Abstract
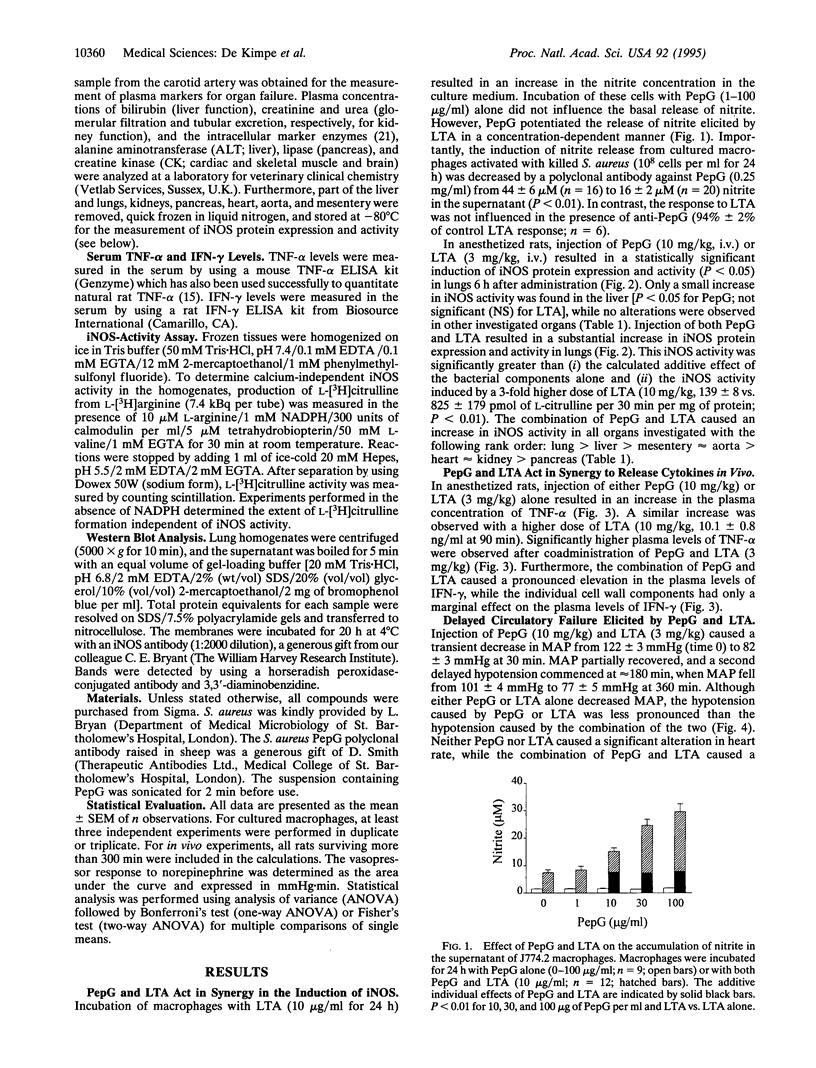
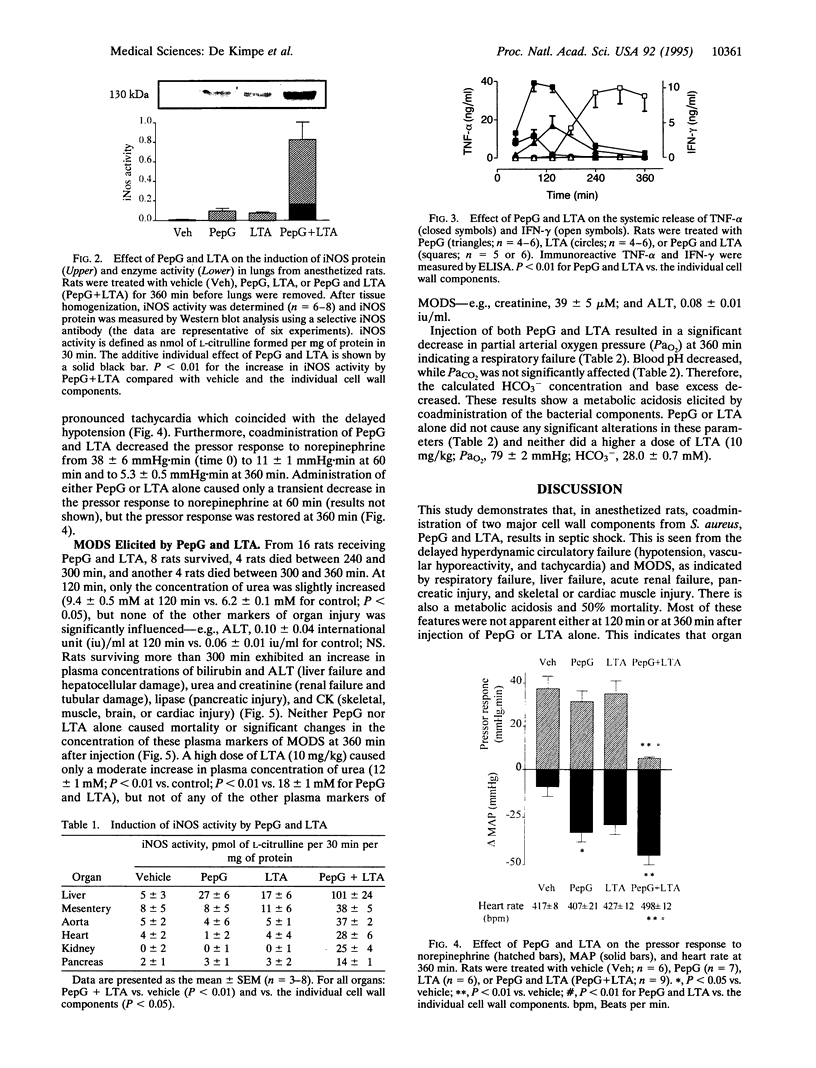
Although the incidence of Gram-positive sepsis has risen strongly, it is unclear how Gram-positive organisms (without endotoxin) initiate septic shock. We investigated whether two cell wall components from Staphylococcus aureus, peptidoglycan (PepG) and lipoteichoic acid (LTA), can induce the inflammatory response and multiple organ dysfunction syndrome (MODS) associated with septic shock caused by Gram-positive organisms. In cultured macrophages, LTA (10 micrograms/ml), but not PepG (100 micrograms/ml), induces the release of nitric oxide measured as nitrite. PepG, however, caused a 4-fold increase in the production of nitrite elicited by LTA. Furthermore, PepG antibodies inhibited the release of nitrite elicited by killed S. aureus. Administration of both PepG (10 mg/kg; i.v.) and LTA (3 mg/kg; i.v.) in anesthetized rats resulted in the release of tumor necrosis factor alpha and interferon gamma and MODS, as indicated by a decrease in arterial oxygen pressure (lung) and an increase in plasma concentrations of bilirubin and alanine aminotransferase (liver), creatinine and urea (kidney), lipase (pancreas), and creatine kinase (heart or skeletal muscle). There was also the expression of inducible nitric oxide synthase in these organs, circulatory failure, and 50% mortality. These effects were not observed after administration of PepG or LTA alone. Even a high dose of LTA (10 mg/kg) causes only circulatory failure but no MODS. Thus, our results demonstrate that the two bacterial wall components, PepG and LTA, work together to cause systemic inflammation and multiple systems failure associated with Gram-positive organisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohach G. A., Fast D. J., Nelson R. D., Schlievert P. M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17(4):251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- Bone R. C. Gram-positive organisms and sepsis. Arch Intern Med. 1994 Jan 10;154(1):26–34. [PubMed] [Google Scholar]
- Card G. L., Jasuja R. R., Gustafson G. L. Activation of arachidonic acid metabolism in mouse macrophages by bacterial amphiphiles. J Leukoc Biol. 1994 Dec;56(6):723–728. doi: 10.1002/jlb.56.6.723. [DOI] [PubMed] [Google Scholar]
- Casey L. C., Balk R. A., Bone R. C. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993 Oct 15;119(8):771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- Danner R. L., Elin R. J., Hosseini J. M., Wesley R. A., Reilly J. M., Parillo J. E. Endotoxemia in human septic shock. Chest. 1991 Jan;99(1):169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- De Kimpe S. J., Hunter M. L., Bryant C. E., Thiemermann C., Vane J. R. Delayed circulatory failure due to the induction of nitric oxide synthase by lipoteichoic acid from Staphylococcus aureus in anaesthetized rats. Br J Pharmacol. 1995 Mar;114(6):1317–1323. doi: 10.1111/j.1476-5381.1995.tb13349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in D-galactosamine-treated mice. Infect Immun. 