with 25-gauge needles in 2.0-mL microcentrifuge tubes. During this process, each crab was kept out of water for no longer than 5 min.
2.4 Determination of Hemocyanin Concentrations
Hemocyanin concentrations were determined using the procedure of Coates et al. (2012). Samples were centrifuged for 10 min at 3000 g and 4°C, and then the supernatant (cell-free hemolymph) was stored at 4°C until analysis (1–2 days). An aliquot of hemolymph was diluted 1:100 in 0.1 M Tris-HCl buffer (pH- 7.5), and absorbance was measured at 280 nm on a UV-160 spectrophotometer (Shimadzu, Columbia, MD). Hemocyanin concentration was calculated using an absorbance of 1.39 for a 1 mg/mL hemocyanin solution in a quartz cuvette (pathlength of 1 cm; Coates et al., 2012).
2.5 Data Analysis
Laboratory Running Wheel Activity
The ClockLab collection system recorded the number of wheel revolutions per minute, and these data were used to generate actograms and Lomb-Scargle periodograms. The number of wheel revolutions was multiplied by the circumference of the wheel to obtain distance moved per minute, and these distances were summed to obtain distance moved per day. The daily sums were averaged over seven day intervals to obtain average daily distance during the two weeks before bleeding and the three weeks after bleeding.
Laboratory Unrestrained Activity
Video files were analyzed for distance moved (cm), linear velocity (cm/s), and angular velocity (degrees/s) at 20-s intervals using Ethovision XT software (Noldus Information Technology Inc., Leesburg, VA). Distance was summed per minute, and these values were used to generate actograms and Lomb-Scargle periodograms using ClockLab. Distance moved per day was calculated, and these sums were averaged over seven day intervals to obtain average daily distance for the two weeks before and the three weeks after the bleeding process. Linear velocity and angular velocity during periods of movement (distance moved > 5 cm per 20 seconds) were averaged for the two weeks prior to and the four weeks after the bleeding process. Linear velocity measured locomotive speed in one direction (net distance moved divided by 20 s between sampling intervals), while angular velocity measured rate of directional change (change in direction of movement between two consecutive samples divided by 20 s; Ethovision XT).
Outdoor Unrestrained Activity
The accelerometers provided acceleration in the three orthogonal axes each minute. The difference between successive x, y, and z coordinates was calculated, and these differences were used to calculate the net acceleration vector. These values were used to generate actograms and Lomb-Scargle periodograms. The daily percent of time active was determined by summing the number of minutes per day with a net vector exceeding 0.02 (determined by frequency histogram analysis to be the threshold value for background noise). The daily percentages were averaged over seven day intervals to obtain weekly values for the two weeks before and the three weeks after the bleeding process.
Hemocyanin Concentration
The percent of original hemocyanin remaining for each animal was calculated as the ratio of final hemocyanin concentration (six weeks post bleeding) to the original hemocyanin concentration (day of bleeding). To distinguish hemocyanin loss due to hemolymph extraction from hemocyanin decline due to other factors, the amount of hemocyanin extracted (volume of hemolymph extracted multiplied by initial hemocyanin concentration) was subtracted from the estimated total hemocyanin (initial hemocyanin concentration multiplied by estimated hemolymph volume); this value was compared to final total hemocyanin (hemocyanin concentration six weeks post-bleeding multiplied by estimated hemolymph volume). In the LCT group, rate of hemocyanin concentration decline (mg*mL−1*week−1) was calculated as the ratio of concentration difference between successive hemolymph samples to the number of weeks between samples.
2.6 Statistical Analyses
Repeated measures ANOVAs, two-way ANOVAs, mixed model ANOVAs, or Student's T-tests (P < 0.05) were performed using Minitab (Minitab Inc., State College, PA) to examine the effects of bleeding on physiological and behavioral parameters. Tukey's HSD post-hoc analyses (P < 0.05) were used to examine differences between means. Lomb-Scargle periodogram analyses were used to determine whether animals expressed significant circatidal (~12.4 h) or daily (~24 h) rhythms each week (peaks exceeding P = 0.001; tidal: 10–14 h range; daily: 22–26 h range; arrhythmic: no significant peaks). Mixed model ANOVAs with repeated measures in one factor were used to compare percentages of animals expressing tidal rhythms in the three activity groups and the rates of hemocyanin decline in the LCT group. Two-way ANOVAs were used to test for effects of environmental conditions (LRW, LU, and OU) and treatment (bled/control) on percent of animals expressing tidal rhythms and percent of hemocyanin remaining in the four treatment groups. Repeated measures ANOVAs were used to compare pre-bleeding activity, linear velocity, and angular velocity to that post-bleeding. Correlational and single linear regression analyses were used to determine relationships between hemocyanin decline and activity, and immediate activity to second week post-bleeding activity.
Results
3.1 Alterations in Behavior: Biological Rhythms, Activity, and Velocity
The bleeding process affected both activity levels and expression of tidal rhythms (Figs. 1–3; Table 2). Bled animals (LRW and OU) decreased their expression of tidal rhythms during the second week post- bleeding (Table 2, Fig. 1). There was a significant interaction of the bleeding treatment by time (F(3, 23) = 9.55, P = 0.005) on the percent of animals expressing tidal rhythms. Specifically, expression of tidal rhythms in bled animals during the second week after bleeding was significantly less (P < 0.05) than it was in bled and control animals before bleeding, during the first week after bleeding, and during the third week after bleeding. During this second week, the percent of animals expressing tidal rhythms decreased by 83% in the OU group and by 60% in the LRW and LU groups. Significant differences between treatment conditions on the expression of tidal behavioral rhythms were not seen (F(2,23) = 1.38, P = 0.305).
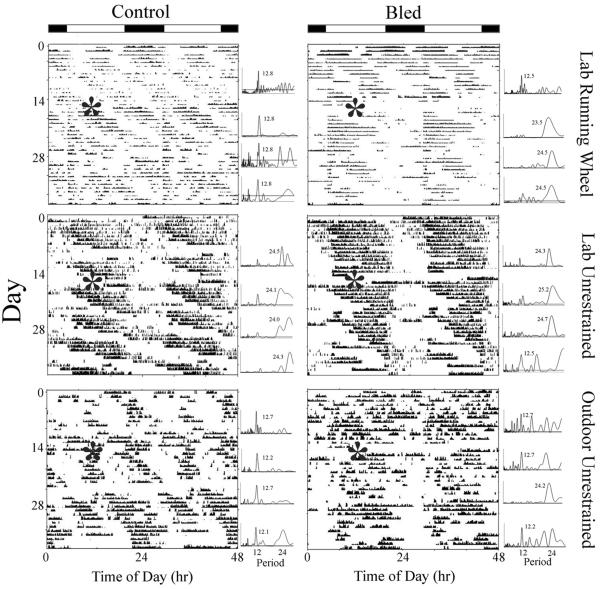
Figure 1. The effects of bleeding on locomotor activity and rhythms in representative L. polyphemus.
