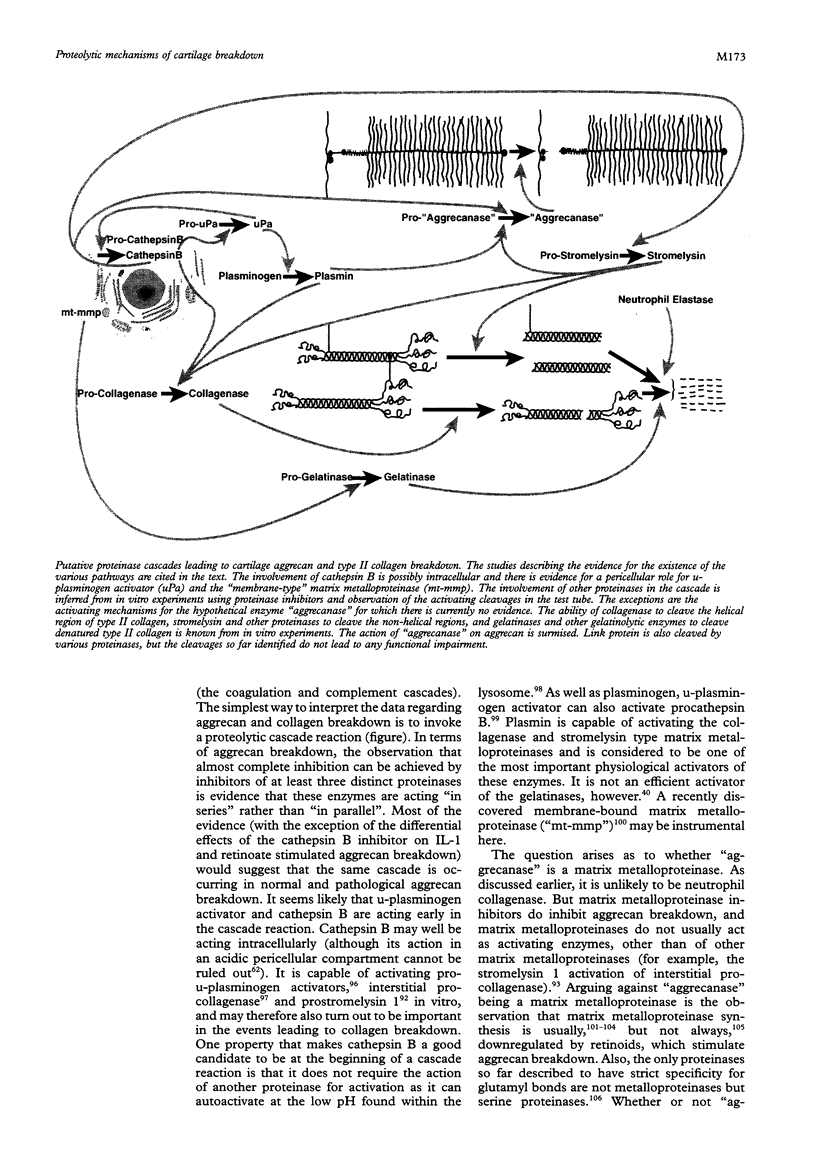
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Meguid S. S., Metcalf B. W., Carr T. J., Demarsh P., DesJarlais R. L., Fisher S., Green D. W., Ivanoff L., Lambert D. M., Murthy K. H. An orally bioavailable HIV-1 protease inhibitor containing an imidazole-derived peptide bond replacement: crystallographic and pharmacokinetic analysis. Biochemistry. 1994 Oct 4;33(39):11671–11677. doi: 10.1021/bi00205a001. [DOI] [PubMed] [Google Scholar]
- Ali S. Y., Evans L., Stainthorpe E., Lack C. H. Characterization of cathepsins in cartilage. Biochem J. 1967 Nov;105(2):549–557. doi: 10.1042/bj1050549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. Y. The degradation of cartilage matrix by an intracellular protease. Biochem J. 1964 Dec;93(3):611–618. doi: 10.1042/bj0930611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews H. J., Plumpton T. A., Harper G. P., Cawston T. E. A synthetic peptide metalloproteinase inhibitor, but not TIMP, prevents the breakdown of proteoglycan within articular cartilage in vitro. Agents Actions. 1992 Sep;37(1-2):147–154. doi: 10.1007/BF01987904. [DOI] [PubMed] [Google Scholar]
- Baici A., Lang A. Effect of interleukin-1 beta on the production of cathepsin B by rabbit articular chondrocytes. FEBS Lett. 1990 Dec 17;277(1-2):93–96. doi: 10.1016/0014-5793(90)80816-2. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Seltzer J. L., Eisen A. Z. Retinoic acid inhibition of collagenase and gelatinase expression in human skin fibroblast cultures. Evidence for a dual mechanism. J Invest Dermatol. 1983 Aug;81(2):162–169. doi: 10.1111/1523-1747.ep12543590. [DOI] [PubMed] [Google Scholar]
- Belch J. J., McArdle B., Madhok R., McLaughlin K., Capell H. A., Forbes C. D., Sturrock R. D. Decreased plasma fibrinolysis in patients with rheumatoid arthritis. Ann Rheum Dis. 1984 Dec;43(6):774–777. doi: 10.1136/ard.43.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birktoft J. J., Breddam K. Glutamyl endopeptidases. Methods Enzymol. 1994;244:114–126. doi: 10.1016/0076-6879(94)44010-7. [DOI] [PubMed] [Google Scholar]
- Blasi F., Stoppelli M. P., Cubellis M. V. The receptor for urokinase-plasminogen activator. J Cell Biochem. 1986;32(3):179–186. doi: 10.1002/jcb.240320303. [DOI] [PubMed] [Google Scholar]
- Bode W., Gomis-Rüth F. X., Stöckler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the 'metzincins'. FEBS Lett. 1993 Sep 27;331(1-2):134–140. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Ehrlich H. P., Wyke A. W. Procollagen: conversion of the precursor to collagen by a neutral protease. Science. 1972 Feb 4;175(4021):544–546. doi: 10.1126/science.175.4021.544. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E. Joint destruction in arthritis: metalloproteinases in the spotlight. Arthritis Rheum. 1991 Sep;34(9):1073–1075. doi: 10.1002/art.1780340902. [DOI] [PubMed] [Google Scholar]
- Brömme D., Steinert A., Friebe S., Fittkau S., Wiederanders B., Kirschke H. The specificity of bovine spleen cathepsin S. A comparison with rat liver cathepsins L and B. Biochem J. 1989 Dec 1;264(2):475–481. doi: 10.1042/bj2640475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunning R. A., Crawford A., Richardson H. J., Opdenakker G., Van Damme J., Russell R. G. Interleukin 1 preferentially stimulates the production of tissue-type plasminogen activator by human articular chondrocytes. Biochim Biophys Acta. 1987 Jun 22;924(3):473–482. doi: 10.1016/0304-4165(87)90163-2. [DOI] [PubMed] [Google Scholar]
