Abstract
Aims—To determine whether expression of CD44 in neoplasia is associated with tumour grade, stage and prognosis.
Methods—The immunohistochemical expression of CD44 was evaluated using the mouse antihuman monoclonal antibody 3G12 which recognises regions shared by all CD44 isoforms to determine whether expression in formalin fixed, paraffin wax embedded tissue correlates with tumour grade, stage or survival in adenocarcinoma of the lung. Thirty one adenocarcinomas of the lung, 16 T2N0 and 15 T2N1, and their nodal metastases were studied.
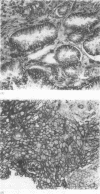
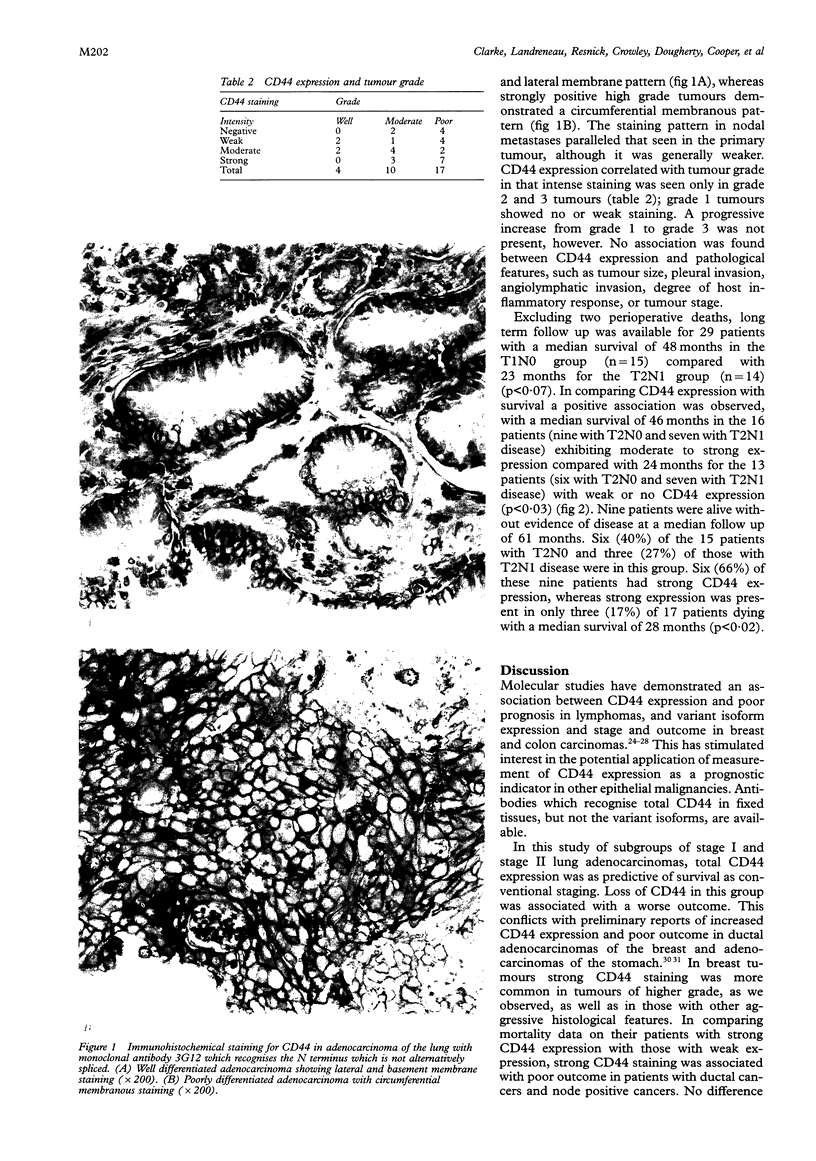
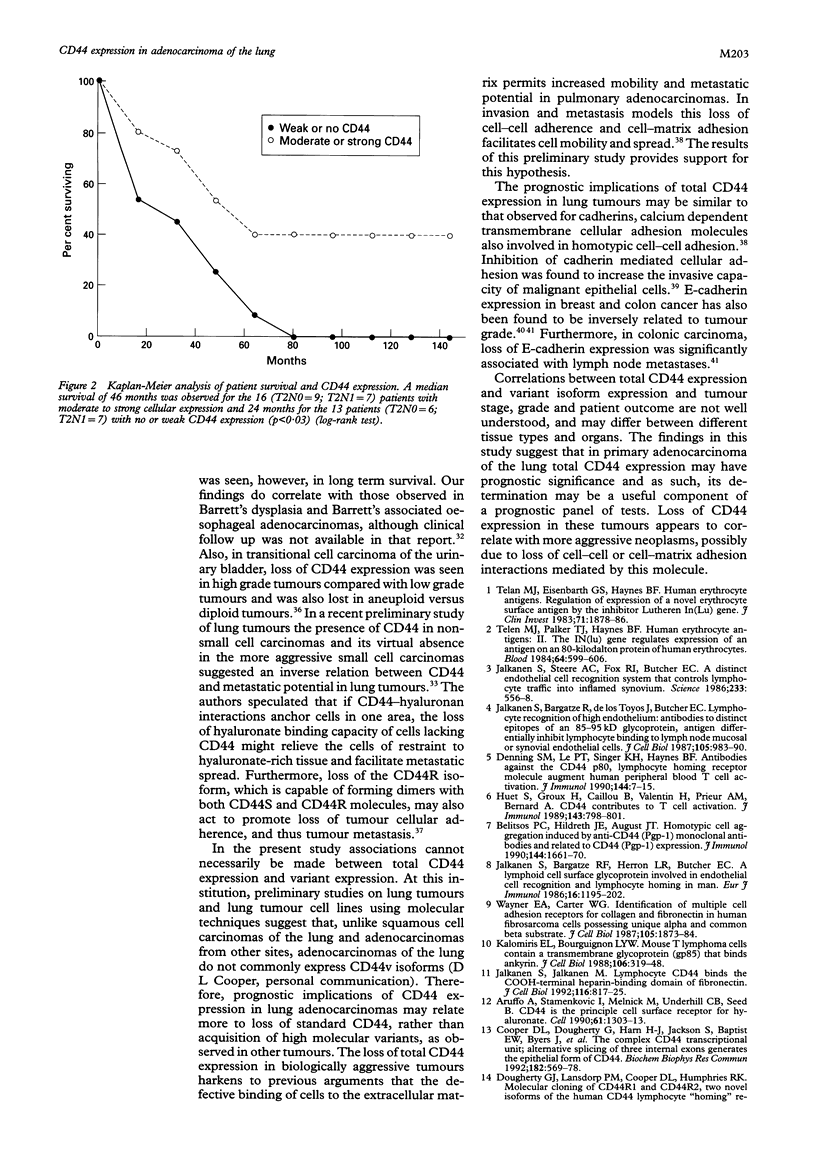
Results—Of the 31 tumours, 25 were positive for the CD44 antigen. CD44 expression correlated with tumour grade, in that intense staining was seen only in moderately and/or poorly differentiated tumours. CD44 did not correlate with nodal status, tumour size, pleural invasion, angiolymphatic invasion, or host inflammatory response, but did correlate with survival. A median survival of 46 months was observed in patients with moderate to strong CD44 expression compared with 24 months for those with no or weak expression. Nine patients were alive without evidence of disease at a median follow up of 61 months. Six (66%) of these nine patients had strong CD44 expression. This contrasts with strong expression in only three (17%) of the 17 patients dying with a median survival of 28 months.
Conclusion—In primary adenocarcinoma of the lung loss of CD44 expression is associated with less favorable outcome and may indicate a more aggressive neoplasm. CD44 may be a useful prognostic marker in lung carcinoma.
