Abstract
Environmental contexts associated with drug use promote craving in humans and drug-seeking in animals. We hypothesized that the basolateral amygdala (BLA) itself, as well as serial connectivity between the basolateral amygdala (BLA) and nucleus accumbens core (NAC core), were required for context-induced renewal of Pavlovian-conditioned alcohol-seeking. Male, Long-Evans rats were trained to discriminate between two conditioned stimuli (CS) - a CS+ that was paired with ethanol (EtOH, 20%, v/v) delivery into a fluid port (0.2 ml/CS+, 3.2 ml/session) and a CS− that was not. Entries into the port during each CS were measured. Next, rats received extinction in a different context where both cues were presented without EtOH. At test, responding to the CS+ and CS− without EtOH was evaluated in the prior training context. Control subjects showed a selective increase in CS+ responding relative to extinction, indicative of renewal. This effect was blocked by pre-test, bilateral inactivation of the BLA using a solution of gamma-amino-butyric-acid receptor agonists (0.1 mM muscimol and 1.0 mM baclofen; M/B; 0.3 µl/side). Renewal was also attenuated following unilateral injections of M/B into the BLA, combined with either M/B, the dopamine D1 receptor antagonist SCH 23390 (0.6 µg/side), or saline infusion in the contralateral NAC core. Hence, unilateral BLA inactivation was sufficient to disrupt renewal, highlighting a critical role for functional activity in the BLA in enabling the reinstatement of alcohol-seeking driven by an alcohol context.
Keywords: ethanol, relapse, reinstatement, rat, addiction, nucleus accumbens, Pavlovian-conditioning
Introduction
Environmental contexts in which drugs of abuse are consumed play an integral role in addiction. For example, drug contexts can elicit robust craving in abstinent drug users (Conklin et al., 2008), and conditioned reactivity triggered by cues that routinely precede drug intake can be strongly modulated by the context in which they are experienced (Collins & Brandon, 2002; Thewissen et al., 2006; Tsiang & Janak, 2006; Chaudhri et al., 2008a). The neurobiological processes that underpin the capacity of drug-contexts to stimulate drug-seeking have been studied using animal models of instrumental conditioning (Crombag & Shaham, 2002; Hamlin et al., 2006; Zironi et al., 2006; Fuchs et al., 2007; Marinelli et al., 2007). However, drug-contexts also influence drug-seeking that is triggered by drug-predictive Pavlovian cues. For example, placement into an alcohol-associated context following extinction in a different context triggers the renewal of alcohol-seeking elicited by a conditioned stimulus (CS) that was previously paired with alcohol in that context: no change in responding is observed to a different CS that does not predict alcohol (Chaudhri et al., 2008b).
The basolateral amygdala (BLA) and nucleus accumbens core (NAC core) have been independently implicated in context-induced renewal of drug- and alcohol-seeking in studies that utilized instrumental conditioning procedures (Fuchs et al., 2008; Chaudhri et al., 2008a; Chaudhri et al., 2009; Marinelli et al., 2010). In addition, the renewal of Pavlovian-conditioned alcohol-seeking is attenuated following bilateral inactivation of the NAC core using a solution of gamma-aminobutyric acid (GABA) receptor agonists (Chaudhri et al., 2010). As the role of the BLA in this effect is not known, we tested the hypothesis that functional activity within the BLA is necessary for context-induced renewal of Pavlovian-conditioned alcohol-seeking (Experiment 1).
Converging evidence indicates that responding driven by Pavlovian cues that predict positive (appetitive) outcomes requires serial connectivity between the BLA and NAC core (Everitt et al., 1991; Di Ciano & Everitt, 2004; Setlow et al., 2002; Ambroggi et al., 2008; Stuber et al., 2011). This hypothesis is typically studied using a functional ‘disconnection’ procedure, in which unilateral pharmacological manipulations or lesions are performed within the BLA and NAC core in contralateral brain hemispheres. Because the BLA-to-NAC core projection is largely ipsilateral (Brog et al., 1993), resulting deficits in behaviour may be attributed to a disruption in amygdalo-striatal processing.
Given that the BLA and NAC core are both involved in renewal, we hypothesized that context-induced renewal of Pavlovian-conditioned alcohol-seeking requires serial connectivity between the BLA and NAC core. First, we determined the impact on renewal of unilaterally inactivating the BLA and NAC in contralateral brain hemispheres (Experiment 2). Second, to determine if renewal is dependent upon interactions between dopamine and glutamatergic afferents from the BLA within the NAC core (Jones et al., 2010; Floresco et al., 1998; Ambroggi et al., 2008) we paired unilateral BLA inactivation with a dopamine D1 receptor antagonist in the contralateral NAC core (Experiment 3a). We also tested the impact of the latter manipulation on Pavlovian-conditioned alcohol-seeking when CS+ trials were paired with alcohol (Experiment 3b).
Materials and Methods
Subjects
Male, Long-Evans rats (Harlan Laboratories, Indianapolis, IN, USA) weighing 220–240 g on arrival were used in all experiments. Rats were housed individually in ventilated polycarbonate cages, in a temperature (20 ± 1 °C) and humidity-controlled vivarium that was maintained on a 12 h light-dark cycle (lights on at 0700 h; behavioural testing conduced during the light phase). Access to food and water was unrestricted, except as described below. All procedures were approved by the Institutional Animal Care and Use Committee at the Ernest Gallo Clinic and Research Center, and are in agreement with recommendations in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission of Life Sciences, National Research Council, 1996), as well as guidelines provided by the National Institutes of Health regarding the care and use of animals for experimental procedures.
Apparatus
Behavioural testing was conducted in 16 operant conditioning chambers housed within ventilated, sound-attenuating melamine cubicles (equipment and software from Med Associates Inc., St. Albans, VT, USA). Each chamber (ENV-009A) was comprised of clear Plexiglas front and back walls, paneled aluminum sidewalls, and a floor made of stainless steel bars that extended from the rear wall to the front of the chamber. Beneath the floor was a steel waste pan containing absorbent bedding. The right wall featured a recessed port containing two circular fluid receptacles (ENV-200R3AM). Ethanol (EtOH) was delivered into one of the receptacles via a 20 mL syringe that was attached to a pump (PHM-100, 3.33 RPM) located outside the melamine cubicle. Entries into the port were measured by interruptions of a photo beam across its entrance. A white chamber light (ENV-215M, 28V, 100 mA) was located centrally near the ceiling on the left wall, which also featured a white noise generator (ENV-225SM, 80–85 dB) and a clicker stimulus (ENV-135M, 76–80 dB). Computers running Med PC IV software controlled the presentation of auditory stimuli and fluid delivery and recorded entries into the fluid port.
Drugs
EtOH solutions (v/v) were made by combining 95% EtOH in tap water. Pharmacological inactivation was conducted using muscimol (GABAA agonist; 0.1 mM; Sigma-Aldrich) and baclofen (GABAB agonist; 1.0 mM; Sigma-Aldrich). A muscimol/baclofen (M/B) solution was prepared by dissolving 5.71 mg of muscimol and 106.85 mg of baclofen into 500 ml of sterile physiological saline (1 ml aliquots stored at −20 °C). These concentrations have been shown to selectively influence behaviour when infused into the NAC core or shell subregions (McFarland & Kalivas, 2001; Fuchs et al., 2004; Floresco et al., 2006; Chaudhri et al., 2008a). Muscimol infused into the brain reduces spontaneous activity of neurons as assessed using electrophysiology, with the most robust effects observed within the first 1.5 hours post-infusion (Arikan et al., 2002). Dopamine D1 receptors were blocked using SCH 23390 hydrochloride (0.6 µg/0.3 µl; Sigma-Aldrich; Chaudhri et al., 2009; Bossert et al., 2007), which was prepared by dissolving 10 mg of SCH 23390 into 5 ml of sterile saline (stored at −20 °C in 200 µl aliquots).
