Abstract
Reproduction is integrated by interaction of neural and hormonal signals converging on hypothalamic neurons for controlling gonadotropin-releasing hormone (GnRH). Kisspeptin, the peptide product of the kiss1 gene and the endogenous agonist for the GRP54 receptor, plays a key role in the regulation of GnRH secretion. In the present study, we investigated the interaction between kisspeptin, estrogen and BMPs in the regulation of GnRH production by using mouse hypothalamic GT1-7 cells. Treatment with kisspeptin increased GnRH mRNA expression and GnRH protein production in a concentrationdependent manner. The expression levels of kiss1 and GPR54 were not changed by kisspeptin stimulation. Kisspeptin induction of GnRH was suppressed by co-treatment with BMPs, with BMP-4 action being the most potent for suppressing the kisspeptin effect. The expression of kisspeptin receptor, GPR54, was suppressed by BMPs, and this effect was reversed in the presence of kisspeptin. It was also revealed that BMP-induced Smad1/5/8 phosphorylation and Id-1 expression were suppressed and inhibitory Smad6/7 was induced by kisspeptin. In addition, estrogen induced GPR54 expression, while kisspeptin increased the expression levels of ERα and ERβ, suggesting that the actions of estrogen and kisspeptin are mutually enhanced in GT1-7 cells. Moreover, kisspeptin stimulated MAPKs and AKT signaling, and ERK signaling was functionally involved in the kisspeptin-induced GnRH expression. BMP-4 was found to suppress kisspeptin-induced GnRH expression by reducing ERK signaling activity. Collectively, the results indicate that the axis of kisspeptin-induced GnRH production is bi-directionally controlled, being augmented by an interaction between ERα/β and GPR54 signaling and suppressed by BMP-4 action in GT1-7 neuron cells.
Keywords: BMP, Estradiol, GnRH, GPR54, Kisspeptin, MAPK
1. Introduction
Reproduction is regulated by a complex interaction of neural and hormonal signals that converge on hypothalamic neurons responsible for the pulsatile secretion of gonadotropin-releasing hormone (GnRH) (Pinilla et al., 2009). Coordination of the GnRH impulse from the hypothalamus and the subsequent secretion of pituitary gonadotropins are critical for the central control of reproductive function (Pinilla et al., 2009). The pattern of GnRH secretion is regulated by the intrinsic oscillatory activity of GnRH neurons and the integration of presynaptic inputs of various neurotransmitters such as kisspeptin and gonadotropin-inhibitory hormone (GnIH) (Tsutsui et al., 2010) and the feedback loop from gonadal steroids (Petersen et al., 2003). Immortalized GnRH-producing GT1-7 cells have proven to be a valuable tool to study the biology of GnRH neurons (Liposits et al., 1991; Wetsel et al., 1991). In females, estrogens act directly or indirectly on the GnRH neuronal network to modulate the final output of GnRH into the median eminence (Garcia-Galiano et al., 2012; Hameed et al., 2011). However, the detailed mechanism by which estrogen exerts positive and negative feedback effects is still controversial.
Progress has been made in elucidation of the indirect mechanism of estrogen-GnRH regulation following the discovery of kisspeptin. Recently, an understanding of the physiological control of the reproductive axis has been advanced by identification of the essential role of kisspeptin. Kisspeptins are products of the kiss1 gene and these peptides are derived from the plasma proteolytic cleavage of the 145-amino-acid gene product. The kisspeptin fragments commonly have a C-terminal decapeptide including Arg-Phe residues that are critical for the biological activity (Clements et al., 2001; Kotani et al., 2001; Ohtaki et al., 2001). Kisspeptin and its receptor, G-protein-coupled receptor 54 (GPR54), are key components in the regulation of GnRH secretion in humans and other mammals (Goodman and Lehman, 2012). Kisspeptin is found in both the peripheral and central nervous systems. In the periphery, kisspeptin has been identified in the testis, ovary, pituitary onadotrope, pancreas, small intestine and placenta (Gaytan et al., 2009; Horikoshi et al., 2003; Muir et al., 2001; Ohtaki et al., 2001; Richard et al., 2008). In the central nervous system, both kiss1 mRNA and kisspeptin protein are abundantly expressed within the hypothalamus in the arcuate nucleus (ARC), anteroventral periventricular nucleus (AVPV) and periventricular nucleus (Gottsch et al., 2004) in rodents. Hypothalamic kiss1 mRNA is predominantly found in the infundibular nucleus, the equivalent of the ARC in primates (Rometo et al., 2007). Accumulated data have shown that central or systemic administration of kisspeptin increases GnRH and gonadotropin secretion in both prepubertal and adult animals, which is crucial for the neuroendocrine regulation of reproduction (Castellano et al., 2005; Funes et al., 2003; Pinilla et al., 2012; Seminara et al., 2003; Tena-Sempere, 2006). GPR54 is also critical for the integrity of reproductive activity, as shown in patients with idiopathic hypogonadotropic hypogonadism and in GPR54-knockout mice (de Roux et al., 2003; Funes et al., 2003; Seminara et al., 2003).
Bone morphogenetic proteins (BMPs), which belong to the transforming growth factor (TGF)-β superfamily, were originally identified as active components in bone extracts that are capable of inducing bone formation at ectopic sites. Recent studies have shown that BMPs are crucial molecules in normal folliculogenesis by regulating gonadotropin-induced steroidogenesis and mitosis in ovarian granulosa cells (Otsuka, 2010; Otsuka et al., 2011; Shimasaki et al., 2004). The major regulatory process by BMPs in ovarian steroidogenesis is control of follicle-stimulating hormone (FSH)-receptor activity, leading to normal follicular development in the ovary. It has also been reported that BMPs are involved in the regulation of various functions of the anterior pituitary including gonadotropin secretion (Otsuka and Shimasaki, 2002; Otsuka et al., 2012; Otsuka, 2013; Takeda et al., 2007; Tsukamoto et al., 2010). Bi-directional communication between the ovary and central nervous system ensures that the neural signal for ovulation occurs at the time point when follicle maturation is completed. The BMP system is also required for the early developmental stage of the hypothalamus for regulating cell identities in the neural tube (Liu and Niswander, 2005; Ulloa and Briscoe, 2007).
We have reported expression of the BMP system including BMP type I and type II receptors in hypothalamic neuron GT1-7 cells (Otani et al., 2009). In that study, it was found that BMPs modulate the suppressive effects of estrogen on GnRH production by regulating estrogen receptor (ER) expression and estrogen-induced MAPK signaling. Interaction of the BMP system and ER action may be linked to hypothalamic GnRH production in an autocrine/paracrine fashion. Hence, the BMP system plays a regulatory role in female reproduction not only by altering gonadotropin sensitivity in ovarian folliculogenesis but also by modulating the hypothalamopituitary-ovarian (HPO) axis. However, the direct effects of BMPs on hypothalamic GnRH production and secretion have yet to be elucidated. Reproduction is regulated by mutual interaction of neural and hormonal factors that direct the production and secretion of GnRH. In the present study, we investigated the effects of BMPs on GnRH production controlled by kisspeptin. We uncovered a novel interaction between BMP-4, kisspeptin and estrogen, which is involved in the control of hypothalamic GnRH production and secretion by GT1-7 neuron cells.