- Burleigh M. C., Barrett A. J., Lazarus G. S. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem J. 1974 Feb;137(2):387–398. doi: 10.1042/bj1370387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttle D. J., Handley C. J., Ilic M. Z., Saklatvala J., Murata M., Barrett A. J. Inhibition of cartilage proteoglycan release by a specific inactivator of cathepsin B and an inhibitor of matrix metalloproteinases. Evidence for two converging pathways of chondrocyte-mediated proteoglycan degradation. Arthritis Rheum. 1993 Dec;36(12):1709–1717. doi: 10.1002/art.1780361210. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Murata M., Knight C. G., Barrett A. J. CA074 methyl ester: a proinhibitor for intracellular cathepsin B. Arch Biochem Biophys. 1992 Dec;299(2):377–380. doi: 10.1016/0003-9861(92)90290-d. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Saklatvala J. Lysosomal cysteine endopeptidases mediate interleukin 1-stimulated cartilage proteoglycan degradation. Biochem J. 1992 Oct 15;287(Pt 2):657–661. doi: 10.1042/bj2870657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttle D. J., Saklatvala J., Tamai M., Barrett A. J. Inhibition of interleukin 1-stimulated cartilage proteoglycan degradation by a lipophilic inactivator of cysteine endopeptidases. Biochem J. 1992 Jan 1;281(Pt 1):175–177. doi: 10.1042/bj2810175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. K., Piccoli D. S., Roberts M. J., Muirden K. D., Hamilton J. A. Effects of tumor necrosis factor alpha and beta on resorption of human articular cartilage and production of plasminogen activator by human articular chondrocytes. Arthritis Rheum. 1990 Apr;33(4):542–552. doi: 10.1002/art.1780330412. [DOI] [PubMed] [Google Scholar]
- Caputo C. B., Sygowski L. A., Wolanin D. J., Patton S. P., Caccese R. G., Shaw A., Roberts R. A., DiPasquale G. Effect of synthetic metalloprotease inhibitors on cartilage autolysis in vitro. J Pharmacol Exp Ther. 1987 Feb;240(2):460–465. [PubMed] [Google Scholar]
- Carmeliet P., Schoonjans L., Kieckens L., Ream B., Degen J., Bronson R., De Vos R., van den Oord J. J., Collen D., Mulligan R. C. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994 Mar 31;368(6470):419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- Cawston T. Blocking cartilage destruction with metalloproteinase inhibitors: a valid therapeutic target? Ann Rheum Dis. 1993 Nov;52(11):769–770. doi: 10.1136/ard.52.11.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah K. S., Stoker N. G., Griffin J. R., Grosveld F. G., Solomon E. Identification and characterization of the human type II collagen gene (COL2A1). Proc Natl Acad Sci U S A. 1985 May;82(9):2555–2559. doi: 10.1073/pnas.82.9.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. U., Tang L. H., Johnson T. L., Pal S., Rosenberg L. C., Reiner A., Poole A. R. Isolation and characterization of a 35,000 molecular weight subunit fetal cartilage matrix protein. J Biol Chem. 1983 Jan 10;258(1):655–661. [PubMed] [Google Scholar]
- Collier S., Ghosh P. The role of plasminogen in interleukin-1 mediated cartilage degradation. J Rheumatol. 1988 Jul;15(7):1129–1137. [PubMed] [Google Scholar]
- Connolly T. J., Clohisy J. C., Shilt J. S., Bergman K. D., Partridge N. C., Quinn C. O. Retinoic acid stimulates interstitial collagenase messenger ribonucleic acid in osteosarcoma cells. Endocrinology. 1994 Dec;135(6):2542–2548. doi: 10.1210/endo.135.6.7988442. [DOI] [PubMed] [Google Scholar]