Ethanol exposure in the home cage
EtOH exposure in the home cage was conducted in order to acclimate rats to the taste and pharmacological effects of EtOH. Two weeks after arrival, water bottles were removed and rats received a solution of 10% EtOH for 24–48 hours. Subsequently, water bottles were replaced and rats were given continuous access to both 10% EtOH and water via two bottles for 11–14 days, followed by 20% EtOH and water for a further 12–14 days. During this phase, EtOH consumption and rat weights were recorded every 24 hours. To maximize significant blood EtOH levels in a short period of time, rats were then given limited access to 20% EtOH for 1 hour/day with water for 23 hours/day for 10–20 days. Throughout pre-exposure, the left/right positions of the water and EtOH bottles on the home cage were alternated daily to mitigate the influence of side preferences. Table 1 provides mean EtOH intakes obtained across the last 2 days of the limited access phase for each experiment.
Table 1. Consumption of 20% ethanol during exposure sessions on the home cage.
Values represent ethanol intake in grams/kilogram averaged (Mean ± SEM) over the last 2 sessions of for each experiment.
| Ethanol intake (g/kg) | |
|---|---|
| Experiment 1 | 1.27 ± 0.12 (n = 10) |
| Experiment 2 | 1.28 ± 0.08 (n = 18) |
| Experiment 3 | 0.60 ± 0.06 (n = 17) |
Surgery
Following EtOH exposure and 4–8 sessions of Pavlovian discrimination training (see below), guide cannulae (26 GA, Plastics One, Roanoke, VA, USA) were implanted to target the BLA (Exp. 1, bilateral) or the BLA and contralateral NAC core in contralateral brain hemispheres (Exp. 2 & 3, unilateral cannula in each region) using standard stereotaxic procedures. Rats with shaved heads were anesthetised with isoflurane and placed in a stereotaxic frame (Kopf, Tujunga, CA). The skull was exposed, and DV coordinates at bregma and lambda were used to estimate a flat skull position. Coordinates for cannula implantation were based on pilot studies as well as our previous research (Chaudhri et al., 2008a): NAC core, AP +1.2, ML ±2.0, DV −3.8; BLA, AP −2.8, ML ±5.1, DV −5.4. Microinfusions utilized 33 gauge injectors (Plastics One, Roanoke, VA, USA) that protruded 3 mm below the base of the cannulae (final DV: NAC core, −6.8; BLA, −8.4). For Experiments 2 & 3, left and right cannula placements were counterbalanced such that each experiment began with equal numbers of subjects with unilateral BLA or NAC core cannulae in either brain hemisphere. Cannulae were anchored to the skull with dental cement and 5 metal screws, and occluded with metal obdurators of the same length (Plastics One, Roanoke, VA, USA). Rats were treated post-surgically with buprenorphine (0.05 mg/kg, i.m. single injection), and monitored daily to ensure regular weight gain. Behavioural training commenced after a minimum of 1 week recovery.
Intracranial microinfusions
During microinfusions rats were lightly restrained and obdurators replaced with 33 gauge injectors that protruded 3 mm below the cannula. Injectors were attached via polyethylene (PE-50) tubing to 10 µl Hamilton syringes that were placed in a syringe pump (Harvard Apparatus, Holliston, MA, USA; PHD 2000). The pump was activated for 1 minute at a rate of 0.3 µl/minute. Injectors were left in place for 2 minutes to allow for diffusion, after which rats were returned to their home-cages within the testing room for 15–20 minutes and then placed into the operant conditioning chambers. All microinfusions were conducted in a volume of 0.3 µl/hemisphere. The same procedure was used for sham injections, wherein injectors were cut to the same length as the cannula and no liquid was infused.
General behavioural procedures
Pavlovian discrimination training (PDT)
Rats were trained to behaviourally discriminate between two auditory conditioned stimuli across daily, 54 minute sessions (Mon-Fri). Session onset was indicated by illumination of the chamber light 1 minute after starting the computer program that controlled session events. Rats received 16 random presentations each of a 10 second white noise and clicker stimulus delivered under the control of a variable-time 67 second schedule. The onset of one stimulus (CS+) coincided with EtOH (0.2 ml, delivered into the fluid receptacle over 6 seconds); the second stimulus (CS−) was presented without EtOH. At the end of each session, ports were checked to ensure that all the EtOH delivered (3.2 ml) had been consumed.
PDT occurred in one of two contexts that were distinct across visual, tactile and olfactory domains (see Janak & Chaudhri, 2010 for illustration of contexts). Context 1 had clear Plexiglas chamber walls, a metal grid floor, and a strawberry scented air freshener, which provided a strong visual and olfactory stimulus, taped to the outside of the chamber door. Context 2 had black chamber walls, a smooth Plexiglas floor, and two sprays of 10% lemon odour misted into the bedding beneath the chamber floor. The context used for training, referred to as Context A, was kept constant across sessions, and designation of the white noise or clicker as the CS+ was counterbalanced across context.
Extinction
At 24 hours after the final PDT session, rats were placed into the context not used for PDT (referred to as Context B) and exposed to the CS+ and CS− as described above for 8 daily (Mon–Fri) 54 minute sessions. The pump was activated for 6 seconds during the CS+, but did not contain a syringe. Consequently, EtOH was not delivered during extinction.
Renewal Tests
At 24 hours after the last extinction session, rats were placed into the prior training context (Context A) and exposed to the CS+ and CS− in a 54 minute session, as described above. During the CS+ the pump was activated for 6 seconds, but did not contain a syringe. Consequently, EtOH was not delivered at test. Before test, rats received a microinfusion according to treatment conditions specified below. Each rat received 2 tests, with consecutive tests separated by additional sessions of PDT in Context A and extinction in Context B (see Table 2 for summary).
Table 2. Summary of the experimental design for each study.
Numbers in parenthesis represent the number of session in each phase. PDT = Pavlovian discrimination training.
| Experimental Phase | ||||||
|---|---|---|---|---|---|---|
| Exp. 1 | PDT (18) Context A |
Extinction (8) Context B |
Test 1 (1) Context A |
PDT (6) Context A |
Extinction (7) Context B |
Test 2 (1) Context A |
| Exp. 2 | PDT (17) Context A |
Extinction (8) Context B |
Test 1 (1) Context A |
PDT (5) Context A |
Extinction (7) Context B |
Test 2 (1) Context A |
| Exp. 3a | PDT (20) Context A |
Extinction (8) Context B |
Test 1 (1) Context A |
PDT (6) Context A |
Extinction (8) Context B |
Test 2 (1) Context A |
| Exp. 3b | PDT (3) Context A |
Test 1 (1) Context A |
PDT (3) Context A |
Test 2 (1) Context A |
||
Experiment 1: Effect of bilateral BLA inactivation on renewal
This experiment tested the hypothesis that the BLA is required for context-induced renewal of Pavlovian-conditioned alcohol-seeking. Rats received 18 PDT sessions in Context A (sham infusion on session 13) and 8 extinction sessions in Context B (saline infusion on session 2). At test in Context A half the rats received bilateral BLA microinfusions of saline and the remainder received bilateral BLA microinfusions of M/B. Treatment conditions for each subject were reversed at test 2, which was conducted after 6 PDT retraining sessions (sham infusion on session 2) and 7 extinction sessions (sham infusion on session 3).