1991 Jun;59(6):2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S. S., Jaffe E. A., Levi R., Kilbourn R. G. Cytokine-activated endothelial cells express an isotype of nitric oxide synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogs with a rank-order of potency characteristic of activated macrophages. Biochem Biophys Res Commun. 1991 Aug 15;178(3):823–829. doi: 10.1016/0006-291x(91)90965-a. [DOI] [PubMed] [Google Scholar]
- Heumann D., Barras C., Severin A., Glauser M. P., Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994 Jul;62(7):2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen J. P., Pyhälä L., Olander R. M., Merimskaya O., Kuzina T., Lysyuk O., Pronin A., Sanin A., Helander I. M., Sarvas M. Biological activities of lipoteichoic acid and peptidoglycan-teichoic acid of Bacillus subtilis 168 (Marburg). J Gen Microbiol. 1993 Nov;139(11):2659–2665. doi: 10.1099/00221287-139-11-2659. [DOI] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Kieft H., Hoepelman A. I., Zhou W., Rozenberg-Arska M., Struyvenberg A., Verhoef J. The sepsis syndrome in a Dutch university hospital. Clinical observations. Arch Intern Med. 1993 Oct 11;153(19):2241–2247. [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994 Mar 1;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman S. N., Wang J., Schwab J. H., Lemasters J. J. Comparison of peptidoglycan-polysaccharide and lipopolysaccharide stimulation of Kupffer cells to produce tumor necrosis factor and interleukin-1. Hepatology. 1994 Apr;19(4):1013–1022. [PubMed] [Google Scholar]
- Nakane A., Okamoto M., Asano M., Kohanawa M., Minagawa T. Endogenous gamma interferon, tumor necrosis factor, and interleukin-6 in Staphylococcus aureus infection in mice. Infect Immun. 1995 Apr;63(4):1165–1172. doi: 10.1128/iai.63.4.1165-1172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natanson C., Danner R. L., Elin R. J., Hosseini J. M., Peart K. W., Banks S. M., MacVittie T. J., Walker R. I., Parrillo J. E. Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureus challenges in a canine model of human septic shock. J Clin Invest. 1989 Jan;83(1):243–251. doi: 10.1172/JCI113866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa J. B., Udekwu A. O., Billiar T. R., Curran R. D., Cerra F. B., Simmons R. L., Peitzman A. B. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg. 1991 Nov;214(5):621–626. doi: 10.1097/00000658-199111000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeding P., Wergeland H., Endresen C., Natås O., Aasjord P. Antibodies to Staphylococcus aureus peptidoglycan and lipoteichoic acid in staphylococcal infections. Scand J Infect Dis Suppl. 1983;41:126–129. [PubMed] [Google Scholar]
- Parrillo J. E. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993 May 20;328(20):1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- Røder B. L., Eriksen N. H., Nielsen L. P., Slotsbjerg T., Rosdahl V. T., Espersen F. No difference in enterotoxin production among Staphylococcus aureus strains isolated from blood compared with strains isolated from healthy carriers. J Med Microbiol. 1995 Jan;42(1):43–47. doi: 10.1099/00222615-42-1-43. [DOI] [PubMed] [Google Scholar]