Larger panels: Actograms are double-plotted, with size and position of black marks indicating the intensity and timing of activity. Asterisks indicate the start of the bleeding process; in the control actograms, the start is marked to facilitate comparison. The LD cycle (14:10) is indicated by light/dark bars at the top. Smaller panels: Lomb-Scargle periodograms to the right of each actogram indicate significant rhythms of activity during successive intervals; horizontal line above x-axis indicates P = 0.001. Number next to peak indicates most significant period of activity within circatidal (12–14 h) or circadian (22–16 h) range.
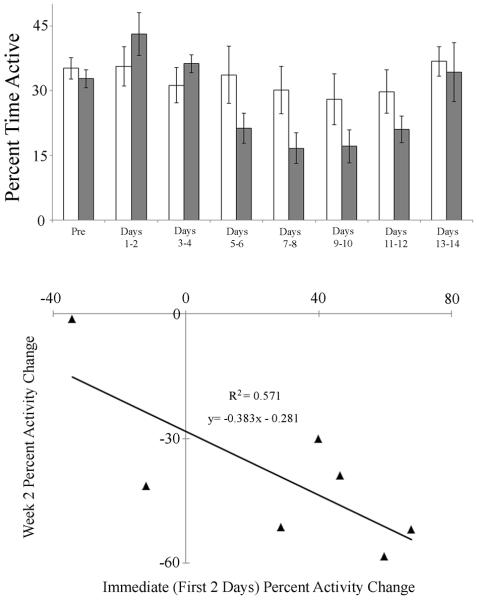
Figure 3. Effects of bleeding on activity (mean ± SEM) in the Outdoor Unrestrained group.
Top: Mean activity; White-Control; Grey-Bled. Pre= 2 days before bleeding; Days 1–14 = post-bleeding. Bottom: Relationship between activity percent change during the first two days post-bleeding to activity percent change during the second week post bleeding.
Table 2.
Percent of L. polyphemus expressing tidal and daily rhythms (τ ± SEM) before and after the bleeding process.
| Experimental Group | Rhythm Type | Pre | Week 1 Post | Week 2 Post | Week 3 Post |
|---|---|---|---|---|---|
| Lab RW Control | Tidal | 86% (12.6 ± 0.3) | 71% (12.1 ± 0.2) | 57% (12.4 ± 0.2) | 57% (12.3 ± 0.3) |
| Daily | 14% (23.8) | 29% (23.0 ± 0.2) | 43% (23.9 ± 0.7) | 43% (24.6 ± 0.1) | |
| Lab RW Bled | Tidal | 83% (12.5 ± 0.1) | 83% (12.5 ± 0.3) | 33% (12.3 ± 0.2) | 83% (12.5 ± 0.2) |
| Daily | 17% (24.3) | 17% (23.5) | 51% (24.3 ± 0.1) | 17% (24.6) | |
| Lab Unrestrained Control | Tidal | 71% (12.6 ± 0.3) | 57% (12.0 ± 0.2) | 57% (12.3 ± 0.2) | 71% (12.6 ± 0.3) |
| Daily | 28% (24.1 ± 0.4) | 42% (23.3 ± 0.4) | 43% (24.3 ± 0.3) | 28% (25.0 ± 0.8) | |
| Lab Unrestrained Bled | Tidal | 83% (12.3 ± 0.1) | 67% (12.7 ± 0.2) | 33% (12.4 ± 1.0) | 83% (12.5 ± 0.2) |
| Daily | 17% (23.2) | 33% (24.5 ± 0.8) | 51% (24.0 ± 0.2) | 17% (23.8) | |
| Outdoor Control | Tidal | 86% (12.5 ± 0.1) | 100% (12.2 ± 0.2) | 86% (12.6 ± 0.2) | 71% (12.2 ± 0.1) |
| Daily | 14% (26) | 0% | 14% (23.9) | 29% (24.0 ± 0.5) | |
| Outdoor Bled | Tidal | 86% (12.7 ± 0.2) | 86% (12.5 ± 0.1) | 14% (11.2) | 86% (12.3 ± 0.1) |
| Daily | 14% (23.7) | 14% (23.1) | 71% (24.4 ± 0.2) | 14% (24.9) |
In the OU group, three of seven bled animals appeared to shift activity patterns to diurnal activity during the second week after the bleeding process (Fig. 1), one appeared to shift to primarily nocturnal activity, and the remaining three did not appear exhibit a preference. In the LU group, four of six animals appeared to express diurnal activity during the second week post-bleeding period, while two animals appeared to express no preference for day/night. In the LRW group, one animal became active primarily during the day, while five animals appeared to express no preference.
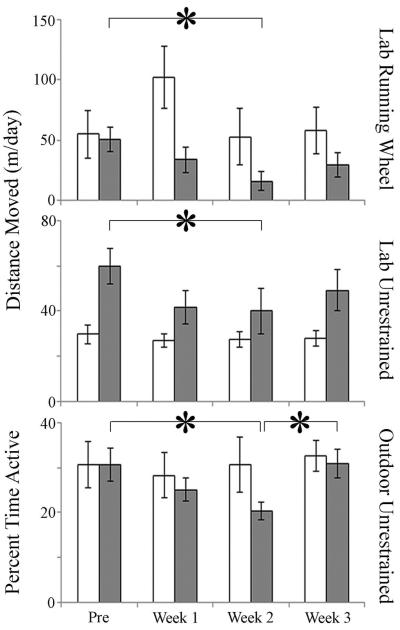
Activity of the three bled groups decreased significantly after the bleeding process (Fig. 2). In the LRW and LU bled groups, there was a significant effect of time on mean daily distance moved (F(3,12) = 3.82, P = 0.039 and F(3,15) = 3.51, P = 0.041, respectively): bled animals decreased the distance they traveled during the second week after the bleeding process (Fig. 2; P < 0.05), while there was no effect of time on daily distance moved in the control groups (LRW: F(3,9) = 0.52, P = 0.678; LU: F(3,15) = 0.514, P = 0.679). In the OU bled group, time after bleeding also significantly affected mean daily activity (F(3,18) = 4.02, P = 0.024; Fig. 2, 3); percent of time spent active decreased (P < 0.05) during the second week after bleeding, while activity in the control group was not affected (F(3,18) = 0.17, P = 0.92). In the OU group, activity returned to pre-bleeding levels during the third week post-bleeding, while activity in the LRW and LU did not fully recover (Fig. 2).
Figure 2. Effects of bleeding on activity (mean ± SEM) in the Laboratory Running Wheel, Laboratory Unrestrained, and Outdoor Unrestrained groups.
White-Control; Grey-Bled. * - P < 0.05. Pre= 2 weeks before bleeding; Weeks 1–3= post-bleeding.