Experiment 2: Effect of contralateral BLA – NAC core inactivation on renewal
This study tested the hypothesis that serial connectivity between the BLA and NAC core is required for context-induced renewal of Pavlovian-conditioned alcohol-seeking. Rats received 17 PDT sessions in Context A (sham infusion on session 13) and 8 extinction sessions in Context B (saline infusion on session 3). At test in Context A, the BLA-to-NAC core projection was functionally ‘disconnected’ for half the rats by unilaterally administering M/B into BLA and the contralateral NAC core. Control subjects received unilateral microinfusions of saline in both regions. Treatment conditions were reversed for each subject at test 2, which was conducted after 5 retraining PDT sessions (sham infusion on session 4) and 7 extinction sessions (sham infusion on session 3).
Experiment 3a: Effect on renewal of unilateral BLA inactivation with unilateral SCH 23390 in the contralateral NAC core
Here we tested the hypothesis that context-induced renewal of Pavlovian-conditioned alcohol-seeking is mediated by convergent DA projections and BLA excitatory projections within the NAC core. Rats received 20 PDT sessions in Context A (sham infusion on session 14) and 8 extinction sessions in Context B (saline infusion on session 4). At test in Context A, rats received one of the following 4 treatment conditions: (1) BLA saline - NAC core saline; (2) BLA saline – NAC core SCH 23390; (3) BLA M/B – NAC core SCH 23390; (4) BLA M/B – NAC core saline. Microinfusions were administered unilaterally into each brain area in each treatment condition. Each rat was then tested a second time, following 6 retraining PDT sessions in Context A (sham infusion on session 5) and 8 extinction sessions in Context B (no sham). At test 2, rats that had previously received treatment condition 1 were tested under condition 3, and vice versa. Likewise, at test 2 rats that had previously received treatment condition 2 were tested under condition 4, and vice versa.
Experiment 3b: Effect on Pavlovian-conditioned alcohol-seeking of unilateral BLA inactivation with unilateral SCH 23390 in the contralateral NAC core
Here we sought to determine if Pavlovian-conditioned alcohol-seeking under conditions in which the CS+ is paired with EtOH is dependent upon the convergence of DA and excitatory BLA inputs in the NAC core. To this end, following the conclusion of Experiment 3a subjects received PDT retraining sessions in Context A. Before the fourth PDT re-training session each rat was administered unilateral BLA and unilateral NAC core microinfusions according to one of the 4 treatment conditions described for Experiment 3a. A second test was then conducted, separated from the first by 3 PDT retraining sessions in Context A. Treatment conditions at tests 1 and test 2 for each rat were largely the same as those assigned in Experiment 3a.
Histology
At the end of each study rats were deeply anesthetised with sodium pentobarbital and perfused transcardially with 9% saline followed by 10% formalin. Brains were removed from the skull and post-fixed in formalin for 24-hours, followed by 25% sucrose for 48–72 hours. Frozen tissue was sectioned (60 µm), mounted onto slides and stained with cresyl violet. Cannula placements were analyzed using light microscopy. Rats were excluded if the injector tips were found to be outside the boundaries of the BLA and/or NAC core as identified by the Paxinos and Watson rat brain atlas (Paxinos G & Watson, 1997). Based on histology, 2 rats were excluded from Experiment 1.
Statistical Analyses
Port entries made into the fluid receptacle were recorded in each session. Baseline differences in behaviour were accounted for by subtracting port entries made during the 10 seconds immediately before a CS from port entries made during the corresponding 10 second CS (normalized port entries). Comparisons across normalized CS+ and normalized CS− responding were used to index behavioural discrimination. In addition, total port entries made during each session are reported.
In each experiment, rats received 2 PDT and 2 extinction phases. Initial PDT and extinction phases were analyzed using analysis of variance (ANOVA) with Session (as per number of PDT or extinction sessions) as a within-subjects repeated measure. For normalized port entries, CS (CS+, CS−) was included as a within-subjects repeated measure. There were no significant differences in behaviour at the end of each PDT or extinction phase. Therefore, data averaged across the last 2 sessions of the PDT and extinction phases before a given treatment were used as the baseline for that condition.
Based on prior research we predicted that placement into the alcohol context at test would cause the renewal of CS+ responding, with no change in CS− responding following saline microinfusion (Chaudhri et al., 2008b; Chaudhri et al., 2010). Thus, planned comparisons (paired samples t-tests) across extinction and test were used to analyze renewal effects for normalized CS+ and normalized CS− in saline-infused rats. Total port entries were not analyzed in this way because in our experience this measure does not consistently change at test, relative to extinction.
There was no significant effect of the side of infusion (i.e., whether infusions were administered into the left or right BLA) for Experiments 2 and 3. Therefore data were collapsed across hemisphere for statistical analyses. For Experiments 1 and 2, treatment effects at test were analyzed using ANOVA with Treatment (as per the experiment) as a within-subjects repeated measure. For normalized responding, CS (CS+, CS−) was included as a second within-subjects repeated measure. Paired samples t-tests were used to pursue significant interactions. In Experiment 3 each subject received 2 out of 4 possible test conditions, thereby negating a true within-subjects design. For this reason Treatment (BLA saline – NAC core saline; BLA M/B – NAC core saline; BLA M/B – NAC core SCH 23390; BLA saline – NAC core SCH 23390) was analyzed as a between-subject factor using ANOVA, and CS (CS+, CS−) was included as a within-subjects repeated measure where appropriate. Significant interactions were investigated using t-tests for independent samples that were adjusted for multiple comparisons using the Bonferroni correction. Analyses were conducted using SPSS (v 19). The Huynh-Feld correction was utilized for data that violated sphericity as detected by Mauchly’s test. The criterion for significance was α = 0.05.
Results
Experiment 1: Effect of bilateral BLA inactivation on renewal
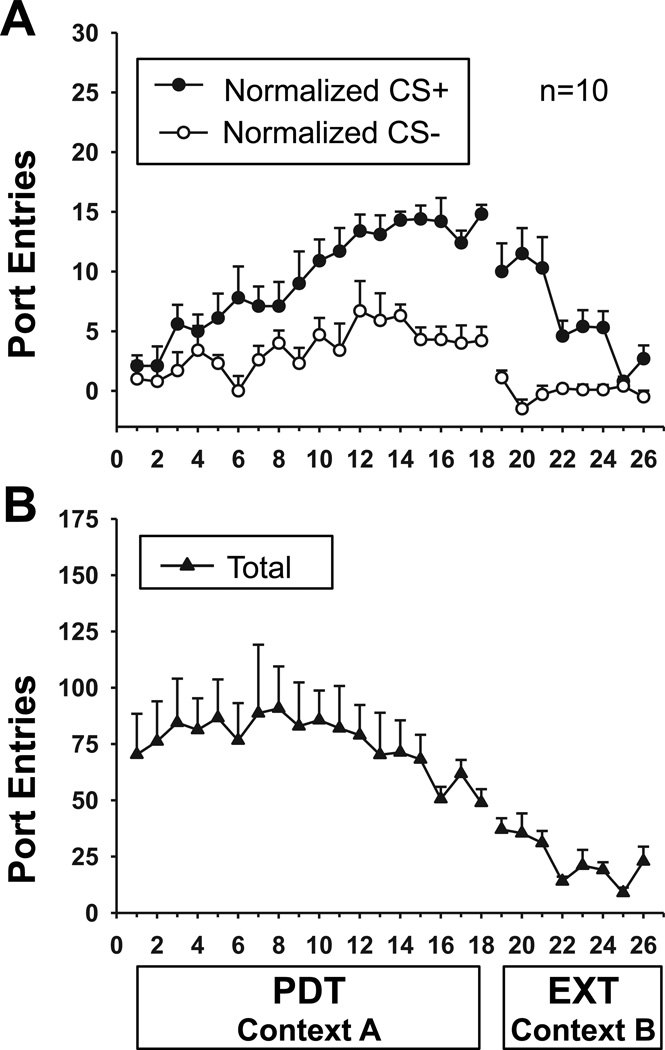
Figure 1 depicts the acquisition and extinction of Pavlovian discrimination training. As the same patterns of behaviour were observed in all three experiments, these data are representative of acquisition and extinction in this procedure. Rats learned to discriminate between the alcohol-predictive CS+ and the CS− across PDT sessions in Context A (Fig 1a). Responding to the CS+ increased across session, whereas responding to the CS− stabilized at a lower level [Session, F17,153 = 6.48, P < 0.0001; CS, F1,9 = 48.22, P < 0.0001; Session × CS, F17,153 = 2.58, P = 0.001]. When alcohol was withheld during extinction in Context B, port entries, particularly those elicited by the CS+, decreased across session [Session, F7,63 = 4.81; P < 0.001; CS, F1,9 = 78.77, P < 0.001; Session × CS, F7,63 = 6.94, P < 0.001]. Total port entries (Fig 1b) made during each session remained consistent across PDT [Session, F17,153 = 1.32, P = 0.19] and decreased across extinction [Session, F7,63 = 3.78, P = 0.002].