2. Materials and methods
2.1. Reagents and supplies
Dulbecco’s Modified Eagle’s Medium (DMEM), dimethylsulfoxide (DMSO), penicillin–streptomycin solution, human metastin (45–54) amide (kisspeptin-10), and 17β-estradiol were purchased from Sigma–Aldrich Co. Ltd. (St. Louis, MO). Mouse hypothalamus total RNA was purchased from Clontech Laboratories, Inc. (Mountain View, CA). Recombinant human BMP-2, -4, -6 and -7 were purchased from R&D Systems Inc. (Minneapolis, MN), the extracellular signal-regulated kinase (ERK) inhibitor U0126 and p38-MAPK inhibitor SB203580 were from Promega Corp. (Madison, WI), the stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK) inhibitor SP600125 was from Biomol Lab. Inc. (Plymouth Meeting, PA), and the AKT inhibitor SH-5 was from Calbiochem (San Diego, CA).
2.2. GT1-7 cell culture
GT1-7 cells were kindly provided by Dr. Pamela L. Mellon, University of California, San Diego, CA. The GT1-7 cells were maintained in DMEM supplemented with 10% fetal calf serum (FCS), penicillin and streptomycin (Sigma–Aldrich Corp.) at 37 °C in a 5% CO2 humidified atmosphere. The culture medium was changed twice a week, and cultures were passaged at ~80% confluence. Changes in cell morphology and growing conditions were carefully monitored under an inverted microscope.
2.3. Measurement of GnRH production
To assess the effects of BMPs on GnRH protein synthesis, GT1-7 cells (2 × 105 viable cells/ml) were cultured in 6-well plates with DMEM containing 10% FCS for 24 h. The medium was then changed to serum-free DMEM and was subsequently treated with the indicated concentrations of kisspeptin. After 24-h culture, the supernatant of the culture media was collected and stored at −80 °C until assay. GnRH concentration (pg/ml) in the conditioned medium was determined by a radioimmunoassay as reported previously (Chappell et al., 2003; Otani et al., 2009). Briefly, 0.1-ml aliquots of each sample were incubated for 48 h with the GnRH antibody EL-14 at 4 °C, after which time 10,000 cpm sample of radio-iodinated GnRH (Amersham Pharmacia, Piscataway, NJ) was added. Following ethanol precipitation of the bound fractions after 48-h incubation, radioactivity was detected by a gamma scintillation counter (Micromedic, Huntsville, AL), and standard curve analysis was performed at each assay. The inter-assay variability and intra-assay variability in the radioimunoassay are 8.7% and 7.7%, respectively.
2.4. RNA extraction, RT-PCR and quantitative real-time PCR analysis
GT1-7 cells (2 × 105 viable cells/ml) were precultured in serumfree DMEM, and the cells were treated with indicated concentrations of kisspeptin in combination with BMPs, estradiol and various chemical inhibitors including U0126, SB203580, SP600125 and SH-5. After 24-h culture, the medium was removed, and total cellular RNAs were extracted using TRIzol® (Invitrogen Corp., Carlsbad, CA) and subsequently quantified by measuring absorbance at 260 nm and then stored at −80 °C until assay. The expression of GnRH, kiss1, GPR54, ERs (ERα and ERβ), BMP ligands (BMP-2, -4, -6 and -7), BMP type I (ALK-2, -3, -4 and -6) and type II (ActRIIA, ActRIIB and BMPRII) receptors, and Smads (Smad6 and 7) was detected by RT-PCR. The extracted RNA (1 μg) was subjected to a RT reaction using First-Strand cDNA Synthesis System® (Invitrogen Corp.) with random hexamer (2 ng/μl), reverse transcriptase (200 U) and deoxynucleotide triphosphate (dNTP; 0.5 mM) at 42 °C for 50 min and at 70 °C for 10 min. Subsequently, hot-start PCR was performed using MgCl2 (1.5 mM), dNTP (0.2 mM) and Taq DNA polymerase (2.5 U) (Invitrogen Corp.). Oligonucleotides used for PCR were custom-ordered from Invitrogen Corp. PCR primer pairs were selected from different exons of the corresponding genes to discriminate PCR products that might arise from possible chromosome DNA contaminants. The primer pairs for mouse BMP ligands, receptors, Id-1, Smads, and the house-keeping gene ribosomal protein- L19 (RPL19) were selected as we previously reported (Otani et al., 2009; Takeda et al., 2007; Tsukamoto et al., 2010). For ERs, GnRH, kiss1 and GPR54 genes, the following sequences were used: ERα, 1523–1543 and 1737–1757 from GenBank accession number NM_007956; ERβ, 701–721 and 1004–1024 from NM_010157; GnRH, 90–110 and 316–336 from BC116897; kiss1, 61–81 and 328–352 from NM_178260; and GPR54, 1959–1980 and 2122–2144 from NM_053244. Aliquots of the PCR products were electrophoresed on 1.5% agarose gels and visualized after ethidium bromide staining. For the quantification of the receptors for BMP-4 (ALK-3 and BMPRII), ERs (ERα and ERβ), GnRH, GPR54, Id-1, kiss1, Smads (Smad6 and Smad7) and RPL19 mRNA levels, real-time PCR was performed using LightCycler-FastStart DNA master SYBR Green I system® (Roche Diagnostic Co., Tokyo, Japan) under each optimized condition of annealing at 59–63 °C with 4 mM MgCl2, following the manufacturer’s protocol. Accumulated levels of fluorescence for each product were analyzed by the second derivative method after melting-curve analysis (Roche Diagnostic Co.), and then, following assay validation by calculating amplification efficiency of each amplicon, the expression levels of target genes were quantified on the basis of standard curve analysis for each product. For each transcript, all treatment groups were quantified simultaneously in a single LightCycler run. To correct for differences in RNA quality and quantity between samples, the expression levels of target gene mRNA were normalized by dividing the quantity of the target gene by the quantity of RPL19 in each sample. The raw data of each target mRNA level (/RPL19) were statistically analyzed as indicated and are shown as fold changes in the figures.
2.5. Western immunoblot analysis
GT1-7 cells (1 × 105 viable cells/ml) were precultured in 12- well plates in serum-free DMEM. The cells were treated with BMPs (100 ng/ml) and kisspeptin (300 nM) either alone or in combination with estradiol (100 nM). After 15- and 60-min stimulation indicated, cells were solubilized in 100 μl RIPA lysis buffer (Upstate Biotechnology, Lake Placid, NY) containing 1 mM Na3VO4, 1mM NaF, 2% SDS and 4% β-mercaptoethanol. The cell lysates were then subjected to SDS–PAGE/immunoblotting analysis as we previously reported (Otani et al., 2009; Tsukamoto et al., 2010), using antiphospho- and anti-total-extracellular signal-regulated kinase (ERK) 1/2 MAPK antibody (Cell Signaling Technology, Inc., Beverly, MA), anti-phospho- and anti-total-p38 MAPK antibody (Cell Signaling Technology, Inc.), anti-phospho- and anti-total-stressactivated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK) MAPK antibody (Cell Signaling Technology, Inc.), anti-phospho- and anti-total-AKT antibody, anti-phospho-Smad1/5/8 (pSmad1/5/8) antibody (Cell Signaling Technology, Inc.), and anti-actin antibody (Sigma–Aldrich Co. Ltd.). The relative integrated density of each protein band was digitized by NIH image J 1.34s.