In the OU group, six of seven bled animals appeared to exhibit normal to high activity levels for one to two days upon return to the water (percent of time active equaled or exceeded mean pre-bleeding levels; Fig. 3, top). This response was also seen in three of seven animals in the LU group, and one of seven animals in the LRW group. However, within the LU group, four of six animals exhibited a period of latency to initiate activity immediately after the bleeding process, with a “quiescent” period ranging 1.5 to 25.5 h (mean ± SE: 16.7 ± 5.5 h) prior to movement for four of the animals. Similarly, six of seven animals of the LRW group had a latency ranging from 2.8 to 21.6 h (8.6 ± 3.5 h) to initiate activity. In the OU group, the initial percent change in activity was significantly negatively correlated to the magnitude of the second-week activity decrease (R2 = 0.57, F(1,6) = 6.64, P < 0.05; Fig. 3, bottom), with animals that exhibited highest initial increases in activity exhibiting larger activity decreases during week two post-bleeding.
Bled animals in the LRW and LU groups moved similar distances (LRW: 50.5 ± 10.7 m/day; LU: 62.3 ± 7.0; t(11) = 0.38, P = 0.715) prior to the bleeding process. During the second week after the bleeding process, LRW animals decreased activity by 66%, while LU animals decreased activity by 33%, with distance moved significantly less in the LRW than in the LU group (t(11) = 2.76, P = 0.04; LRW: 10.2 ± 2.9 m; LU: 42.8 ± 11.5 m). Similar to LU animals, OU animals decreased overall activity by 33% during the second week post-bleeding. Within each group, neither prosomal width nor percent of hemolymph extracted was correlated with percent reduction in activity during the second week post-bleeding for the three activity groups (prosomal width: OU: r(6) = 0.127, P = 0.811; LRW: r(6) = 0.361, P = 0.55; LU: r(6) = −0.275, P = 0.60; percent extracted: OU: r(6) = −0.308, P = 0.50; LRW: r(6) = 0.644, P = 0.24; LU: r(6) = –0.581, P = 0.23).
Linear and Angular Velocity
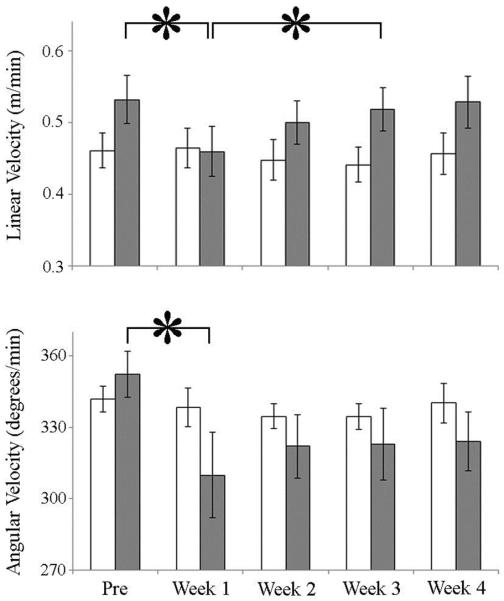
Linear and angular velocities were monitored in the LU group to assess changes in locomotor behaviors. While moving within the LU tank system, animals tended to exhibit three types of behaviors: traversals of the rectangular arenas, circles around the arena, or back and forth movements along one wall of the arena. Animals making both rapid traversals and circling movements exhibited high linear and angular velocity, while animals moving primarily along one side of their arenas exhibited lower velocities. During the 14 days before the bleeding process, both bled and control animals had similar linear (t(9) = 1.5, P = 0.167) and angular (t(12) = 1.02, P = 0.331) velocities. Bled animals decreased both linear (F(4,20) = 6.4, P = 0.002) and angular (F(4,20) = 2.99, P = 0.044) velocity during the first week after the bleeding process (Fig.4). While linear velocity returned to pre-bleeding levels during the third week post-bleeding, angular velocity remained suppressed (Fig. 4). In the control group, there was no effect of time on linear (F(4,24) = 0.93, P = 0.461) or angular (F(4,24) = 0.34, P = 0.849) velocity. Within the context of the LU system, the reductions in linear velocity suggest both slower locomotor rate during tank traversals and increased time spent moving along one side of the arena, while decreased angular velocity suggests slower rotational rates while circling arenas.
Figure 4. Effects of bleeding on linear and angular velocity in the Laboratory Unrestrained group.
White bars: controls; grey bars: bled; *- P < 0.05. Pre= 2 weeks before bleeding; Weeks 1–4= post-bleeding.
3.2 Alterations in Physiology
Hemocyanin
Hemocyanin loss was significantly affected by both bleeding (F(1,39) = 10.10, P = 0.003) and treatment condition (F(4,44) = 7.26, P = 0.001). Post-hoc analyses indicated that, six weeks post-bleeding, the percent of initial hemocyanin concentrations (pre-bleeding) remaining in the LRW, LU, and OU bled groups was significantly less than those of the corresponding control groups (P < 0.05). Further, hemocyanin percent losses in the LRW, LU, and LCT groups significantly exceeded those of the outdoor unrestrained group (P < 0.05; Fig. 5). The LRW bled and LU bled groups lost approximately 70% of the original concentration of hemocyanin, while the OU group lost 40%. On the day of the bleeding process, hemocyanin concentration did not differ significantly between bled and control animals of the four treatment groups (F(7,37) = 1.5, P = 0.2; data not shown).
Figure 5. Percent of original hemocyanin concentration remaining six weeks post-bleeding.
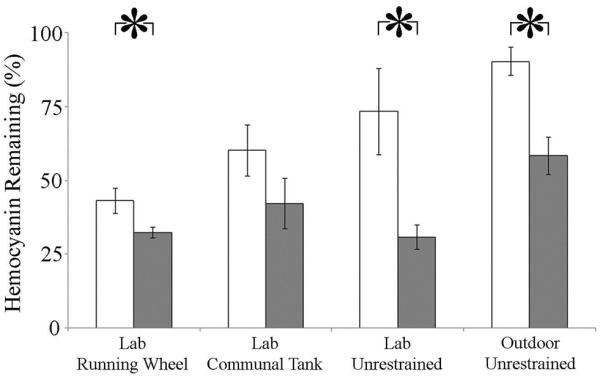
White bars: controls; grey bars: bled. *- P < 0.05.
When amount of hemocyanin lost as a result of the bleeding process was subtracted from initial hemocyanin levels, the hemocyanin reductions that occurred during the six weeks after the bleeding process did not differ between bled and control animals for the four groups combined (F(1,37) = 2.72, P = 0.107; data not shown), nor between bled and control animals within each treatment group (F(3,37) = 0.84, P = 0.479). Treatment conditions, however, affected total hemocyanin loss (F(3, 37) = 9.98, P < 0.001); specifically, subsequent losses in the OU group (bled and control) were significantly less than those laboratory groups.