- Stadler J., Curran R. D., Ochoa J. B., Harbrecht B. G., Hoffman R. A., Simmons R. L., Billiar T. R. Effect of endogenous nitric oxide on mitochondrial respiration of rat hepatocytes in vitro and in vivo. Arch Surg. 1991 Feb;126(2):186–191. doi: 10.1001/archsurg.1991.01410260074010. [DOI] [PubMed] [Google Scholar]
- Suffredini A. F. Current prospects for the treatment of clinical sepsis. Crit Care Med. 1994 Jul;22(7):S12–S18. [PubMed] [Google Scholar]
- Suffredini A. F., Fromm R. E., Parker M. M., Brenner M., Kovacs J. A., Wesley R. A., Parrillo J. E. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989 Aug 3;321(5):280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- Sverko V., Marotti T., Gavella M., Lipovac V., Hrsak I. Side-effects of peptidoglycan monomer (PGM) treatment in mice. Immunopharmacology. 1994 Nov-Dec;28(3):193–199. doi: 10.1016/0162-3109(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Szabó C., Wu C. C., Gross S. S., Thiemermann C., Vane J. R. Interleukin-1 contributes to the induction of nitric oxide synthase by endotoxin in vivo. Eur J Pharmacol. 1993 Nov 30;250(1):157–160. doi: 10.1016/0014-2999(93)90634-t. [DOI] [PubMed] [Google Scholar]
- Takada H., Kawabata Y., Arakaki R., Kusumoto S., Fukase K., Suda Y., Yoshimura T., Kokeguchi S., Kato K., Komuro T. Molecular and structural requirements of a lipoteichoic acid from Enterococcus hirae ATCC 9790 for cytokine-inducing, antitumor, and antigenic activities. Infect Immun. 1995 Jan;63(1):57–65. doi: 10.1128/iai.63.1.57-65.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C. Epidemiology of staphylococcal infections--a USA perspective. J Chemother. 1994 Apr;6 (Suppl 2):61–65. [PubMed] [Google Scholar]
- Timmerman C. P., Mattsson E., Martinez-Martinez L., De Graaf L., Van Strijp J. A., Verbrugh H. A., Verhoef J., Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993 Oct;61(10):4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Verhoef J., Wilkinson B. J., Peterson P. K. Biology and clinical significance of peptidoglycan antibody response in staphylococcal infections. Scand J Infect Dis Suppl. 1983;41:117–125. [PubMed] [Google Scholar]
- Wakabayashi G., Gelfand J. A., Jung W. K., Connolly R. J., Burke J. F., Dinarello C. A. Staphylococcus epidermidis induces complement activation, tumor necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia. Comparison to Escherichia coli. J Clin Invest. 1991 Jun;87(6):1925–1935. doi: 10.1172/JCI115218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wizemann T. M., Gardner C. R., Laskin J. D., Quinones S., Durham S. K., Goller N. L., Ohnishi S. T., Laskin D. L. Production of nitric oxide and peroxynitrite in the lung during acute endotoxemia. J Leukoc Biol. 1994 Dec;56(6):759–768. doi: 10.1002/jlb.56.6.759. [DOI] [PubMed] [Google Scholar]