2.6. Statistical analysis
All results are shown as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. The data were subjected to ANOVA or the unpaired t-test (StatView 5.0 software, Abacus Concepts, Inc., Berkeley, CA). If differences were detected by ANOVA, Tukey–Kramer’s post hoc test was used to determine which means differed (StatView 5.0 software). P values <0.05 were accepted as statistically significant.
3. Results
First, expression of the kisspeptin system in GT1-7 cells was confirmed by RT-PCR. As shown in Fig. 1A, in addition to GnRH expression, kiss1, GPR54, ERα and ERβ were clearly expressed in mouse hypothalamic GT1-7 neuron cells. To clarify the existence of kisspeptin and BMP system in vivo, we examined the expression of these molecules in the mouse hypothalamus. As shown in Fig. 1B, the GnRH-kisspepetin system, including GnRH, kiss1, GPR54, ERα and ERβ, BMP ligands (BMP-2, -4, -6 and -7), BMP type I (ALK-2, -3, -4 and -6) and BMP type II (BMPRII, ActRIIA and Act- RIIB), and inhibitory Smads (Smad6 and 7) were detected in the mouse hypothalamus. Although kisspeptin expression is not demonstrated in GnRH neurons in vivo, GPR54 expression is present and influences GnRH secretion and expression. To examine the functional role of kisspeptin in GnRH production by GT1-7 cells, cells were treated with kisspeptin (0.1–1 μM) and GnRH concentration in the serum-free medium was determined by RIA. As shown in Fig. 1C, kisspeptin treatment significantly increased GnRH release in a concentration-dependent manner. The mRNA level of GnRH was also increased by kisspeptin (0.01–3 μM) treatment (Fig. 1D), whereas the expression levels of kiss1 and GPR54 were not altered by kisspeptin (0.03–3 μM) treatment (Fig. 1E).
Fig. 1.
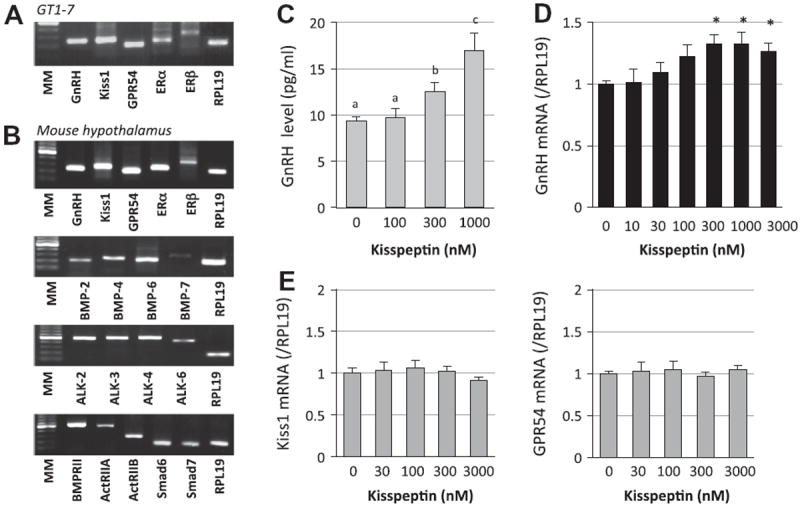
Expression of kisspeptin system and kisspeptin actions on GnRH production by GT1-7 cells. (A) Total cellular RNAs were extracted from GT1-7 cells. The expression of GnRH (247 bp), kiss1 (392 bp), GPR54 (186 bp), ERα (235 bp), ERβ (324 bp) and the housekeeping gene RPL19 (195 bp) in GT1-7 cells was examined by RT-PCR. Aliquots of PCR products were electrophoresed on 1.5% agarose gel and visualized by ethidium bromide staining, and the results shown are representative of those obtained from three independent experiments. MM indicates molecular weight marker. (B) The expression of GnRH, kiss1, GPR54, ERα, ERβ, BMP ligands including BMP-2 (212 bp), -4 (241 bp), -6 (224 bp) and -7 (287 bp), BMP receptors including ALK-2 (483 bp), -3 (510 bp), -4 (528 bp), -6 (456 bp), BMPRII (522 bp), ActRIIA (492 bp) and ActRIIB (271 bp), and Smad6 (165 bp) and Smad 7 (169 bp) in the mouse hypothalamus was also examined by RT-PCR. C) GT1-7 cells (2 × 105 cells/ml) were treated with indicated concentrations of kisspeptin in serum-free conditions. After 24-h culture, the culture media were collected and GnRH concentrations (pg/ml) were determined by a radioimmunoassay. (D and E) Cells (2 × 105 cells/ml) were treated with indicated concentrations of kisspeptin in serum-free conditions for 24 h. Total cellular RNA was extracted and mRNA levels of GnRH, kiss1 and GPR54 were examined by real-time RT-PCR. The expression levels of target genes were standardized by RPL19 level in each sample. Results in all panels are shown as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. The results were analyzed by ANOVA (C–E) and, when a significant effect due to treatment was observed, subsequent comparisons of group means were conducted. For each result within a panel, the values with different superscript letters are significantly different at P < 0.05. * P < 0.05 vs. control group.
Of note, the stimulating effects of kisspeptin on GnRH mRNA levels were suppressed by co-treatment with BMPs (100 ng/ml) including BMP-2, -4, -6 and BMP-7 (Fig. 2A). BMP-4 action was the most potent inhibitor of GnRH induction by kisspeptin. It was also revealed that the expression of the kisspeptin receptor, GPR54, was potently suppressed by treatment with BMP-2, -4, -6 and -7 (Fig. 2B). Interestingly, the BMP actions that suppress GPR54 expression were significantly reversed by co-treatment with kisspeptin (300 nM).
Fig. 2.