Keywords: CD44, adhesion molecule, lung cancer
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993 Jan;68(1):4–17. [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Behrens J., Mareel M. M., Van Roy F. M., Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989 Jun;108(6):2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belitsos P. C., Hildreth J. E., August J. T. Homotypic cell aggregation induced by anti-CD44(Pgp-1) monoclonal antibodies and related to CD44(Pgp-1) expression. J Immunol. 1990 Mar 1;144(5):1661–1670. [PubMed] [Google Scholar]
- Denning S. M., Le P. T., Singer K. H., Haynes B. F. Antibodies against the CD44 p80, lymphocyte homing receptor molecule augment human peripheral blood T cell activation. J Immunol. 1990 Jan 1;144(1):7–15. [PubMed] [Google Scholar]
- Dorudi S., Sheffield J. P., Poulsom R., Northover J. M., Hart I. R. E-cadherin expression in colorectal cancer. An immunocytochemical and in situ hybridization study. Am J Pathol. 1993 Apr;142(4):981–986. [PMC free article] [PubMed] [Google Scholar]
- Dougherty G. J., Cooper D. L., Memory J. F., Chiu R. K. Ligand binding specificity of alternatively spliced CD44 isoforms. Recognition and binding of hyaluronan by CD44R1. J Biol Chem. 1994 Mar 25;269(12):9074–9078. [PubMed] [Google Scholar]
- Dougherty G. J., Landorp P. M., Cooper D. L., Humphries R. K. Molecular cloning of CD44R1 and CD44R2, two novel isoforms of the human CD44 lymphocyte "homing" receptor expressed by hemopoietic cells. J Exp Med. 1991 Jul 1;174(1):1–5. doi: 10.1084/jem.174.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn L., Dougherty G., Finley G., Meisler A., Becich M., Cooper D. L. Alternative splicing of CD44 pre-mRNA in human colorectal tumors. Biochem Biophys Res Commun. 1994 Apr 29;200(2):1015–1022. doi: 10.1006/bbrc.1994.1551. [DOI] [PubMed] [Google Scholar]
- Gamallo C., Palacios J., Suarez A., Pizarro A., Navarro P., Quintanilla M., Cano A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol. 1993 Apr;142(4):987–993. [PMC free article] [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Heider K. H., Hofmann M., Hors E., van den Berg F., Ponta H., Herrlich P., Pals S. T. A human homologue of the rat metastasis-associated variant of CD44 is expressed in colorectal carcinomas and adenomatous polyps. J Cell Biol. 1993 Jan;120(1):227–233. doi: 10.1083/jcb.120.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Rudy W., Zöller M., Tölg C., Ponta H., Herrlich P., Günthert U. CD44 splice variants confer metastatic behavior in rats: homologous sequences are expressed in human tumor cell lines. Cancer Res. 1991 Oct 1;51(19):5292–5297. [PubMed] [Google Scholar]
- Huet S., Groux H., Caillou B., Valentin H., Prieur A. M., Bernard A. CD44 contributes to T cell activation. J Immunol. 1989 Aug 1;143(3):798–801. [PubMed] [Google Scholar]
- Jalkanen S. T., Bargatze R. F., Herron L. R., Butcher E. C. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986 Oct;16(10):1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992 Feb;116(3):817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Joensuu H., Söderström K. O., Klemi P. Lymphocyte homing and clinical behavior of non-Hodgkin's lymphoma. J Clin Invest. 1991 May;87(5):1835–1840. doi: 10.1172/JCI115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Steere A. C., Fox R. I., Butcher E. C. A distinct endothelial cell recognition system that controls lymphocyte traffic into inflamed synovium. Science. 1986 Aug 1;233(4763):556–558. doi: 10.1126/science.3726548. [DOI] [PubMed] [Google Scholar]