Fig 1. The acquisition and extinction of Pavlovian conditioned alcohol seeking.
Data represent average (Mean ± SEM) responding in each session of Pavlovian discrimination training (PDT) in Context A and extinction (EXT) in context B. In each session subjects received 16 CS+ and 16 CS− presentations according to an independent VT-67 sec schedule. During PDT each CS+ was paired with 0.2 ml of 20% EtOH (3.2 ml total). A Normalized port entries made during the CS+ (filled circles) and the CS− (open circles) across session. Data were normalized to account for differences in baseline activity by subtracting port entries during the 10-seconds immediately before each CS from the corresponding CS. B Total port entries across session.
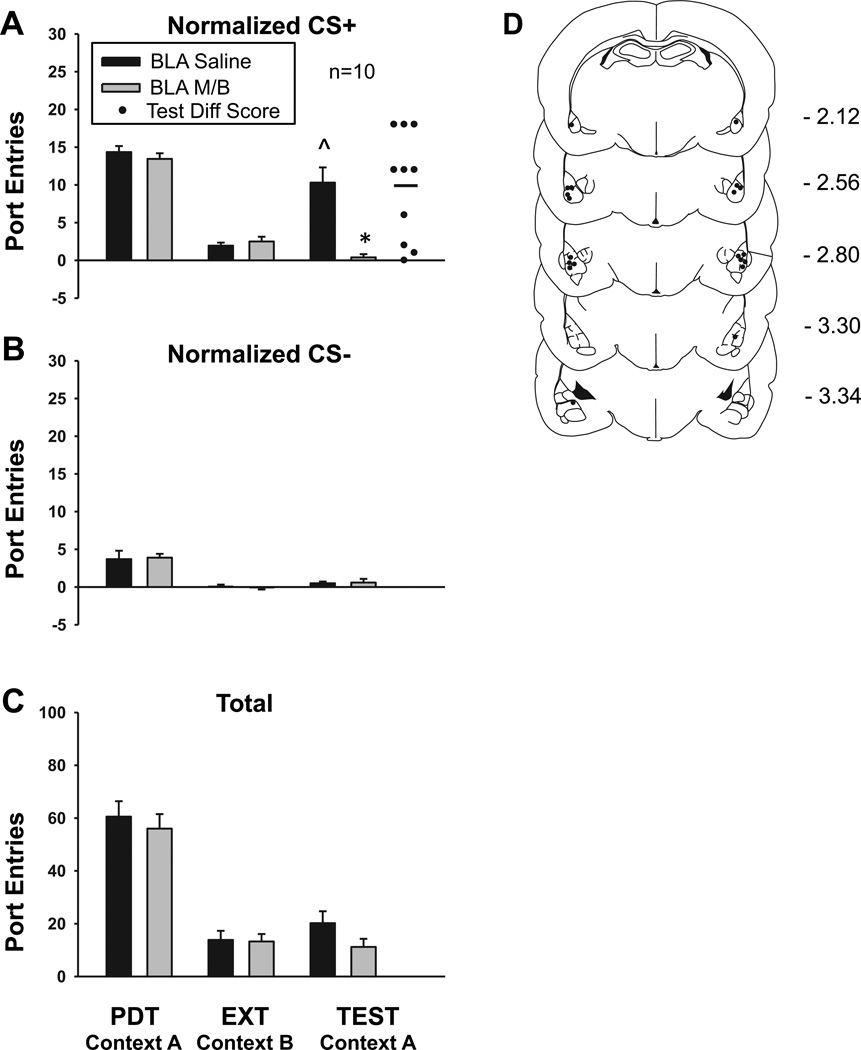
Bilateral BLA inactivation blocked context-induced renewal of Pavlovian-conditioned alcohol-seeking. Figure 2 depicts data obtained from renewal tests following saline or M/B microinfusions, as well as PDT and extinction baselines obtained by averaging data across the last 2 sessions of each phase before a corresponding test. Compared to extinction in Context B, presenting the CS+ and CS− without EtOH in Context A caused a significant increase in CS+ responding following saline microinfusion [Fig 2a; t9 = −4.00, P = 0.003], with no change in CS− responding [Fig 2b; t9 = −1.00, P = 0.34]. Compared to saline, BLA inactivation significantly reduced CS+ responding, with no effect on CS− responding [Treatment, F1,9 = 19.06, P = 0.002; CS, F1,9 = 27.25, P = 0.01; Treatment × CS, F1,9 = 16.73, P = 0.003]. Paired-samples t-tests verified a significant difference in CS+ responding across treatment conditions at test [t9 = 4.36, P = 0.002]. Responding during each CS was directly compared within each treatment condition to assess if discrimination between the cues remained intact at test. This analysis found that rats made more port-entries during the CS+ than the CS− following saline [t9 = 4.84, P = 0.001], but not after BLA inactivation [t9 = −0.26, P = 0.80].
Fig 2. Bilateral BLA inactivation blocks context-induced renewal of Pavlovian conditioned alcohol seeking.
For this and subsequent graphs, data represent average (Mean ± SEM) responding across the last 2 sessions of Pavlovian discrimination training (PDT) in Context A and extinction (EXT) in Context B, before the corresponding renewal test. At test in Context A subjects were pretreated with saline (filled bars) or M/B (gray bars) bilaterally into the BLA, using a within-subject design. They received CS+ and CS− presentations as during PDT, but no EtOH was delivered. A Normalized port entries during the CS+. For this and subsequent graphs filled circles represent a difference score obtained by subtracting normalized CS+ responding following M/B treatment from normalized CS+ responding after saline treatment. The horizontal bar represents the average of this difference score. B Normalized port entries during the CS−. C Total port entries. D Placement of injector tips within the BLA. For this and subsequent graphs distance from bregma is indicated to the right of each coronal section. ^ P = 0.003, BLA Saline EXT vs. BLA Saline TEST; * P = 0.002, BLA Saline TEST vs. BLA M/B TEST
A difference score calculated by subtracting normalized CS+ responding following BLA inactivation from normalized CS+ responding following saline revealed that each subject responded more in the latter condition (Fig 2a). The mean of this measure (Mean = 9.90, SEM ± 2.27) was significantly greater than zero [t9 = 4.36, P = 0.002].