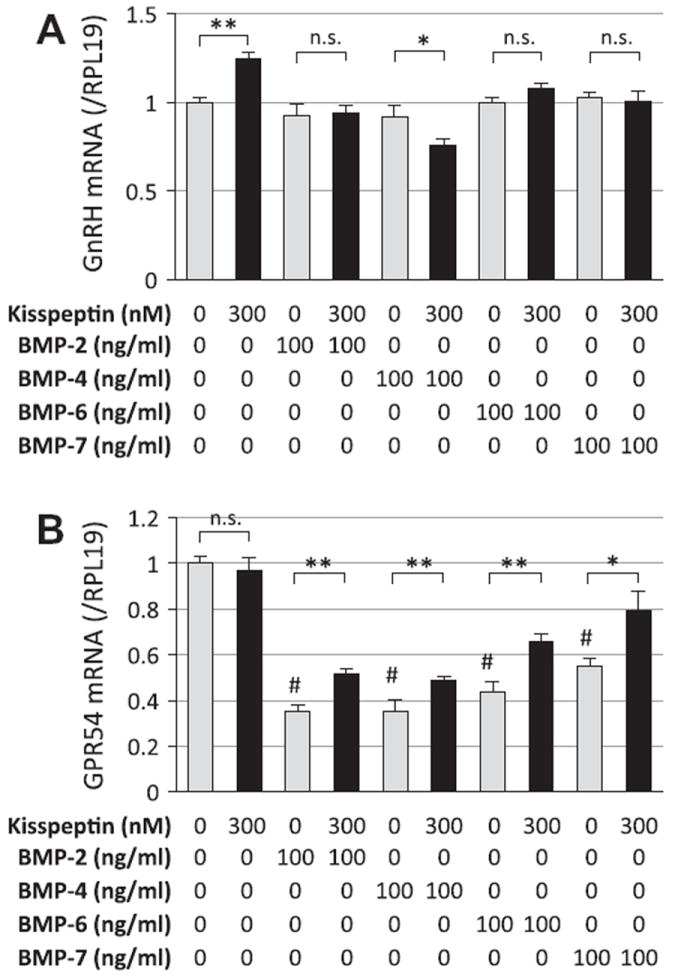
Kisspeptin and BMP actions on GnRH and GPR54 expression by GT1-7 cells. GT1-7 cells (2 × 105 cells/ml) were treated with indicated concentrations of kisspeptin (300 nM) in combination with BMP-2, -4, -6 and -7 (100 ng/ml) in serum-free conditions for 24 h. Total cellular RNA was extracted and (A) GnRH and (B) GPR54 mRNA levels were examined by real-time RT-PCR. The expression levels of target genes were standardized by RPL19 level in each sample. Results in all panels are shown as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. The results were analyzed by ANOVA and unpaired t-test and, when a significant effect due to treatment was observed (P < 0.05), subsequent comparisons of group means were conducted. * P < 0.05 and ** P < 0.01 between the indicated groups; #P < 0.05 vs. control group. n.s., not significant.
To describe the mechanism by which kisspeptin interferes with the effects of BMPs, the downstream signals of BMP receptors, Smad1/5/8 phosphorylation and Id-1 transcription, were examined (Fig. 3). As shown in Fig. 3A, treatments with BMP-2 and -4 (100 ng/ml) readily stimulated Smad1/5/8 phosphorylation in GT1-7 cells; however, BMP-induced Smad1/5/8 phosphorylation, in particular that induced by BMP-4, was suppressed by co-treatment with kisspeptin (300 nM). To confirm the effects of kisspeptin on BMP receptor signaling, Id-1 mRNA levels were examined by real-time PCR analysis. In accordance with the results of immunoblots for Smad1/5/8, Id-1 mRNA activation induced by BMP-2, -4, -6 and -7 (100 ng/ml) was suppressed by kisspeptin (300 nM) (Fig. 3B). Of note, the suppressive effect of kisspeptin on BMP-2-induced Id-1 expression was less potent than that on Id-1 expression induced by BMP-4, -6 and -7.
Fig. 3.
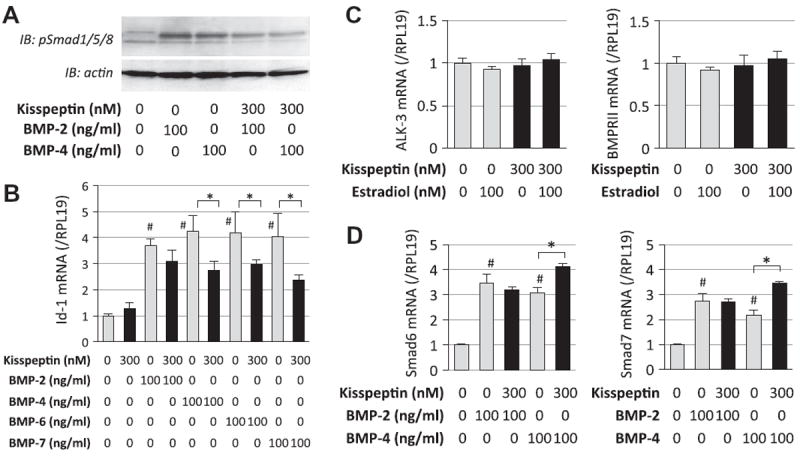
Effects of kisspeptin on BMP-Smad signaling in GT1-7 cells. (A) GT1-7 cells (1 × 105 cells/ml) were precultured in serum-free conditions in the absence or presence of kisspeptin (300 nM) for 24 h, and then stimulated with BMP-2 and -4 (100 ng/ml). After 60-min culture with BMP treatment, cells were lysed and subjected to SDS–PAGE/immunoblotting (IB) analysis using anti-phospho-Smad1/5/8 (pSmad1/5/8) and anti-actin antibodies. The results shown are representative of those obtained from three independent experiments. (B) Cells (2 × 105 cells/ml) were treated with BMP-2, -4, -6 and -7 (100 ng/ml) in combination with kisspeptin (300 nM) in serum-free conditions for 24 h. Total cellular RNA was extracted and Id-1 mRNA level was examined by real-time RT-PCR. The expression levels of target genes were standardized by RPL19 level in each sample. (C) Cells (2 × 105 cells/ml) were treated with kisspepetin (300 nM) in combination with estradiol (100 nM) in serum-free conditions for 24 h. Total cellular RNA was extracted and ALK-3 and BMPRII mRNA levels were examined by real-time RT-PCR. D) Cells were treated with kisspeptin (300 nM) in the presence of BMP-2 and -4 (100 ng/ml) in serum-free conditions for 24 h. Total cellular RNA was extracted and Smad6 and Smad7 mRNA levels were examined by real-time RT-PCR. Results in all panels are shown as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. The results were analyzed by ANOVA and, when a significant effect due to treatment was observed (P < 0.05), subsequent comparisons of group means were conducted. #P < 0.05 vs. control group; and * P < 0.05 between the indicated groups.
To investigate the molecular mechanism by which kisspeptin (300 nM) regulates BMP activities in GT1-7 cells, the expression changes in ALK-3 and BMPRII, which are major type I and type II receptors for BMP-2 and -4, respectively, were examined in the presence or absence of estradiol (100 nM). As shown in Fig. 3C, kisppeptin treatment had no effect on ALK-3 or BMPRII mRNA levels regardless of the presence of estradiol. The effects on inhibitory Smads, including Smad6 and Smad7, were also examined. As shown in Fig. 3D, mRNA levels of Smad6 and Smad7 were increased in the presence of BMP-2 and -4. Kisspeptin treatment had no significant effect on Smad6 and Smad7 mRNA expression in the presence of BMP-2; however, it was notable that kisspeptin significantly increased Smad6 and Smad7 levels in the presence of BMP-4. Thus, kisspeptin regulates BMP activity by increasing inhibitory Smads expression in GT1-7 cells.