In the LCT group, there was a significant effect of the bleeding process on hemocyanin concentration loss (F(1, 32) = 10.95, P = 0.002; data not shown), with a significant interaction of the bleeding treatment by time (F(3, 32) = 6.32, P = 0.002). Particularly, highest hemocyanin reductions in bled animals occurred immediately after the bleeding process (bled: 13.44 ± 2.8 mg*mL−1week−1, control: 2.31 ± 1.4 mg*mL−1 week −1). This immediate loss was significantly greater than that in both bled and control animals before bleeding (bled: 4.48 ± 1.4 mg*mL−1 week −1, control: 1.89 ± 2.1 mg*mL−1 week −1) and during weeks 2–6 post bleeding (bled: 2.87 ± 0.7 mg*mL−1 week −1, control: 2.17 ± 0.7 mg*mL−1 week −1).
Mortality
There were five total mortalities (18%) among bled animals (Table 1), with 42% mortality in the LCT, 0% mortality in the OU group, and 14% mortality in the LRW and LU groups. Mortalities in the LCT and LU groups occurred on the third day after return to water, while the mortality in the LRW group occurred on day two. The mean percent of hemolymph extracted in the five animals that died (15.9 ± 1.8%) did not significantly differ from that extracted from the remaining animals (19.8 ± 1.2%; t(27) = −1.85; P = 0.09).
Discussion
In this study, we found that the biomedical bleeding process causes several sub-lethal behavioral and physiological changes. The most obvious behavioral effects were immediate (within one week) decreases in walking speed and latent (one week post) reductions in both overall activity and the expression of tidal rhythms. The greatest impact of bleeding on Limulus physiology was an immediate and sustained decline in hemocyanin concentrations.
Behavioral Effects
Activity Levels
The bleeding process caused, after a one-week delay, a period of reduced activity: during the second week after the bleeding process, animals decreased activity (distance moved and percent of time active) between 33% and 66%. Similarly, the stone crab (Menippe mercenaria, Davis et al., 1978) decreases activity for ten days after the fishery practice of declawing. Further, discarded (undersized) Norwegian lobsters (Nephrops norvegicus; Harris and Ulmestrand, 2004) exhibit diminished swimming performance for eight to fifteen days following harvest stress (trawl capture plus 1 h of emersion), activity reductions attributed to the energy costs of initial activity during capture, effects of aerial exposure, and recovery from trawl-related injuries. However, in N. norvegicus, the reduction in swimming ability is apparent immediately after harvest processes, a contrast to what we found in L. polyphemus.
While it is likely that the period of reduced activity in L. polyphemus is the result of the combination of blood loss, thermal stress, desiccation, and aerial exposure, the reason for the one week delay prior to a decline in activity is not clear. Animals varied in their initial behavioral responses to the bleeding process, with six of seven animals in the OU group, four of six animals in the LU group, and one of six animals in the LRW group exhibiting high activity levels immediately after re-immersion (Fig.1, LU bled, OU bled; Fig. 3) and then, after a 1 week delay, exhibiting a reduction in activity. The significant negative correlation between the immediate activity increase and the second week activity decrease in the OU group may suggest that the second week activity deficit is possibly a response to the initial damage from the bleeding process compounded by the depletion of energetic resources that may occur during initial high activity output. This initial activity may be a manifestation of an escape response (L. polyphemus, Rudloe and Hernnkind, 1976); alternatively, it may reflect disorientation incurred by the bleeding process (L. polyphemus; Kurz and James-Pirri, 2002) or foraging efforts to replace lost energy reserves (Cancer pagurus; Patterson et al., 2009). In contrast, the second-week activity decrease is likely to be due to an impact of the bleeding process that is more long-term; however, future work is needed to clarify the mechanisms responsible.
These results conflict with those of Rudloe (1983), who found no difference in overall activity between bled and control animals during the 28 days following hemolymph extraction. Several reasons may account for this discrepancy. Firstly, Rudloe (1983) examined activity in animals after a bleeding process that only included 3 h of aerial exposure, while our 52 h procedure more closely resembles the procedure typically used to extract blood for LAL. Secondly, in the Rudloe (1983) study, activity was assessed by the number of deflections of two rods suspended above a pool containing several animals, and thus individual activity was not examined; in contrast, our three separate activity monitoring systems enabled continuous collection of activity data from each individual animal, with the lowest resolution of one sample/minute. Finally, Rudloe (1983) compared activity between bled and control animals during a 2 h window each day over 28 days, while we used several weeks of continuous activity when making comparisons. Overall, our study both more closely replicated an actual biomedical bleeding process and allowed a closer monitoring of individual behaviors.
The degree of activity decline and recovery may have been differentially affected by treatment conditions in this study. Specifically, that complete recovery of activity levels only occurred in the OU group was most likely due to the fact that they had access to food, while the LU and LRW groups were not fed. Starvation both prolongs stress recovery periods (oyster, Crassostrea gigas, Li et al., 2009b) and decreases locomotion (Cancer pagurus, Ansell, 1973). Additionally, though the smaller animals of the LRW group exhibited the same trend in activity decline as did animals of both unrestrained groups, they appeared to have a larger activity loss (66%). However, among controls, LRW animals had the largest decreases in hemocyanin concentration, suggesting that conditions of the LRW system may have been more stressful than those of the LU and OU systems. As such, whether the smaller size of these animals or the experimental conditions affected the magnitude of the activity decline cannot be determined by this study, and it would be useful to compare activity of animals of varied sizes that are maintained in the same data collection system.
Expression of Tidal Rhythms
Decreased expression of tidal rhythms occurred contemporaneously with the decrease in activity levels. In the OU group, the transition from bimodal to unimodal behavioral patterns appeared to be due to a temporary disappearance of one of the two daily bouts of activity (four of five animals; Fig. 1, OU bled), with three animals appearing to be primarily active during the daytime high tide and one animal appearing to be active during the nighttime high tides. These transitions may serve as means of energy conservation, and may be directly related to the decreased overall activity during the second week after the bleeding process. Similarly, concomitant decreases in expression of tidal rhythms and overall activity occurs when L. polyphemus is exposed to decreased water temperature (4–11°C; Chabot and Watson, 2010). Additionally, in our laboratory animals that initially expressed primarily daily patterns of activity, the duration of the activity bout shortened during the second week after the bleeding process (Fig. 1, LU bled), and the loss of rhythmicity that occurred in three of the bled laboratory animals appeared to be a correlate of reduced overall activity.
Velocity
In the LU group, both the linear and angular velocity of the animals' movements significantly decreased during the first week after the bleeding process. Similarly, the great scallop, Pecten maximus, has significant reductions in swimming velocity during the first 24 h after dredge capture and aerial exposure (20 min; Jenkins and Brand, 2001). This transient effect is attributed to physical exhaustion after capture (Jenkins and Brand, 2001), while, in other species, immediate behavioral alterations appear to be caused by the physical trauma of harvest practices (Stoner, 2012a, b). However, the factors responsible for these immediate behavioral changes in L. polyphemus deserve further investigation.