- DINGLE J. T. Studies on the mode of action of excess of vitamin A. 3. Release of a bound protease by the action of vitamin A. Biochem J. 1961 Jun;79:509–512. doi: 10.1042/bj0790509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalet-Fumeron V., Guinec N., Pagano M. In vitro activation of pro-cathepsin B by three serine proteinases: leucocyte elastase, cathepsin G, and the urokinase-type plasminogen activator. FEBS Lett. 1993 Oct 18;332(3):251–254. doi: 10.1016/0014-5793(93)80643-9. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Bréard J., Chess L., Krane S. M. Participation of monocyte-macrophages and lymphocytes in the production of a factor that stimulates collagenase and prostaglandin release by rheumatoid synovial cells. J Clin Invest. 1979 Nov;64(5):1386–1392. doi: 10.1172/JCI109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen S. J., Rajput B., Reich E. The human tissue plasminogen activator gene. J Biol Chem. 1986 May 25;261(15):6972–6985. [PubMed] [Google Scholar]
- Demers L. M., Kleerekoper M. Recent advances in biochemical markers of bone turnover. Clin Chem. 1994 Nov;40(11 Pt 1):1994–1995. [PubMed] [Google Scholar]
- Dessau W., Sasse J., Timpl R., Jilek F., von der Mark K. Synthesis and extracellular deposition of fibronectin in chondrocyte cultures. Response to the removal of extracellular cartilage matrix. J Cell Biol. 1978 Nov;79(2 Pt 1):342–355. doi: 10.1083/jcb.79.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T. Cartilage maintenance in osteoarthritis: interaction of cytokines, NSAID and prostaglandins in articular cartilage damage and repair. J Rheumatol Suppl. 1991 Mar;28:30–37. [PubMed] [Google Scholar]
- Dingle J. T., Dingle T. T. The site of cartilage matrix degradation. Biochem J. 1980 Aug 15;190(2):431–438. doi: 10.1042/bj1900431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge G. R., Pidoux I., Poole A. R. The degradation of type II collagen in rheumatoid arthritis: an immunoelectron microscopic study. Matrix. 1991 Nov;11(5):330–338. doi: 10.1016/s0934-8832(11)80204-0. [DOI] [PubMed] [Google Scholar]
- Dodge G. R., Poole A. R. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989 Feb;83(2):647–661. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Long-Fox A., Charles P., Katsikis P., Brennan F. M., Walker J., Bijl H., Ghrayeb J. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993 Dec;36(12):1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Ellis A. J., Curry V. A., Powell E. K., Cawston T. E. The prevention of collagen breakdown in bovine nasal cartilage by TIMP, TIMP-2 and a low molecular weight synthetic inhibitor. Biochem Biophys Res Commun. 1994 May 30;201(1):94–101. doi: 10.1006/bbrc.1994.1673. [DOI] [PubMed] [Google Scholar]
- FELL H. B., DINGLE J. T. Studies on the mode of action of excess of vitamin A. 6. Lysosomal protease and the degradation of cartilage matrix. Biochem J. 1963 May;87:403–408. doi: 10.1042/bj0870403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELL H. B., MELLANBY E. The effect of hypervitaminosis A on embryonic limb bones cultivated in vitro. J Physiol. 1952 Mar;116(3):320–349. doi: 10.1113/jphysiol.1952.sp004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery C. R., Lark M. W., Sandy J. D. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992 Jan 15;267(2):1008–1014. [PubMed] [Google Scholar]
- Fontana A., Hengartner H., Weber E., Fehr K., Grob P. J., Cohen G. Interleukin 1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):49–53. doi: 10.1007/BF00541245. [DOI] [PubMed] [Google Scholar]