Next, the interaction between kisspeptin and estrogen action was studied. Treatment with kisspeptin (100 nM) significantly increased the expression levels of ERα and ERβ mRNAs (Fig. 4A). Treatment with estradiol (100 nM) suppressed GnRH mRNA expression (Fig. 4B, left) and GnRH protein levels in the media (Fig. 4C) by GT1-7 cells as a negative feedback effect, while estradiol significantly increased kisspeptin receptor GPR54 mRNA expression levels in GT1-7 cells (Fig. 4B, right). These findings suggest that the actions of estrogen and kisspeptin are mutually enhanced in GT1-7 cells.
Fig. 4.
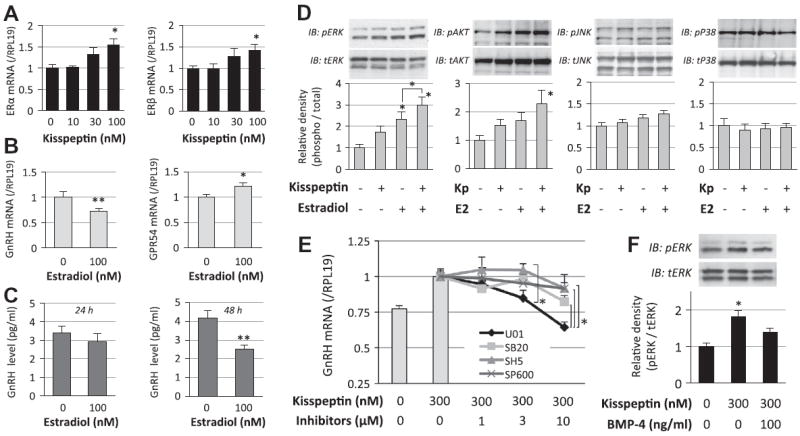
Interaction of kisspeptin and estrogen receptor signaling in GnRH induction by GT1-7 cells. (A and B) GT1-7 cells (2 × 105 cells/ml) were treated with kisspeptin (10–100 nM) and estradiol (100 nM) in serum-free conditions for 24 h. Total cellular RNA was extracted and ERα, ERβ, GnRH and GPR54 mRNA levels were examined by realtime RT-PCR. The expression levels of target genes were standardized by RPL19 level in each sample. (C) Cells (2 × 105 cells/ml) were treated with indicated concentrations of estradiol in serum-free conditions. After 24-h and 48-h culture, the culture media were collected and GnRH concentrations (pg/ml) were determined by a radioimmunoassay. (D and F) Cells (1 × 105 cells/ml) were precultured in serum-free conditions in the absence or presence of estradiol (E2; 100 nM) and BMP-4 (100 ng/ml) for 24 h, and then stimulated with kisspeptin (Kp; 300 nM). After 15-min culture with kisspeptin treatment, cells were lysed and subjected to SDS–PAGE/immunoblotting (IB) analysis using anti-phospho-ERK1/2 (pERK) and anti-total-ERK1/2 (tERK), and anti-phospho-AKT (pAKT) and anti-total-AKT (tAKT), anti-phospho-JNK (pJNK) and anti-total JNK (tJNK), and anti-phospho-p38 (pP38) and anti-total-p38 (tP38) antibodies. The results shown are representative of those obtained from three independent experiments. The relative integrated density of each protein band was digitized, in which the phosphorylated levels were normalized by the total levels, and then the results were expressed as fold changes. (E) Cells (2 × 105 cells/ml) were treated with MAPK and AKT inhibitors (1–10 μM), including U0126 (U01), SB203580 (SB20), SP600125 (SP600) and SH-5 (SH5), in combination with kisspeptin (300 nM) in serum-free conditions for 24 h. Total cellular RNA was extracted and GnRH mRNA levels were examined by real-time RT-PCR. Results in all panels are shown as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. The results were analyzed by ANOVA (A, D–F) and unpaired t-test (B and C) and when a significant effect due to treatment was observed (P < 0.05), subsequent comparisons of group means were conducted. * P < 0.05 and ** P < 0.01 vs. control group and between the indicated groups.
We further investigated the underlying mechanism by which BMP-4 suppressed kisspeptin-induced GnRH expression. As shown in Fig. 4D, kisspeptin (300 nM) stimulated ERK and AKT phosphorylation and the actions of kisspeptin were enhanced in the presence of estradiol (100 nM), although it failed to increase P38 and JNK phosphorylation in GT1-7 cells. As shown in Fig. 4E, treatment with chemical inhibitors for MAPKs and AKT, including U0126, SB203580, SP600125 and SH5 (1–10 μM), revealed that kisspeptin-induced GnRH mRNA expression was suppressed by the ERK inhibitor (U0126), suggesting that ERK signaling is functionally involved in the kisspeptin-induced GnRH expression. As shown in Fig. 4F, BMP-4 treatment (100 ng/ml) was found to suppress kisspeptin-induced ERK phosphorylation in GT1-7 cells. It was thus thought that BMP-4 suppresses kisspeptin-induced GnRH production by inhibiting ERK activation.
4. Discussion
The mechanism by which estrogen acts on the GnRH regulatory system has yet to be clarified. Direct actions on GnRH neurons as well as indirect actions through other linked neurons via transsynaptic interaction seem to be involved in this machinery. It was revealed that kisspeptin stimulated GnRH synthesis and the GnRH induction was suppressed by BMP-4 in mouse hypothalamic GT1-7 cells, suggesting the presence of a mutual balance between the actions of kisspeptin and BMP-4 for regulating GnRH (Fig. 5). In this interaction, estrogen upregulated GPR54 expression, and kisspeptin, in turn, increased the expression levels of ERα and ERβ in addition to the role of estrogen as a GnRH repressor of negative feedback. On the other hand, GPR54 expression was suppressed by BMPs. The suppressive effect of BMPs on GPR54 expression was reversed by kisspeptin via upregulation of inhibitory Smad6/7 expression. Hence, in GT1-7 neuron cells, the axis of kisspeptin-induced GnRH production is bi-directionally controlled, being augmented by the estrogen-to-GPR54 pathway and being suppressed by BMP-4-to-Smad1/5/8 signaling (Fig. 5).
Fig. 5.