- Joensuu H., Klemi P. J., Toikkanen S., Jalkanen S. Glycoprotein CD44 expression and its association with survival in breast cancer. Am J Pathol. 1993 Sep;143(3):867–874. [PMC free article] [PubMed] [Google Scholar]
- Kalomiris E. L., Bourguignon L. Y. Mouse T lymphoma cells contain a transmembrane glycoprotein (GP85) that binds ankyrin. J Cell Biol. 1988 Feb;106(2):319–327. doi: 10.1083/jcb.106.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R., Terpe H. J., Stauder R., Marston W. L., Stark H., Günthert U. Expression and modulation of CD44 variant isoforms in humans. J Cell Biol. 1994 Jan;124(1-2):71–82. doi: 10.1083/jcb.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y., Tarin D. Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet. 1992 Oct 31;340(8827):1053–1058. doi: 10.1016/0140-6736(92)93077-z. [DOI] [PubMed] [Google Scholar]
- Penno M. B., August J. T., Baylin S. B., Mabry M., Linnoila R. I., Lee V. S., Croteau D., Yang X. L., Rosada C. Expression of CD44 in human lung tumors. Cancer Res. 1994 Mar 1;54(5):1381–1387. [PubMed] [Google Scholar]
- Picker L. J., Nakache M., Butcher E. C. Monoclonal antibodies to human lymphocyte homing receptors define a novel class of adhesion molecules on diverse cell types. J Cell Biol. 1989 Aug;109(2):927–937. doi: 10.1083/jcb.109.2.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy W., Hofmann M., Schwartz-Albiez R., Zöller M., Heider K. H., Ponta H., Herrlich P. The two major CD44 proteins expressed on a metastatic rat tumor cell line are derived from different splice variants: each one individually suffices to confer metastatic behavior. Cancer Res. 1993 Mar 15;53(6):1262–1268. [PubMed] [Google Scholar]
- Screaton G. R., Bell M. V., Jackson D. G., Cornelis F. B., Gerth U., Bell J. I. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E., Bishop J. M. Expression of CD44 is repressed in neuroblastoma cells. Mol Cell Biol. 1991 Nov;11(11):5446–5453. doi: 10.1128/mcb.11.11.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Guo Y. J., Stamenkovic I. Distinct effects of two CD44 isoforms on tumor growth in vivo. J Exp Med. 1991 Oct 1;174(4):859–866. doi: 10.1084/jem.174.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K. K., Ellis L. M., Saya H. Expression of CD44R1 adhesion molecule in colon carcinomas and metastases. Lancet. 1993 Mar 20;341(8847):725–726. doi: 10.1016/0140-6736(93)90490-8. [DOI] [PubMed] [Google Scholar]
- Telen M. J., Eisenbarth G. S., Haynes B. F. Human erythrocyte antigens. Regulation of expression of a novel erythrocyte surface antigen by the inhibitor Lutheran In(Lu) gene. J Clin Invest. 1983 Jun;71(6):1878–1886. doi: 10.1172/JCI110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telen M. J., Palker T. J., Haynes B. F. Human erythrocyte antigens: II. The In(Lu) gene regulates expression of an antigen on an 80-kilodalton protein of human erythrocytes. Blood. 1984 Sep;64(3):599–606. [PubMed] [Google Scholar]
- Thomas L., Byers H. R., Vink J., Stamenkovic I. CD44H regulates tumor cell migration on hyaluronate-coated substrate. J Cell Biol. 1992 Aug;118(4):971–977. doi: 10.1083/jcb.118.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousem S. A., Ray L., Paradis I. L., Dauber J. A., Griffith B. P. Potential role of dendritic cells in bronchiolitis obliterans in heart-lung transplantation. Ann Thorac Surg. 1990 Mar;49(3):424–428. doi: 10.1016/0003-4975(90)90248-5. [DOI] [PubMed] [Google Scholar]