- Fosang A. J., Last K., Neame P. J., Murphy G., Knäuper V., Tschesche H., Hughes C. E., Caterson B., Hardingham T. E. Neutrophil collagenase (MMP-8) cleaves at the aggrecanase site E373-A374 in the interglobular domain of cartilage aggrecan. Biochem J. 1994 Dec 1;304(Pt 2):347–351. doi: 10.1042/bj3040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Hardingham T. E., Murphy G., Hamilton J. A. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991 Aug 25;266(24):15579–15582. [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Last K., Hardingham T. E., Murphy G., Hamilton J. A. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J Biol Chem. 1992 Sep 25;267(27):19470–19474. [PubMed] [Google Scholar]
- Gross J., Nagai Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1197–1204. doi: 10.1073/pnas.54.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander A. P., Atkins R. M., Eastwood D. M., Dieppe P. A., Elson C. J. Human cartilage is degraded by rheumatoid arthritis synovial fluid but not by recombinant cytokines in vitro. Clin Exp Immunol. 1991 Jan;83(1):52–57. doi: 10.1111/j.1365-2249.1991.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander A. P., Heathfield T. F., Webber C., Iwata Y., Bourne R., Rorabeck C., Poole A. R. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994 Apr;93(4):1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Hughes C. E., Caterson B., Fosang A. J., Roughley P. J., Mort J. S. Monoclonal antibodies that specifically recognize neoepitope sequences generated by 'aggrecanase' and matrix metalloproteinase cleavage of aggrecan: application to catabolism in situ and in vitro. Biochem J. 1995 Feb 1;305(Pt 3):799–804. doi: 10.1042/bj3050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. E., Caterson B., White R. J., Roughley P. J., Mort J. S. Monoclonal antibodies recognizing protease-generated neoepitopes from cartilage proteoglycan degradation. Application to studies of human link protein cleavage by stromelysin. J Biol Chem. 1992 Aug 15;267(23):16011–16014. [PubMed] [Google Scholar]
- Ilic M. Z., Handley C. J., Robinson H. C., Mok M. T. Mechanism of catabolism of aggrecan by articular cartilage. Arch Biochem Biophys. 1992 Apr;294(1):115–122. doi: 10.1016/0003-9861(92)90144-l. [DOI] [PubMed] [Google Scholar]
- Knudsen B. S., Harpel P. C., Nachman R. L. Plasminogen activator inhibitor is associated with the extracellular matrix of cultured bovine smooth muscle cells. J Clin Invest. 1987 Oct;80(4):1082–1089. doi: 10.1172/JCI113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Schmitt M., Goretzki L., Chucholowski N., Calvete J., Kramer M., Günzler W. A., Jänicke F., Graeff H. Cathepsin B efficiently activates the soluble and the tumor cell receptor-bound form of the proenzyme urokinase-type plasminogen activator (Pro-uPA). J Biol Chem. 1991 Mar 15;266(8):5147–5152. [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 1975 Sep-Oct;18(5):475–483. doi: 10.1002/art.1780180507. [DOI] [PubMed] [Google Scholar]
- LUCY J. A., DINGLE J. T., FELL H. B. Studies on the mode of action of excess of vitamin A. 2. A possible role of intracellular proteases in the degradation of cartilage matrix. Biochem J. 1961 Jun;79:500–508. doi: 10.1042/bj0790500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R., Kim S. J., Angel P., Roberts A. B., Sporn M. B., Karin M., Wilder R. L. Interleukin-1 stimulates and all-trans-retinoic acid inhibits collagenase gene expression through its 5' activator protein-1-binding site. Mol Endocrinol. 1990 Jul;4(7):973–980. doi: 10.1210/mend-4-7-973. [DOI] [PubMed] [Google Scholar]