A comparison across treatment conditions at test indicated a significant reduction in total port entries (Fig 2c) following BLA inactivation [Treatment, F1,9 = 12.66, P = 0.006]. However, when port-entries made during the CS+ were subtracted from total port entries, there was no significant difference between treatment conditions [saline, Mean = 9.90, SEM ± 3.07; M/B, Mean = 10.30, SEM ± 3.21; F 1,9 = 0.04, P = 0.90]. Thus, the effect of treatment on total port-entries is attributable to a specific reduction in CS+ responding following bilateral BLA inactivation. The location of microinfusions within the BLA is represented in Figure 2d.
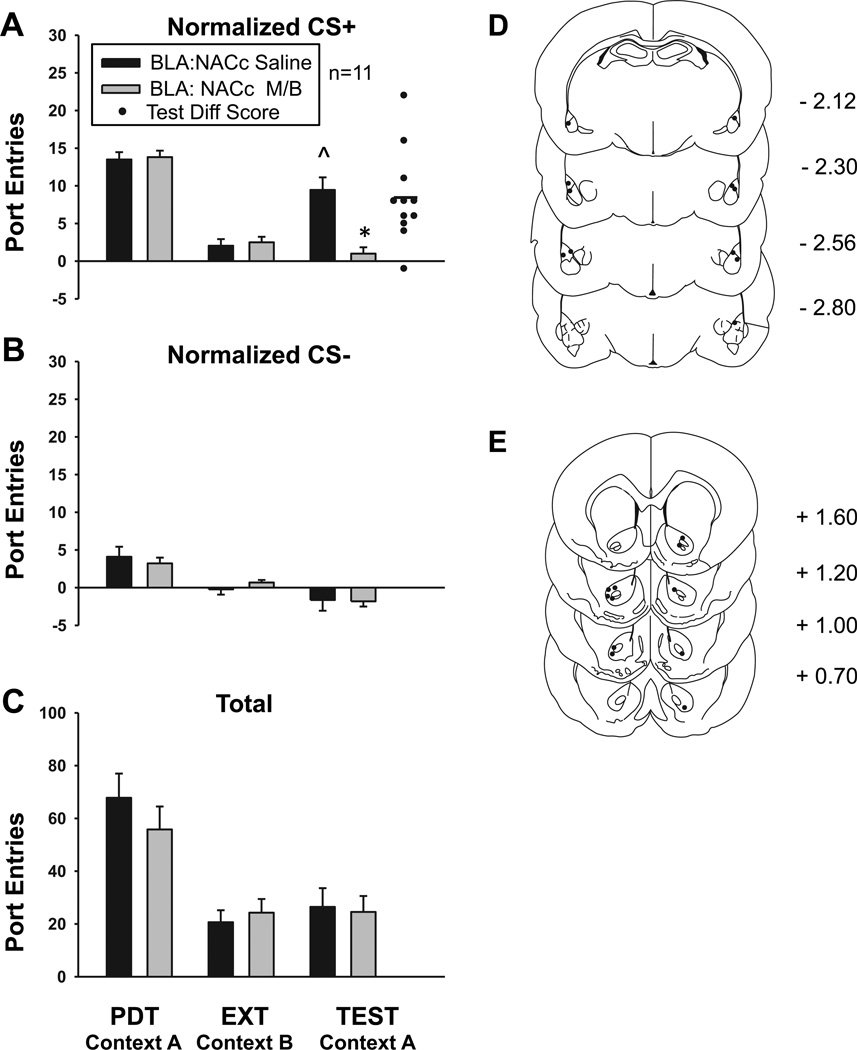
Experiment 2: Effect of contralateral BLA – NAC core inactivation on renewal
Functionally ‘disconnecting’ the BLA-to-NAC core pathway reduced context-induced renewal of Pavlovian-conditioned alcohol-seeking (Fig 3). Compared to extinction, CS+ responding increased significantly at test in saline pretreated subjects [Fig 3a; t10 = 6.07, P < 0.001], with no change in CS− responding [Fig 3b; t10 = −1.29, P = 0.23]. ANOVA conducted on test data revealed that while rats continued to discriminate between the two cues [CS, F1,10 = 13.57, P = 0.004], inactivation caused a significant reduction in CS+ responding [Treatment, F1,10 = 42.83, P < 0.001; Treatment × CS, F1,10 = 13.73, P = 0.004]. Paired samples t-tests verified a significant difference between treatment conditions in CS+ responding [t10 = 4.54, P = 0.001], with no difference in CS− responding [t10 = 0.14, P = 0.89]. Rats in both treatment conditions responded more to the CS+ than the CS− at test [Saline, t10 = 5.57, P < 0.001; M/B, t10 = 3.20, P = 0.01].
Fig 3. Contralateral BLA – NAC core inactivation reduces context-induced renewal of Pavlovian conditioned alcohol seeking.
Before the renewal test in Context A subjects received unilateral infusions of either saline (filled bars) or M/B (gray bars) into the contralateral BLA and NAC core, using a within-subjects design. A Normalized port entries during the CS+. B Normalized port entries made during the CS−. C Total port entries. D Placement of injector tips within the BLA. E Placement of injector tips within the NAC core. ^ P < 0.001 BLA-NAC core Saline EXT vs. BLA-NAC core Saline TEST; * P = 0.001 BLA-NAC core Saline TEST vs. BLA-NAC core M/B
As in Experiment 1, the majority of subjects (10 out of 11) responded more to the CS+ following saline, compared to M/B microinfusion (Diff score, Fig 3a). The mean of the difference score (Mean = 8.45, SEM ± 1.86) was significantly greater than zero [t10 = 4.54, P = 0.001]. There was no difference in total port entries across treatment conditions at test [Fig 3c; F1,10 = 0.45, P = 0.52]. The location of microinfusions within the BLA and NAC core are represented in Figures 3d and 3e, respectively.