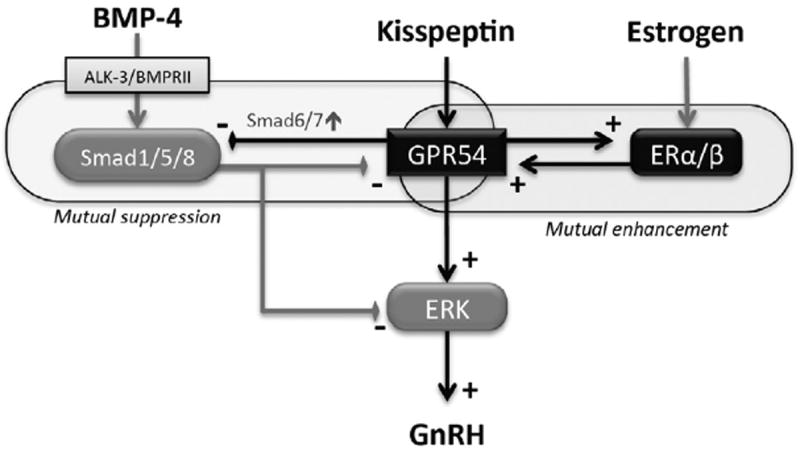
Interaction of kisspeptin, estrogen and BMP-4 in regulation of GnRH production by GT1-7 cells. Kisspeptin stimulates GnRH production via GPR54 and its downstream signaling of the ERK pathway in GT1-7 cells. Estrogen upregulates GPR54 expression, while kisspeptin increases the expression levels of ERα/β, suggesting that the actions of estrogen and kisspeptin are mutually enhanced. Kisspeptin effects on GnRH induction were suppressed by BMP-4, by inhibiting ERK phosphorylation and GPR54 expression. On the other hand, by upregulating inhibitory Smads6/7, kisspeptin suppressed Smad1/5/8 phosphorylation and Id-1 expression induced by BMP-4 through the ALK-3/BMPRII receptor. In GT1-7 neuron cells, the axis of kisspeptin-induced GnRH production is augmented by an interaction between ERα/β and GPR54 and it is also negatively controlled by BMP-4.
Our results showing that estrogen acts directly on GT1-7 neurons to suppress GnRH expression as well as to enhance the GPR54 pathway have added more complexity to the system for gonadotropin regulation. Estrogen also has been shown to regulate kiss1 mRNA differentially in various nuclei that exert major physiological activity in the forebrain (Jacobi et al., 2007). Estrogen stimulation promotes kiss1 expression in the ventromedial hypothalamus AVPV, which is implicated in positive feedback regulation of the luteinizing hormone (LH) surge, whereas it reduces kiss1 expression in the ARC, which is implicated in negative feedback regulation of GnRH in female reproduction (Smith et al., 2005). In the present study, a mutual interaction of the expression control of ERs and GPR54 was shown by GT1-7 cells, in which both ERα and ERβ mRNAs were increased by kisspeptin, while GPR54 expression and the GPR54-induced ERK1/2 signaling were enhanced by estradiol. Expression of ERα in GnRH neurons is not clearly demonstrated, but GnRH neurons do express ERβ and the estrogen membrane receptor GPR30 (Kenealy et al., 2011; Terasawa and Kenealy, 2012). It was also reported that estrogen elicited circadian profiles of GPR54 expression in vitro possibly through ERβ action (Tonsfeldt et al., 2011). The upregulation of GRP54 was likely involved in the stimulation of estradiol-induced GnRH secretion as a positive feedback induced by estrogen (Tonsfeldt et al., 2011). These results suggest that sensitivity of GnRH secretion to kisspeptin is partly dependent on estrogen activity. Elevated gonadal estrogen level may increase kisspeptinergic tone at the same time as increasing GnRH neuronal sensitivity.
Interestingly, effects of kisspeptin on GnRH induction were suppressed by co-treatment with BMPs, with BMP-4 action effectively suppressing induction of GnRH expression by kisspeptin (Fig. 5). A complex interaction of neural and hormonal signals including BMPs and kisspeptin may integrate the neuronal activity for GnRH secretion. The neuroregulatory factor TGF-β1 secreted by astrocytes is a putative candidate for modulation of GnRH secretion (Buchanan et al., 2000; Galbiati et al., 1996; Melcangi et al., 1995; Zwain et al., 2002). A regulatory role of TGF-α originating from astrocytes in GnRH release has also been demonstrated (Ma et al., 1992; Ma et al., 1997; Ojeda et al., 2000). In the present study, expression of the BMP system including BMP ligands and key receptors was shown in the hypothalamus. Given the findings that several BMPs elicit a neurotropic capacity for dopaminergic neurons in cooperation with other members of the TGF-β superfamily through glial cells (Jordan et al., 1997; Reiriz et al., 1999; Samanta et al., 2007), hypothalamic GnRH synthesis may be functionally associated with BMP activity in a neural network in the hypothalamus.
The BMP system has been shown to play critical roles in initial development of the anterior pituitary (Scully and Rosenfeld, 2002), and BMPs modulate GnRH responses in gonadotropes (Takeda et al., 2012). BMP-4 is necessary for the first step of pituitary organogenesis including the proliferation of Rathke’s pouch, which gives rise to Pit-1 lineage cells. BMP-4 not only governs pituitary organogenesis but also plays a role in the pathogenesis of differentiated pituitary lineages (Otsuka et al., 2012; Tsukamoto et al., 2010). The BMP system also acts as a key molecule in the early developmental stages of the hypothalamus in the neural tube in conjunction with hedgehog activity (Liu and Niswander, 2005; Ulloa and Briscoe, 2007). Hypothalamic neuronal differentiation is regulated by a series of activities induced by sonic hedgehog (Shh) and BMP-7 via specification of hypothalamic progenitors (Ohyama et al., 2008). The elaborate neural patterning seems to be achieved by integrated antagonism between Shh and BMP actions. In addition, it was recently reported that BMP type IB receptor (ALK-3) signaling is critical for postnatal establishment of the hypothalamic circuit from ARC to multiple nuclei, which regulate energy homeostasis and feeding behavior (Peng et al., 2012).
The receptors for TGF-β superfamily members consist of type I and type II receptors, each of which exhibits Ser/Thr kinase activity. Among the several combinations of BMP ligands and receptors, BMP-4 readily binds to ALK-3 and/or ALK-6 and preferentially binds to BMPRII (Shimasaki et al., 2004). Since ALK-6 is not expressed in GT1-7 cells (Otani et al., 2009), the receptor pairs of ALK-3/BMPRII are likely to be the major functional complex for BMP-4. Our data demonstrated that kisspeptin suppressed BMPSmad1/5/8 signaling by augmenting the expression of inhibitory Smad6 and/or Smad7 in GT1-7 cells. The expression of GPR54 mRNA was potently suppressed by BMPs, and the suppressive effects of BMPs on GnRH expression were reversed in the presence of kisspeptin. It was also revealed that BMP-4-activated Smad1/5/8 phosphorylation and Id-1 expression were suppressed by kisspeptin-induced increase of inhibitory Smad6/7. Smad6/7 expression was increased by kisspeptin, particularly in the presence of BMP-4. This new interaction between the BMP-Smad pathway and kisspeptin-GPR54 signaling may be involved in the control of GnRH-neuronal activity.