- Leizer T., Clarris B. J., Ash P. E., van Damme J., Saklatvala J., Hamilton J. A. Interleukin-1 beta and interleukin-1 alpha stimulate the plasminogen activator activity and prostaglandin E2 levels of human synovial cells. Arthritis Rheum. 1987 May;30(5):562–566. doi: 10.1002/art.1780300511. [DOI] [PubMed] [Google Scholar]
- Leong W. S., Russell R. G., Caswell A. M. Stimulation of cartilage resorption by extracellular ATP acting at P2-purinoceptors. Biochim Biophys Acta. 1994 Nov 11;1201(2):298–304. doi: 10.1016/0304-4165(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Van Hoef B., Nelles L., Collen D. Plasminogen activation with single-chain urokinase-type plasminogen activator (scu-PA). Studies with active site mutagenized plasminogen (Ser740----Ala) and plasmin-resistant scu-PA (Lys158----Glu). J Biol Chem. 1990 Mar 25;265(9):5232–5236. [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Brundage R., Siegal G. P., Terranova V., Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981 Nov;41(11 Pt 1):4629–4636. [PubMed] [Google Scholar]
- Lohmander L. S., Neame P. J., Sandy J. D. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993 Sep;36(9):1214–1222. doi: 10.1002/art.1780360906. [DOI] [PubMed] [Google Scholar]
- Lohnes D., Dierich A., Ghyselinck N., Kastner P., Lampron C., LeMeur M., Lufkin T., Mendelsohn C., Nakshatri H., Chambon P. Retinoid receptors and binding proteins. J Cell Sci Suppl. 1992;16:69–76. doi: 10.1242/jcs.1992.supplement_16.9. [DOI] [PubMed] [Google Scholar]
- Loulakis P., Shrikhande A., Davis G., Maniglia C. A. N-terminal sequence of proteoglycan fragments isolated from medium of interleukin-1-treated articular-cartilage cultures. Putative site(s) of enzymic cleavage. Biochem J. 1992 Jun 1;284(Pt 2):589–593. doi: 10.1042/bj2840589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy B., Cleasby A., Hassell A. M., Longley K., Luther M. A., Weigl D., McGeehan G., McElroy A. B., Drewry D., Lambert M. H. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science. 1994 Jan 21;263(5145):375–377. doi: 10.1126/science.8278810. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Faure M. P., McCollum R., Mineau F., Cloutier J. M., Pelletier J. P. Plasmin, plasminogen activators and inhibitor in human osteoarthritic cartilage. J Rheumatol. 1991 Dec;18(12):1863–1871. [PubMed] [Google Scholar]
- Matsunaga Y., Saibara T., Kido H., Katunuma N. Participation of cathepsin B in processing of antigen presentation to MHC class II. FEBS Lett. 1993 Jun 21;324(3):325–330. doi: 10.1016/0014-5793(93)80144-j. [DOI] [PubMed] [Google Scholar]
- Mayer M. Biochemical and biological aspects of the plasminogen activation system. Clin Biochem. 1990 Jun;23(3):197–211. doi: 10.1016/0009-9120(90)90601-p. [DOI] [PubMed] [Google Scholar]
- Meats J. E., Elford P. R., Bunning R. A., Russell R. G. Retinoids and synovial factor(s) stimulate the production of plasminogen activator by cultured human chondrocytes. A possible role for plasminogen activator in the resorption of cartilage in vitro. Biochim Biophys Acta. 1985 Jan 28;838(1):161–169. doi: 10.1016/0304-4165(85)90262-4. [DOI] [PubMed] [Google Scholar]
- Mimuro J., Schleef R. R., Loskutoff D. J. Extracellular matrix of cultured bovine aortic endothelial cells contains functionally active type 1 plasminogen activator inhibitor. Blood. 1987 Sep;70(3):721–728. [PubMed] [Google Scholar]
- Mochan E., Uhl J. Elevations in synovial fluid plasminogen activator in patients with rheumatoid arthritis. J Rheumatol. 1984 Apr;11(2):123–128. [PubMed] [Google Scholar]