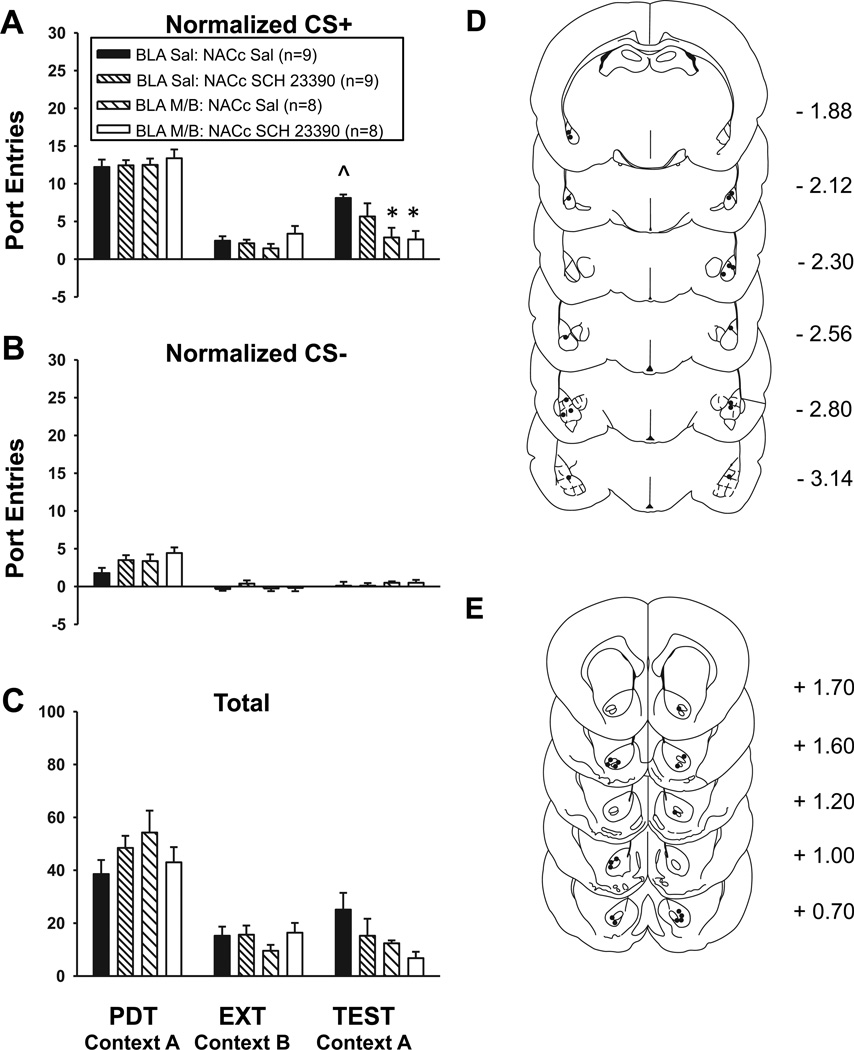
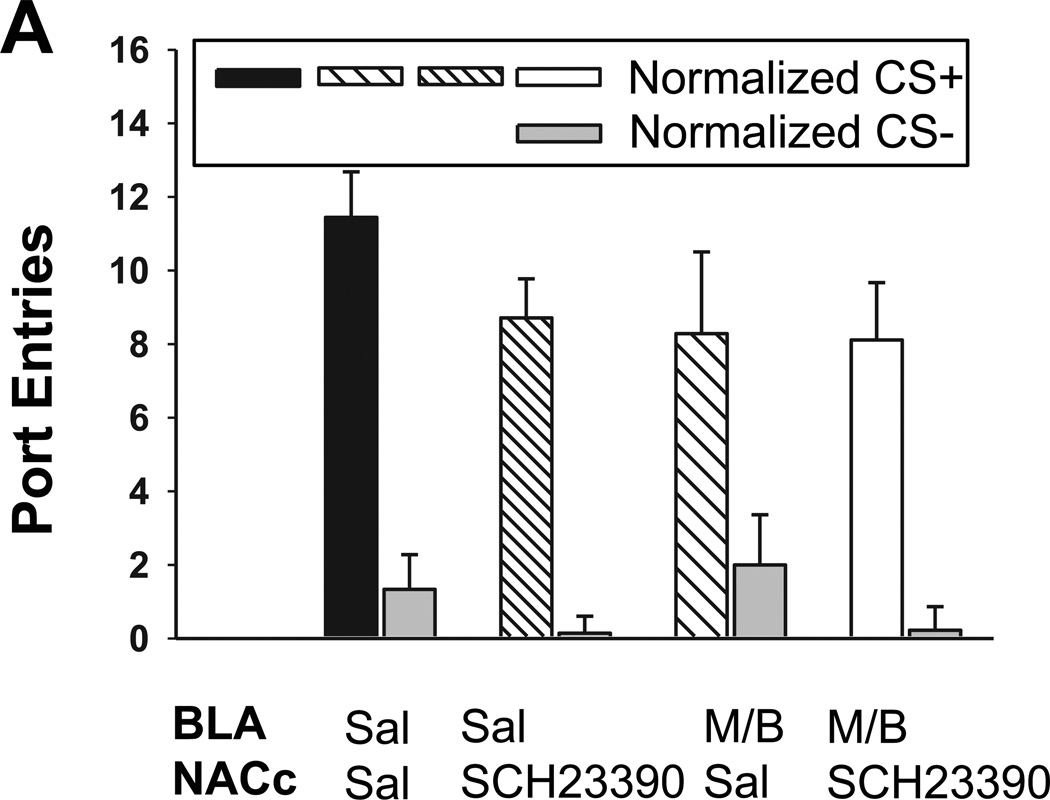
Experiment 3a: Effect on renewal of unilateral BLA inactivation with unilateral SCH 23390 or saline in the contralateral NAC core
Unilateral BLA inactivation together with either SCH 23390 or saline in the contralateral NAC core reduced context-induced renewal of Pavlovian-conditioned alcohol-seeking (Fig 4). Compared to extinction, CS+ responding increased at test following saline [Fig 4a; t8 = −6.40, P < 0.001], with no change in CS− responding [Fig 4b; t8 = −1.10, P = 0.30]. ANOVA revealed that while rats responded more to the CS+ than the CS− at test [CS, F1,30 = 51.65, P < 0.001], there was a significant impact of treatment condition [Treatment, F1,30 = 3.14, P = 0.04] on CS+ responding [CS × Treatment, F1,30 = 5.01, P = 0.006]. Normalized CS+ responding at test was further analyzed with t-tests for independent samples that were adjusted for multiple comparisons using the Bonferroni correction. This analysis indicated significant differences between the following groups: BLA saline – NAC core saline vs. BLA M/B – NAC core SCH 23390 (P = 0.03), and BLA saline – NAC core saline vs. BLA M/B – NAC core saline (P = 0.04). There was no statistically significant impact of treatment condition on total port entries at test [Fig 4c; F3,30 = 2.42, P = 0.09]. The location of microinfusions within the BLA and NAC core are represented in Figures 4d and 4e, respectively.
Fig 4. Context-induced renewal of Pavlovian conditioned alcohol seeking is reduced by unilateral BLA inactivation, and by unilateral BLA inactivation with unilateral SCH 23390 in the contralateral NAC core.
Treatment conditions at test are indicated in the legend. A Normalized port entries during the CS+. B Normalized port entries made during the CS−. C Total port entries. D Placement of injector tips within the BLA. E Placement of injector tips within the NAC core. ^ P < 0.001, BLA-NAC core Saline EXT vs. BLA-NAC core Saline TEST; * P = 0.03, significant difference between BLA saline–NAC core saline vs. BLA M/B–NAC core SCH 23390; * P = 0.04, significant difference between BLA saline–NAC core saline vs. BLA M/B–NAC core saline.
Experiment 3b: Effect on Pavlovian-conditioned alcohol-seeking of unilateral BLA inactivation with unilateral SCH 23390 or saline in the contralateral NAC core
Compared to saline-infused controls, there was no effect of unilateral SCH 23390 in the NAC core, or unilateral BLA inactivation alone or in combination with SCH 23390 in the contralateral NAC core on Pavlovian-conditioned alcohol-seeking when the CS+ was paired with alcohol (Fig 5). At test rats responded more to the CS+ compared to the CS− [CS, F1,28 = 82.78, P < 0.001]. There was no impact of treatment condition on responding to either the CS+ or the CS− [Treatment, F3,28 = 1.34, P = 0.28; CS × Treatment, F3,28 = 0.78, P = 0.52].
Fig 5. No effect of unilateral BLA inactivation or contralateral BLA M/B - NAC core SCH23390 on Pavlovian conditioned alcohol seeking.
At test rats received PDT sessions in Context A where CS+ presentations were paired with EtOH. Samples sizes for each treatment condition as the same as in Figure 4. Data represent average (Mean ± SEM) normalized port entries during the CS+ and CS−. Treatment conditions at test are indicated in the legend.