Our linear velocity findings contrast with those of Kurz and James-Pirri (2002), who found no significant difference between movement rates of bled and non-bled female L. polyphemus returned to Nauset Estuary (Cape Cod, MA) after hemolymph extraction. This discrepancy could have been caused by the more intensive bleeding procedure we used: Kurz and James-Pirri (2002) performed a hemolymph extraction with a maximum of 30 min aerial exposure, while we used a high-stress bleeding treatment more closely analogous to the biomedical bleeding procedure (Hurton and Berkson, 2006). The heightened physiological impact of the high-stress treatment (higher mortality; Hurton and Berkson, 2006) is attributed to the synergistic effect of multiple stressors (hemolymph extraction, thermal stress, and aerial exposure); similarly, the presence of these compound stressors may evoke a heightened behavioral effect. However, Kurz and James-Pirri (2002) also observed that the bled females lacked a directional preference towards spawning beaches, contrasted to the directed movement patterns of controls towards these spawning beaches. Kurz and James-Pirri (2002) suggested this behavioral discrepancy may be caused by disorientation incurred by the bleeding process. Whether the velocity and activity changes we documented were laboratory manifestations of the movement patterns Kurz and James-Pirri observed in the wild remains to be determined and further work is also necessary in order to ascertain the connection between bleeding stress and relatively long-term alterations in behavior.
Physiological Effects
Hemocyanin Concentration Decrease
The bleeding process caused a prolonged period of hemocyanin loss: six weeks after the bleeding process, three of the bled groups exhibited significantly greater losses in hemocyanin concentration than the control animals in those groups (Fig. 5). Despite feedings, bled animals in the OU had only 60% of their original hemocyanin concentration six weeks after being bled. In contrast, OU controls lost just 10% of their original hemocyanin concentration. As the outdoor group experienced the most naturalistic conditions (freshly flowing bay water, sediment, and access to food), these results suggest that hemocyanin recovery in the wild may require a prolonged (>6 week) period. Similarly, James-Pirri et al. (2012) found significantly reduced total hemolymph protein in bled animals, compared to both wild caught and captive controls, 17 days after bleeding (James-Pirri et al., 2012), with hemolymph protein of bled animals approximately 20% less than that of controls. As hemocyanin constitutes over 90% of total hemolymph protein (Ding et al., 2005), our results support the suggestion that the bleeding process has a lingering impact on L. polyphemus hemolymph quality (James-Pirri et al., 2012), with no indication of recovery over six weeks even in animals that were fed.
The fact that the highest hemocyanin reductions occurred in the laboratory groups may be due to the both captivity stress and the lack of feeding. Not surprisingly, in the LCT group, hemolymph extraction caused significant hemocyanin loss during the first week after the process, indicating an immediate effect of the process of hemolymph quality. However, rates of decline between bled and control animals were similar during the following weeks, suggesting subsequent losses were most likely due to captivity stress (Coates et al., 2012); similarly, once hemocyanin loss due to hemolymph extraction was subtracted, net hemocyanin losses in all groups during the weeks following the extraction were equal, further suggesting that captivity stressors perpetuated these additional losses. Within the laboratory groups, the lack of feeding most likely exacerbated hemocyanin loss, as starvation can decrease total hemolymph protein through hemocyanin catabolism (Cancer maenas, Uglow, 1969) and slow protein synthesis (Li et al., 2009; Sokolova et al., 2012), an energetically demanding process (Hand and Hardewig, 1996).
Mortality Rates
The 18% total mortality across our four bled groups is lower than that reported by Leschen and Correia (30%; 2010) and by Hurton and Berkson (29%; 2006) for females that underwent either a 40% blood extraction (Hurton and Berkson, 2006) or a hemolymph extraction at a biomedical company (Associates of Cape Cod; Leschen and Correia, 2010) combined with 24–48 h aerial exposure. The low mortality in the LU and LRW groups, and the lack of mortality in the OU group, may be related to the percent of hemolymph we extracted (15–28%). Similarly, Hurton and Berkson (2006) found 0–6% mortality after a 48 h high stress process with 20–30% hemolymph extraction. However, the five deaths in our experiment appeared to be unrelated to percent of hemolymph extracted, with mean percent extracted for these five animals within the range of the overall mean extraction of 19.8 ± 1.2%. Additionally, the magnitude of the second week activity change did not appear to be related to the amount of hemolymph extracted, suggesting that, possibly, additional factors of the process (for example, air exposure or thermal stress) may be responsible for the observed behavioral and physiological effects.
The apparent variation in mortality among our four groups may be related to treatment conditions, notably the presence of feeding in the OU group and the weekly blood samples in the LCT groups. However, the disparate degrees of thermal stress in the bleeding processes may have also affected mortality: the laboratory groups experienced a maximum of 37°C while the outdoor unrestrained group experienced a maximum of 28°C. Thermal stress alone influences mortality and vigor in Liocarcinus depurator (Giomo et al., 2008) and Nephrops norvegicus (Lund et al., 2009), and, possibly, the higher temperature in our laboratory groups partially accounts for the increased mortality. The latency to initiate activity that occurred in 11 of the 14 animals of our laboratory groups may similarly be related to the degree of thermal stress these animals experienced, with both increased desiccation and accumulation of metabolic waste products potentially responsible for limiting activity until animals regained sufficient water volume. Both length of emersion (Pandalus platyceros, Stoner, 2012) and degree of thermal stress are positively correlated to degree of immediate behavioral and physiological impairments post-exposure in Panulirus cygnus (Paterson et al., 2005), Nephrops norvegicus (Ridgway et al., 2006), and Homarus americanus (Basti et al., 2010), and it would be useful to further investigate the importance of these variables on post-harvest behavior of L. polyphemus. Importantly, both the percent of hemolymph we extracted and the degree of stress to which we exposed the animals may be less than that of standard biomedical practices (ASMFC, 1998; Leschen and Correia, 2010), and the behavioral effects that we observed may underestimate the effects of a complete biomedical bleeding process with a full 30–40% blood extraction.
4.3 Ecological Implications
The changes we observed in activity levels, movement velocity, and expression of tidal rhythms may interfere with daily L. polyphemus activities, which would be particularly pronounced during the spawning season. Spawning necessitates several energetically costly trips to the intertidal zone (Leschen et al., 2006); larger females tend to make more excursions to the intertidal zone, often making multiple trips within the same week (Leschen et al., 2006). An activity deficit, such as that caused by biomedical bleeding, may either influence the number of those trips, or it may influence the timing of those trips. In the case of the latter, females may delay spawning activity while they are recuperating, and this could reduce their spawning output. In addition, modifications in the expression of tidal rhythms may alter the timing of excursions to mating beaches and cause a reduction in the probability they would find males with which to mate. As females are preferentially harvested (76%; Rutecki et al., 2003), these behavioral alterations during the spawning season may partially account for declining populations in heavily harvested regions (James-Pirri, 2012), specifically the declining proportions of females at spawning beaches (1 female: 8.5–14 males in Pleasant Bay), reduced egg abundances (James-Pirri, 2012), and the occurrence of single females attempting to spawn (James-Pirri, 2012).