- Murphy G. J., Murphy G., Reynolds J. J. The origin of matrix metalloproteinases and their familial relationships. FEBS Lett. 1991 Sep 2;289(1):4–7. doi: 10.1016/0014-5793(91)80895-a. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., Reynolds J. J. Characterization of a specific antiserum to rabbit stromelysin and demonstration of the synthesis of collagenase and stromelysin by stimulated rabbit articular chondrocytes. Coll Relat Res. 1986 Oct;6(4):351–363. doi: 10.1016/s0174-173x(86)80005-x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Ward R., Gavrilovic J., Atkinson S. Physiological mechanisms for metalloproteinase activation. Matrix Suppl. 1992;1:224–230. [PubMed] [Google Scholar]
- Nguyen Q., Liu J., Roughley P. J., Mort J. S. Link protein as a monitor in situ of endogenous proteolysis in adult human articular cartilage. Biochem J. 1991 Aug 15;278(Pt 1):143–147. doi: 10.1042/bj2780143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q., Murphy G., Hughes C. E., Mort J. S., Roughley P. J. Matrix metalloproteinases cleave at two distinct sites on human cartilage link protein. Biochem J. 1993 Oct 15;295(Pt 2):595–598. doi: 10.1042/bj2950595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon J. S., Bottomley K. M., Broadhurst M. J., Brown P. A., Johnson W. H., Lawton G., Marley J., Sedgwick A. D., Wilkinson S. E. Potent collagenase inhibitors prevent interleukin-1-induced cartilage degradation in vitro. Int J Tissue React. 1991;13(5):237–241. [PubMed] [Google Scholar]
- Oweida S. W., Ku D. N., Lumsden A. B., Kam C. M., Powers J. C. In vivo determination of the anticoagulant effect of a substituted isocoumarin (ACITIC). Thromb Res. 1990 Apr 15;58(2):191–197. doi: 10.1016/0049-3848(90)90176-d. [DOI] [PubMed] [Google Scholar]
- Page A. E., Fuller K., Chambers T. J., Warburton M. J. Purification and characterization of a tripeptidyl peptidase I from human osteoclastomas: evidence for its role in bone resorption. Arch Biochem Biophys. 1993 Nov 1;306(2):354–359. doi: 10.1006/abbi.1993.1523. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Ionescu M., Swan A., Dieppe P. A. Changes in cartilage metabolism in arthritis are reflected by altered serum and synovial fluid levels of the cartilage proteoglycan aggrecan. Implications for pathogenesis. J Clin Invest. 1994 Jul;94(1):25–33. doi: 10.1172/JCI117314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings N. D., Barrett A. J. Evolutionary families of peptidases. Biochem J. 1993 Feb 15;290(Pt 1):205–218. doi: 10.1042/bj2900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht M. P., Resnick D. MR imaging of articular cartilage: current status and future directions. AJR Am J Roentgenol. 1994 Aug;163(2):283–290. doi: 10.2214/ajr.163.2.8037016. [DOI] [PubMed] [Google Scholar]
- Reinemer P., Grams F., Huber R., Kleine T., Schnierer S., Piper M., Tschesche H., Bode W. Structural implications for the role of the N terminus in the 'superactivation' of collagenases. A crystallographic study. FEBS Lett. 1994 Jan 31;338(2):227–233. doi: 10.1016/0014-5793(94)80370-6. [DOI] [PubMed] [Google Scholar]
- Riccio A., Grimaldi G., Verde P., Sebastio G., Boast S., Blasi F. The human urokinase-plasminogen activator gene and its promoter. Nucleic Acids Res. 1985 Apr 25;13(8):2759–2771. doi: 10.1093/nar/13.8.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan A. D., Mason P., Mach L., Mort J. S. Rat procathepsin B. Proteolytic processing to the mature form in vitro. J Biol Chem. 1992 Aug 5;267(22):15993–15999. [PubMed] [Google Scholar]