The neuronal mechanism through which estrogen regulates GnRH neurons is a key for elucidating reproductive control of the HPOaxis. In our study, kisspeptin stimulated MAPKsand AKT signaling, particularly in the presence of estrogen, in which ERK1/2 signaling was functionally involved in the kisspeptin-induced GnRH expression. It was also revealed that BMP-4 suppressed kisspeptin-induced GnRH expression by reducing ERK signaling activity. There have been several reports on signaling of kisspeptin for GnRH release. The pulsatile pattern of GnRH release, which results in the intermittent release of gonadotropins from the pituitary, is critical for maintenance of reproductive function. Ozcan et al. revealed the pattern of kisspeptin-induced intracellular signaling and the role of protein kinase C (PKC) in the intracellular signaling cascade by fluorescence calcium imaging using GT1-7 cells (Ozcan et al., 2011), and their results suggested the importance of intracellular calcium flux downstream from GPR54 through the PKC signaling pathway. Novaira et al. reported an interesting finding that the negative regulation of GnRH secretion by estrogen was antagonized by kisspeptin (Novaira et al., 2009). In our study, GnRHresponse to kisspeptin was weaker than the previous reports (Novaira et al., 2009; Tonsfeldt et al., 2011). This could be due to the culture condition with serum-free medium for 24 h in our experiments, although this condition was chosen to simplify the interaction betweenBMP and a genomic effect of ER. Much earlier time points and serum inclusion might be suitable to see higher responsiveness of GnRH. Further approaches to the intracellular signaling pathway revealed the involvement of ERK1/2 and phosphatidylinositol-3-kinase (PI3K)- AKT pathways in the effects of kisspeptin onGnRHregulation (Novaira et al., 2009). This work demonstrated that the kisspeptin-GPR54 system plays a role not only in stimulating GnRH secretion but also in positive regulation of GnRH mRNA levels via MAPK and AKT signaling in hypothalamic neurons. Taken together, both MAPK and AKT pathways seem to be substantially linked to the activation of GnRH regulation by kisspeptin and estrogen.
Collectively, the results indicate that the axis of kisspeptin-induced GnRH production was positively controlled by an interaction between ERα/β and GPR54 and, on the other hand, negatively regulated by BMP-4 signaling in GT1-7 cells (Fig. 5). Considering that GT1-7 cells exhibit daily changes in cellular peptide expression and GnRH secretion in response to kisspeptin and other hormones (Zhao and Kriegsfeld, 2009), the regulatory mechanism of GnRH modulated by intrinsic circadian cycles may also be taken account for approaching the role in the reproductive axis. However, we cannot fully translate this interaction shown in a GT1-7 cell model into the physiological situation in vivo because of the lack of ERα in native GnRH neurons (Herbison and Pape, 2001; Herbison, 2008). Given that kisspeptin neurons predominantly express ERα with a modest fraction expressing ERβ (Pinilla et al., 2012), it can be postulated that kisspeptin neurons are also involved in the regulatory mechanism of estrogen for GnRH secretion in the presence of BMP-4. Further studies on the molecular mechanism are needed to elucidate the detailed mechanism of hypothalamic release of GnRH and the in vivo role of the BMP system in the physiologic gonadotropin surge and onset of puberty. A mutual interaction of kisspeptin, estrogen and BMP-4 may be a key for fine-tuning of GnRH regulation under the dynamic influence of estrogen level.
Acknowledgments
We thank Steven A. Cardenas for excellent technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research, Kurozumi Medical Foundation and Foundation for Growth Science, and by Public Health Service Grants R01 HD37568 and U54 012303 to M.A.L. C.G.-K. was supported by Grant T32 HD 007203.
Abbreviations
- ActRII
activin type II receptor
- ALK
activin receptor-like kinase
- ARC
arcuate nucleus
- AVPV
anteroventral periventricular nucleus
- BMP
bone morphogenetic protein
- BMPRII
BMP type II receptor
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinase
- FSH
follicle-stimulating hormone
- GPR54
G-protein-coupled receptor 54
- GnIH
gonadotropin-inhibitory hormone
- GnRH
gonadotropin-releasing hormone
- JNK
c-Jun NH2-terminal kinase
- LH
luteinizing hormone
- MAPK
mitogen-activated protein kinase
References
- Buchanan CD, Mahesh VB, Brann DW. Estrogen-astrocyte-luteinizing hormone-releasing hormone signaling: a role for transforming growth factor-beta(1) Biol Reprod. 2000;62:1710–1721. doi: 10.1095/biolreprod62.6.1710. [DOI] [PubMed] [Google Scholar]
- Castellano JM, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci. 2003;23:11202–11213. doi: 10.1523/JNEUROSCI.23-35-11202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements MK, et al. FMRFamide-related neuropeptides are agonists of the orphan G-protein-coupled receptor GPR54. Biochem Biophys Res Commun. 2001;284:1189–1193. doi: 10.1006/bbrc.2001.5098. [DOI] [PubMed] [Google Scholar]
- de Roux N, et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes S, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Galbiati M, et al. Transforming growth factor-beta and astrocytic conditioned medium influence luteinizing hormone-releasing hormone gene expression in the hypothalamic cell line GT1. Endocrinology. 1996;137:5605–5609. doi: 10.1210/endo.137.12.8940390. [DOI] [PubMed] [Google Scholar]
- Garcia-Galiano D, Pinilla L, Tena-Sempere M. Sex steroids and the control of the Kiss1 system: developmental roles and major regulatory actions. J Neuroendocrinol. 2012;24:22–33. doi: 10.1111/j.1365-2826.2011.02230.x. [DOI] [PubMed] [Google Scholar]
- Gaytan F, et al. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab. 2009;296:E520–E531. doi: 10.1152/ajpendo.90895.2008. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology. 2012;153:5105–5118. doi: 10.1210/en.2012-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Hameed S, Jayasena CN, Dhillo WS. Kisspeptin and fertility. J Endocrinol. 2011;208:97–105. doi: 10.1677/JOE-10-0265. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- Horikoshi Y, et al. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88:914–919. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- Jacobi JS, et al. 17-Beta-estradiol directly regulates the expression of adrenergic receptors and kisspeptin/GPR54 system in GT1-7 GnRH neurons. Neuroendocrinology. 2007;86:260–269. doi: 10.1159/000107770. [DOI] [PubMed] [Google Scholar]
- Jordan J, et al. Bone morphogenetic proteins: neurotrophic roles for midbrain dopaminergic neurons and implications of astroglial cells. Eur J Neurosci. 1997;9:1699–1709. doi: 10.1111/j.1460-9568.1997.tb01527.x. [DOI] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Terasawa E. Rapid action of estradiol in primate GnRH neurons: the role of estrogen receptor alpha and estrogen receptor beta. Steroids. 2011;76:861–866. doi: 10.1016/j.steroids.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani M, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Liposits Z, et al. Morphological characterization of immortalized hypothalamic neurons synthesizing luteinizing hormone-releasing hormone. Endocrinology. 1991;129:1575–1583. doi: 10.1210/endo-129-3-1575. [DOI] [PubMed] [Google Scholar]
- Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- Ma YJ, et al. Transforming growth factor-alpha gene expression in the hypothalamus is developmentally regulated and linked to sexual maturation. Neuron. 1992;9:657–670. doi: 10.1016/0896-6273(92)90029-d. [DOI] [PubMed] [Google Scholar]
- Ma YJ, et al. Hypothalamic astrocytes respond to transforming growth factor-alpha with the secretion of neuroactive substances that stimulate the release of luteinizing hormone-releasing hormone. Endocrinology. 1997;138:19–25. doi: 10.1210/endo.138.1.4863. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, et al. Type 1 astrocytes influence luteinizing hormonereleasing hormone release from the hypothalamic cell line GT1-1: is transforming growth factor-beta the principle involved? Endocrinology. 1995;136:679–686. doi: 10.1210/endo.136.2.7835301. [DOI] [PubMed] [Google Scholar]
- Muir AI, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Novaira HJ, et al. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol Cell Endocrinol. 2009;311:126–134. doi: 10.1016/j.mce.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki T, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Das R, Placzek M. Temporal progression of hypothalamic patterning by a dual action of BMP. Development. 2008;135:3325–3331. doi: 10.1242/dev.027078. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, et al. Glia-to-neuron signaling and the neuroendocrine control of female puberty. Recent Prog Horm Res. 2000;55:197–223. discussion 223–224. [PubMed] [Google Scholar]
- Otani H, et al. Regulation of GNRH production by estrogen and bone morphogenetic proteins in GT1-7 hypothalamic cells. J Endocrinol. 2009;203:87–97. doi: 10.1677/JOE-09-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F. Multiple endocrine regulation by bone morphogenetic protein system. Endocr J. 2010;57:3–14. doi: 10.1507/endocrj.k09e-310. [DOI] [PubMed] [Google Scholar]
- Otsuka F. Multifunctional bone morphogenetic protein system in endocrinology. Acta Med Okayama. 2013;67:75–86. doi: 10.18926/AMO/49665. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Shimasaki S. A novel function of bone morphogenetic protein-15 in the pituitary: selective synthesis and secretion of FSH by gonadotropes. Endocrinology. 2002;143:4938–4941. doi: 10.1210/en.2002-220929. [DOI] [PubMed] [Google Scholar]
- Otsuka F, McTavish KJ, Shimasaki S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol Reprod Dev. 2011;78:9–21. doi: 10.1002/mrd.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F, et al. BMP action in the pituitary: its possible role in modulating somatostatin sensitivity in pituitary tumor cells. Mol Cell Endocrinol. 2012;349:105–110. doi: 10.1016/j.mce.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Ozcan M, et al. Kisspeptin-10 elicits triphasic cytosolic calcium responses in immortalized GT1-7 GnRH neurones. Neurosci Lett. 2011;492:55–58. doi: 10.1016/j.neulet.2011.01.054. [DOI] [PubMed] [Google Scholar]
- Peng CY, et al. BMP receptor 1A regulates development of hypothalamic circuits critical for feeding behavior. J Neurosci. 2012;32:17211–17224. doi: 10.1523/JNEUROSCI.2484-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- Pinilla L, et al. Delayed puberty in spontaneously hypertensive rats involves a primary ovarian failure independent of the hypothalamic KiSS-1/GPR54/GnRH system. Endocrinology. 2009;150:2889–2897. doi: 10.1210/en.2008-1381. [DOI] [PubMed] [Google Scholar]
- Pinilla L, et al. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92:1235–1316. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- Reiriz J, et al. Bone morphogenetic protein-2 promotes dissociated effects on the number and differentiation of cultured ventral mesencephalic dopaminergic neurons. J Neurobiol. 1999;38:161–170. [PubMed] [Google Scholar]
- Richard N, et al. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol. 2008;20:381–393. doi: 10.1111/j.1365-2826.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- Rometo AM, et al. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- Samanta J, et al. BMPR1a signaling determines numbers of oligodendrocytes and calbindin-expressing interneurons in the cortex. J Neurosci. 2007;27:7397–7407. doi: 10.1523/JNEUROSCI.1434-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully KM, Rosenfeld MG. Pituitary development: regulatory codes in mammalian organogenesis. Science. 2002;295:2231–2235. doi: 10.1126/science.1062736. [DOI] [PubMed] [Google Scholar]
- Seminara SB, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, et al. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- Smith JT, et al. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Takeda M, et al. Effects of peroxisome proliferator-activated receptor activation on gonadotropin transcription and cell mitosis induced by bone morphogenetic proteins in mouse gonadotrope LβT2 cells. J Endocrinol. 2007;194:87–99. doi: 10.1677/JOE-07-0138. [DOI] [PubMed] [Google Scholar]
- Takeda M, et al. Interaction between gonadotropin-releasing hormone and bone morphogenetic protein-6 and -7 signaling in LbetaT2 gonadotrope cells. Mol Cell Endocrinol. 2012;348:147–154. doi: 10.1016/j.mce.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena-Sempere M. KiSS-1 and reproduction: focus on its role in the metabolic regulation of fertility. Neuroendocrinology. 2006;83:275–281. doi: 10.1159/000095549. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Kenealy BP. Neuroestrogen, rapid action of estradiol, and GnRH neurons. Front Neuroendocrinol. 2012;33:364–375. doi: 10.1016/j.yfrne.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonsfeldt KJ, et al. Oestrogen induces rhythmic expression of the Kisspeptin-1 receptor GPR54 in hypothalamic gonadotrophin-releasing hormone-secreting GT1-7 cells. J Neuroendocrinol. 2011;23:823–830. doi: 10.1111/j.1365-2826.2011.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto N, et al. Effects of bone morphogenetic protein (BMP) on adrenocorticotropin production by pituitary corticotrope cells: involvement of up-regulation of BMP receptor signaling by somatostatin analogs. Endocrinology. 2010;151:1129–1141. doi: 10.1210/en.2009-1102. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, et al. Discovery and evolutionary history of gonadotrophininhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. J Neuroendocrinol. 2010;22:716–727. doi: 10.1111/j.1365-2826.2010.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa F, Briscoe J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle. 2007;6:2640–2649. doi: 10.4161/cc.6.21.4822. [DOI] [PubMed] [Google Scholar]
- Wetsel WC, et al. Metabolism of pro-luteinizing hormone-releasing hormone in immortalized hypothalamic neurons. Endocrinology. 1991;129:1584–1595. doi: 10.1210/endo-129-3-1584. [DOI] [PubMed] [Google Scholar]
- Zhao S, Kriegsfeld LJ. Daily changes in GT1-7 cell sensitivity to GnRH secretagogues that trigger ovulation. Neuroendocrinology. 2009;89:448–457. doi: 10.1159/000192370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwain IH, et al. A role for hypothalamic astrocytes in dehydroepiandrosterone and estradiol regulation of gonadotropin-releasing hormone (GnRH) release by GnRH neurons. Neuroendocrinology. 2002;75:375–383. doi: 10.1159/000059434. [DOI] [PubMed] [Google Scholar]