The extended periods of low hemocyanin levels that we found may impact L. polyphemus fitness in the natural habitat. Hemocyanins have multiple physiological functions in invertebrates, including sclerotization and maintenance of cuticle integrity (Adachi et al., 2005; Terwilliger, 2007), wound repair, osmoregulation (Paul and Pirow, 1998), and involvement in the immune response (Coates et al., 2011). Reductions of this protein, combined with a reported four month deficit in amebocytes caused by the bleeding process (Novitsky, 1984), may result in a weakened organism, one that is both less able to contend with additional stresses and that exhibits increased susceptibility to infection. Further, the sustained declines in hemocyanin concentration that we found may partially account for the increased (10–11%) probability of mortality during the first two years post-bleeding in animals returned to the wild (Rudloe, 1983).
In summary, L. polyphemus females have decreased overall activity and expression of tidal rhythms during the second week after bleeding, decreased linear and angular movement velocities in the first week after the bleeding process, and long-term (>6 week) declines in hemocyanin concentrations. These results suggest that L. polyphemus experiences sub-lethal effects of the bleeding process, which, along with high mortality rates in females (Hurton and Berkson, 2006; Leschen and Correia, 2010), may partially account for the changing population characteristics in areas of heavy biomedical harvest. Whether these behavioral and physiological changes occur, or are possibly heightened, in the wild deserves further investigation in order to fully assess the implications of the harvest process for L. polyphemus spawning behaviors. Maintenance of populations of L. polyphemus is essential not only for the ecosystem as a whole, including subsistence of shorebird populations (Baker et al., 2004), but also for several commercial sectors. The use of horseshoe crabs in the production of LAL is of global importance, and a continued harvest is important in meeting the demands for LAL. However, to maintain the integrity of the stock needed to supply the industry, adaptive or flexible management strategies may need to be considered. In areas of population decline, harvest limits during the spawning season may help to minimize any potential population-level consequences incurred by individual behavioral and physiological changes.
Acknowledgments
This project could not have been completed without the assistance of Steven Simpson, Katherine Fondo, Alexandria Santry, Kyle Kenyon, Tyler Remillard, and Megan Cooper, who helped with animal collection, maintenance, and data collection. Special thanks to Alicia Franklin for animal care and feeding, and to the staff at Jackson Estuarine Laboratory, especially Dave Shay, for assistance with technical aspects. We also thank the anonymous reviewers and The Biological Bulletin editors for helpful feedback. Financial support for this project was provided by New Hampshire Sea Grant Award (#12-092) to CCC and RLA, NSF (IOS to CCC and WHW III), Plymouth State University College of Graduate Studies, and the New Hampshire IDeA Network of Biological Research Excellence with grants from the National Institute of General Medical Sciences (1P20GM030360) National Institutes of Health.
Abbreviations
- LAL
(Limulus Amebocyte Lysate)
Literature Cited
- Adachi K, Endo H, Watanabe T, Nishioka T, Hirata T. Hemocyanin in the exoskeleton of crustaceans: enzymatic properties and immunolocalization. Pigment Cell Res. 2005;18:136–143. doi: 10.1111/j.1600-0749.2005.00217.x. [DOI] [PubMed] [Google Scholar]
- Aguzzi J, Chiesa JJ, Abelló P, Diez-Noguera A. Temporal modification of cardiac rhythmicity in Nephrops norvegicus (Crustacea: Decapoda) in relation to trawl capture stress. Sci. Mar. 2005;69:369–374. [Google Scholar]
- Ansell AD. Changes in oxygen consumption, heart rate and ventilation accompanying starvation in the decapod crustacean Cancer pagurus. Neth. J. Sea Res. 1973;7:455–475. [Google Scholar]
- Armstrong P, Conrad M. Blood collection from the American horseshoe crab, Limulus polyphemus. J. Vis. Exp. 2008;20:958. doi: 10.3791/958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASMFC . Interstate Fishery Management Plan for Horseshoe Crab. 1998. Fishery management report no.32 of the Atlantic States Marine Fisheries Commission. [Google Scholar]
- ASMFC . 2012 review of the fishery management plan in 2011 for horseshoe crab (Limulus polyphemus) 2012. [Google Scholar]
- Baker A, Gonzalez P, Piersma T, Niles L, Nascimento I, Atkinson P, Clark N, Minton C, C., Peck M, Aarts G. Rapid population decline in red knots: fitness consequences of decreased refueling rates and late arrive in Delaware Bay. Proc. R. Soc. Lond. 2004;271:875–882. doi: 10.1098/rspb.2003.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RB, Jr., Powers MK, Howard H, Kass L. Migration of Limulus for mating: relation to lunar phase, tide height, and sunlight. Biol. Bull. 1986;171:310–329. [Google Scholar]
- Barrento S, Marques A, Vaz-Pires P, Nunes ML. Live shipment of immersed crabs Cancer pagurus from England to Portugal and recovery in stocking tanks: stress parameter characterization. ICES J. Mar. Sci. 2010;67:435–443. [Google Scholar]
- Basti D, Bricknell I, Hoyt K, Chang E, Halteman W, Bouchard D. Factors affecting post-capture survivability of lobster Homarus americanus. Dis. Aquat. Org. 2010;90:153–166. doi: 10.3354/dao02205. [DOI] [PubMed] [Google Scholar]
- Bergmann M, Taylor AC, Moore PG. Physiological stress in decapod crustaceans (Munida rugosa and Liocarcinus depurator) discarded in the Clyde Nephrops fishery. J. Exp. Mar. Biol. Ecol. 2001;259:215–229. doi: 10.1016/s0022-0981(01)00231-3. [DOI] [PubMed] [Google Scholar]
- Botton ML. Diet and food preferences of the adult horseshoe crab Limulus polyphemus in Delaware Bay, New Jersey, USA. Mar. Biol. 1984a;81:199–207. [Google Scholar]
- Botton ML. The importance of predation by horseshoe crabs, Limulus polyphemus, to an intertidal sand flat community. J. Mar. Res. 1984b;42:139–161. [Google Scholar]
- Brown RS, Caputi N. Factors affecting the recapture of undersize western rock lobster Panulirus cygnus George returned by fishermen to the sea. Fish. Res. 1983;2:103–128. [Google Scholar]