Discussion
The present findings demonstrate that alcohol contexts can reliably stimulate the renewal of Pavlovian-conditioned alcohol-seeking. This effect requires functional activity within the BLA, as bilateral BLA inactivation blocks renewal. Similar reductions in renewal are obtained following unilateral inactivation of the BLA and NAC core in contralateral hemispheres and, surprisingly, following unilateral inactivation of the BLA alone. Conversely, responding triggered by a Pavlovian alcohol cue that is paired with alcohol is not affected by unilateral BLA inactivation, unilateral SCH 23390 in the NAC core, or by unilateral BLA inactivation paired with SCH 23390 in the contralateral NAC core. While these results preclude conclusions about the requirement of serial connectivity between the BLA and NAC core in mediating context-induced renewal of Pavlovian-conditioned alcohol-seeking, they suggest that this phenomenon is highly reliant on functional activity within the BLA.
The interpretation of these data requires a consideration of the learning mechanisms that are believed to mediate context-induced renewal effects. When Pavlovian conditioning and extinction training are conducted in different environmental contexts, the context becomes the most reliable predictor for when a CS will be paired with an unconditioned stimulus (US). It has been proposed that in the renewal procedure responding to a CS increases at test because the training context triggers a memory of the CS-US relationship that was acquired in that context (Bouton, 2004). An attenuation of renewal following a neuropharmacological manipulation may therefore result from interference of the capacity of the context to signal this CS-US relationship. However, even with this process intact, a decrease in renewal would be observed if the CS-US memory could not be adequately retrieved. Lastly, a reduction in renewal of appetitive conditioning may also be attributable to a decrease in the conditioned incentive properties of the Pavlovian CS. Our investigation of the role of the BLA and of the BLA-to-NAC core projection in renewal was guided by a consideration of these processes. Behavioural responding to conditioned appetitive cues relies on connectivity within the BLA-to-NAC core projection (Everitt et al., 1991; Di Ciano & Everitt, 2004; Setlow et al., 2002; Ambroggi et al., 2008; Stuber et al., 2011). However, the BLA also receives afferent projections from areas of the brain that process contextual information, such as the ventral hippocampus (McDonald, 1998; Hobin et al., 2006). Thus, the BLA may be a point of convergence where contextual information integrates with, and modulates, neural circuits that mediate responding to discrete, appetitive cues.
We have shown previously that inactivating the NAC core reduces context-induced renewal of Pavlovian-conditioned alcohol-seeking (Chaudhri et al., 2010). This result parallels findings from renewal studies using instrumental drug conditioning procedures, which support a role for the NAC core in the renewal of operant drug-seeking (Fuchs et al., 2008; Chaudhri et al., 2008a; Chaudhri et al., 2009). We have also found that NAC core inactivation reduces CS+ responding using a variation of the present task in which responding to an alcohol predictive CS+ without alcohol delivery is tested in a context that was never paired with alcohol (Chaudhri et al., 2010), suggesting that the NAC core may be required for responding to Pavlovian-conditioned alcohol cues regardless of the context in which they are experienced. Thus, the involvement of the NAC core in context-induced renewal of Pavlovian-conditioned alcohol-seeking may derive from its role in mediating responding to the alcohol-predictive CS+ when it is encountered in the alcohol context.
The present finding that functional activity within the BLA is necessary for the renewal of Pavlovian-conditioned alcohol-seeking is in accord with research showing that BLA inactivation reduces context-induced reinstatement of instrumental cocaine seeking (Fuchs et al., 2005). Bilateral BLA inactivation in the present study also decreased the total number of port-entries at test, although there was no difference across treatment conditions in the number of port-entries that occurred outside CS+ presentations (i.e., total port-entries minus CS+ responding). Thus, impaired locomotor activity is an unlikely explanation for the reduction in renewal, a conclusion that is supported by the finding that BLA inactivation does not decrease locomotor activity in an open field (Woods & Ettenberg, 2004). Furthermore, BLA inactivation in the present study did not reduce total port entries below response levels observed during extinction. Thus, inactivating the BLA appears to have prevented the selective increase in responding to a Pavlovian, alcohol-predictive cue that was renewed by placement into an alcohol context. Context-induced renewal of instrumental sucrose- and alcohol- seeking is correlated with increased neuronal activation in the BLA (Hamlin et al., 2006; Marinelli et al., 2007) and requires opioid receptor activation in the BLA (Marinelli et al., 2010). Future research should determine if opioid receptor activation within the BLA is also required for the renewal of Pavlovian-conditioned alcohol-seeking.
Emerging research suggests that serial connectivity between the BLA and NAC core is required for the capacity of Pavlovian-conditioned stimuli to guide instrumental behaviour. However, the present data cannot confirm a role for this pathway in context-induced renewal of alcohol-seeking. We found that functionally ‘disconnecting’ the BLA-to-NAC core projection via unilaterally inactivating the BLA and NAC core in contralateral brain hemispheres, or by unilaterally inactivating the BLA and blocking dopamine D1 receptors in the contralateral NAC core impaired context-induced renewal of alcohol-seeking. However, ipsilateral BLA-to-NAC core inactivation also reduced CS responding at test (see Supplementary Fig 1). Furthermore, while blocking dopamine D1 receptors unilaterally within the NAC did not have an impact on renewal, unilateral BLA inactivation caused a significant reduction in renewal. Altogether, these findings preclude the conclusion that serial connectivity between the BLA and NAC core mediates renewal within this protocol.
Interestingly, unilateral BLA inactivation does not prevent the renewal of instrumental cocaine-seeking (Fuchs et al., 2007) or instrumental responding for sucrose guided by a discriminative CS (Ambroggi et al., 2008). One explanation for this discrepancy is that contextual renewal of behaviours acquired through Pavlovian learning may be more dependent on the BLA than contextual renewal of behaviours acquired through instrumental learning. Thus, disrupting BLA function in one brain hemisphere might be sufficient to induce a behavioural deficit in responses elicited by Pavlovian-conditioned stimuli during renewal tests. Since unilateral BLA inactivation did not impair responding to the CS+ when it was paired with alcohol, it may be that the BLA is particularly important for the contextual renewal of behaviours that are guided by the memory of the CS-US association. In a renewal test, this memory is retrieved by the contextual cues that comprise the training context. If the BLA is a region where neural circuits that process information about contexts interface with projections that mediate responding to discrete cues, then manipulations within one BLA hemisphere may be sufficient to disrupt context-evoked memories of CS-US associations.
Several lines of evidence suggest that contextual information is processed at the level of the BLA. Conditioned associations between drugs and drug-associated environmental contexts are mediated by, and involve neural plasticity within the BLA (Gremel & Cunningham, 2008; 2009; 2010; Rademacher et al., 2010). In addition, experiments conducted using animal models of fear-conditioning have found that unilateral, excitotoxic BLA lesions conducted after the acquisition of fear-conditioning selectively impair conditioned freezing in response to a shock-associated context, but have no effect on freezing in response to a shock-predictive CS (Flavell & Lee, 2012). These results suggest that the BLA might be particularly important for the expression of context-mediated behaviours, and this interpretation concurs with our finding that unilateral BLA inactivation reduces context-induced renewal of Pavlovian-conditioned alcohol-seeking.
Alternately, a unilateral BLA manipulation may be effective in producing behavioural change if the manipulation has an impact, either directly or indirectly, upon the contralateral BLA. This question has been addressed in at least two studies that obtained conflicting results. In one study, relative to sham lesions, unilateral BLA lesions did not affect c-Fos expression in the contralateral BLA that was triggered by placement into a shock-associated context, suggesting that there is no functional interaction between the two BLA nuclei (Flavell & Lee, 2012). However, a different study found that unilateral BLA infusions of muscimol that impair memory retrieval in a conditioned defeat paradigm in Syrian hamsters also reduced c-Fos expression in the contralateral BLA (Markham et al., 2010). Thus, temporarily inactivating the BLA in one hemisphere might induce a contralateral BLA deficit, via either direct or indirect interhemispheric connectivity between the two BLA nuclei. Notably, sparse intramygdaloid connectivity through the anterior commissure has been reported (Martinez-Lorenzana et al., 2004).