- Saklatvala J., Pilsworth L. M., Sarsfield S. J., Gavrilovic J., Heath J. K. Pig catabolin is a form of interleukin 1. Cartilage and bone resorb, fibroblasts make prostaglandin and collagenase, and thymocyte proliferation is augmented in response to one protein. Biochem J. 1984 Dec 1;224(2):461–466. doi: 10.1042/bj2240461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Flannery C. R., Neame P. J., Lohmander L. S. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992 May;89(5):1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Neame P. J., Boynton R. E., Flannery C. R. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991 May 15;266(14):8683–8685. [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994 Jul 7;370(6484):61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Shinmei M., Ito K., Matsuyama S., Yoshihara Y., Matsuzawa K. Joint fluid carboxy-terminal type II procollagen peptide as a marker of cartilage collagen biosynthesis. Osteoarthritis Cartilage. 1993 Apr;1(2):121–128. doi: 10.1016/s1063-4584(05)80027-5. [DOI] [PubMed] [Google Scholar]
- THOMAS L. Reversible collapse of rabbit ears after intravenous papain, and prevention of recovery by cortisone. J Exp Med. 1956 Aug 1;104(2):245–252. doi: 10.1084/jem.104.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai M., Matsumoto K., Omura S., Koyama I., Ozawa Y., Hanada K. In vitro and in vivo inhibition of cysteine proteinases by EST, a new analog of E-64. J Pharmacobiodyn. 1986 Aug;9(8):672–677. doi: 10.1248/bpb1978.9.672. [DOI] [PubMed] [Google Scholar]
- Van Noorden C. J., Smith R. E., Rasnick D. Cysteine proteinase activity in arthritic rat knee joints and the effects of a selective systemic inhibitor, Z-Phe-AlaCH2F. J Rheumatol. 1988 Oct;15(10):1525–1535. [PubMed] [Google Scholar]
- Van Noorden C. J., Vogels I. M., Everts V., Beertsen W. Localization of cathepsin B activity in fibroblasts and chondrocytes by continuous monitoring of the formation of a final fluorescent reaction product using 5-nitrosalicylaldehyde. Histochem J. 1987 Sep;19(9):483–487. doi: 10.1007/BF01675418. [DOI] [PubMed] [Google Scholar]
- Venn M., Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977 Apr;36(2):121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Wolf G. The molecular basis of the inhibition of collagenase by vitamin A. Nutr Rev. 1992 Oct;50(10):292–294. doi: 10.1111/j.1753-4887.1992.tb02468.x. [DOI] [PubMed] [Google Scholar]
- Wu J. J., Lark M. W., Chun L. E., Eyre D. R. Sites of stromelysin cleavage in collagen types II, IX, X, and XI of cartilage. J Biol Chem. 1991 Mar 25;266(9):5625–5628. [PubMed] [Google Scholar]
- Wu Y. N., Gadina M., Tao-Cheng J. H., Youle R. J. Retinoic acid disrupts the Golgi apparatus and increases the cytosolic routing of specific protein toxins. J Cell Biol. 1994 May;125(4):743–753. doi: 10.1083/jcb.125.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D., Homandberg G. A. Fibronectin fragments bind to and penetrate cartilage tissue resulting in proteinase expression and cartilage damage. Biochim Biophys Acta. 1993 Sep 8;1182(2):189–196. doi: 10.1016/0925-4439(93)90140-v. [DOI] [PubMed] [Google Scholar]
- Yocum S. A., Lopresti-Morrow L. L., Gabel C. A., Milici A. J., Mitchell P. G. Bafilomycin A1 inhibits IL-1-stimulated proteoglycan degradation by chondrocytes without affecting stromelysin synthesis. Arch Biochem Biophys. 1995 Feb 1;316(2):827–835. doi: 10.1006/abbi.1995.1111. [DOI] [PubMed] [Google Scholar]