- Carmichael RH, Rutecki D, Valiela I. Abundance and population structure of the Atlantic horseshoe crab Limulus polyphemus in Pleasant Bay, Cape Cod. Mar. Ecol. Prog. Ser. 2003;246:225–239. [Google Scholar]
- Chabot CC, Kent J, Watson WH., III Circatidal and circadian rhythms of activity in Limulus polyphemus. Biol. Bull. 2004;207:72–75. doi: 10.2307/1543630. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Betournay SH, Braley NR, Watson WH., III Endogenous rhythms of locomotion in the American horseshoe crab, Limulus polyphemus. J. Exp. Mar. Biol. Ecol. 2007;345:79–89. [Google Scholar]
- Chabot CC, Skinner SJ, Watson WH., III Rhythms of locomotion expressed by Limulus polyphemus, the American horseshoe crab: I. Synchronization by artificial tides. Biol. Bull. 2008;215:34–45. doi: 10.2307/25470681. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Watson WH., III Circatidal rhythms of locomotion in the American horseshoe crab Limulus polyphemus: Underlying mechanisms and cues that influence them. Curr. Zool. 2010;56:499–517. [Google Scholar]
- Chabot CC, Yelle JF, O'Donnell CB, Watson WH., III The effects of water pressure, temperature, and current cycles on circatidal rhythms expressed by the American horseshoe crab, Limulus polyphemus. Mar. Freshw. Behav. Physiol. 2011;44:43–60. [Google Scholar]
- Coates CJ, Kelly SM, Nairn J. Possible role of phosphatidylserine-hemocyanin interaction in the innate immune response of Limulus polyphemus. Dev. Comp. Immunol. 2011;35:155–163. doi: 10.1016/j.dci.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Coates CJ, Bradford EL, Krome CA, Nairn J. Effect of temperature on biochemical and cellular properties of captive Limulus polyphemus. Aquaculture. 2012;334:30–38. [Google Scholar]
- Cohen JA, Brockmann HJ. Breeding activity and mate selection in the horseshoe crab Limulus polyphemus. Bull Mar Sci. 1983;33:274–281. [Google Scholar]
- Davis GE, Baughman DS, Chapman JD, MacArthur D, Pierce AC. Mortality associated with declawing stone crabs, Menippe mercenaria. U.S. National Park Service; 1978. Report T-522. [Google Scholar]
- Ding JL, Tan KC, Thangamani S, Kusuma N, Seow WK, Bui THH, Wang J, Ho B. Spatial and temporal coordination of expression of immune genes during Pseudomonas infection of the horseshoe crab, Carcinoscorpius rotundicauda. Genes Immun. 2005;6:557–574. doi: 10.1038/sj.gene.6364240. [DOI] [PubMed] [Google Scholar]
- Fanjul-Moles ML, Bosques-Tistler T, Prieto-Sagredo J, Castañón-Cervantes O, Fernández-Rivera-Río L. Effect of variation in photoperiod and light intensity on oxygen consumption, lactate concentration, and behavior in crayfish Procambarus clarkii and Procambarus digueti. Comp. Biochem. Physiol. 1998;119A:263–269. doi: 10.1016/s1095-6433(97)00413-3. [DOI] [PubMed] [Google Scholar]
- Giomo F, Raicevich S, Giovanardi O, Pranovi F, Di Muro P, Beltramini M. Catch me in winter! Seasonal variation in air temperature severely enhances physiological stress and mortality of species subjected to sorting operations and discarded during annual fishing activities. Hydrobiologia. 2008;606:195–202. [Google Scholar]
- Hand SC, Hardewig I. Downregulation of cellular metabolism during environmental stress: mechanisms and implications. Annu. Rev. Physiol. 1996;58:539–563. doi: 10.1146/annurev.ph.58.030196.002543. [DOI] [PubMed] [Google Scholar]
- Harris RR, Ulmestrand M. Discarding Norway lobster (Nephrops norvegicus L.) through low salinity layers – mortality and damage seen in simulation experiments. ICES J. Mar. Sci. 2004;61:127–139. [Google Scholar]
- Harris RR, Andrews MB. Physiological changes in Norway lobster Nephrops norvegicus (L.) escaping and discarded from commercial trawls on the West Coast of Scotland: II. Disturbances in haemolymph respiratory gases, tissue metabolites and swimming performance after capture and during recovery. J. Exp. Mar. Biol. Ecol. 2005;320:195–210. [Google Scholar]
- Haupt P, Brouwer SL, Branch GM, Gade G. Effects of exposure to air on the escape behavior and haemolymph chemistry of the South African Cape lobster, Jasus lalandii. Fish. Res. 2006;81:210–218. [Google Scholar]
- Hurton L, Berkson J, Smith S. Estimation of total hemolymph volume in the horseshoe crab Limulus polyphemus. Mar. Freshw. Behav. Physiol. 2005;38:139–147. [Google Scholar]
- Hurton L, Berkson J. Potential causers of mortality for horseshoe crabs (Limulus polyphemus) during the biomedical bleeding process. Fish. Bull. 2006;104:293–298. [Google Scholar]
- James-Pirri MJ, Veillette PA, Leschen AS. Selected hemolymph constituents of captive, biomedically bled, and wild caught adult female American horseshoe crabs (Limulus polyphemus) Mar. Freshw. Behav. Physiol. 2012;45:281–289. [Google Scholar]
- James-Pirri MJ. Assessment of spawning horseshoe crabs (Limulus polyphemus) at Cape Cod National Seashore, 2008–2009. Natural Resource Technical Report NPS/CACO/NRTR-2012/573. 2012 [Google Scholar]
- Jenkins SR, Brand AR. The effect of dredge capture on the escape response of the great scallop, Pecten maximus (L.): implications for the survival of undersized discards. J. Exp. Mar. Biol. Ecol. 2001;266:33–50. [Google Scholar]
- Kasten P, Flores AAV. Disruption of endogenous tidal rhythms of larval release linked to food supply and heat stress in an intertidal barnacle. Mar. Ecol. Prog. Ser. 2013;472:185–198. [Google Scholar]
- Kim WS, Huh HT, Huh SH, Lee TW. Effects of salinity on endogenous rhythm of the Manila clam, Ruditapes philippinarum (Bivalvia: Veneridae) Mar. Biol. 2001;138:157–162. [Google Scholar]
- King TL, Eackles MS, Spidle AP, Brockmann HJ. Regional differentiation and sex-biased dispersal among populations of the horseshoe crab Limulus polyphemus. Trans. Am. Fish. Soc. 2005;134:441–465. [Google Scholar]
- Krauter JN, Fegley SR. Vertical disturbance of sediments by horseshoe crabs (Limulus polyphemus) during their spawning season. Estuaries. 1994;17:288–294. [Google Scholar]
- Kurz W, James-Pirri MJ. The impact of biomedical bleeding on horseshoe crabs, Limulus polyphemus, movement patterns on Cape Cod, Massachusetts. Mar. Freshw. Behav. Physiol. 2002;35:261–268. [Google Scholar]
- Lee WJ. Intensive use of an intertidal mudflat by foraging adult American horseshoe crabs Limulus polyphemus in the Great Bay estuary, New Hampshire. Curr. Zool. 2010;56:611–617. [Google Scholar]