As well, BLA neurons that project ipsilaterally to other brain regions that innervate the contralateral hemisphere could also be important for renewal. For example, reciprocal connections between the BLA and orbitofrontal cortex (OFC) and BLA and dorsomedial prefrontal cortex (dm PFC) have been implicated in the renewal of instrumental responding for cocaine (Lasseter et al., 2011; Fuchs et al., 2007). Similarly, while the amygdalostriatal projection is largely ipsilateral, there is a smaller, contralateral amygdalostriatal projection (Kelley et al., 1982). Thus, unilateral BLA inactivation could dampen activity both within ipsilateral BLA-to-NAC core projections, as well as contralateral BLA-to-NAC core pathways, leading to a reduction in renewal. In summary, the context-induced renewal effects reported here might be reliant upon functional integrity within both interhemispheric projections and intrahemispheric projections that originate from the BLA.
It is intriguing that in the present experiments manipulations that reduced renewal had no effect on responding elicited by the CS+ when it was paired with alcohol during retraining (Figures 4 & 5), particularly because we have found previously that inactivating the NAC core bilaterally causes a small but significant reduction in CS+ responding under these conditions (Chaudhri et al., 2010). These results support the argument that reductions in renewal following unilateral BLA inactivation paired with either saline or SCH 23390 in the contralateral NAC core are not attributable to deficits in locomotion. They also suggest that BLA projections to the NAC core are not involved in the consumption of alcohol. However, the latter possibility warrants further investigation, given the finding that opioid receptor antagonists in the BLA reduce operant ethanol self-administration (Hyytia & Kiianmaa, 2001), which suggests that the BLA is important for motivation to consume alcohol. To the extent that renewal might be influenced by motivation to consume alcohol, manipulations within this structure could impact renewal, even though ethanol is not delivered during the renewal test.
The relevance of renewal effects observed in animal models to drug addiction in humans is highlighted by clinical data showing that conditioned reactivity to drug predictive cues in humans is susceptible to renewal (Collins & Brandon, 2002; Thewissen et al., 2006). Our findings support the emerging consensus that extinguishing reactivity to drug cues during treatment may not be a viable long-term strategy against relapse, given that extinguished responses can return outside the extinction context (Conklin, 2006). The present data establish context-induced renewal of Pavlovian-conditioned alcohol-seeking as a reliable phenomenon that is highly dependent upon the BLA. Given the present evidence that context-mediated alcohol-seeking is susceptible to unilateral BLA manipulations, rendering the contralateral disconnection approach ineffective, future research should consider additional techniques, such as optogenetic circuit mapping, to identify the critical amygdala projection(s) that is necessary for context-induced renewal of Pavlovian conditioned alcohol-seeking.
Supplementary Material
Acknowledgments
The National Institute of Alcohol Abuse and Alcoholism (RO1 AA014925; PHJ) provided funding for this research. NC is the recipient of a Chercheurs-Boursiers award from Fonds de la recherché en santé Quebec, and is a member of the FRQS-funded CSBN/GRNC.
Abbreviations
- BLA
Basolateral amygdale
- NAC core
Nucleus accumbens core
- EtOH
Ethanol
- CS
Conditioned stimulus
- PDT
Pavlovian discrimination training
- M/B
Muscimol/baclofen
- SCH 23390
R(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride
References
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikan R, Blake NM, Erinjeri JP, Woolsey TA, Giraud L, Highstein SM. A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. J Neurosci Methods. 2002;118:51–57. doi: 10.1016/s0165-0270(02)00143-7. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Cone JJ, Janak PH. Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci. 2008a;28:2288–2298. doi: 10.1111/j.1460-9568.2008.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry. 2008b;64:203–210. doi: 10.1016/j.biopsych.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology (Berl) 2009;207:303–314. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BN, Brandon TH. Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. J Consult Clin Psychol. 2002;70:390–397. [PubMed] [Google Scholar]
- Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol. 2006;14:12–19. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: the effects of environments on smokers’ cue-reactivity. Exp Clin Psychopharmacol. 2008;16:207–214. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O’Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Flavell CR, Lee JL. Post-training unilateral amygdala lesions selectively impair contextual fear memories. Learn Mem. 2012;19:256–263. doi: 10.1101/lm.025403.111. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci. 2006;26:2449–2457. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci. 1998;10:1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Roles of the nucleus accumbens and amygdala in the acquisition and expression of ethanol-conditioned behavior in mice. J Neurosci. 2008;28:1076–1084. doi: 10.1523/JNEUROSCI.4520-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharmacology. 2009;34:1443–1453. doi: 10.1038/npp.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Effects of disconnection of amygdala dopamine and nucleus accumbens N-methyl-d-aspartate receptors on ethanol-seeking behavior in mice. Eur J Neurosci. 2010;31:148–155. doi: 10.1111/j.1460-9568.2009.07044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Blatchford KE, McNally GP. Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience. 2006;143:25–38. doi: 10.1016/j.neuroscience.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Janak PH, Chaudhri N. The Potent Effect of Environmental Context on Relapse to Alcohol-Seeking After Extinction. Open Addict J. 2010;3:76–87. doi: 10.2174/1874941001003010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Day JJ, Aragona BJ, Wheeler RA, Wightman RM, Carelli RM. Basolateral amygdala modulates terminal dopamine release in the nucleus accumbens and conditioned responding. Biol Psychiatry. 2010;67:737–744. doi: 10.1016/j.biopsych.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat--an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Wells AM, Xie X, Fuchs RA. Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2011;36:711–720. doi: 10.1038/npp.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Le AD. Opioid receptors in the basolateral amygdala but not dorsal hippocampus mediate context-induced alcohol seeking. Behav Brain Res. 2010;211:58–63. doi: 10.1016/j.bbr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Li Z, Le AD. Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur J Neurosci. 2007;26:2815–2823. doi: 10.1111/j.1460-9568.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- Markham CM, Taylor SL, Huhman KL. Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn Mem. 2010;17:109–116. doi: 10.1101/lm.1633710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lorenzana G, Jimenez JR, Condes-Lara M. Interamygdaloid connection of basolateral nucleus through the anterior commissure in the rat. Neurosci Lett. 2004;366:154–157. doi: 10.1016/j.neulet.2004.05.026. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Rosenkranz JA, Morshedi MM, Sullivan EM, Meredith GE. Amphetamine-associated contextual learning is accompanied by structural and functional plasticity in the basolateral amygdala. J Neurosci. 2010;30:4676–4686. doi: 10.1523/JNEUROSCI.6165-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Holland PC, Gallagher M. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive pavlovian second-order conditioned responses. Behav Neurosci. 2002;116:267–275. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewissen R, Snijders SJ, Havermans RC, van den Hout M, Jansen A. Renewal of cue-elicited urge to smoke: implications for cue exposure treatment. Behav Res Ther. 2006;44:1441–1449. doi: 10.1016/j.brat.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Tsiang MT, Janak PH. Alcohol seeking in C57BL/6 mice induced by conditioned cues and contexts in the extinction-reinstatement model. Alcohol. 2006;38:81–88. doi: 10.1016/j.alcohol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Woods VE, Ettenberg A. Increased amphetamine-induced locomotion during inactivation of the basolateral amygdala. Behav Brain Res. 2004;149:33–39. doi: 10.1016/s0166-4328(03)00212-2. [DOI] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak PH. Context is a trigger for relapse to alcohol. Behav Brain Res. 2006;167:150–155. doi: 10.1016/j.bbr.2005.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.