- Leschen AS, Grady SP, Valiela I. Fecundity and spawning of the Atlantic horseshoe crab, Limulus polyphemus, in Pleasant Bay, Cape Cod, Massachusetts, USA. Mar. Ecol. 2006;27:54–65. [Google Scholar]
- Leschen AS, Correia SJ. Mortality in female horseshoe crabs (Limulus polyphemus) from biomedical bleeding and handling: implications for fisheries management. Mar. Freshw. Behav. Physiol. 2010;43:135–147. [Google Scholar]
- Li Y, Qin JG, Li X, Benkendorff K. Spawning-dependent stress responses in Pacific oysters Crassostrea gigas: a simulated bacterial challenge in oysters. Aquaculture. 2009a;293:164–171. [Google Scholar]
- Li Y, Qin JG, Li X, Benkendorff K. Spawning-dependent stress response to food deprivation in Pacific oyster Crassostrea gigas. Aquaculture. 2009b;286:309–317. [Google Scholar]
- Lund HS, Wang T, Chang ES, Pedersen LF, Taylor EW, Pedersen PB, McKenzie DJ. Recovery by the Norway lobster Nephrops norvegicus (L.) from the physiological stresses of trawling: Influence of season and live-storage position. J. Exp. Mar. Biol. Ecol. 2009;373:124–132. [Google Scholar]
- Malkoski V. Massachusetts 2010 compliance report to the Atlantic States Marine Fisheries Commission-Horseshoe Crab. Massachusetts Division of Marine Fisheries. 2010 [Google Scholar]
- Novitsky TJ. Discovery to commercialization: the blood of the horseshoe crab. Oceanus. 1984;27:19–26. [Google Scholar]
- Novitsky TJ. Biomedical applications of Limulus Amebocyte Lysate. In: Tanacredi JT, Botton ML, Smith D, editors. Biology and Conservation of Horseshoe Crabs. Springer Science+Business Media, LLC; New York, NY: 2009. pp. 315–329. [Google Scholar]
- Parsons DM, Eggleston DB. Indirect effects of recreational fishing on behavior of the spiny lobster Panulirus argus. Mar. Ecol. Prog. Ser. 2005;303:235–244. [Google Scholar]
- Paterson BD, Spanoghe PT. Stress indicators in marine decapod crustaceans, with particular reference to the grading of western rock lobsters (Panulirus cygnus) during commercial handling. Mar. Freshw. Res. 1997;48:829–834. [Google Scholar]
- Paterson BD, Spanoghe PT, Davidson GW, Hosking W, Nottingham S, Jussila J, Evans LH. Predicting survival of western rock lobsters Panulirus cygnus using discriminant analysis of haemolymph parameters taken immediately following simulated handling treatments. N. Z. J. Mar. Freshw. Res. 2005;39:1129–1143. [Google Scholar]
- Patterson L, Dick JTA, Elwood RW. Physiological stress responses in the edible crab, Cancer pagurus, to the fishery practice of de-clawing. Mar. Biol. 2007;152:265–272. [Google Scholar]
- Patterson L, Dick JTA, Elwood RW. Claw removal and feeding ability in the edible crab, Cancer pagurus: implications for fishery practice. Appl. Anim. Behav. Sci. 2009;116:302–305. [Google Scholar]
- Paul RJ, Pirow R. The physiological significance of respiratory proteins in invertebrates. Zoology. 1998;100:298–306. [Google Scholar]
- Ridgway ID, Taylor AC, Atkinson RJA, Chang ES, Neil DM. Impact of capture method and trawl duration on the health status of the Norway lobster, Nephrops norvegicus. J. Exp. Mar. Biol. Ecol. 2006a;339:135–147. [Google Scholar]
- Ridgway I, Taylor AC, Atkinson RJA, Stentiford GD, Chang ES, Neil DM. Morbidity and mortality in Norway lobsters, Nephrops norvegicus: physiological, immunological and pathological effects of aerial exposure. J. Exp. Mar. Biol. Ecol. 2006b;328:251–264. [Google Scholar]
- Rudloe A, Herrnkind WF. Orientation of Limulus polyphemus in the vicinity of breeding beaches. Mar. Behav. Physiol. 1976;4:75–89. [Google Scholar]
- Rudloe A. The effect of heavy bleeding on mortality of the horseshoe crab, Limulus polyphemus, in the natural environment. J. Invertebr. Pathol. 1983;42:167–176. [Google Scholar]
- Rutecki D, Carmichael RH, Valiela I. Magnitude of harvest of Atlantic horseshoe crabs, Limulus polyphemus, in Pleasant Bay, Massachusetts. Estuaries. 2004;27:179–187. [Google Scholar]
- Schaller SY, Chabot CC, Watson WH., III Seasonal movements of American horseshoe crabs Limulus polyphemus in the Great Bay Estuary, New Hampshire (USA) Curr. Zool. 2010;56:587–598. [Google Scholar]
- Smith GG, Ritar AJ. Effect of physical disturbance on reproductive performance in the spiny lobster, Jasus edwardsii. N. Z. J. Mar. Freshw. Res. 2005;39:317–324. [Google Scholar]
- Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA. Energy homeostasis as in integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012;79:1–15. doi: 10.1016/j.marenvres.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Stoner AW. Evaluating vitality and predicting mortality in the spot prawn, Pandalus platyceros, using reflex behaviors. Fish. Res. 2012a;119:108–114. [Google Scholar]
- Stoner AW. Assessing stress and predicting mortality in economically significant crustaceans. Rev. Fish. Sci. 2012b;20:111–135. [Google Scholar]
- Styrishave B, Rasmussen AD, Depledge MH. The influence of bulk and trace metals on the circadian rhythm of heart rates in freshwater crayfish, Astacus astacus. Mar. Pollut. Bull. 1995;31:87–92. [Google Scholar]
- Terwilliger NB. Hemocyanins and the immune response: defense against the dark arts. Integr. Comp. Biol. 2007;47:662–665. doi: 10.1093/icb/icm039. [DOI] [PubMed] [Google Scholar]
- Theriault M, VanLeeuwen J, Morrison M, Cawthorn R. Risk factors for the development of shell disease in impounded populations of the American lobster, Homarus americanus. J. Shellfish Res. 2008;27:1239–1245. [Google Scholar]
- Uglow RF. Haemolymph protein concentrations in portunid crabs - II. The effects of imposed fasting on Carcinus maenas. Comp. Biochem. Physiol. 1969;31:959–967. doi: 10.1016/0010-406x(69)91046-9. [DOI] [PubMed] [Google Scholar]
- Vermeer GK. Effects of air exposure on desiccation rate, hemolymph chemistry, and escape behavior of the spiny lobster, Panulirus argus. Fish. Bull. 1987;85:45–51. [Google Scholar]
- Walls EA, Berkson J. Effects of blood extraction on horseshoe crabs (Limulus polyphemus) Fish. Bull. 2003;101:457–459. [Google Scholar]
- Watson WH, III, Chabot CC. High resolution tracking of adult horseshoe crabs Limulus polyphemus in a New Hampshire estuary using fixed array ultrasonic telemetry. Curr. Zool. 2010;56:599–610. [Google Scholar]